Abiotic Stress in Crop Production
Abstract
1. Introduction
2. Abiotic Stresses and Crops
2.1. Heat Stress
2.1.1. Europe Is Experiencing the Hottest Summers in Recorded History
2.1.2. The Temperature Optimum for Most Crops Grown in Europe Is Exceeded for Weeks during the Season
2.1.3. Role of High Temperatures in Crop Production
2.1.4. High-Temperature Signaling in Model Plant and Crops
2.2. Drought Stress
2.2.1. High Temperatures without Precipitation Are Causing Drought across Europe
2.2.2. Impact of Drought on Different Crops at Different Developmental Stages
2.2.3. Drought in Plant Physiology
2.2.4. The Role of Root Growth in Drought Resistance
2.2.5. Roots and Hydrotropism
2.2.6. Long-Distance Signaling of Water Deficit
2.2.7. Other Mechanisms of Drought Resistance
2.3. Salt Stress
2.3.1. Plants Are Variable in Their Resistance to Salt
2.3.2. Key Mechanism for Salt-Stress Resistance
2.3.3. Salt-Stress Signaling and Role of ROS
2.4. Cold Stress
2.4.1. Crop Sensitivity to Low Temperatures
2.4.2. Low-Temperature Signaling
2.4.3. Role of Redox Changes in Cold Signaling
3. Similarities among Abiotic Stresses and Their Potential Crosstalk
3.1. Meta-Analysis of Stress-Responsive Genes
3.2. Subcellular Localization of Products of Genes Involved in Abiotic Stress Response
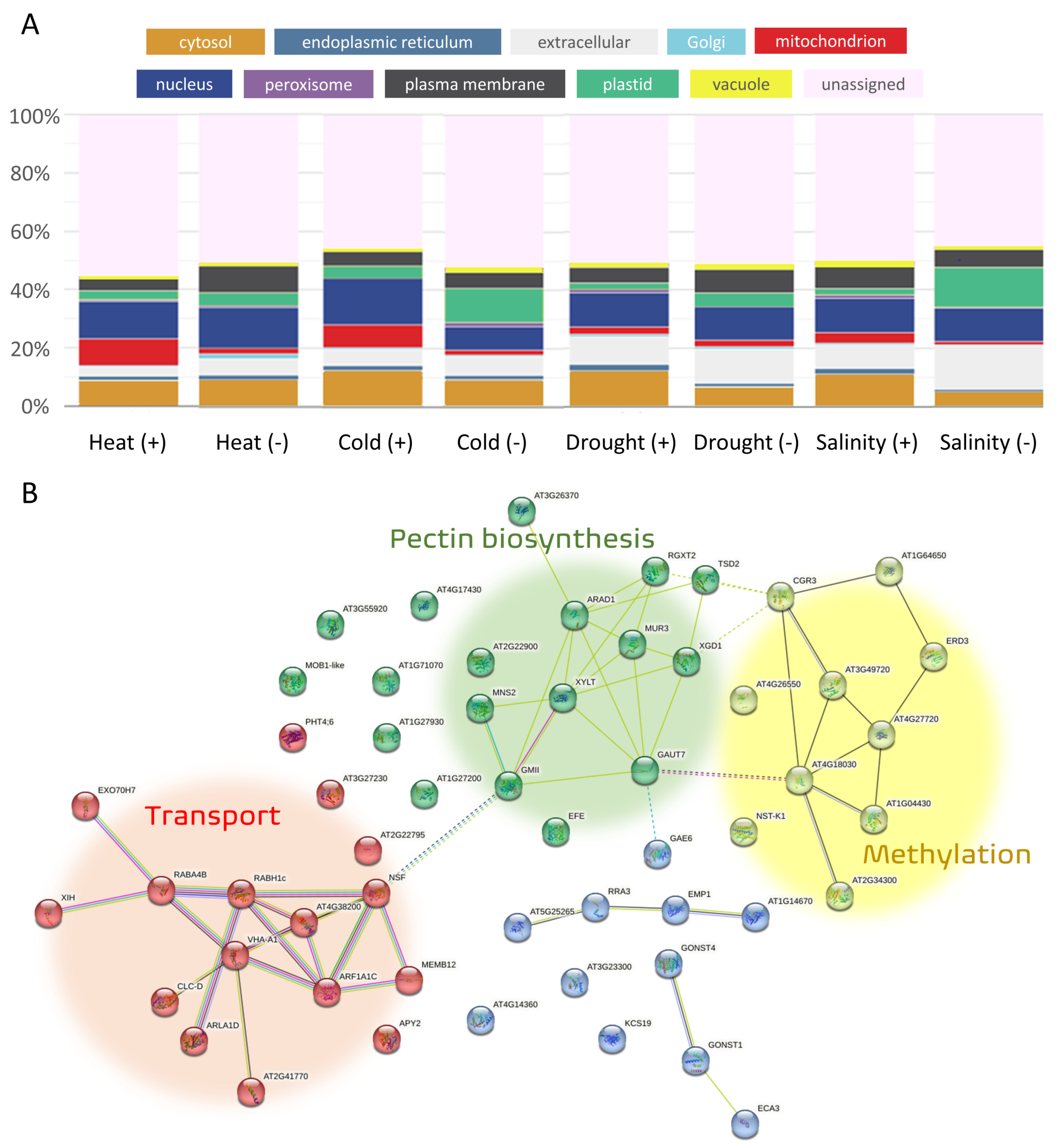
3.3. Functions of Common Genes Responding Identically to at Least Three Different Abiotic Stresses
3.4. Specific Universal Stress Responsive Genes Affected by Heat, Cold, Drought, and Salinity
4. Stress and Metabolites
4.1. Primary Metabolites
4.1.1. Amino Acids and Analogues
4.1.2. Organic Acids
4.1.3. Carbohydrates
4.1.4. Sugar Alcohols
4.2. Secondary Metabolites
4.2.1. Phenolics
4.2.2. Terpenes
4.2.3. Nitrogen/Sulfur-Containing Compounds
4.3. Phytohormones
| Hormone | Stress | Effect | Organism | Publication |
|---|---|---|---|---|
| ABA | heat | improved antioxidant system, lower MDA | wheat | [387] |
| heat | higher yield | rice | [388] | |
| water stress | stomata closure, microtubules | thale cress | [120] | |
| osmotic stress | stomata closure | barley | [122] | |
| salinity | higher yield and water-use efficiency (WUE) | tomato | [389] | |
| cold | activation of CBF regulon | grapevine | [390] | |
| cold | improved antioxidant system | tomato | [391] | |
| AUX | heat | increased yield | wheat | [392] |
| heat | improved embryo development | rapeseed | [393] | |
| drought | decreased ROS, lower electrolyte leakage (EL) | soya | [394] | |
| osmotic stress | lower EL and MDA, increased chlorophyll | tobacco | [395] | |
| salinity | root growth | thale cress | [396] | |
| salinity | root growth | maize | [397] | |
| cold | increased proline, saccharides | rapeseed | [398] | |
| BR | heat | improved growth, increased proline | wheat | [399] |
| heat | improved antioxidant system | tomato | [400] | |
| drought | improved antioxidant system, ABA content | tomato | [373] | |
| osmotic stress | improved antioxidant system, ABA content | grapevine | [401] | |
| osmotic stress | higher survival, improved root growth | cotton | [402] | |
| salinity | higher WUE, increased proline | bean | [403] | |
| cold stress | photoprotection | tomato | [404] | |
| cold stress | improved antioxidant system, lower EL and MDA | tomato | [405] | |
| CK | heat | higher yield | wheat | [406] |
| heat | higher survival | thale cress | [407] | |
| heat | improved photosynthesis, higher proline | rice | [408] | |
| heat/drought | impaired photosynthesis, lower relative water content (RWC) | tomato | [409] | |
| drought | decreased survival, lower RWC | thale cress | [381] | |
| drought | higher yield | rice | [383] | |
| drought | improved antioxidant system | tobacco | [382] | |
| salinity/drought | decreased survival | thale cress | [410] | |
| salinity | improved photosynthesis, lower MDA | tomato | [411] | |
| salinity | improved photosynthesis and growth, lower EL | rice | [412] | |
| cold stress | induction of cold-responsive genes | maize | [413] | |
| cold stress | increased and also decreased survival | thale cress | [414] | |
| ET | heat | lower membrane oxidation and EL, higher biomass | rice | [415] |
| heat | higher pollen quality | tomato | [416] | |
| salinity | increased ROS, inhibited root growth | rice | [417] | |
| salinity | increased ROS | tobacco | [418] | |
| salinity | increased sensitivity to stress | cucurbits | [419] | |
| salinity | improved Na/K homeostasis | thale cress | [420] | |
| drought | drought-induced senescence | maize | [421] | |
| drought | increased survival | rice | [422] | |
| drought | lower yield | barley | [423] | |
| drought | lower yield | maize | [424] | |
| cold stress | increased survival | grapevine | [378] | |
| cold stress | repressed CBF | thale cress | [377] | |
| GA | heat | positive role in thermomorphogenesis | thale cress | [376] |
| heat | higher EL, impaired photosynthesis | barley | [425] | |
| drought | decreased RWC | tomato | [426] | |
| drought | lower yield and pigments | cereals | [427] | |
| salinity | root differentiation/decreased tolerance | thale cress | [428] | |
| cold | increased EL, impaired antioxidant system | maize | [429] | |
| cold | decreased CBF expression | thale cress | [430] | |
| cold | decreased EL and MDA, mitigated stress | tomato | [431] | |
| JA | heat | improved photosynthesis | wheat | [432] |
| heat | increased survival, improved photosynthesis | thale cress | [433] | |
| drought | increased biomass, higher water content | tomato | [434] | |
| drought | higher antioxidant system, increased proline | sweet potato | [435] | |
| salinity | decreased Na+ concentration | barley | [436] | |
| salinity | increased proline, higher tolerance | sorghum | [437] | |
| cold | increased ABA, lower EL, improved photosynthesis | tomato | [371] | |
| cold | increased sugars, decreased browning index | peach fruit | [438] | |
| SA | heat | improved antioxidant system, lower MDA | wheat | [387] |
| heat | protected from pollen abortion, decreased ROS | rice | [439] | |
| drought | lower EL and MDA, higher RWC | barley | [440] | |
| drought | increased yield | tomato | [441] | |
| salinity | improved antioxidant system, lower Na+ level | potato | [442] | |
| salinity | increased yield | tomato | [443] | |
| cold | improved photosynthesis, lower EL and ROS | wheat | [444] | |
| cold | lower EL, improved antioxidant system | grapevine | [445] | |
| SL | heat/cold | higher ABA content, increased resistance | tomato | [372] |
| heat | higher germination, higher proline level, lower MDA | lupine | [446] | |
| drought | improved growth, higher chlorophyll, higher RWC | barley | [447] | |
| drought | improved photosynthesis, lower ROS | wheat | [448] | |
| salinity | improved antioxidant system and growth | tomato | [449] | |
| salinity | improved antioxidant system and photosynthesis | cucumber | [450] | |
| cold | lower ROS and MDA, increased proline | mung bean | [451] | |
| cold | improved antioxidant system and photosynthesis | rapeseed | [452] |
4.4. Other Growth Regulators
5. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, K.; Matsui, A.; Rasheed, S.; Seki, M. Recent Advances in the Characterization of Plant Transcriptomes in Response to Drought, Salinity, Heat, and Cold Stress. F1000Research 2019, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- United Nations. 2023. Available online: https://www.un.org (accessed on 29 November 2022).
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of Abiotic Stress on Plants: A Systems Biology Perspective. BMC Plant Biol. 2011, 11, 1–14. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Ge, Y.; Du, X.; Wang, H. Growing Prevalence of Heat over Cold Extremes with Overall Milder Extremes and Multiple Successive Events. Commun. Earth Environ. 2022, 3, 73. [Google Scholar] [CrossRef]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The Effects of Climate Extremes on Global Agricultural Yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef]
- FAO. 2023. Available online: https://www.fao.org/faostat/ (accessed on 13 January 2023).
- Turco, M.; Rosa-Cánovas, J.J.; Bedia, J.; Jerez, S.; Montávez, J.P.; Llasat, M.C.; Provenzale, A. Exacerbated Fires in Mediterranean Europe Due to Anthropogenic Warming Projected with Non-Stationary Climate-Fire Models. Nat. Commun. 2018, 9, 3821. [Google Scholar] [CrossRef]
- Ionita, M.; Nagavciuc, V.; Kumar, R.; Rakovec, O. On the Curious Case of the Recent Decade, Mid-Spring Precipitation Deficit in Central Europe. Clim. Atmos. Sci. 2020, 3, 49. [Google Scholar] [CrossRef]
- Liu, H.; Bruce, D.R.; Sissons, M.; Able, A.J.; Able, J.A. Genotype-dependent Changes in the Phenolic Content of Durum under Water-deficit Stress. Cereal Chem. 2018, 95, 59–78. [Google Scholar] [CrossRef]
- Ceglar, A.; Zampieri, M.; Toreti, A.; Dentener, F. Observed Northward Migration of Agro-Climate Zones in Europe Will Further Accelerate Under Climate Change. Earths Future 2019, 7, 1088–1101. [Google Scholar] [CrossRef]
- Zhao, J.; Bindi, M.; Eitzinger, J.; Ferrise, R.; Gaile, Z.; Gobin, A.; Holzkämper, A.; Kersebaum, K.-C.; Kozyra, J.; Kriaučiūnienė, Z.; et al. Priority for Climate Adaptation Measures in European Crop Production Systems. Eur. J. Agron. 2022, 138, 126516. [Google Scholar] [CrossRef]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jägermeyr, J. Severity of Drought and Heatwave Crop Losses Tripled over the Last Five Decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Deutscher Wetterdienst. 2022. Available online: https://www.dwd.de (accessed on 13 December 2022).
- Li, D.; Zaman, W.; Lu, J.; Niu, Q.; Zhang, X.; Ayaz, A.; Saqib, S.; Yang, B.; Zhang, J.; Zhao, H.; et al. Natural Lupeol Level Variation among Castor Accessions and the Upregulation of Lupeol Synthesis in Response to Light. Ind. Crops Prod. 2023, 192, 116090. [Google Scholar] [CrossRef]
- Wiik, L.; Ewaldz, T. Impact of Temperature and Precipitation on Yield and Plant Diseases of Winter Wheat in Southern Sweden 1983–2007. Crop Prot. 2009, 28, 952–962. [Google Scholar] [CrossRef]
- Song, Y.; Linderholm, H.W.; Wang, C.; Tian, J.; Huo, Z.; Gao, P.; Song, Y.; Guo, A. The Influence of Excess Precipitation on Winter Wheat under Climate Change in China from 1961 to 2017. Sci. Total Environ. 2019, 690, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Khan, S.; Hussain, N.; Hanjra, M.A.; Akbar, S. Characterizing Soil Salinity in Irrigated Agriculture Using a Remote Sensing Approach. Phys. Chem. Earth Parts A/B/C 2013, 55–57, 43–52. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, J.; Zhou, X.; Li, F. Effects of Shallow Groundwater Table and Fertilization Level on Soil Physico-Chemical Properties, Enzyme Activities, and Winter Wheat Yield. Agric. Water Manag. 2018, 208, 307–317. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, Y.; Meng, F.; Fan, C.; Zhang, M. Identification of Maize Leaf Diseases Using Improved Deep Convolutional Neural Networks. IEEE Access 2018, 6, 30370–30377. [Google Scholar] [CrossRef]
- Lv, X.; Chen, S.; Wang, Y. Advances in Understanding the Physiological and Molecular Responses of Sugar Beet to Salt Stress. Front. Plant Sci. 2019, 10, 1431. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.-Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield Under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- di Caterina, R.; Giuliani, M.M.; Rotunno, T.; de Caro, A.; Flagella, Z. Influence of Salt Stress on Seed Yield and Oil Quality of Two Sunflower Hybrids. Ann. Appl. Biol. 2007, 151, 145–154. [Google Scholar] [CrossRef]
- Keipp, K.; Hütsch, B.W.; Ehlers, K.; Schubert, S. Drought Stress in Sunflower Causes Inhibition of Seed Filling Due to Reduced Cell-extension Growth. J. Agron. Crop Sci. 2020, 206, 517–528. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought Stress Had a Predominant Effect over Heat Stress on Three Tomato Cultivars Subjected to Combined Stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Zhao, T.; Ottosen, C.-O.; Rosenqvist, E.; Wu, Z. Physiological Analysis and Transcriptome Sequencing Reveal the Effects of Combined Cold and Drought on Tomato Leaf. BMC Plant Biol. 2019, 19, 377. [Google Scholar] [CrossRef]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early Growth Stage Characterization and the Biochemical Responses for Salinity Stress in Tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef]
- Unterberger, C.; Brunner, L.; Nabernegg, S.; Steininger, K.W.; Steiner, A.K.; Stabentheiner, E.; Monschein, S.; Truhetz, H. Spring Frost Risk for Regional Apple Production under a Warmer Climate. PLoS ONE 2018, 13, e0200201. [Google Scholar] [CrossRef]
- Elferjani, R.; Soolanayakanahally, R. Canola Responses to Drought, Heat, and Combined Stress: Shared and Specific Effects on Carbon Assimilation, Seed Yield, and Oil Composition. Front. Plant Sci. 2018, 9, 1224. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. (Eds.) Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2019; In Press. [Google Scholar]
- NOAA. 2023. Available online: https://www.noaa.gov (accessed on 11 January 2023).
- COPERNICUS. 2023. Available online: https://www.copernicus.eu (accessed on 30 January 2023).
