An Acidic Heteropolysaccharide Isolated from Pueraria lobata and Its Bioactivities
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
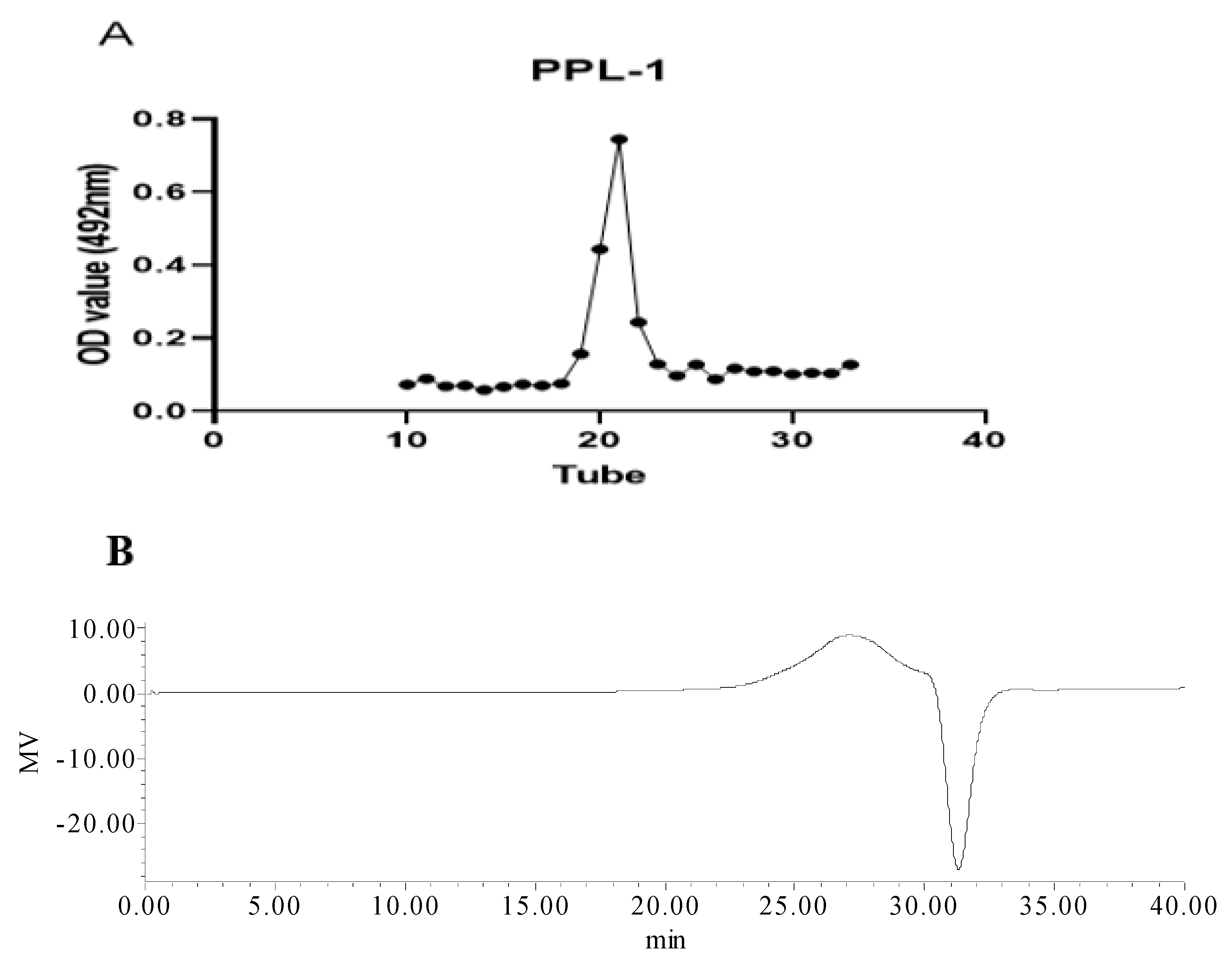
2.1.1. Isolation and Colourimetric Analysis of PPL-1
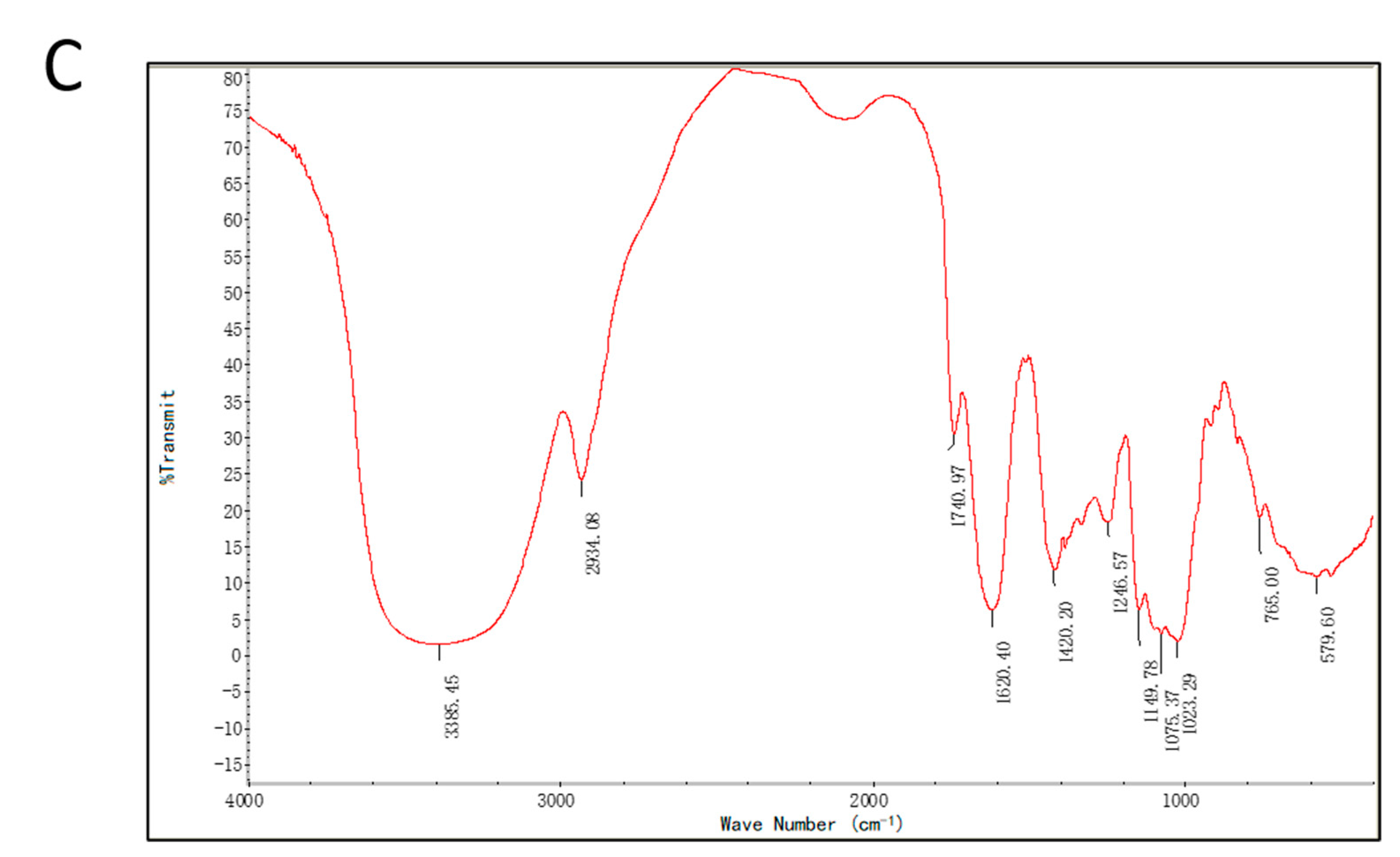
2.1.2. Average Mw and FTIR Spectrum of PPL-1
2.1.3. Monosaccharide Composition Analysis of PPL-1
2.1.4. Glycosidic Linkages of PPL-1
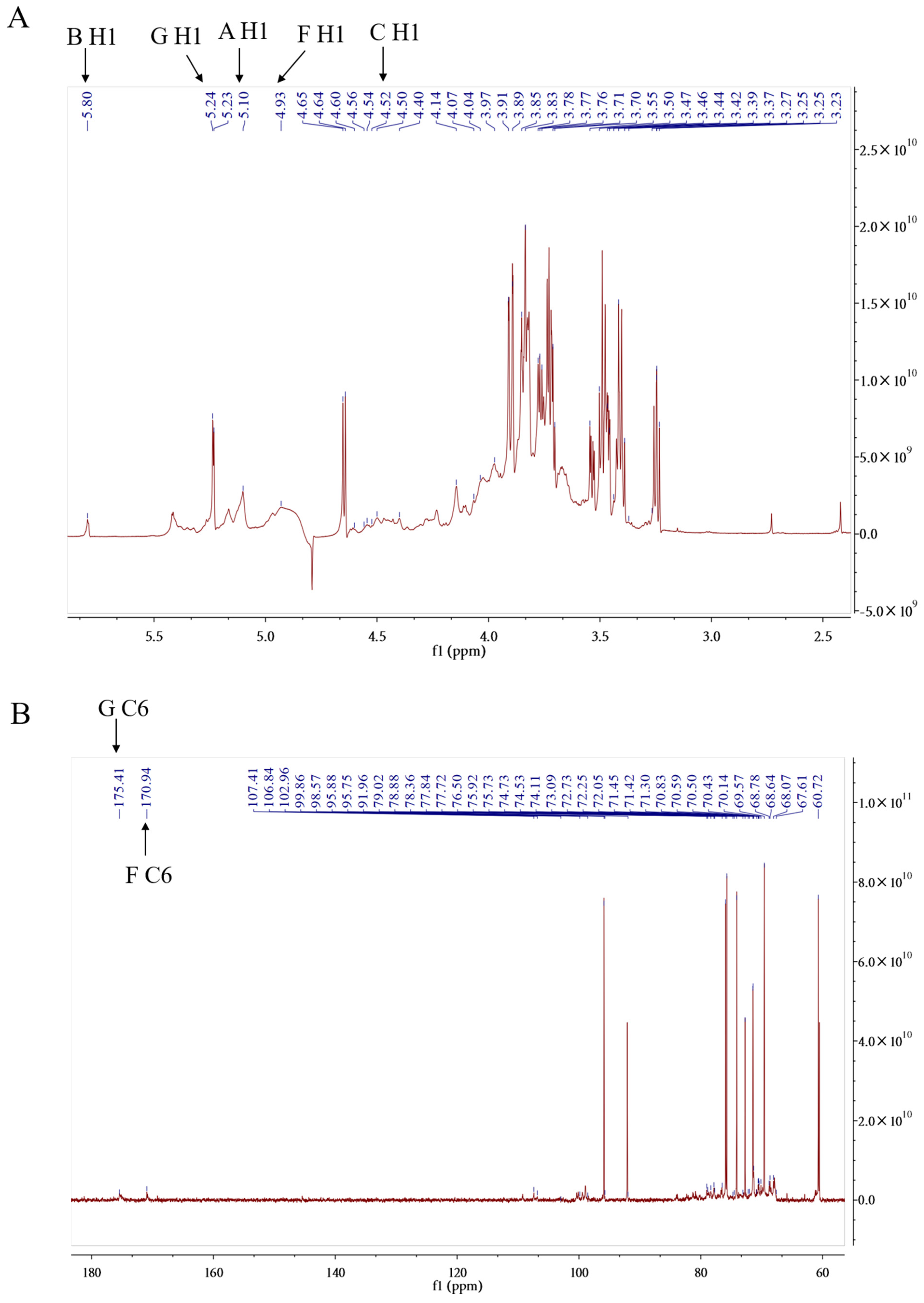
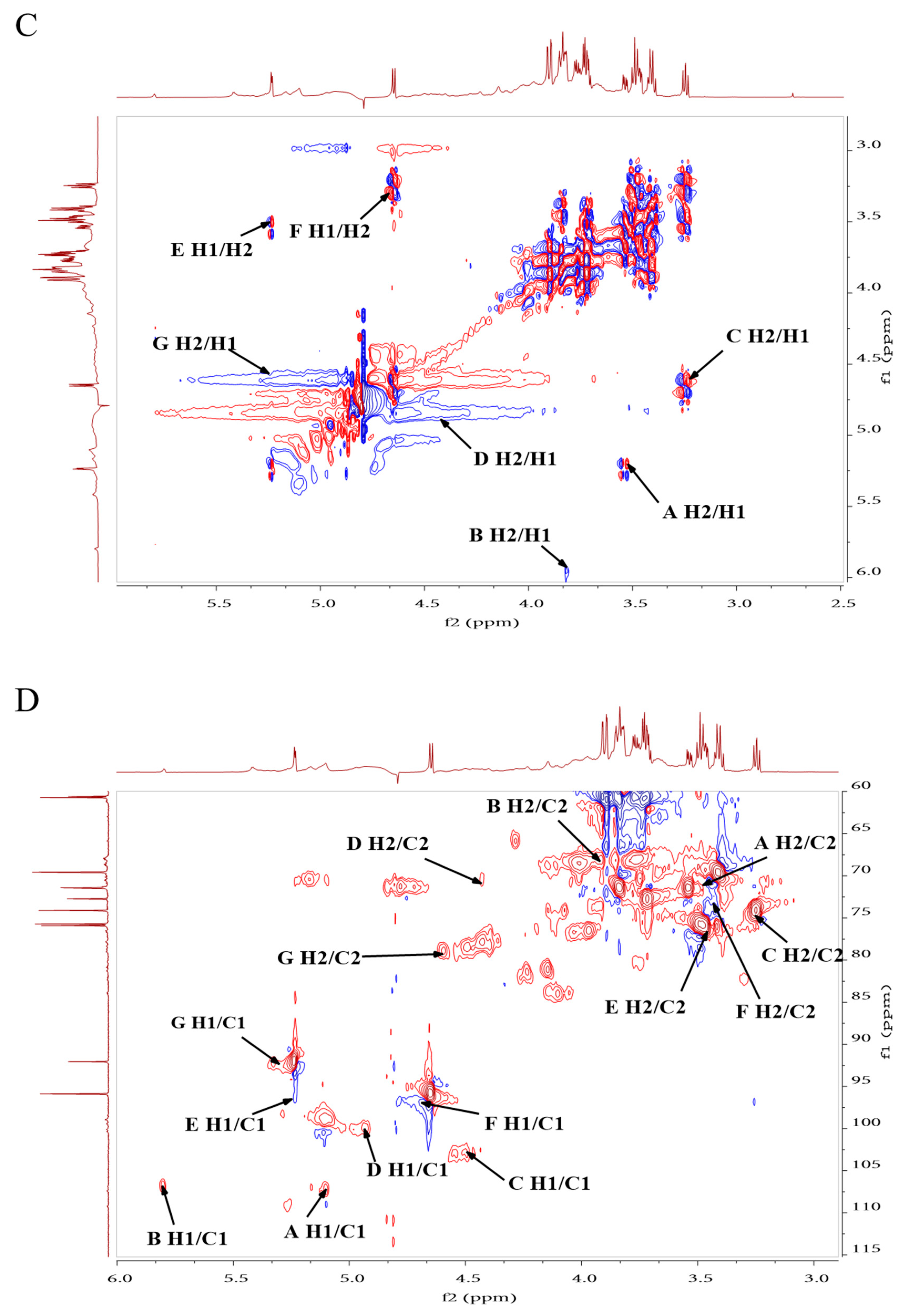
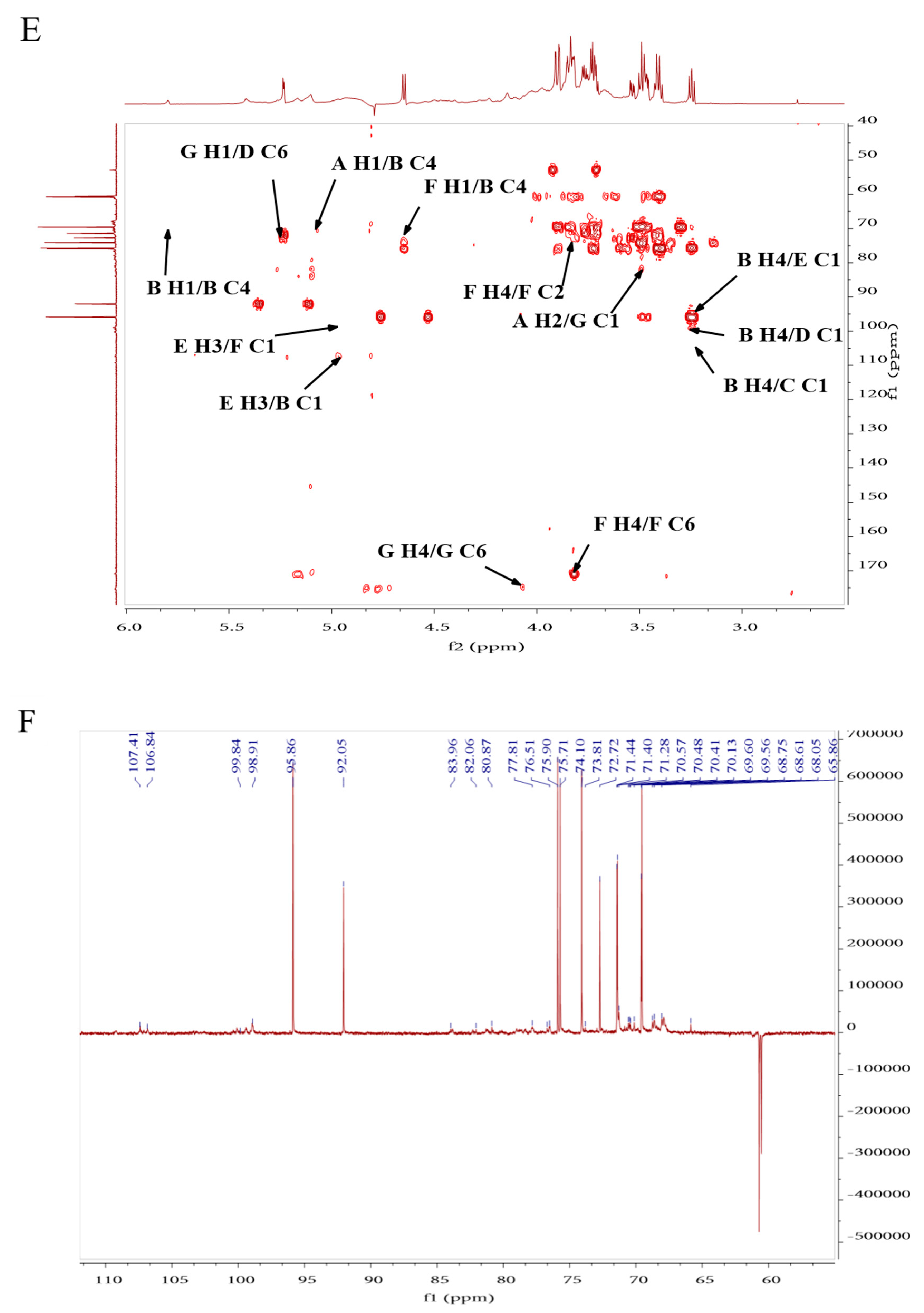
2.1.5. NMR (Nuclear Magnetic Resonance) Analysis of PPL-1
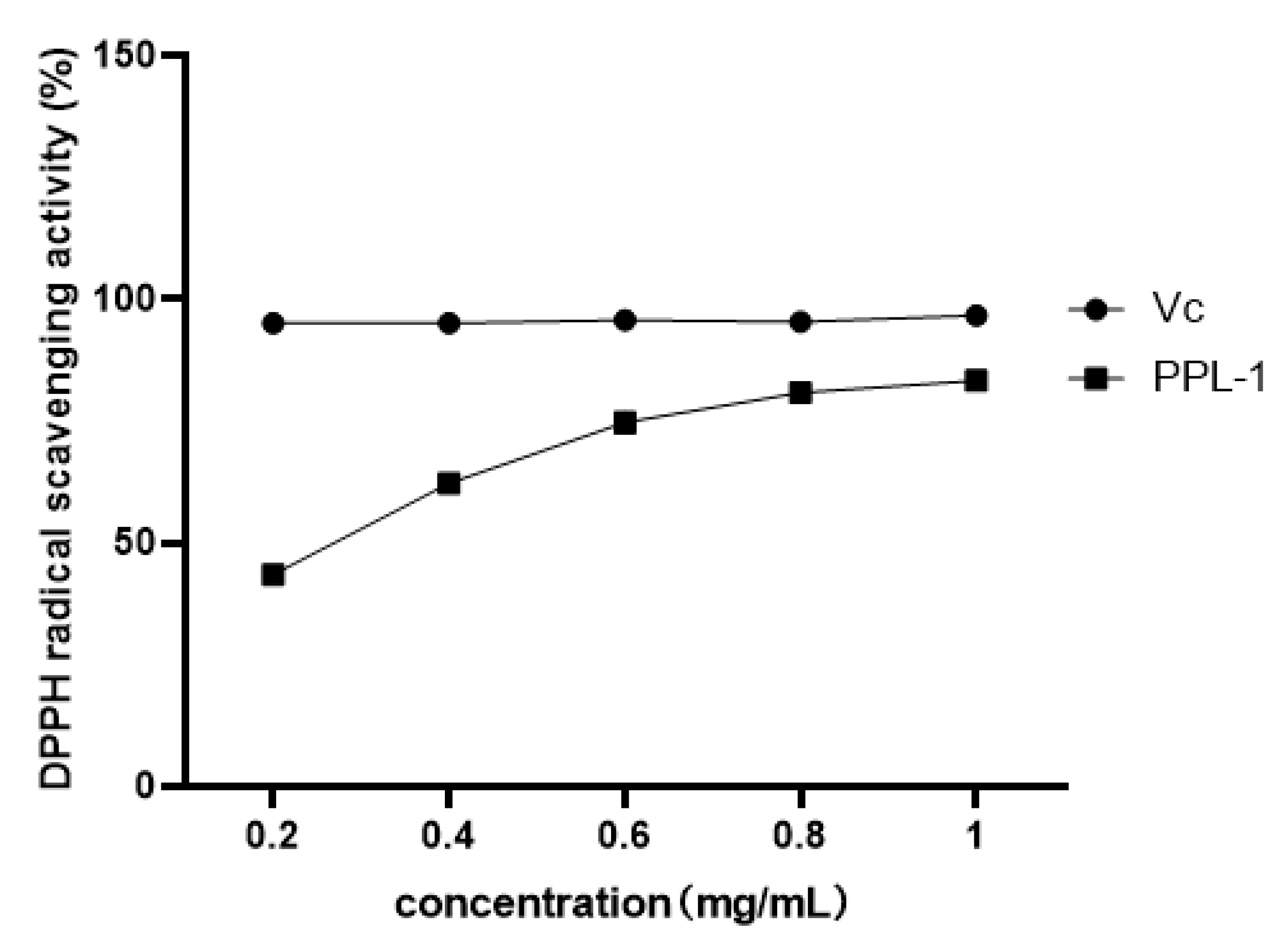
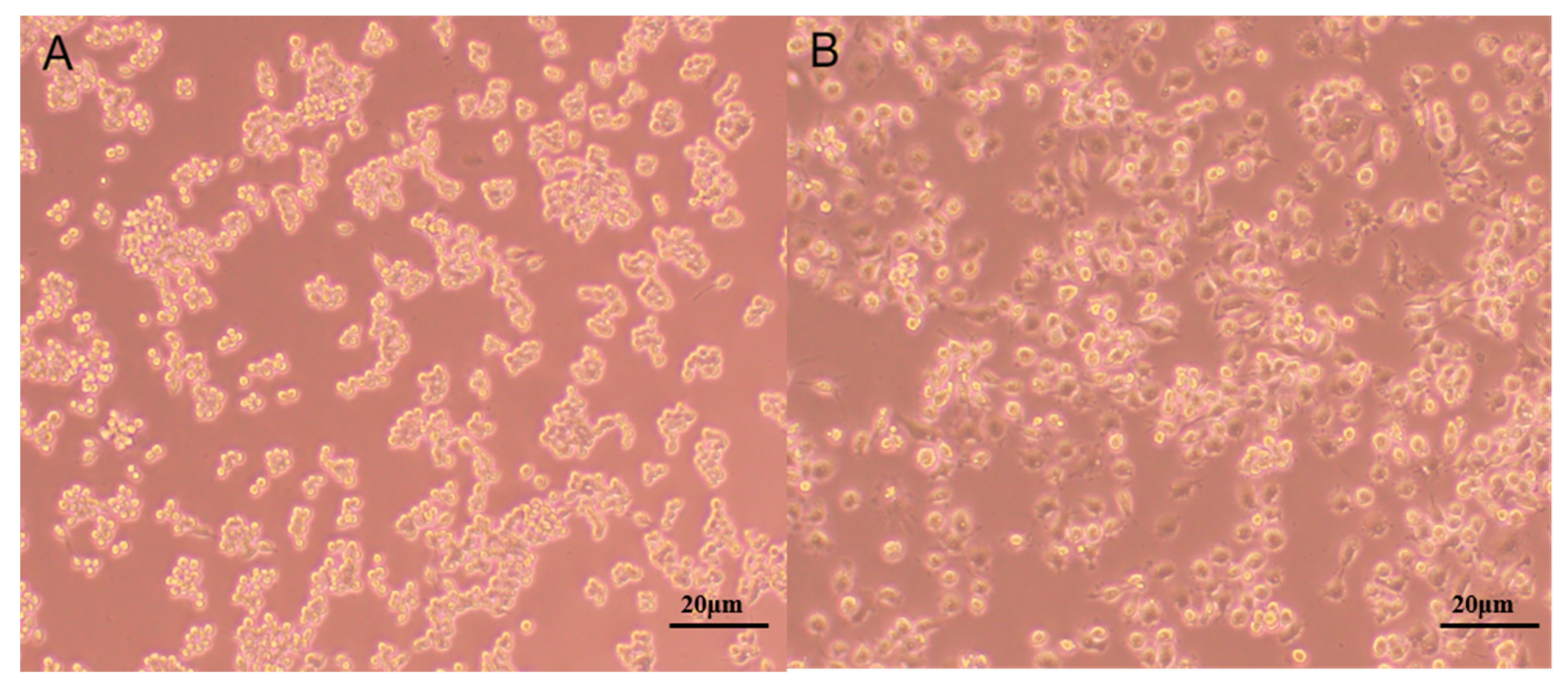
2.1.6. Antioxidant and Immunomodulatory Activity of PPL-1
2.2. Discussion
2.3. Conclusions
3. Materials and Methods
3.1. Reagents
3.2. Preparation of the Polysaccharides
3.3. Colourimetric Analyses
3.4. Determination of Average Mw
3.5. Infrared (IR) Spectrum Analysis
3.6. Monosaccharide Composition
3.7. Glycosidic Linkages
3.8. Nuclear Magnetic Resonance (NMR) Analysis
3.9. DPPH Radical Scavenging Activity of PPL-1
3.10. Culture of RAW 264.7 Cells
3.10.1. Effects of PPL-1 on RAW 264.7 Cell Viability via MTT Assay
3.10.2. Effects of PPL-1 on the Production of NO in RAW 264.7 Cells
3.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.F.; Wang, L.; Wu, Y.Z.; Song, S.Y.; Min, H.Y.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef]
