Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review
Abstract
1. Introduction
2. AD Pathomechanisms
2.1. Anatomical and Pathomorphological Macroscopic Changes
2.2. Amyloid Plaques and Tau Protein
2.3. Oxidative Stress
2.4. Cholinergic Changes
2.5. Genetic Changes
2.6. Mitochondrial Dysfunction
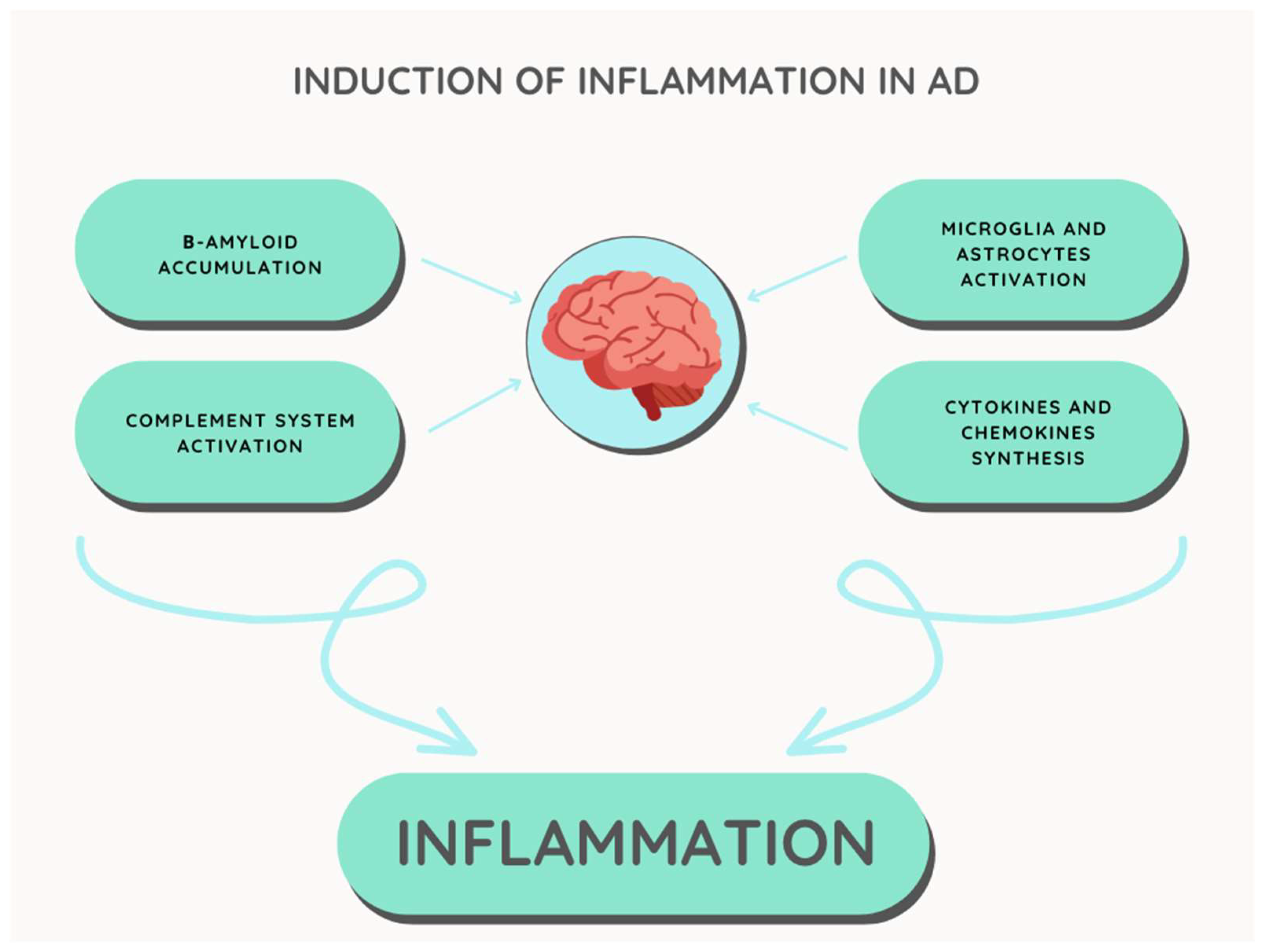
3. Inflammatory Processes in Alzheimer’s Disease
3.1. β-Amyloid Amyloid Hypothesis
3.2. Microglia and Astrocytes
3.3. Complement System
3.4. Cytokines
3.5. Cyclooxygenase Activity
4. Diagnostics of the Inflammatory Process in AD
4.1. Determination of C-Reactive Protein Concentration
4.2. Determination of Pro-Inflammatory Cytokines Concentration and Complement System Activity
4.3. Histological and Pathomorfological Methods
4.4. Genetic Diagnostics
5. Treatment of Inflammation in AD
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Toodayan, N. Professor Alois Alzheimer (1864–1915): Lest we forget. J. Clin. Neurosci. 2016, 31, 47–55. [Google Scholar] [CrossRef]
- D’Cruz, M.M.; Banerjee, D. The person is not the disease—Revisiting Alzheimer’s dementia after 120 years. J. Geriatr. Ment. Health 2021, 8, 136. [Google Scholar]
- Janoutová, J.; Kovalová, M.; Machaczka, O.; Ambroz, P.; Zatloukalová, A.; Němček, K.; Janout, V. Risk Factors for Alzheimer’s Disease: An Epidemiological Study. Curr. Alzheimer Res. 2021, 18, 372–379. [Google Scholar] [CrossRef]
- Qiu, C.; Kivipelto, M.; Von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2022, 11, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Dumurgier, J.; Tzourio, C. Epidemiology of neurological diseases in older adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26, S177–S183. [Google Scholar]
- Dos Santos, P.; Leide, C.; Ozela, P.F.; de Fatima de Brito Brito, M.; Pinheiro, A.A.; Padilha, E.C.; Braga, F.S.; de Paula, D.S.; Carlos, H.T.; dos Santos, C.B.R.; et al. Alzheimer’s disease: A review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr. Med. Chem. 2018, 25, 3141–3159. [Google Scholar] [CrossRef]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef]
- Tangaro, S.; Amoroso, N.; Boccardi, M.; Bruno, S.; Chincarini, A.; Ferraro, G.; Frisoni, G.B.; Maglietta, R.; Redolfi, A.; Rei, L.; et al. Alzheimers Disease Neuroimaging Initiative. Automated voxel-by-voxel tissue classification for hippocampal segmentation: Methods and validation. Phys. Med. 2014, 30, 878–887. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kamboj, P.; Goswami, K.; Ahuja, K.J.J.A.P.R. Pathophysiology and management of Alzheimer’s disease: An overview. J. Anal. Pharm. Res. 2018, 7, 1. [Google Scholar] [CrossRef]
- Nagata, K.; Yamazaki, T.; Takano, D.; Maeda, T.; Fujimaki, Y.; Nakase, T.; Sato, Y. Cerebral circulation in aging. Ageing Res. Rev. 2016, 30, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Shreya, S. In-Silico Approaches of Polyphenols and In-Vivo Evaluation of Neuroprotective Effects of Eugenia Jambolana Leaves Extract for Anticholinesterase and Antioxidant Activities. Ph.D. Thesis, Karpagam College of Pharmacy, Coimbatore, India, 2021. [Google Scholar]
- Salminen, A.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Inflammation in Alzheimer’s disease: Amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 2009, 87, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, M.; Jang, S. Molecular characteristics of amyloid precursor protein (APP) and its effects in cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef]
- Mitra, A.; Sarkar, N. Sequence and structure-based peptides as potent amyloid inhibitors: A review. Arch. Biochem. Biophys. 2020, 695, 108614. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s disease, oligomers, and inflammation. J. Alzheimer’s Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef]
- Jankovska, N.; Olejar, T.; Matej, R. Extracellular amyloid deposits in Alzheimer’s and Creutzfeldt–Jakob disease: Similar behavior of different proteins? Int. J. Mol. Sci. 2020, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Tuppo, E.E.; Arias, H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005, 37, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Pendin, D.; Fasolato, C.; Basso, E.; Filadi, R.; Greotti, E.; Galla, L.; Gomiero, C.; Leparulo, A.; Redolfi, N.; Scremin, E.; et al. Familial Alzheimer’s disease presenilin-2 mutants affect Ca 2+ homeostasis and brain network excitability. Aging Clin. Exp. Res. 2021, 33, 1705–1708. [Google Scholar] [CrossRef]
- Rossi, A.; Galla, L.; Gomiero, C.; Zentilin, L.; Giacca, M.; Giorgio, V.; Calì, T.; Pozzan, T.; Greotti, E.; Pizzo, P. Calcium signaling and mitochondrial function in presenilin 2 knock-out mice: Looking for any loss-of-function phenotype related to Alzheimer’s disease. Cells 2021, 10, 204. [Google Scholar] [CrossRef]
- Guan, P.-P.; Cao, L.-L.; Wang, P. Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef]
- Sebastián-Serrano, Á.; de Diego-García, L.; Díaz-Hernández, M. The neurotoxic role of extracellular tau protein. Int. J. Mol. Sci. 2018, 19, 998. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Yu, K.K.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Wang, J.; Ren, W.X.; Wang, S.; Yu, X.Q.; Kim, J.S. Fluorescent imaging of reactive oxygen and nitrogen species associated with pathophysiological processes. Chem 2020, 6, 832–866. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Gospodaryov, D.V.; Lushchak, V.I. Homeostasis of carbohydrates and reactive oxygen species is critically changed in the brain of middle-aged mice: Molecular mechanisms and functional reasons. BBA Adv. 2023, 3, 100077. [Google Scholar] [CrossRef]
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018, 115, 351–360. [Google Scholar] [CrossRef]