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; ul Haque, E.; et al. The Fingerprints of Climate Warming on Cereal Crops Phenology and Adaptation Options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef]
- Tashiro, T.; Wardlaw, I. The Response to High Temperature Shock and Humidity Changes Prior to and During the Early Stages of Grain Development in Wheat. Funct. Plant Biol. 1990, 17, 551. [Google Scholar] [CrossRef]
- Otero, E.A.; Miralles, D.J.; Benech-Arnold, R.L. Development of a Precise Thermal Time Model for Grain Filling in Barley: A Critical Assessment of Base Temperature Estimation Methods from Field-Collected Data. Field Crops Res. 2021, 260, 108003. [Google Scholar] [CrossRef]
- Arnold, C.Y. Predicting Stages of Sweet Corn (Zea mays L.) Development1. J. Am. Soc. Hortic. Sci. 1974, 99, 501–505. [Google Scholar] [CrossRef]
- Deligios, P.A.; Farci, R.; Sulas, L.; Hoogenboom, G.; Ledda, L. Predicting Growth and Yield of Winter Rapeseed in a Mediterranean Environment: Model Adaptation at a Field Scale. Field Crops Res. 2013, 144, 100–112. [Google Scholar] [CrossRef]
- Kenter, C.; Hoffmann, C.M.; Märländer, B. Effects of Weather Variables on Sugar Beet Yield Development (Beta vulgaris L.). Eur. J. Agron. 2006, 24, 62–69. [Google Scholar] [CrossRef]
- van Dam, J.; Kooman, P.L.; Struik, P.C. Effects of Temperature and Photoperiod on Early Growth and Final Number of Tubers in Potato (Solanum tuberosum L.). Potato Res. 1996, 39, 51–62. [Google Scholar] [CrossRef]
- Greer, D.H. Canopy Growth and Development Processes in Apples and Grapevines. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 313–369. [Google Scholar]
- Sato, S.; Peet, M.M.; Thomas, J.F. Physiological Factors Limit Fruit Set of Tomato (Lycopersicon Esculentum Mill.) under Chronic, Mild Heat Stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Chimenti, C.A.; Hall, A.J.; Sol López, M. Embryo-Growth Rate and Duration in Sunflower as Affected by Temperature. Field Crops Res. 2001, 69, 81–88. [Google Scholar] [CrossRef]
- Heide, O.M.; Rivero, R.; Sønsteby, A. Temperature Control of Shoot Growth and Floral Initiation in Apple (Malus × Domestica Borkh.). CABI Agric. Biosci. 2020, 1, 8. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the Heat Stress Response in Plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Khan, A.H.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature Stress in Crops: Male Sterility, Yield Loss and Potential Remedy Approaches. Plant Biotechnol. J. 2022, 21, 680–697. [Google Scholar] [CrossRef]
- Locato, V.; de Gara, L. Programmed Cell Death in Plants: An Overview. Methods Mol. Biol. 2018, 1743, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar Estimates of Temperature Impacts on Global Wheat Yield by Three Independent Methods. Nat. Clim. Chang. 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Morrison, M.J.; Stewart, D.W. Heat Stress during Flowering in Summer Brassica. Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Aiqing, S.; Somayanda, I.; Sebastian, S.V.; Singh, K.; Gill, K.; Prasad, P.V.V.; Jagadish, S.V.K. Heat Stress during Flowering Affects Time of Day of Flowering, Seed Set, and Grain Quality in Spring Wheat. Crop Sci. 2018, 58, 380–392. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Jagadish, S.V.K. Quantifying the Impact of Heat Stress on Pollen Germination, Seed Set, and Grain Filling in Spring Wheat. Crop Sci. 2019, 59, 684–696. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Wang, X.; Gao, J.; Luo, N.; Meng, Q.; Wang, P. Dissecting the Critical Stage in the Response of Maize Kernel Set to Individual and Combined Drought and Heat Stress around Flowering. Environ. Exp. Bot. 2020, 179, 104213. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Xu, C.; Wang, X.; Luo, N.; Wei, D.; Meng, Q.; Wang, P. Mitigating Heat Impacts in Maize (Zea mays L.) during the Reproductive Stage through Biochar Soil Amendment. Agric. Ecosyst. Environ. 2021, 311, 107321. [Google Scholar] [CrossRef]
- Lamers, J.; van der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Vu, L.D.; Gevaert, K.; de Smet, I. Feeling the Heat: Searching for Plant Thermosensors. Trends Plant Sci. 2019, 24, 210–219. [Google Scholar] [CrossRef]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.M.; Goloubinoff, P. The Heat Shock Response in Moss Plants Is Regulated by Specific Calcium-Permeable Channels in the Plasma Membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, X.; Li, B.; Zhao, L. Cyclic Nucleotide-Gated Ion Channel 6 Mediates Thermotolerance in Arabidopsis Seedlings by Regulating Nitric Oxide Production via Cytosolic Calcium Ions. BMC Plant Biol. 2019, 19, 368. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELS 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Harbaoui, M.; ben Saad, R.; ben Halima, N.; Choura, M.; Brini, F. Structural and Functional Characterisation of Two Novel Durum Wheat Annexin Genes in Response to Abiotic Stress. Funct. Plant Biol. 2018, 45, 542. [Google Scholar] [CrossRef] [PubMed]
- Berka, M.; Luklová, M.; Dufková, H.; Berková, V.; Novák, J.; Saiz-Fernández, I.; Rashotte, A.M.; Brzobohatý, B.; Černý, M. Barley Root Proteome and Metabolome in Response to Cytokinin and Abiotic Stimuli. Front. Plant Sci. 2020, 11, 590337. [Google Scholar] [CrossRef] [PubMed]
- Hyeon Jeong, J.; Joo Jung, W.; Weon Seo, Y. Genome-Wide Identification and Expression Analysis of the Annexin Gene Family in Rye (Secale cereale L.). Gene 2022, 838, 146704. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A Calcium-Binding Protein, Rice Annexin OsANN1, Enhances Heat Stress Tolerance by Modulating the Production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Lu, Y.; Ouyang, B.; Zhang, J.; Wang, T.; Lu, C.; Han, Q.; Zhao, S.; Ye, Z.; Li, H. Genomic Organization, Phylogenetic Comparison and Expression Profiles of Annexin Gene Family in Tomato (Solanum lycopersicum). Gene 2012, 499, 14–24. [Google Scholar] [CrossRef]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes Function as Thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M.; Kim, R.J.-A.; Moore, C.M.; Chen, M. Daytime Temperature Is Sensed by Phytochrome B in Arabidopsis through a Transcriptional Activator HEMERA. Nat. Commun. 2019, 10, 140. [Google Scholar] [CrossRef]
- Yang, J.; Qu, X.; Ji, L.; Li, G.; Wang, C.; Wang, C.; Zhang, Y.; Zheng, L.; Li, W.; Zheng, X. PIF4 Promotes Expression of HSFA2 to Enhance Basal Thermotolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 6017. [Google Scholar] [CrossRef]
- Hayes, S. PIF4 Plays a Conserved Role in Solanum lycopersicum. Plant Physiol. 2019, 181, 838–839. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kato, T.; Yamashino, T.; Murakami, M.; Mizuno, T. Characterization of a Set of Phytochrome-Interacting Factor-Like BHLH Proteins in Oryza sativa. Biosci. Biotechnol. Biochem. 2007, 71, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, X.; Qian, J.; Li, Q.; Tao, H.; Chen, J. The Phytochrome-Interacting Family of Transcription Factors in Maize (Zea mays L.): Identification, Evolution, and Expression Analysis. Acta Physiol. Plant. 2019, 41, 8. [Google Scholar] [CrossRef]
- Jung, J.H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A Prion-like Domain in ELF3 Functions as a Thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.; Deng, W.; Clausen, J.; Oliver, S.; Boden, S.; Hemming, M.; Trevaskis, B. Barley (Hordeum vulgare) Circadian Clock Genes Can Respond Rapidly to Temperature in an EARLY FLOWERING 3 -Dependent Manner. J. Exp. Bot. 2016, 67, 5517–5528. [Google Scholar] [CrossRef] [PubMed]
- Ochagavía, H.; Prieto, P.; Zikhali, M.; Griffiths, S.; Slafer, G.A. Earliness Per Se by Temperature Interaction on Wheat Development. Sci. Rep. 2019, 9, 2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Covington, M.F.; Fankhauser, C.; Chory, J.; Wagner, D.R. ELF3 Encodes a Circadian Clock-Regulated Nuclear Protein That Functions in an Arabidopsis PHYB Signal Transduction Pathway. Plant Cell 2001, 13, 1293. [Google Scholar] [CrossRef]
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular Mechanisms of the Plant Heat Stress Response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature Stress and Redox Homeostasis in Agricultural Crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Liu, Y.; Zhou, W.; Yan, B.; Yang, J.; Shen, Y. Overexpression of BcHsfA1 Transcription Factor from Brassica Campestris Improved Heat Tolerance of Transgenic Tobacco. PLoS ONE 2018, 13, e0207277. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Das, J.R.; Mathur, S. Exploring the Master Regulator Heat Stress Transcription Factor HSFA1a-Mediated Transcriptional Cascade of HSFs in the Heat Stress Response of Tomato. J. Plant Biochem. Biotechnol. 2021, 30, 878–888. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Shao, H.; Wang, G.; Zhang, Y.; Zhang, Y.; Zhao, L.; Guo, X.; Sheteiwy, M.S. ZmHsf05, a New Heat Shock Transcription Factor from Zea mays L. Improves Thermotolerance in Arabidopsis Thaliana and Rescues Thermotolerance Defects of the Athsfa2 Mutant. Plant Sci. 2019, 283, 375–384. [Google Scholar] [CrossRef]
- Bi, H.; Miao, J.; He, J.; Chen, Q.; Qian, J.; Li, H.; Xu, Y.; Ma, D.; Zhao, Y.; Tian, X.; et al. Characterization of the Wheat Heat Shock Factor TaHsfA2e-5D Conferring Heat and Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2784. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhou, Y.; Liu, Z.; Zhang, L.; Song, G.; Guo, Z.; Wang, W.; Qu, X.; Zhu, Y.; Yang, D. An Alternatively Spliced Heat Shock Transcription Factor, OsHSFA2dI, Functions in the Heat Stress-Induced Unfolded Protein Response in Rice. Plant Biol. 2014, 17, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sarkar, N.K.; Grover, A. Hsp70, sHsps and Ubiquitin Proteins Modulate HsfA6a-mediated Hsp101 Transcript Expression in Rice (Oryza sativa L.). Physiol. Plant. 2021, 173, 2055–2067. [Google Scholar] [CrossRef]
- Liu, J.; Sun, N.; Liu, M.; Liu, J.; Du, B.; Wang, X.; Qi, X. An Autoregulatory Loop Controlling Arabidopsis HsfA2 Expression: Role of Heat Shock-Induced Alternative Splicing. Plant Physiol. 2013, 162, 512–521. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Zhang, H.; Zhao, B.; Liu, Z.; Duan, S.; Meng, X.; Li, G.; Guo, X. Alternative Splicing of TaHsfA2-7 Is Involved in the Improvement of Thermotolerance in Wheat. Int. J. Mol. Sci. 2023, 24, 1014. [Google Scholar] [CrossRef]
- Kruszka, K.; Pacak, A.; Swida-Barteczka, A.; Nuc, P.; Alaba, S.; Wroblewska, Z.; Karlowski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Transcriptionally and Post-Transcriptionally Regulated MicroRNAs in Heat Stress Response in Barley. J. Exp. Bot. 2014, 65, 6123–6135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, G.; Fu, C.; Duan, S.; Hu, D.; Guo, X. Genome-Wide Identification, Transcriptome Analysis and Alternative Splicing Events of Hsf Family Genes in Maize. Sci. Rep. 2020, 10, 8073. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mesihovic, A.; Jiménez-Gómez, J.M.; Röth, S.; Gebhardt, P.; Bublak, D.; Bovy, A.; Scharf, K.; Schleiff, E.; Fragkostefanakis, S. Natural Variation in HsfA2 Pre-mRNA Splicing Is Associated with Changes in Thermotolerance during Tomato Domestication. New Phytol. 2020, 225, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. NPJ Clean. Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Samarah, N.H.; Alqudah, A.M.; Amayreh, J.A.; McAndrews, G.M. The Effect of Late-Terminal Drought Stress on Yield Components of Four Barley Cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Webber, H.; Ewert, F.; Olesen, J.E.; Müller, C.; Fronzek, S.; Ruane, A.C.; Bourgault, M.; Martre, P.; Ababaei, B.; Bindi, M.; et al. Diverging Importance of Drought Stress for Maize and Winter Wheat in Europe. Nat. Commun. 2018, 9, 4249. [Google Scholar] [CrossRef]
- Mickky, B.; Aldesuquy, H.; Elnajar, M. Effect of Drought on Yield of Ten Wheat Cultivars Linked with Their Flag Leaf Water Status, Fatty Acid Profile and Shoot Vigor at Heading. Physiol. Mol. Biol. Plants 2020, 26, 1111–1117. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought Stress in Sunflower: Physiological Effects and Its Management through Breeding and Agronomic Alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, T.J.; Al-Abdallat, A.; Thomas, W.T.B.; Bull, H.J.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley Yield Formation under Abiotic Stress Depends on the Interplay between Flowering Time Genes and Environmental Cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef]
- Dufková, H.; Berka, M.; Psota, V.; Brzobohatý, B.; Černý, M. Environmental Impacts on Barley Grain Composition and Longevity. J. Exp. Bot. 2022, 74, 1609–1628. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agron. Crop Sci. 2016, 203, 81–102. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; ISBN 978-1-60535-255-8. [Google Scholar]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.; Siddique, K.H.M.; Zhuang, W.; et al. Developing Drought-smart, Ready-to-grow Future Crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously Applied Polyamines Increase Drought Tolerance of Rice by Improving Leaf Water Status, Photosynthesis and Membrane Properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Li, H.-J.; Wang, Y.-F.; Zhao, C.-F.; Yang, M.; Wang, G.-X.; Zhang, R.-H. The Quantitative Proteomic Analysis Provides Insight into the Effects of Drought Stress in Maize. Photosynthetica 2021, 59, 1–11. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and Genomics of Root System Variation in Adaptation to Drought Stress in Cereal Crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Zörb, C.; Geilfus, C.-M. Drought and Crop Yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of Root System Architecture by DEEPER ROOTING 1 Increases Rice Yield under Drought Conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Feng, X.; Jia, L.; Cai, Y.; Guan, H.; Zheng, D.; Zhang, W.; Xiong, H.; Zhou, H.; Wen, Y.; Hu, Y.; et al. ABA-inducible DEEPER ROOTING Improves Adaptation of Maize to Water Deficiency. Plant Biotechnol. J. 2022, 20, 2077. [Google Scholar] [CrossRef]
- Sun, C.; Liang, W.; Yan, K.; Xu, D.; Qin, T.; Fiaz, S.; Kear, P.; Bi, Z.; Liu, Y.; Liu, Z.; et al. Expression of Potato StDRO1 in Arabidopsis Alters Root Architecture and Drought Tolerance. Front. Plant Sci. 2022, 13, 836063. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Rehman, O.U.; Muzammil, S.; Léon, J.; Naz, A.A.; Rasool, F.; Ali, G.M.; Zafar, Y.; Khan, M.R. Evolution of Deeper Rooting 1-like Homoeologs in Wheat Entails the C-Terminus Mutations as Well as Gain and Loss of Auxin Response Elements. PLoS ONE 2019, 14, e0214145. [Google Scholar] [CrossRef]