- Zhu, X.M.; Xu, R.; Wang, H.; Chen, J.Y.; Tu, Z.C. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A Review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Fang, Q.Y.; Nie, Q.X.; Hu, J.L.; Yang, C.; Huang, T.; Li, H.; Nie, S.P. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef]
- Zeng, P.J.; Li, J.; Chen, Y.L.; Zhang, L.J. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar]
- Yip, K.M.; Xu, J.; Tong, W.S.; Zhou, S.S.; Yi, T.; Zhao, Z.Z.; Chen, H.B. Ultrasound-Assisted Extraction May Not Be a Better Alternative Approach than Conventional Boiling for Extracting Polysaccharides from Herbal Medicines. Molecules 2016, 21, 1569. [Google Scholar] [CrossRef]
- Li, Z.J.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Ji, X.L.; Peng, Q.; Yuan, Y.P.; Shen, J.; Xie, X.Y.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphusjujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Cui, X.W.; Wang, S.Y.; Cao, H.; Guo, H.; Li, Y.J.; Xu, F.X.; Zhang, M.M.; Xi, X.Z.; Han, C.C. A Review: The Bioactivities and Pharmacological Applications of Polygonatumsibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, M.; Li, H.; Zhan, Q.; Lai, F.; Wu, H. Structural characterization and immunomodulatory activity of a novel polysaccharide from Pueraria lobata (Willd.) Ohwiroot. Int. J. Biol. Macromol. 2020, 154, 1556–1564. