Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice
Abstract
1. Introduction
2. Results
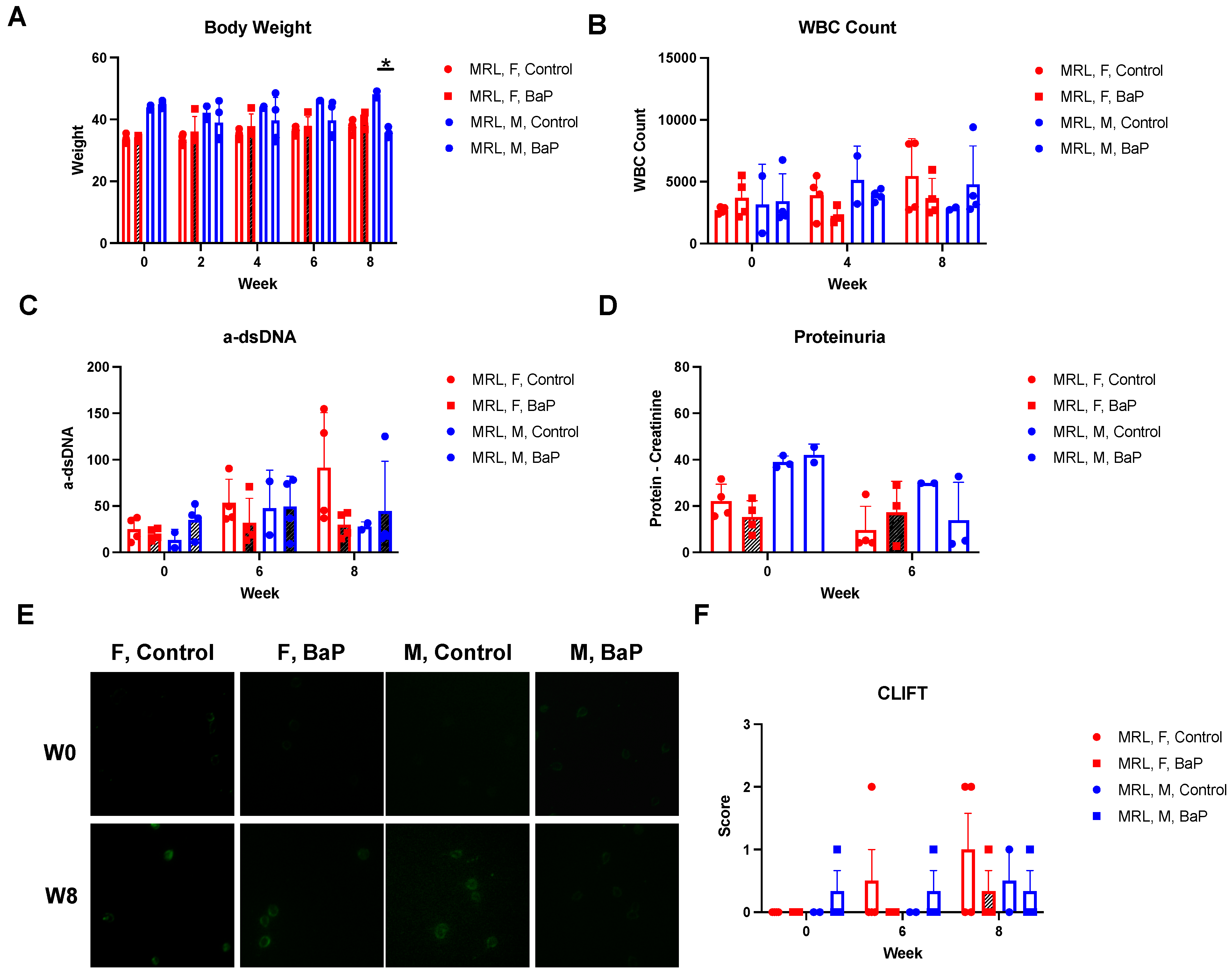
