Over-Expression of LEDGF/p75 in HEp-2 Cells Enhances Autoimmune IgG Response in Patients with Benign Prostatic Hyperplasia—A Novel Diagnostic Approach with Therapeutic Consequence?
Abstract
1. Introduction
2. Results
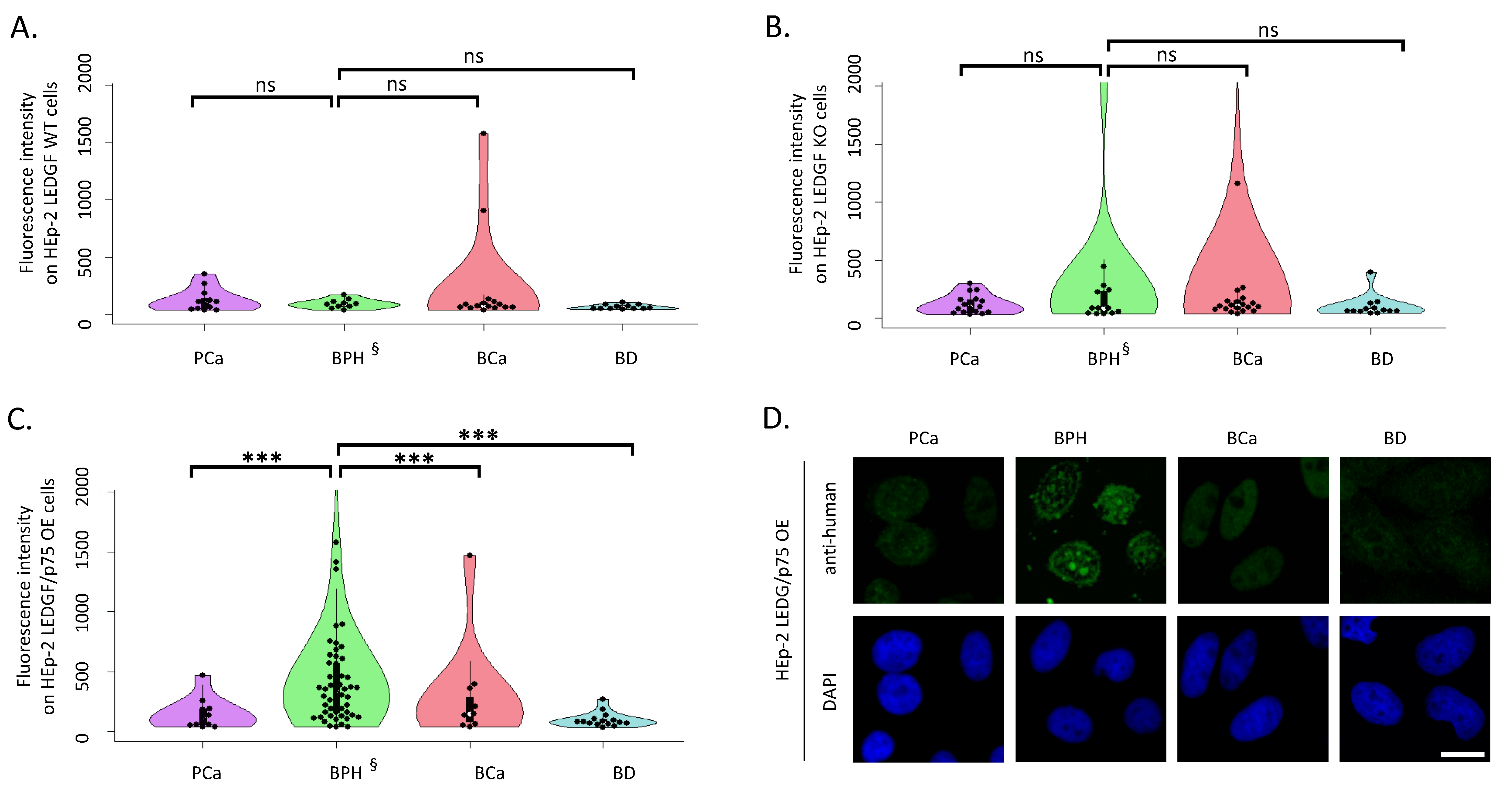
2.1. LEDGF/p75 Over-Expression Increases autoAb Binding of Patients with BPH
2.2. LEDGF/p75 Over-Expression Increases the Frequency of Nucleolar and Speckled Patterns in Patients with BPH
2.3. Patients with BPH Show a Significantly Increased Prevalence of autoAbs against dsDNA in LIA
2.4. Patients with BPH Showed autoAbs to mDNA Detected by CLIFT
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Generation of LEDGF-Modified Cell Clones
4.3. Cell Lines and Culture
4.4. Detection of autoAbs by IFA
4.5. Detection of autoAbs by Line Immunoassay
4.6. Analysis of autoAbs to mDNA by IFA
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, T.; Zhang, J.; Gutierrez, I.; Elliot, M.L.; Yamada, B.; Heeb, M.J.; Sheets, S.M.; Wu, X.; Casiano, C.A. Antinuclear Autoantibodies in Prostate Cancer: Immunity to LEDGF/P75, a Survival Protein Highly Expressed in Prostate Tumors and Cleaved during Apoptosis. Prostate 2005, 62, 14–26. [Google Scholar] [CrossRef]
- Ochs, R.L.; Mahler, M.; Basu, A.; Rios-Colon, L.; Sanchez, T.W.; Andrade, L.E.; Fritzler, M.J.; Casiano, C.A. The Significance of Autoantibodies to DFS70/LEDGFp75 in Health and Disease: Integrating Basic Science with Clinical Understanding. Clin. Exp. Med. 2016, 16, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Ochs, R.L.; Muro, Y.; Si, Y.; Ge, H.; Chan, E.K.L.; Tan, E.M. Autoantibodies to DFS 70 Kd/Transcription Coactivator P75 in Atopic Dermatitis and Other Conditions. J. Allergy Clin. Immunol. 2000, 105, 1211–1220. [Google Scholar] [CrossRef]
- Basu, A.; Rojas, H.; Banerjee, H.; Cabrera, I.B.; Perez, K.Y.; De León, M.; Casiano, C.A. Expression of the Stress Response Oncoprotein LEDGF/P75 in Human Cancer: A Study of 21 Tumor Types. PLoS ONE 2012, 7, e30132. [Google Scholar] [CrossRef] [PubMed]
- Bhargavan, B.; Fatma, N.; Chhunchha, B.; Singh, V.; Kubo, E.; Singh, D.P. LEDGF Gene Silencing Impairs the Tumorigenicity of Prostate Cancer DU145 Cells by Abating the Expression of Hsp27 and Activation of the Akt/ERK Signaling Pathway. Cell Death Dis. 2012, 3, e316. [Google Scholar] [CrossRef]
- Daugaard, M.; Baude, A.; Fugger, K.; Povlsen, L.K.; Beck, H.; Sørensen, C.S.; Petersen, N.H.T.; Sorensen, P.H.B.; Lukas, C.; Bartek, J.; et al. LEDGF (P75) Promotes DNA-End Resection and Homologous Recombination. Nat. Struct. Mol. Biol. 2012, 19, 803–810. [Google Scholar] [CrossRef]