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, B.R.; Lee, H.K.; Kim, Y.J.; Park, M.J.; Kim, S.Y.; Lee, M.K.; Hong, J.T.; Kim, Y.; Han, S.B. A polysaccharide isolated from Pueraria lobata enhances maturation of murine dendritic cells. Int. J. Biol. Macromol. 2013, 52, 184–191. [Google Scholar] [CrossRef]
- Xu, C.; Qin, N.B.; Yan, C.Y.; Wang, S.M. Isolation, purification, characterization and bioactivities of a glucan from the root of: Pueraria lobata. Food Funct. 2018, 9, 2644–2652. [Google Scholar] [CrossRef]
- Wang, Z.B.; Chen, B.B.; Luo, L.; Yan, J.K. Fractionation, physicochemical characteristics and biological activities of polysaccharides from Pueraria lobata roots. J. Taiwan Inst. Chem. Eng. 2016, 67, 54–60. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Wang, X.Y.; Chen, K.; Zhang, K.Y.; Zhang, L.F.; Fei, C.Z.; Wang, C.M.; Liu, Y.C.; Xue, F.Q.; et al. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. Int. J. Biol. Macromol. 2020, 158, 740–749. [Google Scholar] [CrossRef]
- Cui, H.X.; Liu, Q.; Tao, Y.Z.; Zhang, H.F.; Zhang, L.N.; Ding, K. Structure and chain conformation of a (1→ 6)-α-d-glucan from the root of Pueraria lobata (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydr. Polym. 2008, 74, 771–778. [Google Scholar] [CrossRef]