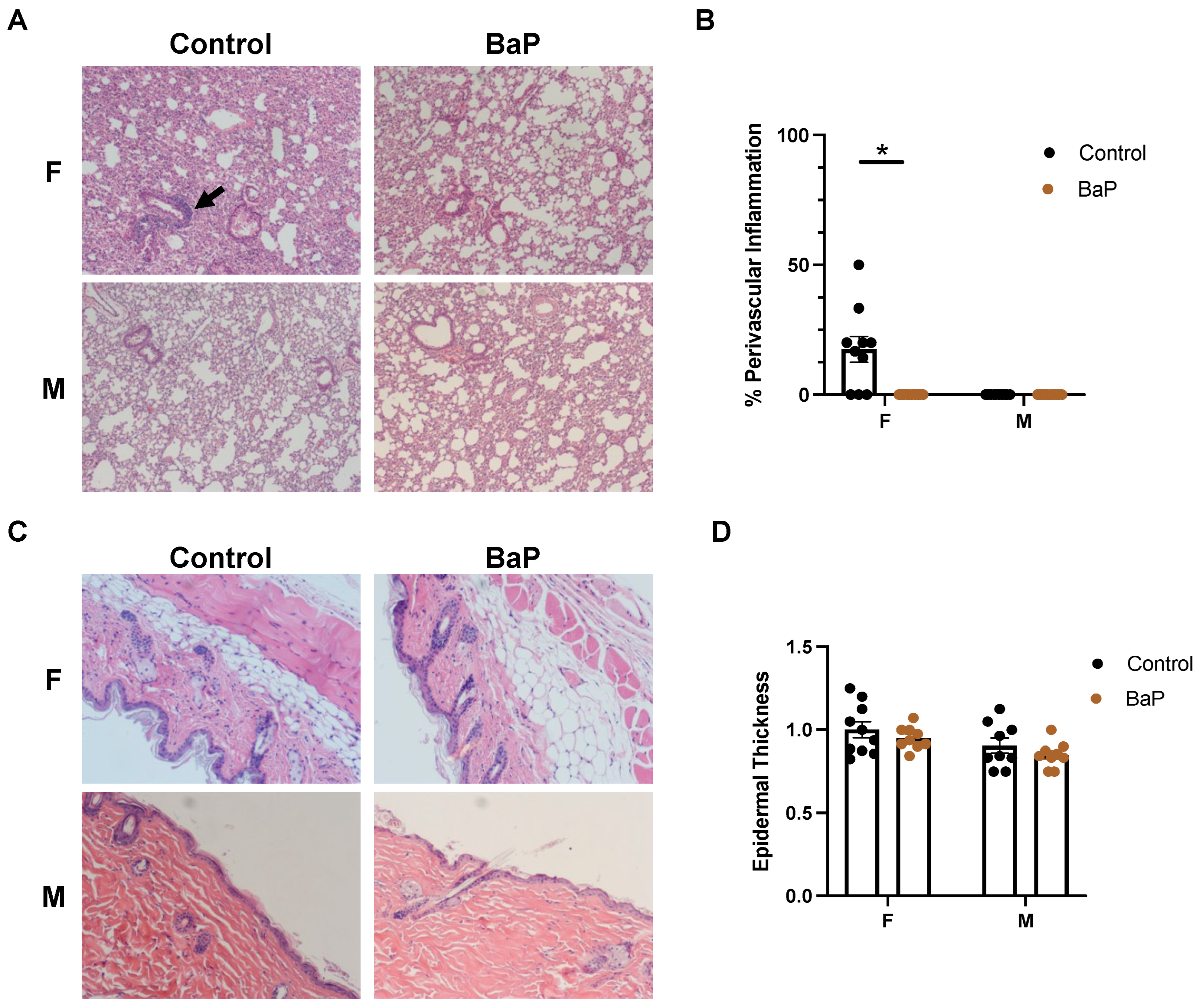
2.1. BaP Exposure Caused Immunosuppression in Female MRL Mice