- McNaull, B.B.A.; Todd, S.; McGuinness, B.; Passmore, A.P. Inflammation and anti-inflammatory strategies for Alzheimer’s disease—A mini-review. Gerontology 2010, 56, 3–14. [Google Scholar] [CrossRef]
- Bennett, R.E.; Robbins, A.B.; Hu, M.; Cao, X.; Betensky, R.A.; Clark, T.; Das, S.; Hyman, B.T. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1289–E1298. [Google Scholar] [CrossRef]
- Ge, M.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of calcium homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in Alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Millán, I.; Piñero-Ramos, J.D.; Lara, I.; Parra-Llorca, A.; Torres-Cuevas, I.; Vento, M. Oxidative stress in the newborn period: Useful biomarkers in the clinical setting. Antioxidants 2018, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Barciszewska, A.-M.; Giel-Pietraszuk, M.; Perrigue, P.M.; Naskręt-Barciszewska, M. Total DNA methylation changes reflect random oxidative DNA damage in gliomas. Cells 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013, 521, 4124–4144. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Cao, F.; Xiao, W.; Zhao, L.; Ding, G.; Wang, Z.Z. Identification of human acetylcholinesterase inhibitors from the constituents of EGb761 by modeling docking and molecular dynamics simulations. Comb. Chem. High Throughput Screen. 2018, 21, 41–49. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Shu, K.; Kang, H.-C. Deep brain stimulation in Alzheimer’s disease: Targeting the nucleus basalis of Meynert. J. Alzheimer’s Dis. 2021, 80, 53–70. [Google Scholar] [CrossRef]
- Dallé, E.; Mabandla, M.V.; Daniels, W.M.U. Dielectric constant and conductivity of blood plasma: Possible novel biomarkers for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2020, 2020, 5756382. [Google Scholar] [CrossRef]
- Gatta, V.; Mengod, G.; Reale, M.; Tata, A.M. Possible correlation between cholinergic system alterations and neuro/inflammation in multiple sclerosis. Biomedicines 2020, 8, 153. [Google Scholar] [CrossRef]
- Vitanova, K.S.; Stringer, K.M.; Benitez, D.P.; Brenton, J.; Cummings, D.M. Dementia associated with disorders of the basal ganglia. J. Neurosci. Res. 2019, 97, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L.; Lim, A.C.; Gordon, H.L.; Yarns, B.C.; Melrose, R.J. Cholinergic receptor binding in unimpaired older adults, mild cognitive impairment, and Alzheimer’s disease dementia. Alzheimer’s Res. Ther. 2022, 14, 25. [Google Scholar] [CrossRef]
- Lebois, E.P.; Thorn, C.; Edgerton, J.R.; Popiolek, M.; Xi, S. Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 2018, 136, 362–373. [Google Scholar] [CrossRef]
- Erskine, D.; Taylor, J.P.; Bakker, G.; Brown, A.J.H.; Tasker, T.; Nathan, P.J. Cholinergic muscarinic M1 and M4 receptors as therapeutic targets for cognitive, behavioural, and psychological symptoms in psychiatric and neurological disorders. Drug Discov. Today 2019, 24, 2307–2314. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, M.; Elder, G.A.; Sano, M.; Holtzman, D.M.; Gandy, S.; Cardozo, C.; Haroutunian, V.; Robakis, N.K.; Cai, D. Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer’s disease pathogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 11965–11970. [Google Scholar] [CrossRef]
- Sienski, G.; Narayan, P.; Bonner, J.M.; Kory, N.; Boland, S.; Arczewska, A.A.; Ralvenius, W.T.; Akay, L.; Lockshin, E.; He, L.; et al. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci. Transl. Med. 2021, 13, eaaz4564. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; Bagyinszky, E.; Youn, Y.C.; An, S.S.A.; Kim, S. APP, PSEN1, and PSEN2 mutations in Asian patients with early-onset Alzheimer disease. Int. J. Mol. Sci. 2019, 20, 4757. [Google Scholar] [CrossRef] [PubMed]
- Vilatela, M.E.A.; López-López, M.; Yescas-Gómez, P. Genetics of Alzheimer’s disease. Arch. Med. Res. 2012, 43, 622–631. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Heims-Waldron, D.; Bezzerides, V.; Guatimosim, S.; Guo, Y.; Gu, F.; Zhou, P.; Lin, Z.; Ma, Q.; et al. Mitochondrial cardiomyopathy caused by elevated reactive oxygen species and impaired cardiomyocyte proliferation. Circ. Res. 2018, 122, 74–87. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and reactive oxygen species in aging and age-related diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Gowda, P.; Reddy, P.H.; Kumar, S. Deregulated mitochondrial microRNAs in Alzheimer’s disease: Focus on synapse and mitochondria. Ageing Res. Rev. 2022, 73, 101529. [Google Scholar] [CrossRef]
- Huang, Y.R.; Liu, R.T. The toxicity and polymorphism of β-amyloid oligomers. Int. J. Mol. Sci. 2020, 21, 4477. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Qu, Y.Q.; Tang, Y.P.; Chen, X.; Lo, H.H.; Qu, L.Q.; Yun, Y.X.; Wong, V.K.W.; Zhang, R.L.; Wang, H.M.; et al. Hyperoside alleviates toxicity of β-amyloid via endoplasmic reticulum-mitochondrial calcium signal transduction cascade in APP/PS1 double transgenic Alzheimer’s disease mice. Redox Biol. 2023, 61, 102637. [Google Scholar] [CrossRef]
- Milane, L.; Dolare, S.; Jahan, T.; Amiji, M. Mitochondrial nanomedicine: Subcellular organelle-specific delivery of molecular medicines. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102422. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Inflammation in Alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 959–961. [Google Scholar] [CrossRef]
- Cichoń, N.; Lach, D.; Dziedzic, A.; Bijak, M.; Saluk, J. The inflammatory processes in atherogenesis. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2017, 42, 125–128. [Google Scholar]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Mauersberger, C.; Schunkert, H.; Sager, H. Inflammation-related risk loci in genome-wide association studies of coronary artery disease. Cells 2021, 10, 440. [Google Scholar] [CrossRef]