- Qian, K.; Tan, T.; Ouyang, H.; Yang, S.L.; Zhu, W.F.; Liu, R.H.; Wen, Q.; Feng, Y.L. Structural characterization of a homopolysaccharide with hypoglycemic activity from the roots of Pueraria lobate. Food Funct. 2020, 11, 7104–7114. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, T.; Mao, G.H.; Zhang, M.; Zheng, D.H.; Feng, W.W.; Wang, W.; Wu, X.Y.; Yang, L.Q. Isolation, purification and characterisation of selenium-containing polysaccharides and proteins in selenium-enriched Radix puerariae. J. Sci. Food Agric. 2014, 94, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Chen, C.; Fu, X. Digestive Property and Bioactivity of Blackberry Polysaccharides with Different Molecular Weights. J. Agric. Food Chem. 2019, 67, 12428–12440. [Google Scholar] [CrossRef]
- Chen, Z.E.; Wufuer, R.; Ji, J.H.; Li, J.F.; Cheng, Y.F.; Dong, C.X.; Taoerdahong, H. Structural Characterization and Immunostimulatory Activity of Polysaccharides from Brassica rapa L. J. Agric. Food Chem. 2017, 65, 9685–9692. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.G.; Amicucci, M.J.; Cheng, Z.; Galermo, A.G.; Lebrilla, C.B. Revisiting monosacc-haride analysis-quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst 2018, 143, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Regenstein, J.M.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2020, 251, 117090. [Google Scholar] [CrossRef]