- Shkolnik, D.; Nuriel, R.; Bonza, M.C.; Costa, A.; Fromm, H. MIZ1 Regulates ECA1 to Generate a Slow, Long-Distance Phloem-Transmitted Ca 2+ Signal Essential for Root Water Tracking in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 8031–8036. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 Mediates Osmotic-Stress-Evoked Ca2+ Increases Vital for Osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Pei, S.; Liu, Y.; Li, W.; Krichilsky, B.; Dai, S.; Wang, Y.; Wang, X.; Johnson, D.M.; Crawford, B.M.; Swift, G.B.; et al. OSCA1 Is an Osmotic Specific Sensor: A Method to Distinguish Ca2+-mediated Osmotic and Ionic Perception. New Phytol. 2022, 235, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Miyazawa, Y. The Mechanosensitive Ca2+ Channel, OSCA1.1, Modulates Root Hydrotropic Bending in Arabidopsis Thaliana. Environ. Exp. Bot. 2022, 197, 104825. [Google Scholar] [CrossRef]
- She, K.; Pan, W.; Yan, Y.; Shi, T.; Chu, Y.; Cheng, Y.; Ma, B.; Song, W. Genome-Wide Identification, Evolution and Expressional Analysis of OSCA Gene Family in Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2022, 23, 13027. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, Y.; Cheng, H.; Hu, Z.; Pei, Z.-M.; Li, Q. Systematic Characterization of the OSCA Family Members in Soybean and Validation of Their Functions in Osmotic Stress. Int. J. Mol. Sci. 2022, 23, 10570. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, P.; Lu, X.; Wang, G.; Wang, Z.; Zhang, Q.; Zhang, X.; Wei, X.; Mei, F.; Wei, L.; et al. Systematic Analysis of the Maize OSCA Genes Revealing ZmOSCA Family Members Involved in Osmotic Stress and ZmOSCA2.4 Confers Enhanced Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 351. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, W.; Li, L.; Miao, R.; Dai, H.; Zhang, J.; Xu, W. Light-Dark Modulates Root Hydrotropism Associated with Gravitropism by Involving Amyloplast Response in Arabidopsis. Cell Rep. 2020, 32, 108198. [Google Scholar] [CrossRef]
- Novák, J.; Černý, M.; Pavlů, J.; Zemánková, J.; Skalák, J.; Plačková, L.; Brzobohatý, B. Roles of Proteome Dynamics and Cytokinin Signaling in Root to Hypocotyl Ratio Changes Induced by Shading Roots of Arabidopsis Seedlings. Plant Cell Physiol. 2015, 56, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, S.; Wang, X.; Dou, L.; Jia, M.; Mao, T.; Guo, Y.; Wang, X. The OPEN STOMATA1–SPIRAL1 Module Regulates Microtubule Stability during Abscisic Acid-Induced Stomatal Closure in Arabidopsis. Plant Cell 2023, 35, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Yuan, W.; Suo, J.; Shi, B.; Zhou, C.; Bai, B.; Bian, H.; Zhu, M.; Han, N. The Barley MiR393 Has Multiple Roles in Regulation of Seedling Growth, Stomatal Density, and Drought Stress Tolerance. Plant Physiol. Biochem. 2019, 142, 303–311. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The BHLH Family Member ZmPTF1 Regulates Drought Tolerance in Maize by Promoting Root Development and Abscisic Acid Synthesis. J. Exp. Bot. 2019, 70, 5471–5486. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen Peroxide Sensor HPCA1 Is an LRR Receptor Kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Chen, L.; Xiao, W.; Yu, J. ZmEREB46, a Maize Ortholog of Arabidopsis WAX INDUCER1/SHINE1, Is Involved in the Biosynthesis of Leaf Epicuticular Very-Long-Chain Waxes and Drought Tolerance. Plant Sci. 2022, 321, 111256. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between Leaf Morpho-Anatomy, Water Status and Cell Membrane Stability in Leaves of Wheat Seedlings Subjected to Severe Soil Drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Maiquetía, M.; Cáceres, A.; Herrera, A. Mycorrhization and Phosphorus Nutrition Affect Water Relations and CAM Induction by Drought in Seedlings of Clusia Minor. Ann. Bot. 2009, 103, 525–532. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant Abiotic Stress Response and Nutrient Use Efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Kotuby-Amacher, J.; Koenig, R.; Kitchen, B. Salinity and Plant Tolerance; Utah State University Extension: Logan, UT, USA, 2000; pp. 1–8. [Google Scholar]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Regni, L.; del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of Four Olive Cultivars During Salt Stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Peng, Y.; Zeng, W.; Zhao, X.; Ding, Y.; Zhuang, Z.; Gao, Q.; Ren, B. Comparative Proteomics of Salt-Tolerant and Salt-Sensitive Maize Inbred Lines to Reveal the Molecular Mechanism of Salt Tolerance. Int. J. Mol. Sci. 2019, 20, 4725. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I.; Stefanova, K.; Munns, R.; Barrett-Lennard, E.G. Salt Tolerance, Date of Flowering and Rain Affect the Productivity of Wheat and Barley on Rainfed Saline Land. Field Crops Res. 2016, 194, 31–42. [Google Scholar] [CrossRef]
- van Straten, G.; Bruning, B.; de Vos, A.C.; González, A.P.; Rozema, J.; van Bodegom, P.M. Estimating Cultivar-Specific Salt Tolerance Model Parameters from Multi-Annual Field Tests for Identification of Salt Tolerant Potato Cultivars. Agric. Water Manag. 2021, 252, 106902. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Meng, L.; Han, J.; Mao, P.; Tian, X.; Zheng, M.; Mur, L.A.J. SOS1 Is a Key Systemic Regulator of Salt Secretion and K+/Na+ Homeostasis in the Recretohalophyte Karelinia Caspia. Environ. Exp. Bot. 2020, 177, 104098. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Lv, W.; Yang, Y. Molecular Mechanisms of Plant Responses to Salt Stress. Front. Plant Sci. 2022, 13, 934877. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis Thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, M.; Zhang, J.; Duan, L.; Li, Z. SOS1 Gene Overexpression Increased Salt Tolerance in Transgenic Tobacco by Maintaining a Higher K+/Na+ Ratio. J. Plant Physiol. 2012, 169, 255–261. [Google Scholar] [CrossRef] [PubMed]
- el Mahi, H.; Pérez-Hormaeche, J.; de Luca, A.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A Critical Role of Sodium Flux via the Plasma Membrane Na+/H+ Exchanger SOS1 in the Salt Tolerance of Rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Wang, Y.; Liang, X.; Zhang, M.; Lu, M.; Guo, Y.; Qin, F.; Jiang, C. The Classical SOS Pathway Confers Natural Variation of Salt Tolerance in Maize. New Phytol. 2022, 236, 479–494. [Google Scholar] [CrossRef]
- Olías, R.; Eljakaoui, Z.; Li, J.U.N.; de Morales, P.A.Z.A.; Marín-Manzano, M.C.; Pardo, J.M.; Belver, A. The Plasma Membrane Na+ /H+ Antiporter SOS1 Is Essential for Salt Tolerance in Tomato and Affects the Partitioning of Na+ between Plant Organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef]
- Egea, I.; Pineda, B.; Ortíz-Atienza, A.; Plasencia, F.A.; Drevensek, S.; García-Sogo, B.; Yuste-Lisbona, F.J.; Barrero-Gil, J.; Atarés, A.; Flores, F.B.; et al. The SlCBL10 Calcineurin B-Like Protein Ensures Plant Growth under Salt Stress by Regulating Na+ and Ca2+ Homeostasis. Plant Physiol. 2018, 176, 1676–1693. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Sim, S.-C.; Kim, K.-N. Calcium Sensor SlCBL4 Associates with SlCIPK24 Protein Kinase and Mediates Salt Tolerance in Solanum lycopersicum. Plants 2021, 10, 2173. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant Cell-Surface GIPC Sphingolipids Sense Salt to Trigger Ca2+ Influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, M.; Sun, J. Overexpression of an Inositol Phosphorylceramide Glucuronosyltransferase Gene IbIPUT1 Inhibits Na+ Uptake in Sweet Potato Roots. Genes 2022, 13, 1140. [Google Scholar] [CrossRef]
- Chung, J.-S.; Zhu, J.-K.; Bressan, R.A.; Hasegawa, P.M.; Shi, H. Reactive Oxygen Species Mediate Na+-Induced SOS1 MRNA Stability in Arabidopsis. Plant J. 2007, 53, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Q.; Park, S.-C.; Wang, X.; Liu, Y.; Zhang, Y.; Tang, W.; Kou, M.; Ma, D. Overexpression of CuZnSOD and APX Enhance Salt Stress Tolerance in Sweet Potato. Plant Physiol. Biochem. 2016, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.-G.; Gilroy, S.; Morris, R.J. A ROS-Assisted Calcium Wave Dependent on the AtRBOHD NADPH Oxidase and TPC1 Cation Channel Propagates the Systemic Response to Salt Stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Toyota, M.; Kim, S.-H.; Hilleary, R.; Gilroy, S. Salt Stress-Induced Ca2+ Waves Are Associated with Rapid, Long-Distance Root-to-Shoot Signaling in Plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xing, L.; Xu, K.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Salt Stress-Induced H2O2 and Ca2+ Mediate K+/Na+ Homeostasis in Pyropia Haitanensis. J. Appl. Phycol. 2020, 32, 4199–4210. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and Challenges in Uncovering Cold Tolerance Regulatory Mechanisms in Plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Mahfoozi, S. Developmental Regulation of Low-Temperature Tolerance in Winter Wheat. Ann. Bot. 2001, 87, 751–757. [Google Scholar] [CrossRef]
- Li, X.; Pu, H.; Liu, F.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Winter Wheat Photosynthesis and Grain Yield Responses to Spring Freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold Stress Effects on Reproductive Development in Grain Crops: An Overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Borjas, A.H.; de Leon, T.B.; Subudhi, P.K. Genetic Analysis of Germinating Ability and Seedling Vigor under Cold Stress in US Weedy Rice. Euphytica 2016, 208, 251–264. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon Improves Chilling Tolerance During Early Growth of Maize by Effects on Micronutrient Homeostasis and Hormonal Balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Lee, K.; Jeong, H.-B.; Cho, M.-C.; Nam, C.-W.; Yang, E.-Y. Physiological Traits of Thirty-Five Tomato Accessions in Response to Low Temperature. Agriculture 2021, 11, 792. [Google Scholar] [CrossRef]
- Nayyar, H.; Bains, T.S.; Kumar, S.; Kaur, G. Chilling Effects during Seed Filling on Accumulation of Seed Reserves and Yield of Chickpea. J. Sci. Food Agric. 2005, 85, 1925–1930. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. The Effects of Cold Stress on the Phenolic Compounds and Antioxidant Capacity of Grapevine (Vitis vinifera L.) Leaves. J. Plant Physiol. 2015, 189, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, K.; Gu, D.; Zhang, S.; Wu, J. Quantifying the Effect of Low-Temperature Events on the Grain Quality Formation of Wheat. J. Cereal Sci. 2021, 100, 103257. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, R.; Xu, K.; Xiao, Y.; Zhang, S.; Tian, J.; Yang, X. Genome-Wide Association Study Reveals the Genetic Basis of Cold Tolerance in Wheat. Mol. Breed. 2020, 40, 36. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Butrón, A.; Rady, M.O.A.; Soengas, P.; Revilla, P. Identification of Quantitative Trait Loci Involved in the Response to Cold Stress in Maize (Zea mays L.). Mol. Breed. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop. Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Webster, T.M.; Grey, T.L.; Scully, B.T.; Johnson, W.C.; Davis, R.F.; Brenneman, T.B. Yield Potential of Spring-Harvested Sugar Beet (Beta Vulgaris) Depends on Autumn Planting Time. Ind. Crops Prod. 2016, 83, 55–60. [Google Scholar] [CrossRef]
- Loel, J.; Hoffmann, C.M. Importance of Growth Stage and Weather Conditions for the Winter Hardiness of Autumn Sown Sugar Beet. Field Crops Res. 2014, 162, 70–76. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse Weather Conditions for European Wheat Production Will Become More Frequent with Climate Change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium Signaling-Mediated Plant Response to Cold Stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef]
- Guo, X.; Liu, D.; Chong, K. Cold Signaling in Plants: Insights into Mechanisms and Regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Kakar, K.U.; Ullah, R.; Yu, S.; Zhang, J.; Shu, Q.-Y.; Ren, X. Genome-Wide Identification, Evolution and Expression Analysis of Cyclic Nucleotide-Gated Channels in Tobacco (Nicotiana tabacum L.). Genomics 2019, 111, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The Glutamate Receptors AtGLR1.2 and AtGLR1.3 Increase Cold Tolerance by Regulating Jasmonate Signaling in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef]
- Nawaz, Z.; Kakar, K.U.; Saand, M.A.; Shu, Q.-Y. Cyclic Nucleotide-Gated Ion Channel Gene Family in Rice, Identification, Characterization and Experimental Analysis of Expression Response to Plant Hormones, Biotic and Abiotic Stresses. BMC Genom. 2014, 15, 853. [Google Scholar] [CrossRef]
- Kakar, K.U.; Nawaz, Z.; Kakar, K.; Ali, E.; Almoneafy, A.A.; Ullah, R.; Ren, X.; Shu, Q.-Y. Comprehensive Genomic Analysis of the CNGC Gene Family in Brassica Oleracea: Novel Insights into Synteny, Structures, and Transcript Profiles. BMC Genom. 2017, 18, 869. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.H.; Maathuis, F.J.M.; Saidi, Y.; Goloubinoff, P. Plasma Membrane Cyclic Nucleotide Gated Calcium Channels Control Land Plant Thermal Sensing and Acquired Thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.; Zhao, C. The Transcription Factor ICE1 Functions in Cold Stress Response by Binding to the Promoters of CBF and COR Genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef]
- Dong, C.-H.; Agarwal, M.; Zhang, Y.; Xie, Q.; Zhu, J.-K. The Negative Regulator of Plant Cold Responses, HOS1, Is a RING E3 Ligase That Mediates the Ubiquitination and Degradation of ICE1. Proc. Natl. Acad. Sci. USA 2006, 103, 8281–8286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Y.; Liu, J.; Li, Z.; Fu, D.; Wu, S.; Li, M.; Yang, Z.; Shi, Y.; Lai, J.; et al. Natural Polymorphism of ZmICE1 Contributes to Amino Acid Metabolism That Impacts Cold Tolerance in Maize. Nat. Plants 2022, 8, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF Regulon by a Complex Low-temperature Regulatory Network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef]
- Park, S.; Gilmour, S.J.; Grumet, R.; Thomashow, M.F. CBF-Dependent and CBF-Independent Regulatory Pathways Contribute to the Differences in Freezing Tolerance and Cold-Regulated Gene Expression of Two Arabidopsis Ecotypes Locally Adapted to Sites in Sweden and Italy. PLoS ONE 2018, 13, e0207723. [Google Scholar] [CrossRef]
- Thomashow, M.F.; Gilmour, S.J.; Stockinger, E.J.; Jaglo-Ottosen, K.R.; Zarka, D.G. Role of the Arabidopsis CBF Transcriptional Activators in Cold Acclimation. Physiol. Plant 2001, 112, 171–175. [Google Scholar] [CrossRef]