- Denver, P.; McClean, P.L. Distinguishing normal brain aging from the development of Alzheimer’s disease: Inflammation, insulin signaling and cognition. Neural Regen. Res. 2018, 13, 1719. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.H.; Zhao, X.J.; Xu, L.; Pan, C.L.; Zhang, Z.Y. Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling. J. Neuroinflammation 2020, 17, 61. [Google Scholar] [CrossRef]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 138–151. [Google Scholar]
- Mohamed Asik, R.; Suganthy, N.; Aarifa, M.A.; Kumar, A.; Szigeti, K.; Mathe, D.; Gulyás, B.; Archunan, G.; Padmanabhan, P. Alzheimer’s disease: A molecular view of β-amyloid induced morbific events. Biomedicines 2021, 9, 1126. [Google Scholar] [CrossRef]
- Leinonen, V.; Koivisto, A.M.; Savolainen, S.; Rummukainen, J.; Sutela, A.; Vanninen, R.; Jääskeläinen, J.E.; Soininen, H.; Alafuzoff, I. Post-mortem findings in 10 patients with presumed normal-pressure hydrocephalus and review of the literature. Neuropathol. Appl. Neurobiol. 2012, 38, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef]
- Nizami, S.; Hall-Roberts, H.; Warrier, S.; Cowley, S.A.; Di Daniel, E. Microglial inflammation and phagocytosis in Alzheimer’s disease: Potential therapeutic targets. Br. J. Pharmacol. 2019, 176, 3515–3532. [Google Scholar] [CrossRef]
- Streit, W.J.; Braak, H.; Del Tredici, K.; Leyh, J.; Lier, J.; Khoshbouei, H.; Eisenlöffel, C.; Müller, W.; Bechmann, I. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 2018, 66, 2550–2562. [Google Scholar] [CrossRef]
- Hommet, C.; Mondon, K.; Camus, V.; Ribeiro, M.J.; Beaufils, E.; Arlicot, N.; Corcia, P.; Paccalin, M.; Minier, F.; Gosselin, T.; et al. Neuroinflammation and β amyloid deposition in Alzheimer’s disease: In vivo quantification with molecular imaging. Dement. Geriatr. Cogn. Disord. 2014, 37, 1–18. [Google Scholar] [CrossRef]
- Tautou, M.; Descamps, F.; Larchanché, P.E.; Buée, L.; El Bakali, J.; Melnyk, P.; Sergeant, N.A. A Polyaminobiaryl-Based β-secretase Modulator Alleviates Cognitive Impairments, Amyloid Load, Astrogliosis, and Neuroinflammation in APPSwe/PSEN1ΔE9 Mice Model of Amyloid Pathology. Int. J. Mol. Sci. 2023, 24, 5285. [Google Scholar] [CrossRef]
- Van Acker, Z.P.; Perdok, A.; Bretou, M.; Annaert, W. The microglial lysosomal system in Alzheimer’s disease: Guardian against proteinopathy. Ageing Res. Rev. 2021, 71, 101444. [Google Scholar] [CrossRef] [PubMed]
- Solé-Domènech, S.; Cruz, D.L.; Capetillo-Zarate, E.; Maxfield, F.R. The endocytic pathway in microglia during health, aging and Alzheimer’s disease. Ageing Res. Rev. 2016, 32, 89–103. [Google Scholar] [CrossRef]
- Nazareth, L.; St John, J.; Murtaza, M.; Ekberg, J. Phagocytosis by peripheral glia: Importance for nervous system functions and implications in injury and disease. Front. Cell Dev. Biol. 2021, 9, 660259. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, E.E.; Green, K.N. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav. Immun. 2017, 61, 1–11. [Google Scholar] [CrossRef]
- Uddin, M.S.; Lim, L.W. Glial cells in Alzheimer’s disease: From neuropathological changes to therapeutic implications. Ageing Res. Rev. 2022, 78, 101622. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.E.; Keppler, K.; Steinbach, S.; Blazquez-Llorca, L.; Herms, J. Fibrillar amyloid plaque formation precedes microglial activation. PLoS ONE 2015, 10, e0119768. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Chansawhang, A.; Karinchai, J.; Phochantachinda, S.; Buranasinsup, S.; Inthachat, W.; Pitchakarn, P.; Chantong, B. Kaempferia parviflora Extracts Protect Neural Stem Cells from Amyloid Peptide-Mediated Inflammation in Co-Culture Model with Microglia. Nutrients 2023, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Liu, C.C.; Wang, S.; Cheng, H.C.; Meadows, C.; Chang, K.C. The Role of Aldose Reductase in Beta-Amyloid-Induced Microglia Activation. Int. J. Mol. Sci. 2022, 23, 15088. [Google Scholar] [CrossRef]
- Avila-Muñoz, E.; Arias, C. When astrocytes become harmful: Functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res. Rev. 2014, 18, 29–40. [Google Scholar] [CrossRef]
- Valenza, M.; Facchinetti, R.; Menegoni, G.; Steardo, L.; Scuderi, C. Alternative targets to fight Alzheimer’s disease: Focus on astrocytes. Biomolecules 2021, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.K.; Kirsch, W.M. From development to dysfunction: Microglia and the complement cascade in CNS homeostasis. Ageing Res. Rev. 2013, 12, 749–756. [Google Scholar] [CrossRef]
- Mcalpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 2021, 595, 701–706. [Google Scholar] [CrossRef]
- Das, S.; Li, Z.; Noori, A.; Hyman, B.T.; Serrano-Pozo, A. Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration. J. Neuroinflamm. 2020, 17, 227. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Tesh, V.L. Complement-mediated lipopolysaccharide release. In Endotoxin in Health and Disease; CRC Press: Boca Raton, FL, USA, 2020; pp. 77–91. [Google Scholar]
- Yaseen, S.; Demopulos, G.; Dudler, T.; Yabuki, M.; Wood, C.L.; Cummings, W.J.; Tjoelker, L.W.; Fujita, T.; Sacks, S.; Garred, P.; et al. Lectin pathway effector enzyme mannan-binding lectin-associated serine protease-2 can activate native complement C3 in absence of C4 and/or C2. FASEB J. 2017, 31, 2210–2219. [Google Scholar] [CrossRef]
- Molins, B.; Romero-Vázquez, S.; Fuentes-Prior, P.; Adan, A.; Dick, A.D. C-reactive protein as a therapeutic target in age-related macular degeneration. Front. Immunol. 2018, 9, 808. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Tacnet-Delorme, P.; Chevallier, S.; Arlaud, G.J. β-amyloid fibrils activate the C1 complex of complement under physiological conditions: Evidence for a binding site for Aβ on the C1q globular regions. J. Immunol. 2001, 167, 6374–6381. [Google Scholar] [CrossRef] [PubMed]