- Yue, H.; Xu, Q.; Bian, G.; Guo, Q.; Fang, Z.; Wu, W. Structure characterization and immunomodulatory activity of a new neutral polysaccharide SMP-0b from Solanum muricatum. Int. J. Biol. Macromol. 2020, 155, 853–860. [Google Scholar] [CrossRef]
- Shi, Z.Y.; An, L.J.; Zhang, S.J.; Li, Z.G.; Li, Y.H.; Cui, J.L.; Zhang, J.; Jin, D.Q.; Tuerhong, M.; Abudukeremu, M.; et al. A heteropolysaccharide purified from leaves of Ilex latifolia displaying immunomodulatory activity in vitro and in vivo. Carbohydr. Polym. 2020, 245, 116469. [Google Scholar] [CrossRef]
- Guo, D.; Yu, K.; Sun, X.Y.; Ouyang, J.M. Structural Characterization and Repair Mechanism of Gracilarialemaneiformis Sulfated Polysaccharides of Different Molecular Weights on Damaged Renal Epithelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 7410389. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.T.; Tian, Q.H.; Xiao, L.X.; Zeng, Z.; Cai, X.T.; Yan, J.Z.; Li, Q.Y. Microwave-Assisted Degradation of Polysaccharide from Polygonatumsibiricum and Antioxidant Activity. J. Food Sci. 2019, 84, 754–761. [Google Scholar] [CrossRef]
- Hu, H.B.; Li, H.M.; Han, M.H.; Cao, Q.; Liang, H.P.; Yuan, R.A.; Sun, J.; Zhang, L.L.; Wu, Y. Chemical modification an antioxidant activity of the polysaccharide from acanthopanaxleucorrhizus. Carbohydr. Res. 2020, 487, 107890. [Google Scholar] [CrossRef]