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-Sensitive Tomato Has a Functional CBF Cold Response Pathway, but a CBF Regulon That Differs from That of Freezing-Tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Longin, C.F.H.; Hahn, V.; Tucker, M.R.; Leiser, W.L. Copy Number Variations of CBF Genes at the Fr-A2 Locus Are Essential Components of Winter Hardiness in Wheat. Plant J. 2017, 89, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Francia, E.; Barabaschi, D.; Tondelli, A.; Laidò, G.; Rizza, F.; Stanca, A.M.; Busconi, M.; Fogher, C.; Stockinger, E.J.; Pecchioni, N. Fine Mapping of a HvCBF Gene Cluster at the Frost Resistance Locus Fr-H2 in Barley. Theor. Appl. Genet. 2007, 115, 1083–1091. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Li, J.; Liu, Q.; Li, Y.-Q.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Cloning and Functional Analysis of a Novel DREB1/CBF Transcription Factor Involved in Cold-Responsive Gene Expression in Zea mays L. Plant Cell Physiol. 2004, 45, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shi, A.; Mou, B. Genome-Wide Identification and Expression Analysis of the CBF/DREB1 Gene Family in Lettuce. Sci. Rep. 2020, 10, 5733. [Google Scholar] [CrossRef]
- An, J.-P.; Yao, J.-F.; Wang, X.-N.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. MdHY5 Positively Regulates Cold Tolerance via CBF-Dependent and CBF-Independent Pathways in Apple. J. Plant Physiol. 2017, 218, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Soltész, A.; Smedley, M.; Vashegyi, I.; Galiba, G.; Harwood, W.; Vágújfalvi, A. Transgenic Barley Lines Prove the Involvement of TaCBF14 and TaCBF15 in the Cold Acclimation Process and in Frost Tolerance. J. Exp. Bot. 2013, 64, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Song, R.-F.; Qiu, Y.-M.; Zheng, S.-Q.; Li, T.-T.; Wu, Y.; Song, C.-P.; Lu, Y.-T.; Yuan, H.-M. Sulfenylation of ENOLASE2 Facilitates H2O2-Conferred Freezing Tolerance in Arabidopsis. Dev. Cell 2022, 57, 1883–1898.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Paeng, S.K.; Ji, M.G.; Kim, W.-Y.; Kim, M.G.; et al. Redox-Dependent Structural Switch and CBF Activation Confer Freezing Tolerance in Plants. Nat. Plants 2021, 7, 914–922. [Google Scholar] [CrossRef]
- Xu, A.; Wei, N.; Hu, H.; Zhou, S.; Huang, Y.; Kong, Q.; Bie, Z.; Nie, W.-F.; Cheng, F. Thioredoxin H2 Inhibits the MPKK5-MPK3 Cascade to Regulate the CBF–COR Signaling Pathway in Citrullus Lanatus Suffering Chilling Stress. Hortic. Res. 2023, 10, uhac256. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of Diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and Drought Stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Ayub, M.; Ashraf, M.Y.; Kausar, A.; Saleem, S.; Anwar, S.; Altay, V.; Ozturk, M. Growth and Physio-Biochemical Responses of Maize (Zea mays L.) to Drought and Heat Stresses. Plant Biosyst. 2021, 155, 535–542. [Google Scholar] [CrossRef]
- Mahalingam, R.; Bregitzer, P. Impact on Physiology and Malting Quality of Barley Exposed to Heat, Drought and Their Combination during Different Growth Stages under Controlled Environment. Physiol. Plant. 2019, 165, 277–289. [Google Scholar] [CrossRef]
- Shahriari, A.G.; Soltani, Z.; Tahmasebi, A.; Poczai, P. Integrative System Biology Analysis of Transcriptomic Responses to Drought Stress in Soybean (Glycine max L.). Genes 2022, 13, 1732. [Google Scholar] [CrossRef]
- Saidi, M.N.; Mahjoubi, H.; Yacoubi, I. Transcriptome Meta-Analysis of Abiotic Stresses-Responsive Genes and Identification of Candidate Transcription Factors for Broad Stress Tolerance in Wheat. Protoplasma 2022. [Google Scholar] [CrossRef] [PubMed]
- Soltanpour, S.; Tarinejad, A.; Hasanpur, K.; Majidi, M. A Meta-Analysis of Microarray Data Revealed Hub Genes and Transcription Factors Involved in Drought Stress Response in Rice (Oryza sativa L.). Funct. Plant Biol. 2022, 49, 898–916. [Google Scholar] [CrossRef]
- Papatheodorou, I.; Moreno, P.; Manning, J.; Fuentes, A.M.-P.; George, N.; Fexova, S.; Fonseca, N.A.; Füllgrabe, A.; Green, M.; Huang, N.; et al. Expression Atlas Update: From Tissues to Single Cells. Nucleic Acids Res. 2019, 48, D77–D83. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [PubMed]
- Sugio, A.; Dreos, R.; Aparicio, F.; Maule, A.J. The Cytosolic Protein Response as a Subcomponent of the Wider Heat Shock Response in Arabidopsis. Plant Cell 2009, 21, 642–654. [Google Scholar] [CrossRef]
- Bieniawska, Z.; Espinoza, C.; Schlereth, A.; Sulpice, R.; Hincha, D.K.; Hannah, M.A. Disruption of the Arabidopsis Circadian Clock Is Responsible for Extensive Variation in the Cold-Responsive Transcriptome. Plant Physiol. 2008, 147, 263–279. [Google Scholar] [CrossRef]
- Hannah, M.A.; Wiese, D.; Freund, S.; Fiehn, O.; Heyer, A.G.; Hincha, D.K. Natural Genetic Variation of Freezing Tolerance in Arabidopsis. Plant Physiol. 2006, 142, 98–112. [Google Scholar] [CrossRef]
- Schlaen, R.G.; Mancini, E.; Sanchez, S.E.; Perez-Santángelo, S.; Rugnone, M.L.; Simpson, C.G.; Brown, J.W.S.; Zhang, X.; Chernomoretz, A.; Yanovsky, M.J. The Spliceosome Assembly Factor GEMIN2 Attenuates the Effects of Temperature on Alternative Splicing and Circadian Rhythms. Proc. Natl. Acad. Sci. USA 2015, 112, 9382–9387. [Google Scholar] [CrossRef]
- Wong, M.M.; Bhaskara, G.B.; Wen, T.-N.; Lin, W.-D.; Nguyen, T.T.; Chong, G.L.; Verslues, P.E. Phosphoproteomics of Arabidopsis Highly ABA-Induced1 Identifies AT-Hook–Like10 Phosphorylation Required for Stress Growth Regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 2354–2363. [Google Scholar] [CrossRef]
- Pandey, N.; Ranjan, A.; Pant, P.; Tripathi, R.K.; Ateek, F.; Pandey, H.P.; Patre, U.V.; Sawant, S.V. CAMTA 1 Regulates Drought Responses in Arabidopsis Thaliana. BMC Genom. 2013, 14, 216. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Nguyen, T.T.; Verslues, P.E. Unique Drought Resistance Functions of the Highly ABA-Induced Clade A Protein Phosphatase 2Cs. Plant Physiol. 2012, 160, 379–395. [Google Scholar] [CrossRef]
- Allu, A.D.; Soja, A.M.; Wu, A.; Szymanski, J.; Balazadeh, S. Salt Stress and Senescence: Identification of Cross-Talk Regulatory Components. J. Exp. Bot. 2014, 65, 3993–4008. [Google Scholar] [CrossRef]
- Guan, Q.; Wu, J.; Yue, X.; Zhang, Y.; Zhu, J. A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in Arabidopsis. PLoS Genet. 2013, 9, e1003755. [Google Scholar] [CrossRef]
- Sun, L.; Dong, S.; Ge, Y.; Fonseca, J.P.; Robinson, Z.T.; Mysore, K.S.; Mehta, P. DiVenn: An Interactive and Integrated Web-Based Visualization Tool for Comparing Gene Lists. Front. Genet. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Sensing the Environment: Key Roles of Membrane-Localized Kinases in Plant Perception and Response to Abiotic Stress. J. Exp. Bot. 2013, 64, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Heazlewood, J.L.; Verboom, R.E.; Tonti-Filippini, J.; Small, I.; Millar, A.H. SUBA: The Arabidopsis Subcellular Database. Nucleic Acids Res. 2007, 35, D213–D218. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wu, H.-C.; Wang, Y.-D.; Liu, C.-H.; Lin, C.-C.; Luo, D.-L.; Jinn, T.-L. PECTIN METHYLESTERASE34 Contributes to Heat Tolerance through Its Role in Promoting Stomatal Movement. Plant Physiol. 2017, 174, 748–763. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of Cell-Wall Pectin Controls the Diurnal Flower-Opening Times in Rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Liebermeister, W.; Noor, E.; Flamholz, A.; Davidi, D.; Bernhardt, J.; Milo, R. Visual Account of Protein Investment in Cellular Functions. Proc. Natl. Acad. Sci. USA 2014, 111, 8488–8493. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum Wheat Seedling Responses to Simultaneous High Light and Salinity Involve a Fine Reconfiguration of Amino Acids and Carbohydrate Metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Derakhshani, Z.; Bhave, M.; Shah, R.M. Metabolic Contribution to Salinity Stress Response in Grains of Two Barley Cultivars with Contrasting Salt Tolerance. Environ. Exp. Bot. 2020, 179, 104229. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of Water Stress on Processing Tomatoes Yield, Quality and Water Use Efficiency with Plastic Mulched Drip Irrigation in Sandy Soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Afrin, T.; Seok, M.; Terry, B.C.; Pajerowska-Mukhtar, K.M. Probing Natural Variation of IRE1 Expression and Endoplasmic Reticulum Stress Responses in Arabidopsis Accessions. Sci. Rep. 2020, 10, 19154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Zhu, D.; Liu, N.; Yan, Y. Endoplasmic Reticulum Subproteome Analysis Reveals Underlying Defense Mechanisms of Wheat Seedling Leaves under Salt Stress. Int. J. Mol. Sci. 2021, 22, 4840. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaitheri Kandoth, P. Tomato BZIP60 MRNA Undergoes Splicing in Endoplasmic Reticulum Stress and in Response to Environmental Stresses. Plant Physiol. Biochem. 2021, 160, 397–403. [Google Scholar] [CrossRef]
- Xiang, Y.; Sun, X.; Gao, S.; Qin, F.; Dai, M. Deletion of an Endoplasmic Reticulum Stress Response Element in a ZmPP2C-A Gene Facilitates Drought Tolerance of Maize Seedlings. Mol. Plant 2017, 10, 456–469. [Google Scholar] [CrossRef]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and Proteomics Reveal Drought-Stress Responses of Leaf Tissues from Spring-Wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef]
- Casartelli, A.; Melino, V.J.; Baumann, U.; Riboni, M.; Suchecki, R.; Jayasinghe, N.S.; Mendis, H.; Watanabe, M.; Erban, A.; Zuther, E.; et al. Opposite Fates of the Purine Metabolite Allantoin under Water and Nitrogen Limitations in Bread Wheat. Plant Mol. Biol. 2019, 99, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Yang, Y.; Liu, S.; Zenda, T.; Liu, X.; Wang, Y.; Li, J.; Duan, H. Comparative Proteomics Analysis of Two Maize Hybrids Revealed Drought-Stress Tolerance Mechanisms. Biotechnol. Biotechnol. Equip. 2020, 34, 763–780. [Google Scholar] [CrossRef]
- Benhassaine-Kesri, G.; Aid, F.; Demandre, C.; Kader, J.-C.; Mazliak, P. Drought Stress Affects Chloroplast Lipid Metabolism in Rape (Brassica napus) Leaves. Physiol. Plant. 2002, 115, 221–227. [Google Scholar] [CrossRef]
- Gu, Y.; He, L.; Zhao, C.; Wang, F.; Yan, B.; Gao, Y.; Li, Z.; Yang, K.; Xu, J. Biochemical and Transcriptional Regulation of Membrane Lipid Metabolism in Maize Leaves under Low Temperature. Front. Plant Sci. 2017, 8, 2053. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.V.; Welti, R. Wheat Leaf Lipids during Heat Stress: I. High Day and Night Temperatures Result in Major Lipid Alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Spicher, L.; Glauser, G.; Kessler, F. Lipid Antioxidant and Galactolipid Remodeling under Temperature Stress in Tomato Plants. Front. Plant Sci. 2016, 7, 167. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, S.; Tao, W.; Zhang, X.; Liu, J.; Sun, J.; Zhang, H.; Pu, L.; Huang, R.; Chen, T. INDETERMINATE SPIKELET1 Recruits Histone Deacetylase and a Transcriptional Repression Complex to Regulate Rice Salt Tolerance. Plant Physiol. 2018, 178, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, N.; Li, X.; Xie, Y.; Xiang, D.; Fu, J.; Shen, J.; Yang, J.; Tu, H.; Li, X.; et al. Reversible Histone H2B Monoubiquitination Fine-Tunes Abscisic Acid Signaling and Drought Response in Rice. Mol. Plant 2019, 12, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; von Koskull-Doring, P.; Bharti, S.; Kumar, P.; Tintschl-Korbitzer, A.; Treuter, E.; Nover, L. Tomato Heat Stress Transcription Factor HsfB1 Represents a Novel Type of General Transcription Coactivator with a Histone-Like Motif Interacting with the Plant CREB Binding Protein Ortholog HAC1[W]. Plant Cell 2004, 16, 1521–1535. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Mallikarjuna, G.; Mallikarjuna, K.; Reddy, M.K.; Kaul, T. Expression of OsDREB2A Transcription Factor Confers Enhanced Dehydration and Salt Stress Tolerance in Rice (Oryza sativa L.). Biotechnol. Lett. 2011, 33, 1689–1697. [Google Scholar] [CrossRef]
- Filyushin, M.A.; Kochieva, E.Z.; Shchennikova, A.V. ZmDREB2.9 Gene in Maize (Zea mays L.): Genome-Wide Identification, Characterization, Expression, and Stress Response. Plants 2022, 11, 3060. [Google Scholar] [CrossRef]
- Pagliarini, R.F.; Marinho, J.P.; Molinari, M.D.C.; Marcolino-Gomes, J.; Caranhoto, A.L.H.; Marin, S.R.R.; Oliveira, M.C.N.; Foloni, J.S.S.; Melo, C.L.P.; Kidokoro, S.; et al. Overexpression of Full-Length and Partial DREB2A Enhances Soybean Drought Tolerance. Agron. Sci. Biotechnol. 2021, 8, 1–21. [Google Scholar] [CrossRef]
- Maki, H.; Sakaoka, S.; Itaya, T.; Suzuki, T.; Mabuchi, K.; Amabe, T.; Suzuki, N.; Higashiyama, T.; Tada, Y.; Nakagawa, T.; et al. ANAC032 Regulates Root Growth through the MYB30 Gene Regulatory Network. Sci. Rep. 2019, 9, 11358. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; El-Kereamy, A.; Kim, S.-H.; Nambara, E.; Rothstein, S.J. ANAC032 Positively Regulates Age-Dependent and Stress-Induced Senescence in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 2029–2046. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Altartouri, B.; Hegde, N.; Duggavathi, R.; Nazarian-Firouzabadi, F.; Kushalappa, A.C. TaNAC032 Transcription Factor Regulates Lignin-Biosynthetic Genes to Combat Fusarium Head Blight in Wheat. Plant Sci. 2021, 304, 110820. [Google Scholar] [CrossRef]
- Zhao, Y.; Miao, J.; He, J.; Tian, X.; Gao, K.; Ma, C.; Tian, X.; Men, W.; Li, H.; Bi, H.; et al. Wheat Heat Shock Factor TaHsfA2d Contributes to Plant Responses to Phosphate Deficiency. Plant Physiol. Biochem. 2022, 185, 178–187. [Google Scholar] [CrossRef]
- Mei, F.; Chen, B.; Li, F.; Zhang, Y.; Kang, Z.; Wang, X.; Mao, H. Overexpression of the Wheat NAC Transcription Factor TaSNAC4-3A Gene Confers Drought Tolerance in Transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 160, 37–50. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-Cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef]
- Capelle, V.; Remoué, C.; Moreau, L.; Reyss, A.; Mahé, A.; Massonneau, A.; Falque, M.; Charcosset, A.; Thévenot, C.; Rogowsky, P.; et al. QTLs and Candidate Genes for Desiccation and Abscisic Acid Content in Maize Kernels. BMC Plant Biol. 2010, 10, 2. [Google Scholar] [CrossRef]
- Asghar, M.A.; Du, J.; Jiang, H.; Li, Y.; Sun, X.; Shang, J.; Liu, J.; Liu, W.; Imran, S.; Iqbal, N.; et al. Shade Pretreatment Enhanced Drought Resistance of Soybean. Environ. Exp. Bot. 2020, 171, 103952. [Google Scholar] [CrossRef]
- Ayaz, M.; Ahmad, R.; Shahzad, M.; Khan, N.; Shah, M.M.; Khan, S.A. Drought Stress Stunt Tomato Plant Growth and Up-Regulate Expression of SlAREB, SlNCED3, and SlERF024 Genes. Sci. Hortic. 2015, 195, 48–55. [Google Scholar] [CrossRef]