- Cedzynski, M.; Swierzko, A.S. Components of the Lectin Pathway of Complement in Solid Tumour Cancers. Cancers 2022, 14, 1543. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef]
- Sung, P.-S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and neurogenesis in Alzheimer’s disease and potential therapeutic approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef]
- Kretzschmar, G.C.; Bumiller-Bini, V.; Gasparetto Filho, M.A.; Zonta, Y.R.; Yu, K.S.T.; de Souza, R.L.R.; Dias-Melicio, L.A.; Boldt, A.B.W. Neutrophil extracellular traps: A perspective of neuroinflammation and complement activation in Alzheimer’s disease. Front. Mol. Biosci. 2021, 8, 630869. [Google Scholar] [CrossRef]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef]
- Wang, Y.B.; Yi-Bin, W.; Xiang, L.; Bing, Y.; Qi, Z.; Fei-Tong, J.; Minghong, W.; Xiangxiang, Z.; Le, K.; Yan, L.; et al. Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis. 2022, 13, 318. [Google Scholar]
- Song, C.; Zhang, Y.; Dong, Y. Acute and subacute IL-1β administrations differentially modulate neuroimmune and neurotrophic systems: Possible implications for neuroprotection and neurodegeneration. J. Neuroinflamm. 2013, 10, 826. [Google Scholar] [CrossRef]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s disease: Pathological significance and molecular pathways. Cells 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Peng, W.; Lu, W.; Jiang, X.; Xiong, C.; Chai, H.; Cai, L.; Lan, Z. Current Progress on Neuroinflammation-mediated Postoperative Cognitive Dysfunction: An Update. Curr. Mol. Med. 2023; in press. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- West, P.K.; McCorkindale, A.N.; Guennewig, B.; Ashhurst, T.M.; Viengkhou, B.; Hayashida, E.; Jung, S.R.; Butovsky, O.; Campbell, I.L.; Hofer, M.J. The cytokines interleukin-6 and interferon-α induce distinct microglia phenotypes. J. Neuroinflamm. 2022, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Haddick, P.C.G.; Larson, J.L.; Rathore, N.; Bhangale, T.R.; Phung, Q.T.; Srinivasan, K.; Hansen, D.V.; Lill, J.R.; Alzheimer’s Disease Genetic Consortium (ADGC); Alzheimer’s Disease Neuroimaging Initiative (ADNI); et al. A common variant of IL-6R is associated with elevated IL-6 pathway activity in Alzheimer’s disease brains. J. Alzheimer’s Dis. 2017, 56, 1037–1054. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef]
- Batista, J.A.; Magalhães, D.A.; Sousa, S.G.; Ferreira, J.D.S.; Pereira, C.M.C.; Lima, J.V.D.N.; de Albuquerque, I.F.; Bezerra, N.L.S.D.; de Brito, T.V.; Monteiro, C.E.D.S.; et al. Polysaccharides derived from Morinda citrifolia Linn reduce inflammatory markers during experimental colitis. J. Ethnopharmacol. 2020, 248, 112303. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xian, X.; Xu, G.; Tan, Z.; Dong, F.; Zhang, M.; Zhang, F. Toll-Like Receptor 4: A Promising Therapeutic Target for Alzheimer’s Disease. Mediat. Inflamm. 2022, 2022, 7924199. [Google Scholar] [CrossRef] [PubMed]
- Fuellen, G.; Liesenfeld, O.; Kowald, A.; Barrantes, I.; Bastian, M.; Simm, A.; Jansen, L.; Tietz-Latza, A.; Quandt, D.; Franceschi, C.; et al. The preventive strategy for pandemics in the elderly is to collect in advance samples & data to counteract chronic inflammation (inflammaging). Ageing Res. Rev. 2020, 62, 101091. [Google Scholar] [PubMed]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflam-mation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Uyar, B.; Palmer, D.; Kowald, A.; Murua Escobar, H.; Barrantes, I.; Möller, S.; Akalin, A.; Fuellen, G. Single-cell analyses of aging, inflammation and senescence. Ageing Res. Rev. 2020, 64, 101156. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Irwin, M.R.; Vitiello, M.V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019, 18, 296–306. [Google Scholar] [CrossRef]
- Muzyka, B.C.; Christie, J.; Collins, B. Laboratory Medicine and Diagnostic Pathology. In Burket’s Oral Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 1037–1058. [Google Scholar]
- Peng, Y.; Gao, P.; Shi, L.; Chen, L.; Liu, J.; Long, J. Central and peripheral metabolic defects contribute to the pathogenesis of Alzheimer’s disease: Targeting mitochondria for diagnosis and prevention. Antioxid. Redox Signal. 2020, 32, 1188–1236. [Google Scholar] [CrossRef]
- Martins, D.A.; Lopes, J.; Martins da Silva, A.; Morais, C.I.; Vasconcelo, J.; Lima, I.; Carneiro, C.; Neves, E. Kappa free light chains: Diagnostic performance in multiple sclerosis and utility in a clinical laboratory. Clin. Chim. Acta 2022, 528, 56–64. [Google Scholar] [CrossRef]
- Tsatsanis, A.; McCorkindale, A.N.; Wong, B.X.; Patrick, E.; Ryan, T.M.; Evans, R.W.; Bush, A.I.; Sutherland, G.T.; Sivaprasadarao, A.; Guennewig, B.; et al. The acute phase protein lactoferrin is a key feature of Alzheimer’s disease and predictor of Aβ burden through induction of APP amyloidogenic processing. Mol. Psychiatry 2021, 26, 5516–5531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Sheng, H.; Li, H.; Wang, R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Chem. 2019, 90, 25–80. [Google Scholar]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383. [Google Scholar] [CrossRef]
- Park, J.-C.; Han, S.-H.; Mook-Jung, I. Peripheral inflammatory biomarkers in Alzheimer’s disease: A brief review. BMB Rep. 2020, 53, 10. [Google Scholar] [CrossRef]