- Cai, L.; Chen, B.; Yi, F.; Zou, S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 140, 907–919. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated polysaccharide from Cyclocaryapaliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z. Extraction, Isolation, Purification, Structural Characterization and Immunomodulatory Activity in Raw264. 7 Cells of the Polysaccharides from Pueraria lobate (willd.) ohwi Root. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2018; p. 17. (In Chinese). [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Chem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Gou, Y.R.; Sun, J.; Liu, J.; Chen, H.; Kan, J.; Qian, C.L.; Zhang, N.F.; Jin, C.H. Structural characterization of a water-soluble purple sweet potato polysaccharide and its effect on intestinal inflammation in mice. J. Funct. Foods. 2019, 61, 103502. [Google Scholar] [CrossRef]
- Amicucci, M.J.; Galermo, A.G.; Nandita, E.; Vo, T.T.; Liu, Y.Y.; Lee, M.; Xu, G.G.; Lebrilla, C.B. A rapid-throughput adaptable method for determining the monosaccharide composition of polysaccharides. Int. J. Mass Spectrom. 2019, 438, 22–28. [Google Scholar] [CrossRef]

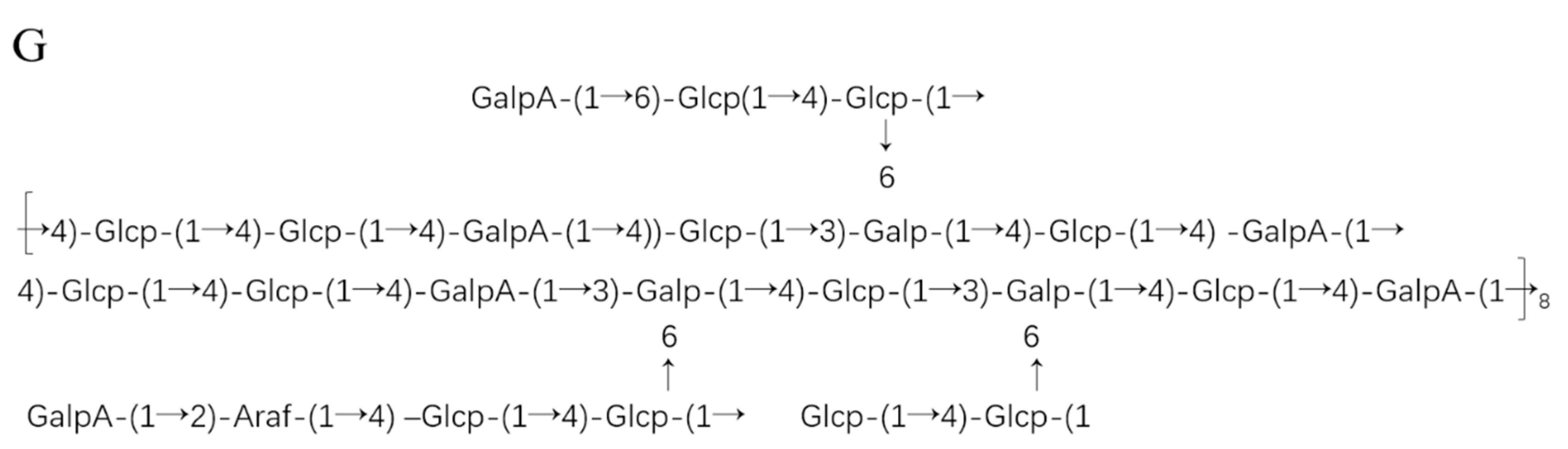

| Monosaccharide | Arabinose | Glucose | Galactose | Galacturonic Acid |
|---|---|---|---|---|
| Retention time (min) | 8.9 | 8.1 | 8.6 | 6.8 |
| Molar ratio (%) | 8 | 63 | 8 | 21 |
| Monosaccharide | Type of Linkage | Sugar Derivatives | Retention Time | Molar (%) |
|---|---|---|---|---|