- Truong, H.A.; Lee, S.; Trịnh, C.S.; Lee, W.J.; Chung, E.-H.; Hong, S.-W.; Lee, H. Overexpression of the HDA15 Gene Confers Resistance to Salt Stress by the Induction of NCED3, an ABA Biosynthesis Enzyme. Front. Plant Sci. 2021, 12, 640443. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zheng, X.; Liu, H.; Able, J.A.; Yang, H.; Zhao, H.; Zhang, M.; Qiao, Y.; Wang, Y.; Liu, M. Effects of Drought Stress on Pollen Sterility, Grain Yield, Abscisic Acid and Protective Enzymes in Two Winter Wheat Cultivars. Front. Plant Sci. 2017, 8, 1008. [Google Scholar] [CrossRef]
- Holsteens, K.; de Jaegere, I.; Wynants, A.; Prinsen, E.L.J.; van de Poel, B. Mild and Severe Salt Stress Responses Are Age-Dependently Regulated by Abscisic Acid in Tomato. Front. Plant Sci. 2022, 13, 269. [Google Scholar] [CrossRef]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated Analysis of the Effects of Cold and Dehydration on Rice Metabolites, Phytohormones, and Gene Transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, J.; Gao, Y.; Zhou, Y.; Chen, M.; Xu, Z.; Fang, Z.; Ma, Y. Genomic Analysis of Isopentenyltransferase Genes and Functional Characterization of TaIPT8 Indicates Positive Effects of Cytokinins on Drought Tolerance in Wheat. Crop J. 2023, 11, 46–56. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In Concert: Orchestrated Changes in Carbohydrate Homeostasis Are Critical for Plant Abiotic Stress Tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Brocard, L.; Immel, F.; Coulon, D.; Esnay, N.; Tuphile, K.; Pascal, S.; Claverol, S.; Fouillen, L.; Bessoule, J.-J.; Bréhélin, C. Proteomic Analysis of Lipid Droplets from Arabidopsis Aging Leaves Brings New Insight into Their Biogenesis and Functions. Front. Plant Sci. 2017, 8, 894. [Google Scholar] [CrossRef] [PubMed]
- Castelló, M.J.; Carrasco, J.L.; Navarrete-Gómez, M.; Daniel, J.; Granot, D.; Vera, P. A Plant Small Polypeptide Is a Novel Component of DNA-Binding Protein Phosphatase 1-Mediated Resistance to Plum Pox Virus in Arabidopsis. Plant Physiol. 2011, 157, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Z.; Zhang, D.; Huang, J.; Wu, C.; Yang, G.; Yan, K.; Zhang, S.; Zheng, C. CYSTM, a Novel Non-Secreted Cysteine-Rich Peptide Family, Involved in Environmental Stresses in Arabidopsis Thaliana. Plant Cell Physiol. 2017, 59, 423–438. [Google Scholar] [CrossRef]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A Novel Plant Defensin-like Gene of Winter Wheat Is Specifically Induced during Cold Acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2019, 225, 87–104. [Google Scholar] [CrossRef]
- Menna, A.; Nguyen, D.; Guttman, D.S.; Desveaux, D. Elevated Temperature Differentially Influences Effector-Triggered Immunity Outputs in Arabidopsis. Front. Plant Sci. 2015, 6, 995. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.M.; Oña, I.; Bai, J.; Garrett, K.A.; Mew, T.; Vera Cruz, C.M.; Leach, J.E. A Benefit of High Temperature: Increased Effectiveness of a Rice Bacterial Blight Disease Resistance Gene. New Phytol. 2009, 185, 568–576. [Google Scholar] [CrossRef]
- Negeri, A.; Wang, G.-F.; Benavente, L.; Kibiti, C.M.; Chaikam, V.; Johal, G.; Balint-Kurti, P. Characterization of Temperature and Light Effects on the Defense Response Phenotypes Associated with the Maize Rp1-D21autoactive Resistance Gene. BMC Plant Biol. 2013, 13, 106. [Google Scholar] [CrossRef]
- Byamukama, E.; Seifers, D.L.; Hein, G.L.; de Wolf, E.; Tisserat, N.A.; Langham, M.A.C.; Osborne, L.E.; Timmerman, A.; Wegulo, S.N. Occurrence and Distribution of Triticum Mosaic Virus in the Central Great Plains. Plant Dis. 2013, 97, 21–29. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, Z.; Zhang, Y.; Li, X.; Hong, Y.; Huang, L.; Liu, S.; Zhang, H.; Li, D.; Song, F. Tomato NAC Transcription Factor SlSRN1 Positively Regulates Defense Response against Biotic Stress but Negatively Regulates Abiotic Stress Response. PLoS ONE 2014, 9, e102067. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant Hormones Are Versatile Chemical Regulators of Plant Growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Liu, B.; Kong, L.; Zhang, Y.; Liao, Y. Gene and Metabolite Integration Analysis through Transcriptome and Metabolome Brings New Insight into Heat Stress Tolerance in Potato (Solanum tuberosum L.). Plants 2021, 10, 103. [Google Scholar] [CrossRef]
- Joshi, J.; Hasnain, G.; Logue, T.; Lynch, M.; Wu, S.; Guan, J.-C.; Alseekh, S.; Fernie, A.R.; Hanson, A.D.; McCarty, D.R. A Core Metabolome Response of Maize Leaves Subjected to Long-Duration Abiotic Stresses. Metabolites 2021, 11, 797. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Impa, S.M.; Krassovskaya, I.; Vennapusa, A.R.; Gill, K.S.; Obata, T.; Jagadish, S.V.K. Enhanced N-metabolites, ABA and IAA -conjugate in Anthers Instigate Heat Sensitivity in Spring Wheat. Physiol. Plant. 2020, 169, 501–514. [Google Scholar] [CrossRef]
- Lecourieux, D.; Kappel, C.; Claverol, S.; Pieri, P.; Feil, R.; Lunn, J.E.; Bonneu, M.; Wang, L.; Gomès, E.; Delrot, S.; et al. Proteomic and Metabolomic Profiling Underlines the Stage- and Time-dependent Effects of High Temperature on Grape Berry Metabolism. J. Integr. Plant Biol. 2020, 62, 1132–1158. [Google Scholar] [CrossRef]
- Qu, M.; Chen, G.; Bunce, J.A.; Zhu, X.; Sicher, R.C. Systematic Biology Analysis on Photosynthetic Carbon Metabolism of Maize Leaf Following Sudden Heat Shock under Elevated CO2. Sci. Rep. 2018, 8, 7849. [Google Scholar] [CrossRef]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.F.; Bovy, A.G. Untargeted Metabolomic Analysis of Tomato Pollen Development and Heat Stress Response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Boero, A.; Escalante, M.; Llanes, A.; Arbona, V.; Gómez-Cádenas, A.; Alemano, S. Comparative Hormonal and Metabolic Profile Analysis Based on Mass Spectrometry Provides Information on the Regulation of Water-Deficit Stress Response of Sunflower (Helianthus annuus L.) Inbred Lines with Different Water-Deficit Stress Sensitivity. Plant Physiol. Biochem. 2021, 168, 432–446. [Google Scholar] [CrossRef]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and Physiological Responses to Progressive Drought Stress in Bread Wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ni, S.-J.; Zhang, G.-P. Transcriptome and Metabolome Analysis Reveals Regulatory Networks and Key Genes Controlling Barley Malting Quality in Responses to Drought Stress. Plant Physiol. Biochem. 2020, 152, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marček, T.; Hamow, K.Á.; Végh, B.; Janda, T.; Darko, E. Metabolic Response to Drought in Six Winter Wheat Genotypes. PLoS ONE 2019, 14, e0212411. [Google Scholar] [CrossRef]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite Profiling of Barley Grains Subjected to Water Stress: To Explain the Genotypic Difference in Drought-Induced Impacts on Malting Quality. Front. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Sprenger, H.; Erban, A.; Seddig, S.; Rudack, K.; Thalhammer, A.; Le, M.Q.; Walther, D.; Zuther, E.; Köhl, K.I.; Kopka, J.; et al. Metabolite and Transcript Markers for the Prediction of Potato Drought Tolerance. Plant Biotechnol. J. 2018, 16, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Moschen, S.; di Rienzo, J.A.; Higgins, J.; Tohge, T.; Watanabe, M.; González, S.; Rivarola, M.; García-García, F.; Dopazo, J.; Hopp, H.E.; et al. Integration of Transcriptomic and Metabolic Data Reveals Hub Transcription Factors Involved in Drought Stress Response in Sunflower (Helianthus annuus L.). Plant Mol. Biol. 2017, 94, 549–564. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Li, C.; Shang, X.; Meng, Y.; et al. Combined Proteomic and Metabolomic Analysis of the Molecular Mechanism Underlying the Response to Salt Stress during Seed Germination in Barley. Int. J. Mol. Sci. 2022, 23, 10515. [Google Scholar] [CrossRef]
- Wan, H.; Qian, J.; Zhang, H.; Lu, H.; Li, O.; Li, R.; Yu, Y.; Wen, J.; Zhao, L.; Yi, B.; et al. Combined Transcriptomics and Metabolomics Analysis Reveals the Molecular Mechanism of Salt Tolerance of Huayouza 62, an Elite Cultivar in Rapeseed (Brassica napus L.). Int. J. Mol. Sci. 2022, 23, 1279. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and Metabolic Contribution to Salinity Stress Responses in Two Rapeseed Cultivars during the Early Seedling Stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Liu, D.; Zou, C.; Wu, P.; Wang, Z.; Wang, Y.; Li, C. Transcriptomic and Metabolomic Analyses Reveal Mechanisms of Adaptation to Salinity in Which Carbon and Nitrogen Metabolism Is Altered in Sugar Beet Roots. BMC Plant Biol. 2020, 20, 138. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, J.; Fu, L.; Wu, L.; Dai, F.; Jiang, L.; Wu, D.; Zhang, G. Ionomic, Metabolomic and Proteomic Analyses Reveal Molecular Mechanisms of Root Adaption to Salt Stress in Tibetan Wild Barley. Plant Physiol. Biochem. 2018, 123, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of Metabolic and Mineral Changes in Response to Salt Stress in Durum Wheat (Triticum Turgidum Ssp. Durum) Genotypes, Which Differ in Salinity Tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Persicke, M.; ElSayed, A.I.; Kalinowski, J.; Dietz, K.-J. Metabolite Profiling at the Cellular and Subcellular Level Reveals Metabolites Associated with Salinity Tolerance in Sugar Beet. J. Exp. Bot. 2017, 68, 5961–5976. [Google Scholar] [CrossRef]
- Cao, D.; Lutz, A.; Hill, C.B.; Callahan, D.L.; Roessner, U. A Quantitative Profiling Method of Phytohormones and Other Metabolites Applied to Barley Roots Subjected to Salinity Stress. Front. Plant Sci. 2017, 7, 2070. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, J.; Cao, J.; Li, X.; Li, S.; Liu, C.; Wang, L. Metabolic Insight into Cold Stress Response in Two Contrasting Maize Lines. Life 2022, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, L.; Zhou, J.; Lv, D.; Zhao, D.; Qin, S. Comparison of Transcriptome and Metabolome Analysis Revealed Differences in Cold Resistant Metabolic Pathways in Different Apple Cultivars under Low Temperature Stress. Hortic. Plant J. 2022. [Google Scholar] [CrossRef]
- Liu, X.; Wei, R.; Tian, M.; Liu, J.; Ruan, Y.; Sun, C.; Liu, C. Combined Transcriptome and Metabolome Profiling Provide Insights into Cold Responses in Rapeseed (Brassica napus L.) Genotypes with Contrasting Cold-Stress Sensitivity. Int. J. Mol. Sci. 2022, 23, 13546. [Google Scholar] [CrossRef]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Bénard, C.; et al. Maize Metabolome and Proteome Responses to Controlled Cold Stress Partly Mimic Early-sowing Effects in the Field and Differ from Those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Hussain, M.A.; Mehmood, S.S.; Zhang, X.; Cheng, Y.; Zou, X.; Lv, Y. Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Cold Tolerance in Rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Garzon, C.D.; Lequart, M.; Rautengarten, C.; Bassard, S.; Sellier-Richard, H.; Baldet, P.; Heazlewood, J.L.; Gibon, Y.; Domon, J.-M.; Giauffret, C.; et al. Regulation of Carbon Metabolism in Two Maize Sister Lines Contrasted for Chilling Tolerance. J. Exp. Bot. 2020, 71, 356–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Li, S.; Zhang, S.; Yang, X. Integrated Transcriptomics and Metabolomics Analyses Provide Insights into Cold Stress Response in Wheat. Crop J. 2019, 7, 857–866. [Google Scholar] [CrossRef]
- Kou, S.; Chen, L.; Tu, W.; Scossa, F.; Wang, Y.; Liu, J.; Fernie, A.R.; Song, B.; Xie, C. The Arginine Decarboxylase Gene ADC1, Associated to the Putrescine Pathway, Plays an Important Role in Potato Cold-Acclimated Freezing Tolerance as Revealed by Transcriptome and Metabolome Analyses. Plant J. 2018, 96, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Suravajhala, P.; Rathnagiri, P.; Sreenivasulu, N. Intriguing Role of Proline in Redox Potential Conferring High Temperature Stress Tolerance. Front. Plant Sci. 2022, 13, 867531. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, X.; Liu, J.; Hou, L.; Liu, H.; Zhao, X. OsProDH Negatively Regulates Thermotolerance in Rice by Modulating Proline Metabolism and Reactive Oxygen Species Scavenging. Rice 2020, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-Induced Synthesis of Proline Confers Tolerance to Water Deficit in Transgenic Wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline Accumulation in Plants. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Guan, C.; Cui, X.; Liu, H.; Li, X.; Li, M.; Zhang, Y. Proline Biosynthesis Enzyme Genes Confer Salt Tolerance to Switchgrass (Panicum virgatum L.) in Cooperation with Polyamines Metabolism. Front. Plant Sci. 2020, 11, 46. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of Proline Function in Higher Plants under Extreme Temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Guerzoni, J.T.S.; Belintani, N.G.; Moreira, R.M.P.; Hoshino, A.A.; Domingues, D.S.; Filho, J.C.B.; Vieira, L.G.E. Stress-Induced Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) Gene Confers Tolerance to Salt Stress in Transgenic Sugarcane. Acta Physiol. Plant. 2014, 36, 2309–2319. [Google Scholar] [CrossRef]
- Karabudak, T.; Bor, M.; Özdemir, F.; Türkan, İ. Glycine Betaine Protects Tomato (Solanum lycopersicum) Plants at Low Temperature by Inducing Fatty Acid Desaturase7 and Lipoxygenase Gene Expression. Mol. Biol. Rep. 2014, 41, 1401–1410. [Google Scholar] [CrossRef]
- Gupta, P.; Rai, R.; Vasudev, S.; Yadava, D.K.; Dash, P.K. Ex-Foliar Application of Glycine Betaine and Its Impact on Protein, Carbohydrates and Induction of ROS Scavenging System during Drought Stress in Flax (Linum Usitatissimum). J. Biotechnol. 2021, 337, 80–89. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhang, M.; Jin, Y.-K.; Jiang, Z.-Z.; Jiang, N.; Wang, Q.; Qu, J.; Guan, S.-Y.; Wang, P.-W. Drought Tolerance in High-Generation Transgenic Maize Inbred Lines Overexpressing the Betaine Aldehyde Dehydrogenase Gene. Cereal Res. Commun. 2021, 49, 183–192. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Murata, N.; Bansal, K.C. Transformation of Tomato with a Bacterial CodA Gene Enhances Tolerance to Salt and Water Stresses. J. Plant Physiol. 2011, 168, 1286–1294. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, X.Y.; Li, F.; Luo, Y.; Wang, W. Overaccumulation of Glycine Betaine Enhances Tolerance to Drought and Heat Stress in Wheat Leaves in the Protection of Photosynthesis. Photosynthetica 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liang, C.; Wang, G.-P.; Luo, Y.; Wang, W. The Protection of Wheat Plasma Membrane under Cold Stress by Glycine Betaine Overproduction. Biol. Plant. 2010, 54, 83–88. [Google Scholar] [CrossRef]