- Stocker, M.; van Herk, W.; El Helou, S.; Dutta, S.; Schuerman, F.A.B.A.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; Moonen, R.; et al. C-reactive protein, procalcitonin, and white blood count to rule out neonatal early-onset sepsis within 36 hours: A secondary analysis of the neonatal procalcitonin intervention study. Clin. Infect. Dis. 2021, 73, e383–e390. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.L.; Poulsen, H.E.; Michaelsen, C.; Kjær, L.K.; Lyngbæk, M.; Andersen, E.S.; Petersen-Bønding, C.; Lemoine, C.; Gillum, M.; Jørgensen, N.R.; et al. Differential time responses in inflammatory and oxidative stress markers after a marathon: An observational study. J. Sport. Sci. 2020, 38, 2080–2091. [Google Scholar] [CrossRef]
- Grondman, I.; Pirvu, A.; Riza, A.; Ioana, M.; Netea, M.G. Biomarkers of inflammation and the etiology of sepsis. Biochem. Soc. Trans. 2020, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Afsana Mim, S.; Afroza Alam Tumpa, M.; Taslim Sarker, M.; Ahmed, M.; Alghamdi, B.S.; Hafeez, A.; Alexiou, A.; Perveen, A.; Md Ashraf, G. Exploring the management approaches of cytokines including viral infection and neuroinflammation for neurological disorders. Cytokine 2022, 157, 155962. [Google Scholar] [CrossRef]
- Imai, R.; Imai, R.; Hori, H.; Itoh, M.; Lin, M.; Niwa, M.; Ino, K.; Ogawa, S.; Ishida, M.; Sekiguchi, A.; et al. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J. Psychiatr. Res. 2018, 102, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, P.; Weinbeer, J.; Herrmann, M.; Oberstein, T.J.; Condic, M.; Lewczuk, P.; Kornhuber, J.; Maler, J.M. Analysis of surface levels of IL-1 receptors and macrophage scavenger receptor I in peripheral immune cells of patients with Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2019, 32, 211–220. [Google Scholar] [CrossRef]
- Prasad, K. AGE–RAGE stress: A changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell. Biochem. 2019, 459, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Terrill-Usery, S.E.; Mohan, M.J.; Nichols, M.R. Amyloid-β (1-42) protofibrils stimulate a quantum of secreted IL-1β despite significant intracellular IL-1β accumulation in microglia. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 2276–2285. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Szydłowska, A.; Skupień, A.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin—A future therapy for neurodegenerative diseases: Theme: Drug discovery, development and delivery in Alzheimer’s disease Guest Editor: Davide Brambilla. Pharm. Res. 2017, 34, 2614–2627. [Google Scholar] [CrossRef]
- Guo, H.; Cao, H.; Cui, X.; Zheng, W.; Wang, S.; Yu, J.; Chen, Z. Silymarin’s inhibition and treatment effects for Alzheimer’s disease. Molecules 2019, 24, 1748. [Google Scholar] [CrossRef]
- Cichacz-Kwiatkowska, B.; Sekita-Krzak, J.; Kot-Bakiera, K.; Jodłowska-Jędrych, B.; Wawryk-Gawda, E. Choroba Alzheimera—Rola badań immunohistochemicznych w diagnostyce choroby. J. Educ. Health Sport 2016, 6, 122–137. [Google Scholar]
- Gholami, M.D.; Sonar, P.; Ayoko, G.A.; Izake, E.L. A highly sensitive SERS quenching nanosensor for the determination of tumor necrosis factor alpha in blood. Sens. Actuators B Chem. 2020, 310, 127867. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Electrochemical immunosensors for the detection of cytokine tumor necrosis factor alpha: A review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Alzheimer’s disease and chronic periodontitis: Is there an association? Geriatr. Gerontol. Int. 2015, 15, 391–404. [Google Scholar] [CrossRef]
- Kisuya, J.; Chemtai, A.; Raballah, E.; Keter, A.; Ouma, C. The diagnostic accuracy of Th1 (IFN-γ, TNF-α, and IL-2) and Th2 (IL-4, IL-6 and IL-10) cytokines response in AFB microscopy smear negative PTB-HIV co-infected patients. Sci. Rep. 2019, 9, 2966. [Google Scholar] [CrossRef]
- Bashir, H.; Ahmad Bhat, S.; Majid, S.; Hamid, R.; Koul, R.K.; Rehman, M.U.; Din, I.; Ahmad Bhat, J.; Qadir, J.; Masood, A. Role of inflammatory mediators (TNF-α, IL-6, CRP), biochemical and hematological parameters in type 2 diabetes mellitus patients of Kashmir, India. Med. J. Islam. Repub. Iran 2020, 34, 5. [Google Scholar] [CrossRef]
- Liu, C.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF cytokines in aging, multiple sclerosis, and dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Jensen, C.S.; Bahl, J.M.; Østergaard, L.B.; Høgh, P.; Wermuth, L.; Heslegrave, A.; Zetterberg, H.; Heegaard, N.H.H.; Hasselbalch, S.G.; Simonsen, A.H. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 2019, 121, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Goetzl, E.J.; Schwartz, J.B.; Elahi, F.M.; Rissman, R.A. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 61–66. [Google Scholar] [CrossRef]
- Dahmani, M.; Cook, J.H.; Zhu, J.C.; Riley, S.P. Contribution of classical complement activation and IgM to the control of Rickettsia infection. Mol. Microbiol. 2021, 116, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Costabel, U.; McDowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical detection of potential microbial antigens in granulomas in the diagnosis of sarcoidosis. J. Clin. Med. 2021, 10, 983. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, C.; Mohiuddin, T.M.; Al-Rawe, M.; Zeppernick, F.; Falcone, F.H.; Meinhold-Heerlein, I.; Hussain, A.F. Multiplex Immunofluorescence: A Powerful Tool in Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 3086. [Google Scholar] [CrossRef] [PubMed]
- Karle, A.C.; Wrobel, M.B.; Koepke, S.; Gutknecht, M.; Gottlieb, S.; Christen, B.; Rubic-Schneider, T.; Pruimboom-Brees, I.; Leber, X.C.; Scharenberg, M.; et al. Anti-brolucizumab immune response as one prerequisite for rare retinal vasculitis/retinal vascular occlusion adverse events. Sci. Transl. Med. 2023, 15, eabq5241. [Google Scholar] [CrossRef]