- Liedtke, V.; Schröder, C.; Roggenbuck, D.; Weiss, R.; Stohwasser, R.; Schierack, P.; Rödiger, S.; Schenk, L. LEDGF/P75 Is Required for an Efficient DNA Damage Response. Int. J. Mol. Sci. 2021, 22, 5866. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, D.H.; Park, G.; Park, S.; Kim, H.-S. Clinical Significance of Anti-Dense Fine Speckled 70 Antibody in Patients with Fibromyalgia. Korean J. Intern. Med. 2019, 34, 426–433. [Google Scholar] [CrossRef]
- Mahler, M.; Parker, T.; Peebles, C.L.; Andrade, L.E.; Swart, A.; Carbone, Y.; Ferguson, D.J.; Villalta, D.; Bizzaro, N.; Hanly, J.G.; et al. Anti-DFS70/LEDGF Antibodies Are More Prevalent in Healthy Individuals Compared to Patients with Systemic Autoimmune Rheumatic Diseases. J. Rheumatol. 2012, 39, 2104–2110. [Google Scholar] [CrossRef]
- Ortiz-Hernandez, G.L.; Sanchez-Hernandez, E.S.; Casiano, C.A. Twenty Years of Research on the DFS70/LEDGF Autoantibody-Autoantigen System: Many Lessons Learned but Still Many Questions. Autoimmun. Highlights 2020, 11, 3. [Google Scholar] [CrossRef]
- Seelig, C.; Bauer, O.; Seelig, H.-P. Autoantibodies Against DFS70/LEDGF Exclusion Markers for Systemic Autoimmune Rheumatic Diseases (SARD). Clin. Lab. 2016, 62, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Röber, N.; Andrade, L.E.C.; Mahler, M. The Clinical Relevance of Anti-DFS70 Autoantibodies. Clin. Rev. Allergy Immunol. 2017, 52, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Meroni, P.L.; Andrade, L.E.; Khamashta, M.; Bizzaro, N.; Casiano, C.A.; Fritzler, M.J. Towards a Better Understanding of the Clinical Association of Anti-DFS70 Autoantibodies. Autoimmun. Rev. 2016, 15, 198–201. [Google Scholar] [CrossRef]
- Malyavantham, K.; Suresh, L. Analysis of DFS70 Pattern and Impact on ANA Screening Using a Novel HEp-2 ELITE/DFS70 Knockout Substrate. Autoimmun. Highlights 2017, 8, 3. [Google Scholar] [CrossRef]
- Vickman, R.E.; Aaron-Brooks, L.; Zhang, R.; Lanman, N.A.; Lapin, B.; Gil, V.; Greenberg, M.; Sasaki, T.; Cresswell, G.M.; Broman, M.M.; et al. TNF Is a Potential Therapeutic Target to Suppress Prostatic Inflammation and Hyperplasia in Autoimmune Disease. Nat. Commun. 2022, 13, 2133. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Ortega, R.; Qian, W.; Casiano, C.A.; Zhang, J.-Y. Preferential Autoimmune Response in Prostate Cancer to Cyclin B1 in a Panel of Tumor-Associated Antigens. J. Immunol. Res. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- O’Rourke, D.J.; DiJohnson, D.A.; Caiazzo, R.J.; Nelson, J.C.; Ure, D.; O’Leary, M.P.; Richie, J.P.; Liu, B.C.-S. Autoantibody Signatures as Biomarkers to Distinguish Prostate Cancer from Benign Prostatic Hyperplasia in Patients with Increased Serum Prostate Specific Antigen. Clin. Chim. Acta 2012, 413, 561–567. [Google Scholar] [CrossRef]