- Zhao, J.; Missihoun, T.D.; Bartels, D. The Role of Arabidopsis Aldehyde Dehydrogenase Genes in Response to High Temperature and Stress Combinations. J. Exp. Bot. 2017, 68, 4295–4308. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Trobacher, C.P.; Shelp, B.J. Arabidopsis Aldehyde Dehydrogenase 10 Family Members Confer Salt Tolerance through Putrescine-Derived 4-Aminobutyrate (GABA) Production. Sci. Rep. 2016, 6, 35115. [Google Scholar] [CrossRef] [PubMed]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against Abiotic Stress Tolerance in Legumes: A Review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic Acids: Versatile Stress-Response Roles in Plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Chevilly, S.; Dolz-Edo, L.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Yenush, L.; Mulet, J.M. Identification of Distinctive Physiological and Molecular Responses to Salt Stress among Tolerant and Sensitive Cultivars of Broccoli (Brassica Oleracea Var. Italica). BMC Plant Biol. 2021, 21, 488. [Google Scholar] [CrossRef]
- Kim, J.-M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S.; et al. Acetate-Mediated Novel Survival Strategy against Drought in Plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, Y. Effect of Various Salt–Alkaline Mixed Stress Conditions on Sunflower Seedlings and Analysis of Their Stress Factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Yu, J.; Du, H.; Xu, M.; Huang, B. Metabolic Responses to Heat Stress under Elevated Atmospheric CO2 Concentration in a Cool-Season Grass Species. J. Am. Soc. Hortic. Sci. 2012, 137, 221–228. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z.; Xiang, Z.; Yang, Z. Exogenous Application of Citric Acid Ameliorates the Adverse Effect of Heat Stress in Tall Fescue (Lolium arundinaceum). Front. Plant Sci. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, M.; Nashed, M. Effect of Foliar Application by Some Antioxidants on Growth and Productivity of Maize under Saline Soil Conditions. J. Plant Prod. 2019, 10, 93–99. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Zepeda, A.C.; Heuvelink, E.; Marcelis, L.F.M. Carbon Storage in Plants: A Buffer for Temporal Light and Temperature Fluctuations. In Silico Plants 2023, 5, diac020. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant Responses to Climate Change: Metabolic Changes under Combined Abiotic Stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, L.; Wu, D.; Liu, S.; Gong, X.; Cui, Z.; Cui, N.; Cao, H.; Rao, L.; Wang, C. Sucrose Transporter AtSUC9 Mediated by a Low Sucrose Level Is Involved in Arabidopsis Abiotic Stress Resistance by Regulating Sucrose Distribution and ABA Accumulation. Plant Cell Physiol. 2015, 56, 1574–1587. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Wang, H.; Zhang, P.; Shi, C. A Novel Sucrose Transporter Gene IbSUT4 Involves in Plant Growth and Response to Abiotic Stress through the ABF-Dependent ABA Signaling Pathway in Sweetpotato. BMC Plant Biol. 2020, 20, 157. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, W.; Gao, J.; Yang, F.; Zhuang, C. Overexpression of the Trehalose-6-Phosphate Phosphatase OsTPP3 Increases Drought Tolerance in Rice. Plant Biotechnol. Rep. 2019, 13, 285–292. [Google Scholar] [CrossRef]
- Lyu, J.I.; Park, J.H.; Kim, J.-K.; Bae, C.-H.; Jeong, W.-J.; Min, S.R.; Liu, J.R. Enhanced Tolerance to Heat Stress in Transgenic Tomato Seeds and Seedlings Overexpressing a Trehalose-6-Phosphate Synthase/Phosphatase Fusion Gene. Plant Biotechnol. Rep. 2018, 12, 399–408. [Google Scholar] [CrossRef]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; del Orozco, M.C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of Drought Tolerance and Grain Yield in Common Bean by Overexpressing Trehalose-6-Phosphate Synthase in Rhizobia. Mol. Plant-Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.-P.; Meghji, M.; Primavesi, L.F.; Paul, M.J.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of Trehalose-6-Phosphate Phosphatase in Maize Ears Improves Yield in Well-Watered and Drought Conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Imran, Q.M.; Yun, B.-W.; Lee, I.-J. Osmoprotective Functions Conferred to Soybean Plants via Inoculation with Sphingomonas Sp. LK11 and Exogenous Trehalose. Microbiol. Res. 2017, 205, 135–145. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Sagar, R.; Geng, Y.; Primavesi, L.F.; Patel, M.K.; Passarelli, M.K.; Gilmore, I.S.; Steven, R.T.; Bunch, J.; Paul, M.J.; et al. Chemical Intervention in Plant Sugar Signalling Increases Yield and Resilience. Nature 2016, 540, 574–578. [Google Scholar] [CrossRef]
- Hisano, H.; Kanazawa, A.; Kawakami, A.; Yoshida, M.; Shimamoto, Y.; Yamada, T. Transgenic Perennial Ryegrass Plants Expressing Wheat Fructosyltransferase Genes Accumulate Increased Amounts of Fructan and Acquire Increased Tolerance on a Cellular Level to Freezing. Plant Sci. 2004, 167, 861–868. [Google Scholar] [CrossRef]
- Tamura, K.; Sanada, Y.; Tase, K.; Kawakami, A.; Yoshida, M.; Yamada, T. Comparative Study of Transgenic Brachypodium Distachyon Expressing Sucrose:Fructan 6-Fructosyltransferases from Wheat and Timothy Grass with Different Enzymatic Properties. Planta 2014, 239, 783–792. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Kesten, C.; Wallmann, A.; Schneider, R.; McFarlane, H.E.; Diehl, A.; Khan, G.A.; van Rossum, B.-J.; Lampugnani, E.R.; Szymanski, W.G.; Cremer, N.; et al. The Companion of Cellulose Synthase 1 Confers Salt Tolerance through a Tau-like Mechanism in Plants. Nat. Commun. 2019, 10, 857. [Google Scholar] [CrossRef]
- Takahashi, D.; Johnson, K.L.; Hao, P.; Tuong, T.; Erban, A.; Sampathkumar, A.; Bacic, A.; Livingston, D.P.; Kopka, J.; Kuroha, T.; et al. Cell Wall Modification by the Xyloglucan Endotransglucosylase/Hydrolase XTH19 Influences Freezing Tolerance after Cold and Sub-zero Acclimation. Plant Cell Environ. 2021, 44, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Seo, Y.S.; Kim, S.J.; Kim, W.T.; Shin, J.S. Constitutive Expression of CaXTH3, a Hot Pepper Xyloglucan Endotransglucosylase/Hydrolase, Enhanced Tolerance to Salt and Drought Stresses without Phenotypic Defects in Tomato Plants (Solanum lycopersicum Cv. Dotaerang). Plant Cell Rep. 2011, 30, 867–877. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.E.; Tanimoto, E.; Inanaga, S. Effects of NaCl on Root Growth and Cell Wall Composition of Two Soya Bean Cultivars with Contrasting Salt Tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Iqbal, M.; Aslam Bharwana, S.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol Alleviates Chromium Toxicity in Wheat Plants in Relation to Growth, Yield, Stimulation of Anti-Oxidative Enzymes, Oxidative Stress and Cr Uptake in Sand and Soil Media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seckin, B.; Sekmen, A.H.; Türkan, İ. An Enhancing Effect of Exogenous Mannitol on the Antioxidant Enzyme Activities in Roots of Wheat Under Salt Stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of Mannitol-Accumulating Transgenic Wheat to Water Stress and Salinity. Plant Physiol. 2003, 131, 1748–1755. [Google Scholar] [CrossRef]
- Patel, K.G.; Mandaliya, V.B.; Mishra, G.P.; Dobaria, J.R.; Thankappan, R. Transgenic Peanut Overexpressing MtlD Gene Confers Enhanced Salinity Stress Tolerance via Mannitol Accumulation and Differential Antioxidative Responses. Acta Physiol. Plant. 2016, 38, 181. [Google Scholar] [CrossRef]
- Yan, L.; Zeng, L.; Raza, A.; Lv, Y.; Ding, X.; Cheng, Y.; Zou, X. Inositol Improves Cold Tolerance Through Inhibiting CBL1 and Increasing Ca2+ Influx in Rapeseed (Brassica napus L.). Front. Plant Sci. 2022, 13, 775692. [Google Scholar] [CrossRef]
- Nisa, Z.; Chen, C.; Yu, Y.; Chen, C.; Mallano, A.I.; Xiang-bo, D.; Xiao-li, S.; Yan-ming, Z. Constitutive Overexpression of Myo-Inositol-1-Phosphate Synthase Gene (GsMIPS2) from Glycine Soja Confers Enhanced Salt Tolerance at Various Growth Stages in Arabidopsis. J. Northeast Agric. Univ. (Engl. Ed.) 2016, 23, 28–44. [Google Scholar] [CrossRef]
- Jain, M.; Tiwary, S.; Gadre, R. Sorbitol-Induced Changes in Various Growth and Biochemici Parameters in Maize. Plant Soil Environ. 2010, 56, 263–267. [Google Scholar] [CrossRef]
- Theerakulp, P.; Gunnula, W. Exogenous Sorbitol and Trehalose Mitigated Salt Stress Damage in Salt-Sensitive but Not Salt-Tolerant Rice Seedlings. Asian J. Crop Sci. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128126899. [Google Scholar]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 517–532. [Google Scholar]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of Genotype and Temperature on Accumulation of Plant Secondary Metabolites in Canadian and Australian Wheat Grown under Controlled Environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, Flavonoids, and Antioxidant Activity Involved in Salt Tolerance in Wheat, Aegilops Cylindrica and Their Amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D.; Naceur, E.A. Differential Responses of Two Maize (Zea mays L.) Varieties to Salt Stress: Changes on Polyphenols Composition of Foliage and Oxidative Damages. Ind. Crops Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids Are Determinants of Freezing Tolerance and Cold Acclimation in Arabidopsis Thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. Role of Terpenes in Plant Defense to Biotic Stress. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 401–417. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Christensen, S.; Schmelz, E.A.; Huffaker, A.; Mcauslane, H.J.; Alborn, H.T.; Romero, M.; Allen, L.H.; Teal, P.E.A. Accumulation of Terpenoid Phytoalexins in Maize Roots Is Associated with Drought Tolerance. Plant Cell Environ. 2015, 38, 2195–2207. [Google Scholar] [CrossRef]
- Bertamini, M.; Grando, M.S.; Zocca, P.; Pedrotti, M.; Lorenzi, S.; Cappellin, L. Linking Monoterpenes and Abiotic Stress Resistance in Grapevines. BIO Web Conf. 2019, 13, 1003. [Google Scholar] [CrossRef]
- Tomescu, D.; Şumălan, R.; Copolovici, L.; Copolovici, D. The Influence of Soil Salinity on Volatile Organic Compounds Emission and Photosynthetic Parameters of Solanum lycopersicum L. Varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of Green Leaf Volatiles and Terpenoids from Solanum lycopersicum Are Quantitatively Related to the Severity of Cold and Heat Shock Treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Brunetti, C.; Loreto, F.; Centritto, M.; Ferrini, F.; Tattini, M. Isoprene Responses and Functions in Plants Challenged by Environmental Pressures Associated to Climate Change. Front. Plant Sci. 2017, 8, 1281. [Google Scholar] [CrossRef] [PubMed]
- Yogendra, K.N.; Sarkar, K.; Kage, U.; Kushalappa, A.C. Potato NAC43 and MYB8 Mediated Transcriptional Regulation of Secondary Cell Wall Biosynthesis to Contain Phytophthora Infestans Infection. Plant Mol. Biol. Rep. 2017, 35, 519–533. [Google Scholar] [CrossRef]
- Eom, S.; Baek, S.-A.; Kim, J.; Hyun, T. Transcriptome Analysis in Chinese Cabbage (Brassica Rapa Ssp. Pekinensis) Provides the Role of Glucosinolate Metabolism in Response to Drought Stress. Molecules 2018, 23, 1186. [Google Scholar] [CrossRef] [PubMed]
- Shunkao, S.; Theerakulpisut, P.; Wanichthanarak, K.; Pongdontri, P.; Thitisaksakul, M. Integrative Physiological and Metabolomics Study Reveals Adaptive Strategies of Wheat Seedlings to Salt and Heat Stress Combination. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Rau, M.R.; Fett-Neto, A.G. Oxidative Stress and Production of Bioactive Monoterpene Indole Alkaloids: Biotechnological Implications. Biotechnol. Lett. 2014, 36, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Jürgens, H.-U.; Ordon, F. Effects of Temperature on the Alkaloid Content of Seeds of Lupinus angustifolius Cultivars. J. Agron. Crop Sci. 2009, 195, 172–177. [Google Scholar] [CrossRef]
- el Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as Growth Regulators During Abiotic Stress Tolerance in Plants. Front. Agron. 2022, 4, 4. [Google Scholar] [CrossRef]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2022, 12, 60. [Google Scholar] [CrossRef]
- Chi, C.; Xu, X.; Wang, M.; Zhang, H.; Fang, P.; Zhou, J.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Strigolactones Positively Regulate Abscisic Acid-Dependent Heat and Cold Tolerance in Tomato. Hortic. Res. 2021, 8, 237. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Jia, C.-G.; Li, Z.; Sun, B.; Zhang, L.-P.; Liu, N.; Wang, Q.-M. Effect of Brassinosteroids on Drought Resistance and Abscisic Acid Concentration in Tomato under Water Stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Altamura, M.M.; Piacentini, D.; della Rovere, F.; Fattorini, L.; Falasca, G.; Betti, C. New Paradigms in Brassinosteroids, Strigolactones, Sphingolipids, and Nitric Oxide Interaction in the Control of Lateral and Adventitious Root Formation. Plants 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening Under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Stavang, J.A.; Gallego-Bartolomé, J.; Gómez, M.D.; Yoshida, S.; Asami, T.; Olsen, J.E.; García-Martínez, J.L.; Alabadí, D.; Blázquez, M.A. Hormonal Regulation of Temperature-Induced Growth in Arabidopsis. Plant J. 2009, 60, 589–601. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene Signaling Negatively Regulates Freezing Tolerance by Repressing Expression of CBF and Type-A ARR Genes in Arabidopsis. Plant Cell. 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, T.; Gan, S.; Ren, X.; Fang, L.; Karungo, S.K.; Wang, Y.; Chen, L.; Li, S.; Xin, H. Ethylene Positively Regulates Cold Tolerance in Grapevine by Modulating the Expression of Ethylene Response Factor 057. Sci. Rep. 2016, 6, 24066. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The Antagonistic Action of Abscisic Acid and Cytokinin Signaling Mediates Drought Stress Response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef]
- Nguyen, K.H.; van Ha, C.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M.; et al. Arabidopsis Type B Cytokinin Response Regulators ARR1, ARR10, and ARR12 Negatively Regulate Plant Responses to Drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Van Ha, C.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Herrera-Estrella, L.; et al. Arabidopsis AHP2, AHP3, and AHP5 Histidine Phosphotransfer Proteins Function as Redundant Negative Regulators of Drought Stress Response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed Leaf Senescence Induces Extreme Drought Tolerance in a Flowering Plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef]