- Mohsenian Sisakht, A.; Karamzade-Ziarati, N.; Jahanbakhshi, A.; Shahpasand, K.; Aghababaei, S.; Ahmadvand, O.; Azar, M.; Fattahi, A.; Zamanzadeh, S. Pathogenic cis p-tau levels in CSF reflects severity of traumatic brain injury. Neurol. Res. 2022, 44, 496–502. [Google Scholar] [CrossRef]
- Sharoar, M.G.; Palko, S.; Ge, Y.; Saido, T.C.; Yan, R. Accumulation of saposin in dystrophic neurites is linked to impaired lysosomal functions in Alzheimer’s disease brains. Mol. Neurodegener. 2021, 16, 45. [Google Scholar] [CrossRef]
- Mittal, P.; Singh, N.; Chaturvedi, S.; Jyoti, A.; Mishra, A.K.; Hazari, P.P. Comprehensive review on design perspective of PET ligands based on β-amyloids, tau and neuroinflammation for diagnostic intervention of Alzheimer’s disease. Clin. Transl. Imaging 2021, 9, 153–175. [Google Scholar] [CrossRef]
- Barton, S.M.; To, E.; Rogers, B.P.; Whitmore, C.; Uppal, M.; Matsubara, J.A.; Pham, W. Inhalable thioflavin S for the detection of amyloid beta deposits in the retina. Molecules 2021, 26, 835. [Google Scholar] [CrossRef]
- Veerhuis, R.; Van Breemen, M.J.; Hoozemans, J.M.; Morbin, M.; Ouladhadj, J.; Tagliavini, F.; Eikelenboom, P. Amyloid β plaque-associated proteins C1q and SAP enhance the Aβ 1–42 peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol. 2003, 105, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Elmaleh, D.R.; Farlow, M.R.; Conti, P.S.; Tompkins, R.G.; Kundakovic, L.; Tanzi, R.E. Developing effective Alzheimer’s disease therapies: Clinical experience and future directions. J. Alzheimer’s Dis. 2019, 71, 715–732. [Google Scholar] [CrossRef]
- Huang, J.; Tao, Q.; Ang, T.F.A.; Farrell, J.; Zhu, C.; Wang, Y.; Stein, T.D.; Lunetta, K.L.; Massaro, J.; Mez, J.; et al. The impact of increasing levels of blood C-reactive protein on the inflammatory loci SPI1 and CD33 in Alzheimer’s disease. Transl. Psychiatry 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, B.; Dwyer, A.; Grenyer, R.; Hodgetts, T.; McLeod, C.; Lorimer, J. Unsettling antibiosis: How might interdisciplinary researchers generate a feeling for the microbiome and to what effect? Palgrave Commun. 2018, 4, 149. [Google Scholar] [CrossRef]
- Friker, L.L.; Scheiblich, H.; Hochheiser, I.V.; Brinkschulte, R.; Riedel, D.; Latz, E.; Geyer, M.; Heneka, M.T. β-amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Rep. 2020, 30, 3743–3754.e6. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.L.; Rusz, D.; Jones, M.L.; Farm-Franks, D.; Jones, B.; Cyr Gluck, J.; Thomas, D.B.; Gleason, K.T.; Welte, K.; Abfalter, J.; et al. The new diagnostic team. Diagnosis 2017, 4, 225–238. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Fernández-Pérez, I.; Espinosa-Fernández, V.; Sánchez-Pérez, A.M.; Ledesma, J.C. Can we treat neuroinflammation in Alzheimer’s disease? Int. J. Mol. Sci. 2020, 21, 8751. [Google Scholar] [CrossRef]
- Scearce-Levie, K.; Sanchez, P.E.; Lewcock, J.W. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat. Rev. Drug Discov. 2020, 19, 447–462. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Isik, A.T.; Soysal, P.; Solmi, M.; Veronese, N. Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer’s disease: A narrative review. Int. J. Geriatr. Psychiatry 2019, 34, 1326–1334. [Google Scholar] [CrossRef]
- Gupta, S.P.; Patil, V.M. Recent studies on design and development of drugs against Alzheimer’s disease (AD) based on inhibition of BACE-1 and other AD-causative agents. Curr. Top. Med. Chem. 2020, 20, 1195–1213. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.F.L.; Cardoso, C.L.; Mateo, C. An immobilized acetylcholinesterase as test system to screen new inhibitor drugs to treat Alzheimer’s disease. Sens. Actuators B Chem. 2019, 278, 196–201. [Google Scholar] [CrossRef]
- Bidzan, L. Farmakologiczne leczenie choroby Alzheimera—Współczesne możliwości. Psychiatria 2020, 17, 87–94. [Google Scholar] [CrossRef]
- Balázs, N.; Bereczki, D.; Ajtay, A.; Oberfrank, F.; Kovács, T. Cholinesterase inhibitors for the treatment of dementia: Real-life data in Hungary. GeroScience 2022, 44, 253–263. [Google Scholar] [CrossRef]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef]
- Niznik, J. Deprescribing of Acetylcholinesterase Inhibitors in Older Adult Nursing Home Residents with Severe Dementia. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2019. [Google Scholar]
- Zaręba, N.; Kepinska, M. The function of transthyretin complexes with metallothionein in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 9003. [Google Scholar] [CrossRef]
- Macleod, R.; Hillert, E.K.; Cameron, R.T.; Baillie, G.S. The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer’s disease. Future Sci. OA 2015, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Z.P.; Song, C.G.; Liu, L.; Zhao, Y.; Du, J.L.; Lai, Y.B.; Cao, X.L.; Ye, W.M.; Zhang, Y.F.; et al. γ-secretase inhibitor disturbs the morphological development of differentiating neurons through affecting Notch/miR-342-5p. Neurosci. Lett. 2022, 778, 136603. [Google Scholar] [CrossRef]
- Jo, D.-G.; Arumugam, T.V.; Woo, H.N.; Park, J.-S.; Tang, S.-C.; Mughal, M.; Hyun, D.-H.; Park, J.-H.; Choi, Y.-H.; Gwon, A.-R.; et al. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 917–925. [Google Scholar] [CrossRef]
- Ugbaja, S.C.; Sanusi, Z.K.; Appiah-Kubi, P.; Lawal, M.M.; Kumalo, H.M. Computational modelling of potent β-secretase (BACE1) inhibitors towards Alzheimer’s disease treatment. Biophys. Chem. 2021, 270, 106536. [Google Scholar] [CrossRef] [PubMed]
- Szaruga, M.; Munteanu, B.; Lismont, S.; Veugelen, S.; Horré, K.; Mercken, M.; Saido, T.C.; Ryan, N.S.; De Vos, T.; Savvides, S.N.; et al. Alzheimer’s-causing mutations shift Aβ length by destabilizing γ-secretase-Aβn interactions. Cell 2021, 184, 2257–2258. [Google Scholar] [CrossRef]