- Langan, R.C. Benign Prostatic Hyperplasia. Prim. Care Clin. Off. Pract. 2019, 46, 223–232. [Google Scholar] [CrossRef]
- Bizzaro, N.; Tonutti, E.; Visentini, D.; Alessio, M.G.; Platzgummer, S.; Morozzi, G.; Antico, A.; Villalta, D.; Piller-Roner, S.; Vigevani, E. Antibodies to the Lens and Cornea in Anti-DFS70-Positive Subjects. Ann. N. Y. Acad. Sci. 2007, 1107, 174–183. [Google Scholar] [CrossRef]
- Kramer, G.; Mitteregger, D.; Marberger, M. Is Benign Prostatic Hyperplasia (BPH) an Immune Inflammatory Disease? Eur. Urol. 2007, 51, 1202–1216. [Google Scholar] [CrossRef]
- Hata, J.; Machida, T.; Matsuoka, K.; Hoshi, S.; Akaihata, H.; Hiraki, H.; Suzuki, T.; Ogawa, S.; Kataoka, M.; Haga, N.; et al. Complement Activation by Autoantigen Recognition in the Growth Process of Benign Prostatic Hyperplasia. Sci. Rep. 2019, 9, 20357. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Keller, A.; Milchram, L.; Harz, C.; Hart, M.; Werth, A.; Lenhof, H.-P.; Weinhäusel, A.; Keck, B.; Wullich, B.; et al. Combination of Autoantibody Signature with PSA Level Enables a Highly Accurate Blood-Based Differentiation of Prostate Cancer Patients from Patients with Benign Prostatic Hyperplasia. PLoS ONE 2015, 10, e0128235. [Google Scholar] [CrossRef] [PubMed]
- Lokant, M.T.; Naz, R.K. Presence of PSA Auto-Antibodies in Men with Prostate Abnormalities (Prostate Cancer/Benign Prostatic Hyperplasia/Prostatitis). Andrologia 2015, 47, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Jandrig, B.; Kunze, R.; Wendler, J.J.; Müller, J.; Schostak, M.; Schimke, I. Autoantibodies Directed Against the Endothelin A Receptor in Patients With Benign Prostatic Hyperplasia: Prostate and Endothelin Receptor Autoantibodies. Prostate 2017, 77, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Luo, C.; Cui, K.; Xiong, T.; Chen, Z. Chronic Inflammation Promotes Proliferation in the Prostatic Stroma in Rats with Experimental Autoimmune Prostatitis: Study for a Novel Method of Inducing Benign Prostatic Hyperplasia in a Rat Model. World J. Urol. 2020, 38, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Wichainun, R.; Kasitanon, N.; Wangkaew, S.; Hongsongkiat, S.; Sukitawut, W.; Louthrenoo, W. Sensitivity and Specificity of ANA and Anti-DsDNA in the Diagnosis of Systemic Lupus Erythematosus: A Comparison Using Control Sera Obtained from Healthy Individuals and Patients with Multiple Medical Problems. Asian Pac. J. Allergy Immunol. 