| Arabinose | (1→2)-Araf | 1,2,4-tri-O-acetyl-3,5-tri-O-methyl arabinitol | 9.3 | 5.42 |
| Glucose | (1→4)-Glcp | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 14.4 | 50.11 |
| (1→)-Glcp | 1,5-tri-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 9.46 | 7.35 | |
| (1→6)-Glcp | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl glucitol | 16.11 | 5.12 | |
| Galactose | (1→3,6)-Galp | 1,3,5,6-tetra-O-acetyl-2,4-tri-O-methyl galactitol | 19.61 | 11.21 |
| Galacturonic Acid | (1→4)-GalpA | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl galactitol | 14.7 | 13.13 |
| (1→)-GalpA | 1,5-tri-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 10.48 | 7.65 |
| Monosaccharide | Molar Ratio | Type of Linkage | H1 | H2 | H3 | H4 | H5 | H6 |
|---|---|---|---|---|---|---|---|---|
| C1 | C3 | C3 | C4 | C5 | C6 | |||
| Arabinose | 1 | (1→2)-linked α-Araf | 5.1 | 3.55 | 3.85 | 3.97 | 4.07 | |
| (A) | 107.41 | 71.45 | 74.73 | 69.57 | 60.72 | |||
| Glucose | 13 | (1→4)-linked α-Glcp | 5.8 | 3.91 | 4.64 | 3.25 | 3.5 | 3.39 |
| (B) | 106.84 | 68.78 | 78.88 | 70.14 | 73.09 | 70.83 | ||
| 2 | (1→)-linked β-Glcp | 4.5 | 3.25 | 4.52 | 3.23 | 3.44 | 3.78 | |
| (C) | 102.96 | 74.11 | 78.36 | 70.5 | 73.09 | 68.07 | ||
| 1 | (1→6)-linked α-Glcp | 4.93 | 4.4 | 3.27 | 3.47 | 3.71 | 3.77 | |
| (D) | 99.86 | 70.43 | 77.84 | 75.92 | 70.59 | 72.25 | ||