- Srivastava, K.; Tiwari, M.; Dubey, A.; Dubey, A. D-Pinitol-A Natural Phytomolecule and its Pharmacological effect. Int. J. Pharm. Life Sci. 2020, 11, 6609–6623. [Google Scholar]
- Shi, L.; Yu, X.T.; Li, H.; Wu, G.S.; Luo, H.R. D-chiro-inositol increases antioxidant capacity and longevity of Caenorhabditis elegans via activating Nrf-2/SKN-1 and FOXO/DAF-16. Exp. Gerontol. 2023, 175, 112145. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Meng, Y.; Yan, X.-J.; Liu, S.; Wang, G.-Q.; Cao, Y.-P. Immunization with Aβ3-10-KLH vaccine improves cognitive function and ameliorates mitochondrial dysfunction and reduces Alzheimer’s disease-like pathology in Tg-APPswe/PSEN1dE9 mice. Brain Res. Bull. 2021, 174, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Ye, C.; Tian, C.; Zhao, D.; Li, H.; Sun, Z.; Miao, Y.; Zhang, Q.; Wang, J.; Dou, Y. Engineered macrophage-biomimetic versatile nanoantidotes for inflammation-targeted therapy against Alzheimer’s disease by neurotoxin neutralization and immune recognition suppression. Bioact. Mater. 2023, 26, 337–352. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Seripa, D.; Imbimbo, B.P. Amyloid-β immunotherapy for alzheimer disease: Is it now a long shot? Ann. Neurol. 2019, 85, 303–315. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef]
- Santos, J.; Lobato, L.; Vale, N. Clinical pharmacokinetic study of latrepirdine via in silico sublingual administration. Silico Pharmacol. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, R.; Bochon, B.; Piontek, A.; Kunert, Ł.; Sobiś, J.; Gorczyca, P.W. Choroba Alzheimera—Nowe strategie leczenia. Psychiatria 2016, 13, 210–214. [Google Scholar]
- Nirogi, R.; Jayarajan, P.; Shinde, A.; Mohammed, A.R.; Grandhi, V.R.; Benade, V.; Goyal, V.K.; Abraham, R.; Jasti, V.; Cummings, J. Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules 2023, 13, 309. [Google Scholar] [CrossRef]
- Qin, C.; Wang, K.; Bai, L.; Shi, G.; Huang, Y.; Li, Y. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: A meta-analytic review on potential mechanisms. Transl. Neurodegener. 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Páez, A. Emerging insights into the role of albumin with plasma exchange in Alzheimer’s disease management. Transfus. Apher. Sci. 2021, 60, 103164. [Google Scholar] [CrossRef]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Shyamasundar, S.; Patnala, R.; Karthikeyan, A.; Arumugam, T.V.; Ling, E.A.; Dheen, S.T. Recent progress in therapeutic strategies for microglia-mediated neuroinflammation in neu-ropathologies. Expert Opin. Ther. Targets 2018, 22, 765–781. [Google Scholar] [CrossRef]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. BioMed Res. Int. 2014, 2014, 309129. [Google Scholar] [CrossRef]
- Kellar, D.; Register, T.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal insulin modulates cerebrospinal fluid markers of neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A randomized trial. Sci. Rep. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Rather, M.A.; Khan, A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Rashid, S.; Alsaffar, R.M.; Kamal, M.A.; Rehman, M.U. Inflammation and Alzheimer’s disease: Mechanisms and therapeutic implications by natural products. Mediat. Inflamm. 2021, 2021, 9982954. [Google Scholar] [CrossRef]
- Ren, S.; Breuillaud, L.; Yao, W.; Yin, T.; Norris, K.A.; Zehntner, S.P.; D’Adamio, L. TNF-α–mediated reduction in inhibitory neurotransmission precedes sporadic Alzheimer’s disease pathology in young Trem2R47H rats. J. Biol. Chem. 2021, 296, 100089. [Google Scholar] [CrossRef]
- Yedke, N.G.; Kumar, P. The Neuroprotective Role of BCG Vaccine in Movement Disorders: A Review. CNS Neurol. Disord. Drug Targets 2023, in press. [Google Scholar] [CrossRef]
- Guha, A.; Husain, M.A.; Si, Y.; Nabors, L.B.; Filippova, N.; Promer, G.; Smith, R.; King, P.H. RNA regulation of inflammatory responses in glia and its potential as a therapeutic target in central nervous system disorders. Glia 2023, 71, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Hirtz, C.; Lehmann, S. Overview of the blood biomarkers in Alzheimer’s disease: Promises and challenges. Rev. Neurol. 2022, 179, 161–172. [Google Scholar] [CrossRef]
- Ou, W.; Yang, J.; Simanauskaite, J.; Choi, M.; Castellanos, D.M.; Chang, R.; Sun, J.; Jagadeesan, N.; Parfitt, K.D.; Cribbs, D.H.; et al. Biologic TNF-α inhibitors reduce microgliosis, neuronal loss, and tau phosphorylation in a transgenic mouse model of tauopathy. J. Neuroinflamm. 2021, 18, 312. [Google Scholar] [CrossRef]
- Zagórska, A.; Czopek, A.; Fryc, M.; Jaromin, A.; Boyd, B.J. Drug Discovery and Development Targeting Dementia. Pharmaceuticals 2023, 16, 151. [Google Scholar] [CrossRef]
- Hosseini-Chegeni, A.; Jazaeri, F.; Yousefi-Ahmadipour, A.; Heidari, M.; Abdollahie, A.; Dehpour, A.R. Thalidomide attenuates the hyporesponsiveness of isolated atria to chronotropic stimulation in BDL rats: The involvement of TNF-α, IL-6 inhibition, and SOCS1 activation. Iran. J. Basic Med. Sci. 2019, 22, 1259. [Google Scholar] [PubMed]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflamma-tion for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef]
- Deng, M.-Y.; Ahmad, K.A.; Han, Q.Q.; Wang, Z.Y.; Shoaib, R.M.; Li, X.Y.; Wang, Y.X. Thalidomide alleviates neuropathic pain through microglial IL-10/β-endorphin signaling pathway. Biochem. Pharmacol. 2021, 192, 114727. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Fatima, M.; Mondal, A.C. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: Rational insights for the therapeutic approaches. J. Clin. Neurosci. 2019, 59, 6–11. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 family cytokines in cancer and immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Lalgudi, V.G.; Shetty, R.; Nischal, K.K.; Ziai, S.; Koaik, M.; Sethu, S. Biochemical and molecular alterations and potential clinical applications of biomarkers in keratoconus. Saudi J. Ophthalmol. 2022, 36, 7. [Google Scholar]