2013, 31, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Schur, P.H. ANA Screening: An Old Test with New Recommendations. Ann. Rheum. Dis. 2010, 69, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Berg, P.A.; Bianchi, F.B.; Bianchi, L.; Burroughs, A.K.; Cancado, E.L.; Chapman, R.W.; Cooksley, W.G.E.; Czaja, A.J.; Desmet, V.J.; et al. International Autoimmune Hepatitis Group Report: Review of Criteria for Diagnosis of Autoimmune Hepatitis. J. Hepatol. 1999, 31, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Gu, X.; Yu, W.Z.; Wang, Z.; Jiao, M. Detection of Serum Antinuclear Antibodies in Lymphoma Patients. Genet. Mol. Res. 2015, 14, 16546–16552. [Google Scholar] [CrossRef]
- Conrad, K.; Ittenson, A.; Reinhold, D.; Fischer, R.; Roggenbuck, D.; Büttner, T.; Bosselmann, H.-P.; Steinbach, J.; Schößler, W. High Sensitive Detection of Double-Stranded DNA Autoantibodies by a Modified Crithidia Luciliae Immunofluorescence Test. Ann. N. Y. Acad. Sci. 2009, 1173, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.; Kushner, I. An Immunofluorescent Method Using Crithidia Luciliae to Detect Antibodies to Double-Stranded DNA. Arthritis Rheum. 1977, 20, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial Form and Function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Becker, Y.; Loignon, R.-C.; Julien, A.-S.; Marcoux, G.; Allaeys, I.; Lévesque, T.; Rollet-Labelle, E.; Benk-Fortin, H.; Cloutier, N.; Melki, I.; et al. Anti-Mitochondrial Autoantibodies in Systemic Lupus Erythematosus and Their Association with Disease Manifestations. Sci. Rep. 2019, 9, 4530. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, D.; Conrad, K.; Reinhold, D. High Sensitive Detection of Double-Stranded DNA Antibodies by a Modified Crithidia Luciliae Immunofluorescence Test May Improve Diagnosis of Systemic Lupus Erythematosus. Clin. Chim. Acta 2010, 411, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Committee on Cancer; Springer: Chicago, IL, USA, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Hiemann, R.; Büttner, T.; Krieger, T.; Roggenbuck, D.; Sack, U.; Conrad, K. Challenges of Automated Screening and Differentiation of Non-Organ Specific Autoantibodies on HEp-2 Cells. Autoimmun. Rev. 2009, 9, 17–22. [Google Scholar] [CrossRef]
- Willitzki, A.; Hiemann, R.; Peters, V.; Sack, U.; Schierack, P.; Rödiger, S.; Anderer, U.; Conrad, K.; Bogdanos, D.P.; Reinhold, D.; et al. New Platform Technology for Comprehensive Serological Diagnostics of Autoimmune Diseases. Clin. Dev. Immunol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 3 June 2020).