- Peleg, Z.; Reguera, M.; Tumimbang, E.; Walia, H.; Blumwald, E. Cytokinin-Mediated Source/Sink Modifications Improve Drought Tolerance and Increase Grain Yield in Rice under Water-Stress. Plant Biotechnol. J. 2011, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Novák, J.; Pavlů, J.; Novák, O.; Nožková-Hlaváčková, V.; Špundová, M.; Hlavinka, J.; Koukalová, Š.; Skalák, J.; Černý, M.; Brzobohatý, B. High Cytokinin Levels Induce a Hypersensitive-like Response in Tobacco. Ann. Bot. 2013, 112, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, J.; Kerchev, P.; Černý, M.; Novák, J.; Berka, M.; Jobe, T.O.; López Ramos, J.M.; Saiz-Fernández, I.; Rashotte, A.M.; Kopriva, S.; et al. Cytokinin Modulates the Metabolic Network of Sulfur and Glutathione. J. Exp. Bot. 2022, 73, 7417–7433. [Google Scholar] [CrossRef]
- Asthir, B.; Kaur, S.; Mann, S.K. Effect of Salicylic and Abscisic Acid Administered through Detached Tillers on Antioxidant System in Developing Wheat Grains under Heat Stress. Acta Physiol. Plant. 2009, 31, 1091–1096. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic Acid Prevents Pollen Abortion under High-Temperature Stress by Mediating Sugar Metabolism in Rice Spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Martínez-Pérez, A.; Albacete, A.; Martínez-Melgarejo, P.A.; Dodd, I.C.; Thompson, A.J.; Mohareb, F.; Estelles-Lopez, L.; Kevei, Z.; Ferrández-Ayela, A.; et al. Overproduction of ABA in Rootstocks Alleviates Salinity Stress in Tomato Shoots. Plant Cell Environ. 2021, 44, 2966–2986. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Pérez, F.J. Abscisic Acid (ABA) and Low Temperatures Synergistically Increase the Expression of CBF/DREB1 Transcription Factors and Cold-Hardiness in Grapevine Dormant Buds. Ann. Bot. 2019, 123, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Nai, G.; Liang, G.; Ma, W.; Lu, S.; Li, Y.; Gou, H.; Guo, L.; Chen, B.; Mao, J. Overexpression VaPYL9 Improves Cold Tolerance in Tomato by Regulating Key Genes in Hormone Signaling and Antioxidant Enzyme. BMC Plant Biol. 2022, 22, 344. [Google Scholar] [CrossRef]
- Abeysingha, D.N.; Ozga, J.A.; Strydhorst, S.; Doyle, P.; Iqbal, M.; Yang, R.; Reinecke, D.M. The Effect of Auxins on Amelioration of Heat Stress-induced Wheat (Triticum aestivum L.) Grain Loss. J. Agron. Crop Sci. 2021, 207, 970–983. [Google Scholar] [CrossRef]
- Dubas, E.; Moravčíková, J.; Libantová, J.; Matušíková, I.; Benková, E.; Żur, I.; Krzewska, M. The Influence of Heat Stress on Auxin Distribution in Transgenic B. Napus Microspores and Microspore-Derived Embryos. Protoplasma 2014, 251, 1077–1087. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, H.J.; Cho, H.S.; Jung, H.W.; Cha, J.-Y.; Yun, D.-J.; Oh, S.-W.; Chung, Y.-S. Overexpression of AtYUCCA6 in Soybean Crop Results in Reduced ROS Production and Increased Drought Tolerance. Plant Biotechnol. Rep. 2019, 13, 161–168. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Q.; Duan, D.; Dong, Q.; Zhao, S.; Zhang, M.; Jing, G.; Liu, C.; van Nocker, S.; Ma, F.; et al. Overexpression of MdIAA9 Confers High Tolerance to Osmotic Stress in Transgenic Tobacco. PeerJ 2019, 7, e7935. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, T.; Korver, R.A.; Testerink, C.S.; ten Tusscher, K.H.W.J. Modeling Halotropism: A Key Role for Root Tip Architecture and Reflux Loop Remodeling in Redistributing Auxin. Development 2016, 143, 3350–3362. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, M.-X.; Liu, R.; Zhang, L.; Hou, X.; Liu, S.; Ding, X.; Jiang, Y.; Xu, J.; Zhang, J.; et al. Abscisic Acid Regulates Auxin Distribution to Mediate Maize Lateral Root Development Under Salt Stress. Front. Plant Sci. 2019, 10, 716. [Google Scholar] [CrossRef]
- Gavelienė, V.; Novickienė, L.; Pakalniškytė, L. Effect of Auxin Physiological Analogues on Rapeseed (Brassica Napus) Cold Hardening, Seed Yield and Quality. J. Plant Res. 2013, 126, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon-Mediated Role of 24-Epibrassinolide in Wheat under High-Temperature Stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Holton, N.; Bishop, G.J.; Núñez, M. Heat Shock Response in Tomato Brassinosteroid Mutants Indicates That Thermotolerance Is Independent of Brassinosteroid Homeostasis. Plant Physiol. Biochem. 2011, 49, 1420–1428. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chen, Z.-Y.; Jiang, Y.; Duan, B.-B.; Xi, Z.-M. Involvement of ABA and Antioxidant System in Brassinosteroid-Induced Water Stress Tolerance of Grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Chen, E.; Zhang, X.; Yang, Z.; Zhang, C.; Wang, X.; Ge, X.; Li, F. BR Deficiency Causes Increased Sensitivity to Drought and Yield Penalty in Cotton. BMC Plant Biol. 2019, 19, 220. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-Epibrassinolide on Growth, Yield, Antioxidant System and Cadmium Content of Bean (Phaseolus vulgaris L.) Plants under Salinity and Cadmium Stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Z.; Wu, M.; Wang, W.; Wang, Y.; Nie, S. Enhanced Brassinosteroid Signaling via the Overexpression of SlBRI1 Positively Regulates the Chilling Stress Tolerance of Tomato. Plant. Sci. 2022, 320, 111281. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Shi, Y.; Cui, Z.; Luo, Y.; Zheng, M.; Chen, J.; Li, Y.; Yin, Y.; Wang, Z. Exogenous Cytokinins Increase Grain Yield of Winter Wheat Cultivars by Improving Stay-Green Characteristics under Heat Stress. PLoS ONE 2016, 11, e0155437. [Google Scholar] [CrossRef] [PubMed]
- Skalák, J.; Černý, M.; Jedelský, P.; Dobrá, J.; Ge, E.; Novák, J.; Hronková, M.; Dobrev, P.; Vanková, R.; Brzobohatý, B. Stimulation of Ipt Overexpression as a Tool to Elucidate the Role of Cytokinins in High Temperature Responses of Arabidopsis Thaliana. J. Exp. Bot. 2016, 67, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar Cytokinins or Brassinosteroids Applications Influence the Rice Plant Acclimatization to Combined Heat Stress. Front. Plant Sci. 2022, 13, 983276. [Google Scholar] [CrossRef]
- Mushtaq, N.; Wang, Y.; Fan, J.; Li, Y.; Ding, J. Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants 2022, 11, 154. [Google Scholar] [CrossRef]
- Tran, L.-S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of AHK1/ATHK1 and Cytokinin Receptor Histidine Kinases in Response to Abscisic Acid, Drought, and Salt Stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Sunmonu, T.O.; Kulkarni, M.G.; Zatloukal, M.; Spichal, L.; Doležal, K.; Van Staden, J. A Novel Inhibitor of Cytokinin Degradation (INCYDE) Influences the Biochemical Parameters and Photosynthetic Apparatus in NaCl-Stressed Tomato Plants. Planta 2014, 240, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an Inflorescence Meristem-Specific Cytokinin Oxidase—OsCKX2 in Rice Reduces Yield Penalty under Salinity Stress Condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural Variation in a Type-A Response Regulator Confers Maize Chilling Tolerance. Nat. Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, N.Y.; Kim, S.; Kang, N.Y.; Novák, O.; Ku, S.-J.; Cho, C.; Lee, D.J.; Lee, E.-J.; Strnad, M.; et al. A Subset of Cytokinin Two-Component Signaling System Plays a Role in Cold Temperature Stress Response in Arabidopsis. J. Biol. Chem. 2010, 285, 23371–23386. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Yang, C.-Y. Ethylene-Mediated Signaling Confers Thermotolerance and Regulates Transcript Levels of Heat Shock Factors in Rice Seedlings under Heat Stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, S.; Chaturvedi, P.; Ghatak, A.; Pressman, E.; Meir, S.; Faigenboim, A.; Rutley, N.; Beery, A.; Harel, A.; Weckwerth, W.; et al. Proteomics of Heat-Stress and Ethylene-Mediated Thermotolerance Mechanisms in Tomato Pollen Grains. Front. Plant Sci. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, G.; Zhao, J.-L.; Zhang, L.-Q.; Ai, L.-F.; Han, Y.-F.; Sun, D.-Y.; Zhang, S.-W.; Sun, Y. The Receptor-Like Kinase SIT1 Mediates Salt Sensitivity by Activating MAPK3/6 and Regulating Ethylene Homeostasis in Rice. Plant Cell 2014, 26, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.J.; Jang, S.J.; Park, K.Y. Inhibition of Biphasic Ethylene Production Enhances Tolerance to Abiotic Stress by Reducing the Accumulation of Reactive Oxygen Species in Nicotiana tabacum. Mol. Cells 2010, 30, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Iglesias-Moya, J.; García, A.; Martínez, J.; Romero, J.; Regalado, J.J.; Martínez, C.; Valenzuela, J.L.; Jamilena, M. Involvement of Ethylene Receptors in the Salt Tolerance Response of Cucurbita Pepo. Hortic. Res. 2021, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Belfield, E.J.; Cao, Y.; Smith, J.A.C.; Harberd, N.P. An Arabidopsis Soil-Salinity–Tolerance Mutation Confers Ethylene-Mediated Enhancement of Sodium/Potassium Homeostasis. Plant Cell 2013, 25, 3535–3552. [Google Scholar] [CrossRef]
- Young, T.E.; Meeley, R.B.; Gallie, D.R. ACC Synthase Expression Regulates Leaf Performance and Drought Tolerance in Maize. Plant J. 2004, 40, 813–825. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional Activation of OsDERF1 in OsERF3 and OsAP2-39 Negatively Modulates Ethylene Synthesis and Drought Tolerance in Rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef]
- Bergner, C.; Teichmann, C. A Role for Ethylene in Barley Plants Responding to Soil Water Shortage. J. Plant Growth Regul. 1993, 12, 67–72. [Google Scholar] [CrossRef]
- Habben, J.E.; Bao, X.; Bate, N.J.; DeBruin, J.L.; Dolan, D.; Hasegawa, D.; Helentjaris, T.G.; Lafitte, R.H.; Lovan, N.; Mo, H.; et al. Transgenic Alteration of Ethylene Biosynthesis Increases Grain Yield in Maize under Field Drought-Stress Conditions. Plant Biotechnol. J. 2014, 12, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Perras, M.R.; Falk, D.E.; Zhang, R.; Pharis, R.P.; Austin Fletcher, R. Relationship between Gibberellins, Height, and Stress Tolerance in Barley (Hordeum vulgare L.) Seedlings. Plant Growth Regul. 2004, 42, 125–135. [Google Scholar] [CrossRef]
- Nir, I.D.O.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 Suppresses Gibberellin Activity, Reduces Whole-Plant Transpiration and Promotes Drought Tolerance in Transgenic Tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Wüthrich, S.; Blösch, R.; Rindisbacher, A.; Cannarozzi, G.; Tadele, Z. Gibberellin Deficiency Confers Both Lodging and Drought Tolerance in Small Cereals. Front. Plant Sci. 2016, 7, 643. [Google Scholar] [CrossRef] [PubMed]
- Augstein, F.; Carlsbecker, A. Salinity Induces Discontinuous Protoxylem via a DELLA-dependent Mechanism Promoting Salt Tolerance in Arabidopsis Seedlings. New Phytol. 2022, 236, 195–209. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in Activities of Antioxidant Enzymes and Their Relationship to Genetic and Paclobutrazol-Induced Chilling Tolerance of Maize Seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, H.; Wei, D.; Ma, H.; Lin, J. Arabidopsis CBF3 and DELLAs Positively Regulate Each Other in Response to Low Temperature. Sci. Rep. 2017, 7, 39819. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The Role of Gibberellins in the Mitigation of Chilling Injury in Cherry Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic Acid Is Required for Plant Acclimation to a Combination of High Light and Heat Stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, H.; Li, Y.; Zhang, N.; Zhang, S. Abscisic Acid Synthesis and Root Water Uptake Contribute to Exogenous Methyl Jasmonate-Induced Improved Tomato Drought Resistance. Plant Biotechnol. Rep. 2022, 16, 183–193. [Google Scholar] [CrossRef]
- Yoshida, C.H.P.; Pacheco, A.C.; de Lapaz, A.M.; Gorni, P.H.; Vítolo, H.F.; Bertoli, S.C. Methyl Jasmonate Modulation Reduces Photosynthesis and Induces Synthesis of Phenolic Compounds in Sweet Potatoes Subjected to Drought. Bragantia 2020, 79, 319–334. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Close, T.J. Large-Scale Expression Profiling and Physiological Characterization of Jasmonic Acid-Mediated Adaptation of Barley to Salinity Stress. Plant Cell Environ. 2007, 30, 410–421. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Yue, W. Exogenous Jasmonic Acid and Humic Acid Increased Salinity Tolerance of Sorghum. Agron. J. 2020, 112, 871–884. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic Acid Treatment Alleviates Chilling Injury in Peach Fruit by Promoting Sugar and Ethylene Metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic Acid Reverses Pollen Abortion of Rice Caused by Heat Stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, M.A.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid Can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Chakma, R.; Biswas, A.; Saekong, P.; Ullah, H.; Datta, A. Foliar Application and Seed Priming of Salicylic Acid Affect Growth, Fruit Yield, and Quality of Grape Tomato under Drought Stress. Sci. Hortic. 2021, 280, 109904. [Google Scholar] [CrossRef]
- Faried, H.N.; Ayyub, C.M.; Wattoo, F.M.; Bashir, M.; Razzaq, K.; Akhtar, G.; Hussain, A.; Ullah, S.; Wattoo, J.I.; Amin, M.; et al. Assessing Salt Tolerance Induction in Potato by Salicylic Acid Using Morpho-Physio-Biochemical, Ionic, and Yield Indices. Potato Res. 2022, 65, 677–691. [Google Scholar] [CrossRef]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Gesunde Pflanz. 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Hydrogen Peroxide and Abscisic Acid Mediate Salicylic Acid-Induced Freezing Tolerance in Wheat. Front. Plant Sci. 2018, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Li, S.-H. Salicylic Acid-Induced Heat or Cold Tolerance in Relation to Ca2+ Homeostasis and Antioxidant Systems in Young Grape Plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Pospíšil, T.; Strnad, M.; Van Staden, J. Synthetic Strigolactone (Rac-GR24) Alleviates the Adverse Effects of Heat Stress on Seed Germination and Photosystem II Function in Lupine Seedlings. Plant Physiol. Biochem. 2020, 155, 965–979. [Google Scholar] [CrossRef]
- Marzec, M.; Daszkowska-Golec, A.; Collin, A.; Melzer, M.; Eggert, K.; Szarejko, I. Barley Strigolactone Signalling Mutant Hvd14.d Reveals the Role of Strigolactones in Abscisic Acid-dependent Response to Drought. Plant Cell Environ. 2020, 43, 2239–2253. [Google Scholar] [CrossRef]