- Ahmadian, K.M.; Cabañas, N.S.; Herrera, C.C.; de Arizon, L.F.; Mir, M.P.; Perich, L.G.; Molas, C.F. Assessment of Tacrolimus Neurotoxicity Measured by Retinal OCT. Transplant. Proc. 2022, 54, 80–86. [Google Scholar] [CrossRef]
- Meyer, N.; Brodowski, L.; von Kaisenberg, C.; Schröder-Heurich, B.; von Versen-Höynck, F. Cyclosporine A and tacrolimus induce functional impairment and inflammatory reactions in endothelial progenitor cells. Int. J. Mol. Sci. 2021, 22, 9696. [Google Scholar] [CrossRef]
- Ramos Campos, E.V.; Proença, P.L.D.F.; Doretto-Silva, L.; Andrade-Oliveira, V.; Fraceto, L.F.; de Araujo, D.R. Trends in nanoformulations for atopic dermatitis treatment. Expert Opin. Drug Deliv. 2020, 17, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Gomez, W.; Morales, R.; Maracaja-Coutinho, V.; Parra, V.; Nassif, M. Down syndrome and Alzheimer’s disease: Common molecular traits beyond the amyloid precursor protein. Aging 2020, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mangano, K.; Martino, G.; Quattropani, M.C.; Pennisi, M.; Bella, R.; Fisicaro, F.; Nicoletti, F.; Petralia, M.C. Characterization of Altered Molecular Pathways in the Entorhinal Cortex of Alzheimer’s Disease Patients and In Silico Prediction of Potential Repurposable Drugs. Genes 2022, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.M.; Esmael, A.; Nassar, A.K.; El-Sherif, M. The relationship between exacerbated diabetic peripheral neuropathy and metformin treatment in type 2 diabetes mellitus. Sci. Rep. 2021, 11, 1940. [Google Scholar] [CrossRef]
- Fu, W.-Y.; Wang, X.; Ip, N.Y. Targeting neuroinflammation as a therapeutic strategy for Alzheimer’s disease: Mechanisms, drug candidates, and new opportunities. ACS Chem. Neurosci. 2018, 10, 872–879. [Google Scholar] [CrossRef]
- Maldonado, M.; Romero-Aibar, J.; Calvo, J. The melatonin contained in beer can provide health benefits, due to its antioxidant, anti-inflammatory and immunomodulatory properties. J. Sci. Food Agric. 2022; in press. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin prevents the dysbiosis of intestinal microbiota in sleep-restricted mice by improving oxidative stress and inhibiting inflammation. Saudi J. Gastroenterol. 2022, 28, 209. [Google Scholar]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s disease, inflammation, and the role of antioxidants. J. Alzheimer’s Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Ardura-Fabregat, A.; Boddeke, E.W.G.M.; Boza-Serrano, A.; Brioschi, S.; Castro-Gomez, S.; Ceyzériat, K.; Dansokho, C.; Dierkes, T.; Gelders, G.; Heneka, M.T.; et al. Targeting neuroinflammation to treat Alzheimer’s disease. CNS Drugs 2017, 31, 1057–1082. [Google Scholar] [CrossRef]
- Kapileshwar, J. Formulation and Evaluation of Flurbiprofen Nanoparticles. Ph.D. Thesis, Jaya College of Paramedical Sciences, Chennai, India, 2019. [Google Scholar]
- Hassan, M.; Ismail, H.; Hammam, O.; Elsayed, A.; Othman, O.; Aly, S. Natural inhibitors for acetylcholinesterase and autophagy modulators as effective antagonists for tau and β-amyloid in Alzheimer’s rat model. Biomarkers 2022, in press. [Google Scholar] [CrossRef]
- Fišar, Z. Linking the Amyloid, Tau, and Mitochondrial Hypotheses of Alzheimer’s Disease and Identifying Promising Drug Targets. Biomolecules 2022, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Binert-Kusztal, Ż.; Starek, M.; Dąbrowska, M. Choroby neurodegeneracyjne—Aspekt farmakoterapeutyczny choroby Alzheimera. Farm. Pol. 2021, 77, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Hathout, R.M.; El-Ahmady, S.; Metwally, A. Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. Nat. Prod. Res. 2018, 32, 2873–2881. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, Y.; Li, Y.P.; Chen, S.H.; Tan, J.H.; Ou, T.M.; Gu, L.Q.; Huang, Z.S. Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2011, 19, 5596–5604. [Google Scholar] [CrossRef]
- Shinzato, T.; Sato, R.; Suzuki, K.; Tomioka, S.; Sogawa, H.; Shulga, S.; Kurita, N. Proposal of therapeutic curcumin derivatives for Alzheimer’s disease based on ab initio molecular simulations. Chem. Phys. Lett. 2020, 738, 136883. [Google Scholar] [CrossRef]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer pathogenesis. Neurobiol. Dis. 2019, 127, 432–448. [Google Scholar] [CrossRef]
- Kałużna, A.; Olczyk, P.; Komosińska-Vassev, K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J. Clin. Med. 2022, 11, 400. [Google Scholar] [CrossRef]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin prevents acute neuroinflammation and long-term memory impairment induced by systemic lipopolysaccharide in mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Shukla, S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1190. [Google Scholar] [PubMed]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy: Possible therapeutic role of curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. https://doi.org/10.3390/ijms24076518
Twarowski B, Herbet M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. International Journal of Molecular Sciences. 2023; 24(7):6518. https://doi.org/10.3390/ijms24076518
Chicago/Turabian StyleTwarowski, Bartosz, and Mariola Herbet. 2023. "Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review" International Journal of Molecular Sciences 24, no. 7: 6518. https://doi.org/10.3390/ijms24076518
APA StyleTwarowski, B., & Herbet, M. (2023). Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. International Journal of Molecular Sciences, 24(7), 6518. https://doi.org/10.3390/ijms24076518