| autoAbs to | BPH (103) n (%) | BCa (116) n (%) | PCa (89) n (%) | BD (60) n (%) |
|---|---|---|---|---|
| LEDGF/p75 HEp-2 | 49 (47.6%) *§ | 9 (7.8%) | 5 (5.6%) | 5 (8.3%) |
| LEDGF KO HEp-2 | 10 (9.7%) | 15 (13.0%) | 10 (11.2%) | 5 (8.3%) |
| LEDGF WT HEp-2 | 8 (7.8%) | 11 (9.5%) | 7 (7.9%) | 5 (8.3%) |
| dsDNA | 35 (34.0%) * | 11 (9.5%) | 16 (18.0%) | 2 (3.3%) |
| mDNA | 35 (34.0%) * | 6 (5.2%) | 5 (5.6%) | 1 (1.7%) |
| LEDGF/p75 HEp-2 + dsDNA | 23 (22.3%) * | 1 (0.9%) | 1 (1.1%) | 0 (0.0%) |
| LEDGF/p75 HEp-2 + mDNA | 26 (25.2%) * | 1 (0.9%) | 1 (1.1%) | 0 (0.0%) |
| dsDNA + mDNA | 28 (27.2%) * | 3 (2.6%) | 4 (4.5%) | 0 (0.0%) |
| Age (Median) [years] | Age Range [years] | Interquartile Range (IQR) [years] | Gender | Male [%] | Tumour Stage | Gleason Score | |
|---|---|---|---|---|---|---|---|
| BPH | 70.0 | 50–88 | 14 | Male | 100% | N/A | N/A |
| BCa | 77.2 | 50–96 | N/A | female + male | 81% | pTa-pT4 | N/A |
| PCa | 64.0 | 43–77 | N/A | male | 100% | pT2a-pT3b | 7a-9b |
| BD | 34.0 | 21–58 | N/A | female + male | 86.7% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liedtke, V.; Rose, L.; Hiemann, R.; Nasser, A.; Rödiger, S.; Bonaventura, A.; Winkler, L.; Sowa, M.; Stöckle, M.; Schierack, P.; et al. Over-Expression of LEDGF/p75 in HEp-2 Cells Enhances Autoimmune IgG Response in Patients with Benign Prostatic Hyperplasia—A Novel Diagnostic Approach with Therapeutic Consequence? Int. J. Mol. Sci. 2023, 24, 6166. https://doi.org/10.3390/ijms24076166
Liedtke V, Rose L, Hiemann R, Nasser A, Rödiger S, Bonaventura A, Winkler L, Sowa M, Stöckle M, Schierack P, et al. Over-Expression of LEDGF/p75 in HEp-2 Cells Enhances Autoimmune IgG Response in Patients with Benign Prostatic Hyperplasia—A Novel Diagnostic Approach with Therapeutic Consequence? International Journal of Molecular Sciences. 2023; 24(7):6166. https://doi.org/10.3390/ijms24076166
Chicago/Turabian StyleLiedtke, Victoria, Laura Rose, Rico Hiemann, Abdullah Nasser, Stefan Rödiger, Alena Bonaventura, Laura Winkler, Mandy Sowa, Michael Stöckle, Peter Schierack, and et al. 2023. "Over-Expression of LEDGF/p75 in HEp-2 Cells Enhances Autoimmune IgG Response in Patients with Benign Prostatic Hyperplasia—A Novel Diagnostic Approach with Therapeutic Consequence?" International Journal of Molecular Sciences 24, no. 7: 6166. https://doi.org/10.3390/ijms24076166
APA StyleLiedtke, V., Rose, L., Hiemann, R., Nasser, A., Rödiger, S., Bonaventura, A., Winkler, L., Sowa, M., Stöckle, M., Schierack, P., Junker, K., & Roggenbuck, D. (2023). Over-Expression of LEDGF/p75 in HEp-2 Cells Enhances Autoimmune IgG Response in Patients with Benign Prostatic Hyperplasia—A Novel Diagnostic Approach with Therapeutic Consequence? International Journal of Molecular Sciences, 24(7), 6166. https://doi.org/10.3390/ijms24076166