| Galactose | 2 | (1→3,6)-linked α-Galp | 5.23 | 3.46 | 4.97 | 4.54 | 3.27 | 3.37 |
| (E) | 95.88 | 76.5 | 98.57 | 77.72 | 67.61 | 67.61 | ||
| Galacturonic Acid | 3 | (1→4)-linked β-GalpA | 4.65 | 3.42 | 3.7 | 3.83 | 4.14 | |
| (F) | 95.75 | 73.09 | 72.73 | 71.42 | 75.73 | 170.93 | ||
| 3 | (1→)-linked α-GalpA | 5.24 | 4.6 | 3.89 | 4.04 | 3.76 | ||
| (G) | 91.96 | 79.02 | 68.64 | 74.53 | 70.59 | 175.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Xue, H.; Tao, Y.; Chen, K.; Li, X.; Wang, M. An Acidic Heteropolysaccharide Isolated from Pueraria lobata and Its Bioactivities. Int. J. Mol. Sci. 2023, 24, 6247. https://doi.org/10.3390/ijms24076247
Zhao S, Xue H, Tao Y, Chen K, Li X, Wang M. An Acidic Heteropolysaccharide Isolated from Pueraria lobata and Its Bioactivities. International Journal of Molecular Sciences. 2023; 24(7):6247. https://doi.org/10.3390/ijms24076247
Chicago/Turabian StyleZhao, Shifan, Hualei Xue, Yijiong Tao, Kai Chen, Xiao Li, and Mi Wang. 2023. "An Acidic Heteropolysaccharide Isolated from Pueraria lobata and Its Bioactivities" International Journal of Molecular Sciences 24, no. 7: 6247. https://doi.org/10.3390/ijms24076247
APA StyleZhao, S., Xue, H., Tao, Y., Chen, K., Li, X., & Wang, M. (2023). An Acidic Heteropolysaccharide Isolated from Pueraria lobata and Its Bioactivities. International Journal of Molecular Sciences, 24(7), 6247. https://doi.org/10.3390/ijms24076247