- Sedaghat, M.; Emam, Y.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Lovisolo, C.; Visentin, I.; Cardinale, F.; Tahmasebi-Sarvestani, Z. The Potential of the Synthetic Strigolactone Analogue GR24 for the Maintenance of Photosynthesis and Yield in Winter Wheat under Drought: Investigations on the Mechanisms of Action and Delivery Modes. Plants 2021, 10, 1223. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Yan, M.; Zhao, Z.; Huang, P.; Wei, L.; Wu, X.; Wang, C.; Liao, W. Strigolactone Is Involved in Nitric Oxide-Enhanced the Salt Resistance in Tomato Seedlings. J. Plant Res. 2022, 135, 337–350. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T. Exogenous Strigolactones Alleviate the Photosynthetic Inhibition and Oxidative Damage of Cucumber Seedlings under Salt Stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Strigolactone Analog (Rac-GR24) Enhances Chilling Tolerance in Mung Bean Seedlings. S. Afr. J. Bot. 2021, 140, 173–181. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen Peroxide Is Involved in Strigolactone Induced Low Temperature Stress Tolerance in Rape Seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of Melatonin on Anti-Oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, M.; Zhang, P.; Liu, L.; Liu, S.; Zhao, C.; Li, X. Exogenous Melatonin Reprograms the Rhizosphere Microbial Community to Modulate the Responses of Barley to Drought Stress. Int. J. Mol. Sci. 2022, 23, 9665. [Google Scholar] [CrossRef] [PubMed]
- El-Yazied, A.A.; Ibrahim, M.F.M.; Ibrahim, M.A.R.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M.; et al. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and Abiotic Stress in Plants: A Complex Relationship1. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amorós, A.; Botella, M.Á. Polyamines and Ethylene Changes during Germination of Different Plant Species under Salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Liu, H.P.; Dong, B.H.; Zhang, Y.Y.; Liu, Z.P.; Liu, Y.L. Relationship between Osmotic Stress and the Levels of Free, Conjugated and Bound Polyamines in Leaves of Wheat Seedlings. Plant Sci. 2004, 166, 1261–1267. [Google Scholar] [CrossRef]
- Lefèvre, I.; Gratia, E.; Lutts, S. Discrimination between the Ionic and Osmotic Components of Salt Stress in Relation to Free Polyamine Level in Rice (Oryza sativa). Plant Sci. 2001, 161, 943–952. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Šveikauskas, V.; Mockevičiūtė, R.; Jankauskienė, J.; Todorova, D.; Sergiev, I.; Jurkonienė, S. Foliar Application of Polyamines Modulates Winter Oilseed Rape Responses to Increasing Cold. Plants 2020, 9, 179. [Google Scholar] [CrossRef]
- Wang, H.; Qin, F. Genome-Wide Association Study Reveals Natural Variations Contributing to Drought Resistance in Crops. Front. Plant Sci. 2017, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Kameniarová, M.; Černý, M.; Novák, J.; Ondrisková, V.; Hrušková, L.; Berka, M.; Vankova, R.; Brzobohatý, B. Light Quality Modulates Plant Cold Response and Freezing Tolerance. Front. Plant Sci. 2022, 13, 887103. [Google Scholar] [CrossRef] [PubMed]
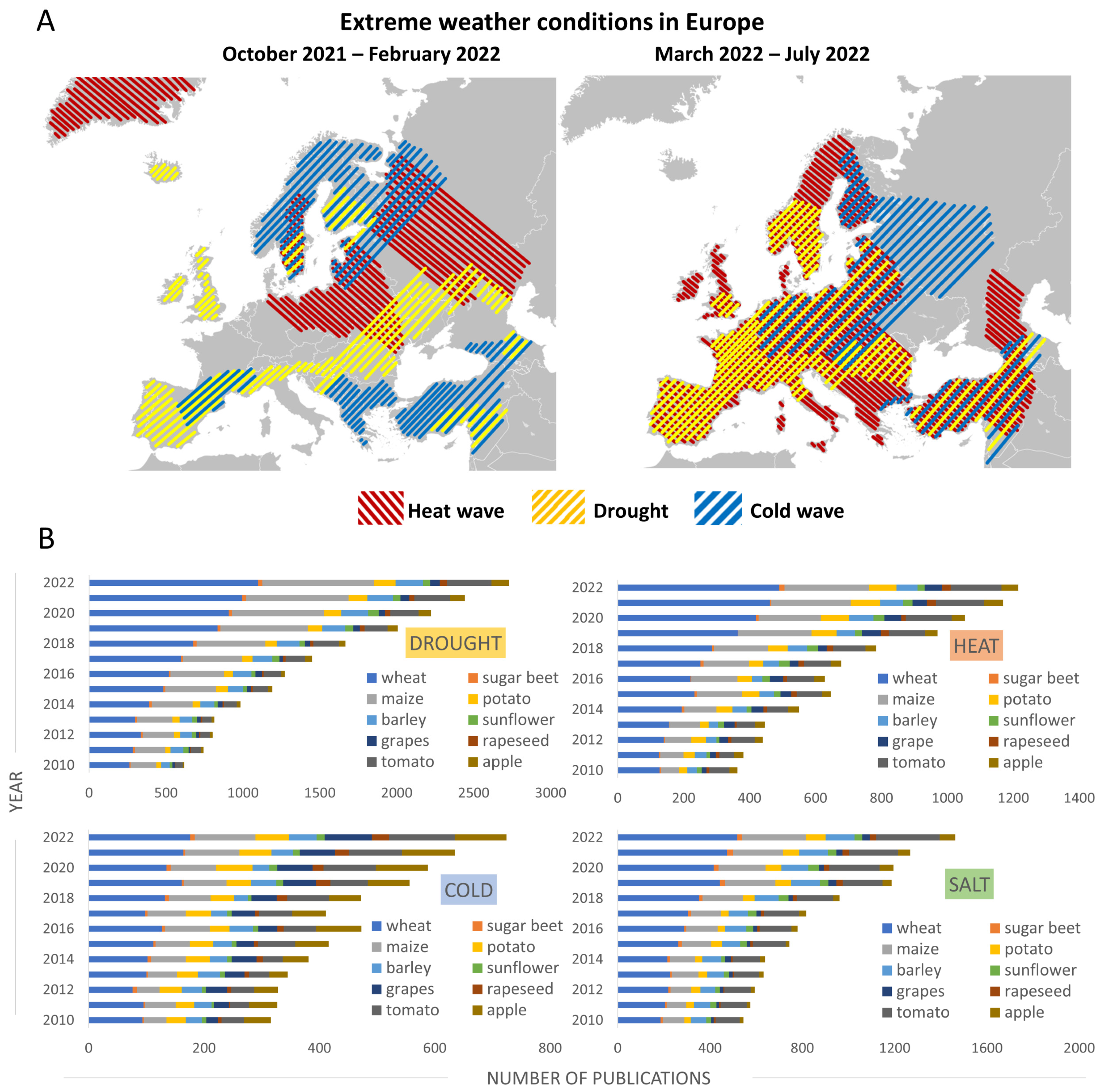

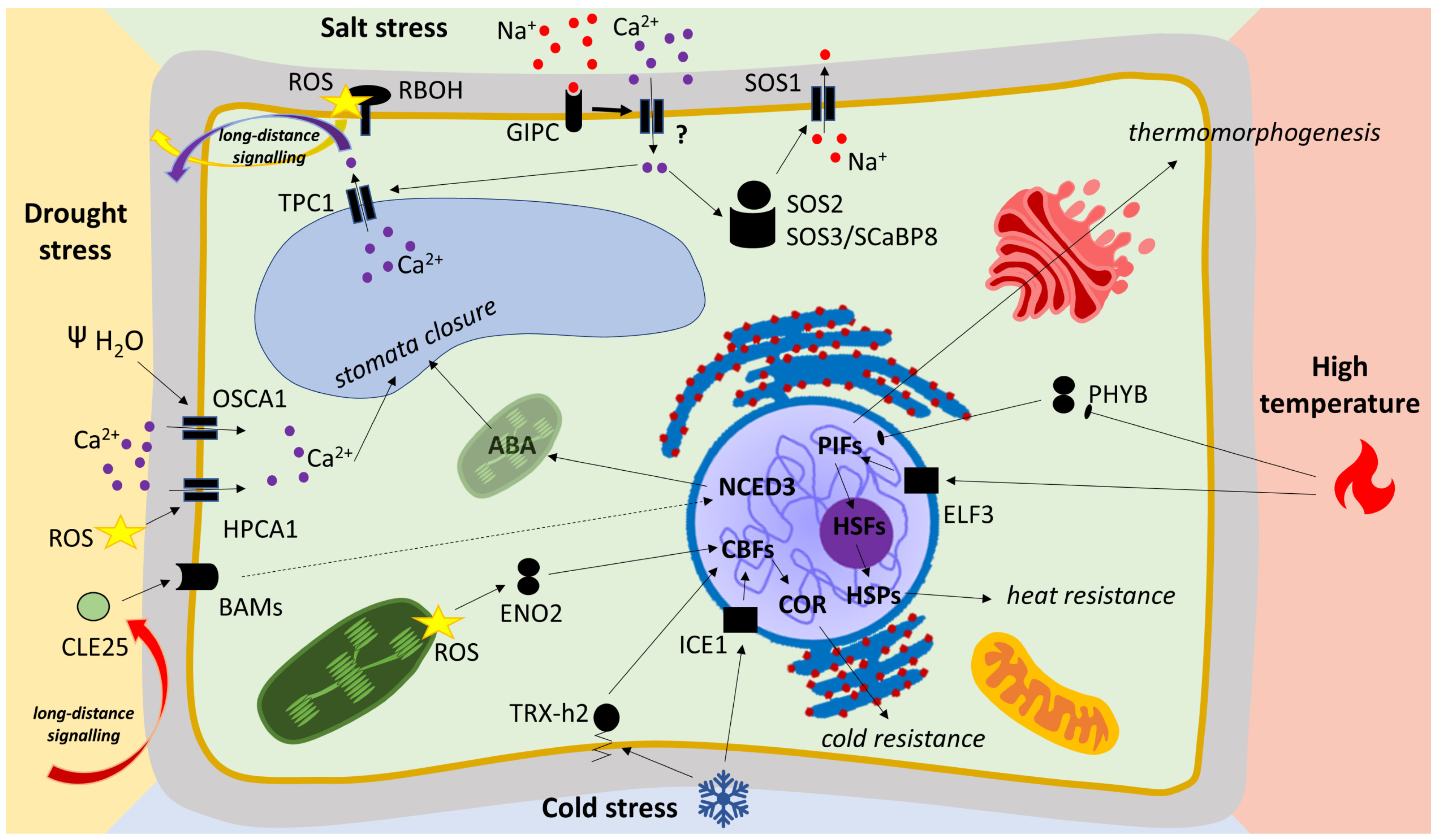
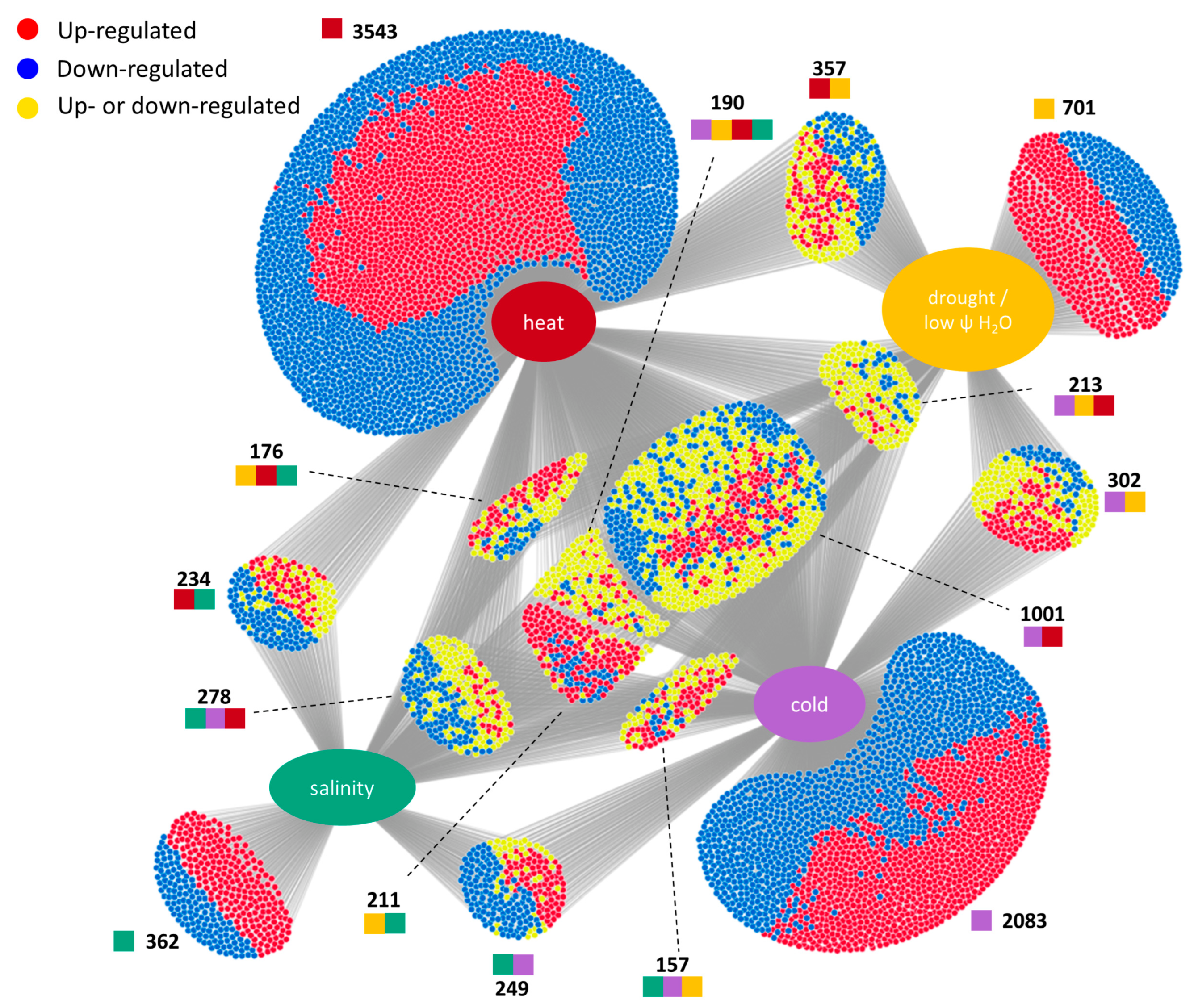
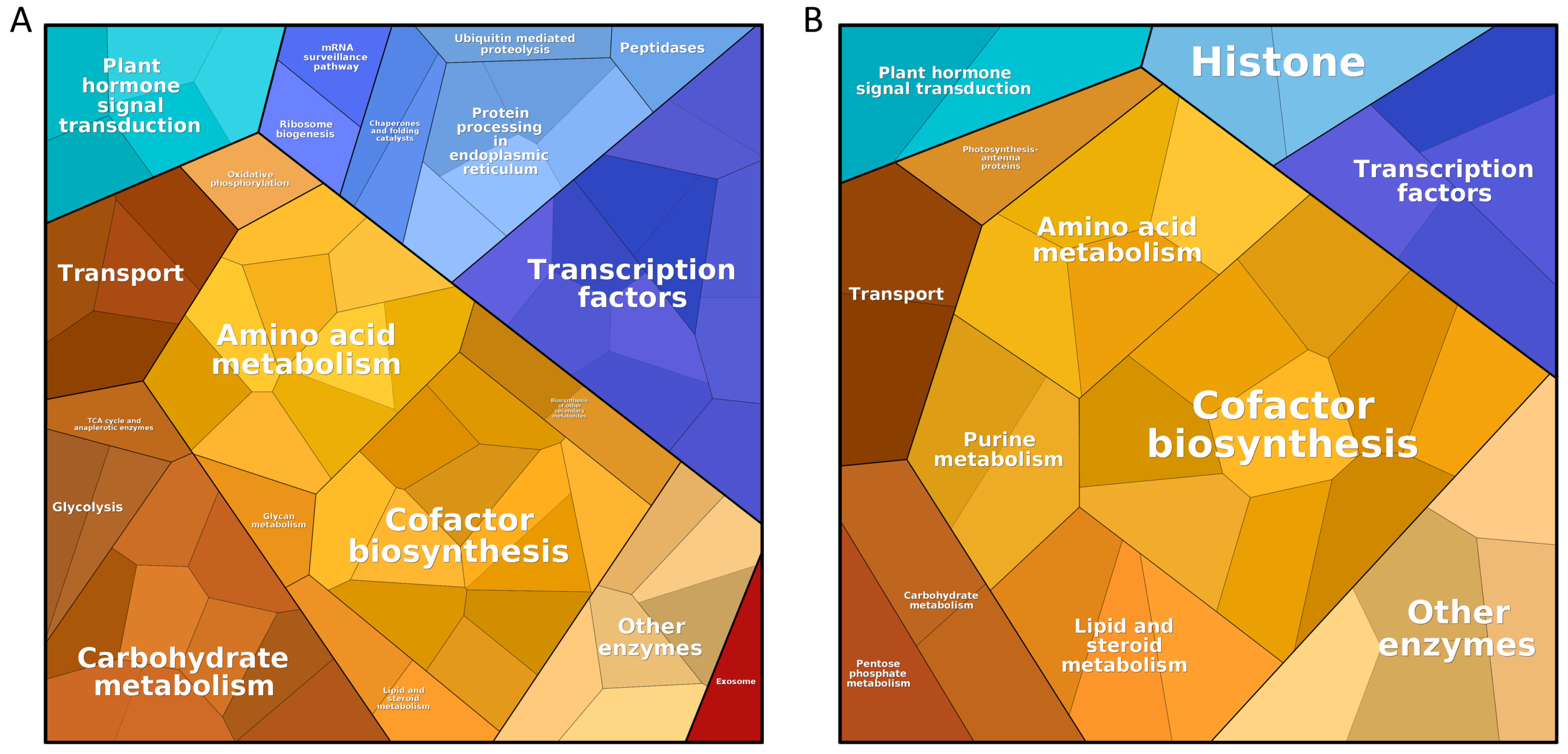
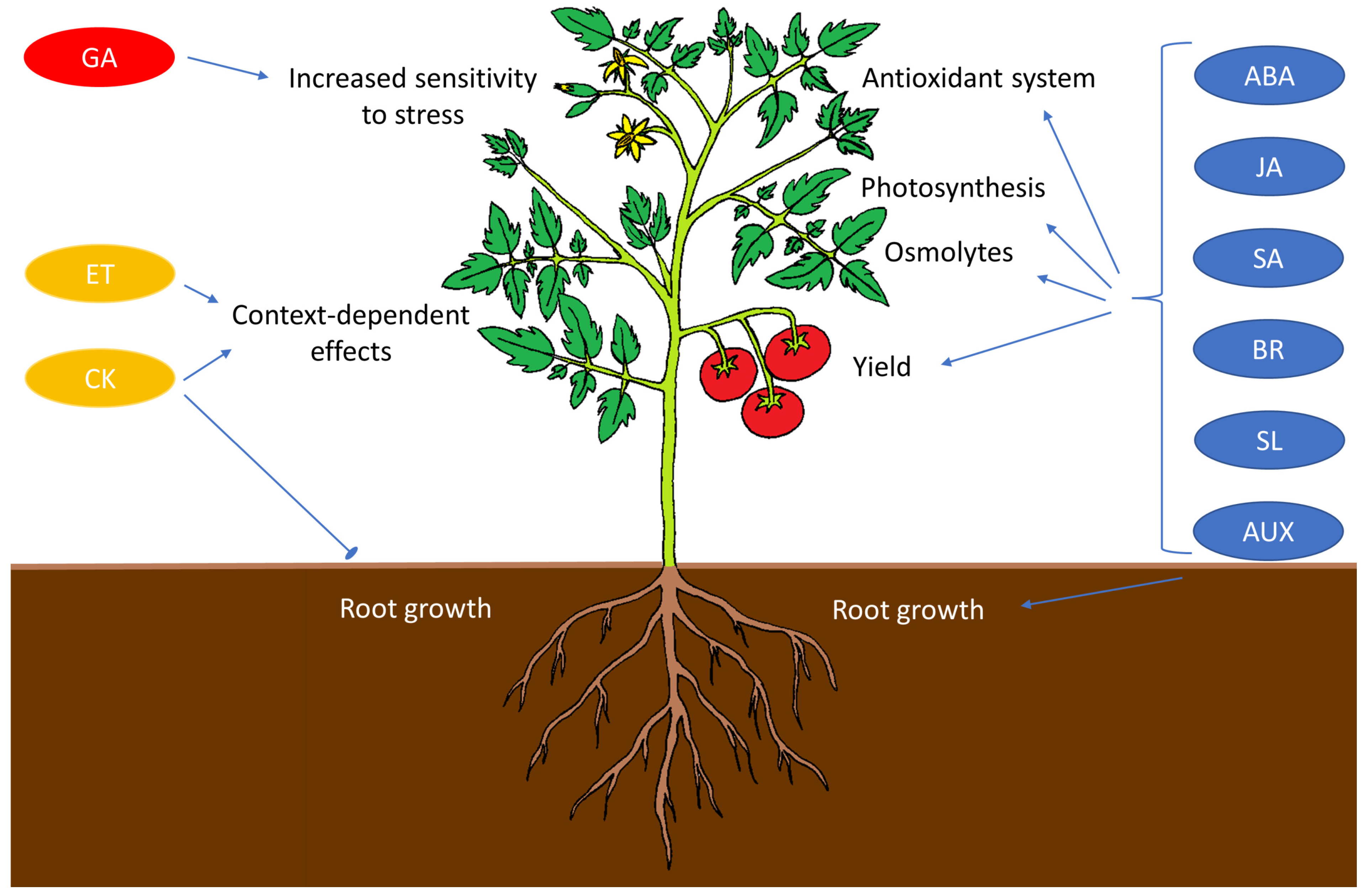
| Crop | Optimal Temperature | Developmental Stage | Publication |
|---|---|---|---|
| Wheat (Triticum aestivum) | 12–22 °C | flowering and grain filling | [37] |
| Barley (Hordeum vulgare) | 25 °C | grain filling | [38] |
| Maize (Zea mays) | 28–32 °C | general | [39] |
| Rapeseed (Brassica napus) | 21–25 °C | general | [40] |
| Sugar beet (Beta vulgaris) | 22–26 °C | growth of the taproot | [41] |
| Potatoes (Solanum tuberosum) | 15–19 °C | tuber growth | [42] |
| Grapes (Vitis vinifera) | 20–40 °C | berry development | [43] |
| Tomatoes (Solanum lycopersicum) | 22–26 °C | fruit set and satisfactory fruit yield | [44] |
| Sunflowers (Helianthus annuum) | 12–20 °C | general | [45] |
| Apples (Malus domestica) | 18–21 °C | shoot growth and floral initiation | [46] |
| Crop | Description of the Experiment | Metabolites Involved in the Response to Stress Conditions |
|---|---|---|
| heat | ||
| potato (Solanum tuberosum) | Metabolome changes after 3 days of heat stress (35 °C) in potato leaves [273]. | ↑ tyrosine, arachidonic acid metabolism, flavone, and flavonol biosynthesis |
| ↓ glutathione, linoleic acid, steroid, fatty acid, phosphonate, and phosphinate metabolism | ||
| maize (Zea mays) | Metabolome change after long-term heat stress in maize leaves. Heat stress started at 30/24 °C, which was increased 2 °C per day for 5 days, then maintained at 37 °C for 12 days [274]. | ↑ tryptophan, threonine, histidine, raffinose, galactinol, lactitol |
| ↓ citrate and trans-3-caffeoyl quinic acid | ||
| wheat (Triticum aestivum) | Two contrasting spring wheat genotypes were exposed to heat stress (34/16 °C, 10 days) during heading. Anthers were collected for metabolic profiling [275]. | ↑ N-based amino acids, ABA, IAA-conjugate |
| ↓ dehydroascorbic acid, quinic acid, 5-Hydroxyindole-3-acetic acid, putrescine, and shikimic acid | ||
| grape (Vitis vinifera) | Metabolomic analysis of high-temperature effect (34/26 °C, 14 days) on grapevine berries [276]. | ↑ lipid metabolism metabolites, lignin, cuticle, vax, GABA, galactinol, vitamins |
| ↓ malic acid, shikimate, sugar phosphate, secondary metabolites, sugars | ||
| maize (Zea mays) | Recovery profiling following sudden heat shock (46 °C, 2 h) regarding metabolites in two maize genotypes grown under ambient or elevated CO2 [277]. | ↑ ribose, valine, asparagine, isoleucine, adipic, 2-oxoglutarate, pyruvate, maltose, malate, trehalose, myo-inositol, starch, citric, fumarate |
| ↓ glycerate, serine, glycine, shikimate, leucine, proline, and sucrose | ||
| tomato (Solanum lycopersicum) | Untargeted metabolomic analyses of tomato pollen after short heat exposure (38 °C, 2 h) [278]. | ↑ flavonoids |
| drought | ||
| sunflower (Helianthus annuus) | Comparison of metabolic profiles of sensitive/tolerant sunflower seedlings subjected to water-deficit stress [279]. | Water-deficit stress-tolerant line accumulated: |
| ↑ anthranilic acid, maleic acid, malonic acid, putative-rhamnose, fructose | ||
| wheat (Triticum aestivum) | Drought effect (up to 10 days after withholding water) on bread wheat metabolism during the flowering stage [280]. | ↑ 1-aminocyclopropane-1-carboxylic acid, Asn, serotonin, GABA, cystine, deoxyuridine, tryptamine, putrescine |
| ↓ glyceric, shikimic, ferulic and succinic acid | ||
| barley (Hordeum vulgare) | Transcriptome and metabolome analysis on the developing grains of two barley genotypes differing in the responses to drought stress [281]. | ↑ amino acids, sugars, abscisic acid, jasmonic acid, ferulate |
| ↓ citrate | ||
| wheat (Triticum aestivum) | Metabolic adjustment of six winter wheat cultivars to drought (induced by withholding watering for 6 days) [282]. | ↑ sugars, malic acid, oxalic acids, proline, threonine, GABA, glutamine, myo-inositol |
| ↓ propanoic acid | ||
| wheat (Triticum aestivum) | Changes in protein and metabolite abundance of two wheat cultivars after 7 days of water deficit [230]. | ↑ purine bases, organic acids, sugars, amino acids |
| ↓ aspartate, glutamate | ||
| barley (Hordeum vulgare) | Metabolic changes in four wild and cultivated barley genotypes contrasting in drought tolerance during grain-filling stage in response to water stress [283]. | ↑ mannitol, L-proline, sucrose, TCA cycle components, quinic acid |
| ↓ 2-ketoglutaric acid | ||
| potato (Solanum tuberosum) | Set of predictive markers for drought tolerance by transcriptomic and metabolomic profiling of 31 potato cultivars [284]. | markers for drought tolerance: |
| ↑ galactaric, galactonic, glyceric, saccharic acid, dopamine, tyramine | ||
| sunflower (Helianthus annuus) | Metabolic pathways related to drought conditions in sunflowers. The response of plants was studied in the early stage of water deficit [285]. | ↑ TCA cycle components, carbohydrates, amino acids, and derivatives proline, tyramine, glycine, malonate, γ-aminobutyrate |
| ↓ amino acid metabolites | ||
| salinity | ||
| barley (Hordeum vulgare) | Metabolic analyses of barley seeds in response to salt stress (24 h, 200 mM NaCl), during the germination process. Two differentially salt-tolerant barley varieties were compared [286]. | ↑ aminoacyl-tRNA biosynthesis, glycine, serine and threonine metabolism, glyoxylate and dicarboxylate metabolism, and porphyrin and chlorophyll metabolism (tolerant) |
| ↑ valine, leucine and isoleucine biosynthesis, biosynthesis of amino acids, alanine, aspartate and glutamate metabolism, glycine, serine and threonine metabolism, and cyanoamino acid metabolism (sensitive) | ||
| rapeseed (Brassica napus) | Molecular mechanism of salt tolerance in rapeseed. Two rapeseed varieties were compared, showing the metabolites common to both [287]. | ↑ glutathione amid, aconitase, glucose, mannose, inositol, epigallocatechin 3-gallate |
| ↓ arginine, citrulline, trimethyl-lysine, acetylaspartate, inositol-triphosphate | ||
| rapeseed (Brassica napus) | Key salt-related metabolites in five different rapeseed cultivars. Salt stress (up to 200 mM NaCl) was applied during the early seedling stage [288]. | ↑ linolenic acid, xanthosine, inosine 5′-monophosphate, adenosine 3′-monophosphate, niacinamide, oleamide, phosphoric acid, etamiphylline (in tolerant cultivars) |
| ↓ 5-hydroxytryptophan, cholesterol, L-aspartic acid, beta-homotreonine, N-p-coumaroyl serotonin, ornithine (in tolerant cultivars) | ||
| sugar beet (Beta vulgaris) | Metabolites involved in the short-term (1 day) and long-term (7 days) salt-stress response (300 mM Na+ treatment) in sugar beet [289]. | ↑ L-malic acid and 2-oxoglutaric acid, amino acids, betaine, melatonin, (S)-2-aminobutyric acid, cis-aconitate, benzoic acid L-malic acid, alpha-ketoglutarate, 2-isopropylmalic acid |
| ↓ sucrose | ||
| barley (Hordeum vulgare) | Ionomic, metabolomic, and proteomic responses in roots of salt-tolerant/sensitive barley accession exposed to salinity stress (200 and 400 mM) [290]. | ↑ fructose, trehalose, sorbitol (in both genotypes), glycine, alanine, valine, inositol, allothreonine, glutamic acid, glycine, cysteine (in tolerant genotypes) |
| ↓ glucose-6-P, fructose-6-P | ||
| durum wheat (Triticum durum) | Metabolic changes in the shoots and roots of five durum-wheat genotypes exposed to the different salt levels [291]. | ↑ proline |
| ↓ organic acids involved in the Krebs cycle, gluconic, quinic, shikimic acid | ||
| sugar beet (Beta vulgaris) | Metabolic adaptation of sugar beet to salt stress (up to 300 mM NaCl) at the cellular and subcellular levels. Metabolites were profiled at 3 h and 14 d after reaching the maximum salinity stress [292]. | ↑ arabinose, gluconolactone, inositol, mannitol, proline, serine, and thymine |
| ↓ lactate, homoserine, adenosine, guanine (early response), fumarate, L-aspartate, gluconate (late response) | ||
| barley (Hordeum vulgare) | The effects of salinity stress (up to 150 mM NaCl) on barley roots through quantitation of polar metabolites [293]. | ↑ 4-hydroxy-proline, alanine, arginine, asparagine, citrulline, glutamine, phenylalanine, proline |
| ↓ putrescine, succinate | ||
| cold | ||
| maize (Zea mays) | Metabolic responses under cold stress (5 °C, 24 h) in the early growth stages of maize. Responses of tolerant and susceptible lines were compared [294]. | Cold-tolerant line accumulated: |
| ↑ guanosine 3′,5′-cyclic monophosphate, quercetin-3-O-(2″′-p-coumaroyl)sophoroside-7-O-glucoside, phloretin, phloretin-2′-O-glucoside, naringenin-7-O-Rutinoside, L-lysine, L-phenylalanine, L-glutamine, sinapyl alcohol, feruloyl tartaric | ||
| apple (Malus domestica) | Molecular mechanism of apple trees in response to freezing injury during winter dormancy. Cold-resistant and cold-sensitive cultivars were compared [295]. | ↑ 4-aminobutyric acid, spermidine, and ascorbic acid (cold-resistant) |
| ↓ oxidized glutathione, vitamin C, glutathione, spermidine (cold-resistant) | ||
| rapeseed (Brassica napus) | Metabolite profiling of cold-treated (−2 °C, 2 h) contrasting rapeseed genotypes focusing on siliques [296]. | ↑ 8-hydroxyguanosine, 9-(arabinosyl)hypoxanthine, inosine, uridine, guanosine 3′,5′-cyclic monophosphate, β-pseudouridine, 4-acetamidobutyric acid, phenylpyruvic acid, 6-hydroxyhexanoic acid, valeric acid, γ-aminobutyric acid, oxalic acid, jasmonic acid (both genotypes) |
| ↑ adenine, riboprine, cytidine, N6-isopentenyladenine (cold-tolerant only) | ||
| maize (Zea mays) | Metabolic responses of maize hybrids could be extrapolated from growth-chamber (gradually decreasing temperature) conditions to early sowing in the field [297]. | ↑ trans-aconitate, coumaroyl hydroxycitrate, chrysoeriol glucosyl rhamnoside, caffeoylquinate, ferruloylquinate, (iso)vitexin, DIBOA-glucoside |
| ↓ malate, glutamine | ||
| rapeseed (Brassica napus) | Cold-responsive metabolites in two contrasting varieties of rapeseed after 1 and 7 days of cold treatment [298]. | Response to cold in both varieties: |
| ↑ trehalose, L-Kynurenine, gamma-tocotrienol, phenyllactic acid, L-Gulonic gamma-lactone | ||
| maize (Zea mays) | Two maize lines with contrasting chilling-tolerance capacities were used to identify the major factors of chilling tolerance. The plants were exposed to 14 °C day/10 °C night for 60 days [299]. | Chilling tolerance in tolerant plants correlated with: |
| ↑ chlorophyll content, glucose-6-phosphate dehydrogenase activity, sucrose-to-starch ratio | ||
| wheat (Triticum aestivum) | Metabolite activity in winter-hardy wheat subjected to cold stress (cold acclimation at 4 °C for 28 days, then freezing at −5 °C for 24 h) [300]. | ↑ aspartic acid O-rutinoside, proline, tyramine, raffinose, gluconic acid, melezitose, mannose, maltotetraose |
| ↓ aspartic acid, lysine, ornithine | ||
| potato (Solanum tuberosum) | Metabolome of the freezing-tolerant Solanum acaule and freezing-sensitive S. tuberosum. The plants were exposed to 4 °C for 14 days, then to gradient freezing at 1 °C/h up to −12 °C [301]. | Chilling tolerance in tolerant plants correlated with: |
| ↑ putrescine, 1-kestose, raffinose, xylose, fucose, isoleucine, tyrosine, valine, benzoic acid, trans-caffeic acid, dehydroascorbic acid, uracil | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. https://doi.org/10.3390/ijms24076603
Kopecká R, Kameniarová M, Černý M, Brzobohatý B, Novák J. Abiotic Stress in Crop Production. International Journal of Molecular Sciences. 2023; 24(7):6603. https://doi.org/10.3390/ijms24076603
Chicago/Turabian StyleKopecká, Romana, Michaela Kameniarová, Martin Černý, Břetislav Brzobohatý, and Jan Novák. 2023. "Abiotic Stress in Crop Production" International Journal of Molecular Sciences 24, no. 7: 6603. https://doi.org/10.3390/ijms24076603
APA StyleKopecká, R., Kameniarová, M., Černý, M., Brzobohatý, B., & Novák, J. (2023). Abiotic Stress in Crop Production. International Journal of Molecular Sciences, 24(7), 6603. https://doi.org/10.3390/ijms24076603