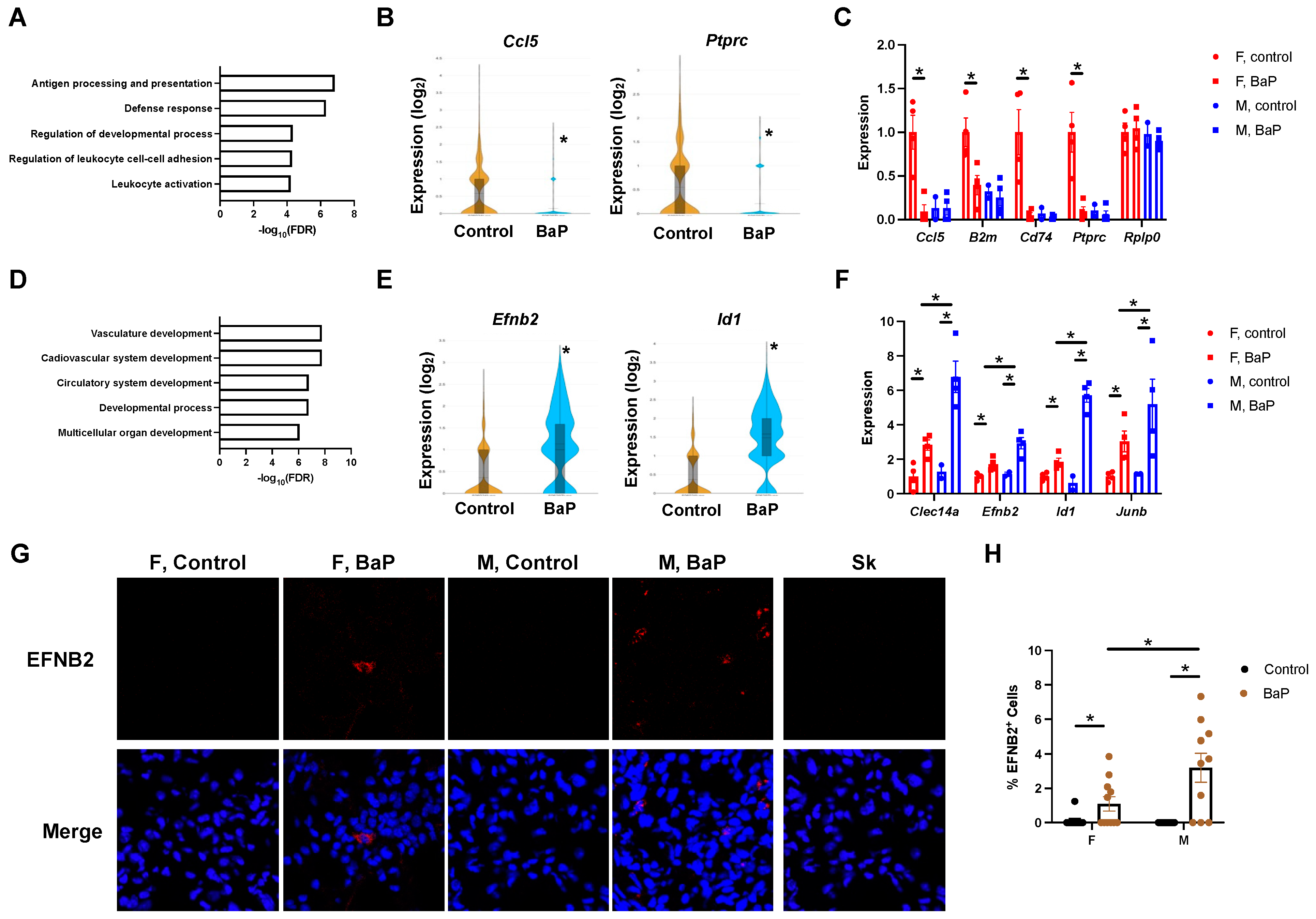
2.2. BaP Treatment Stimulated the Expression of Vasculature Genes in the Lungs of MRL Mice
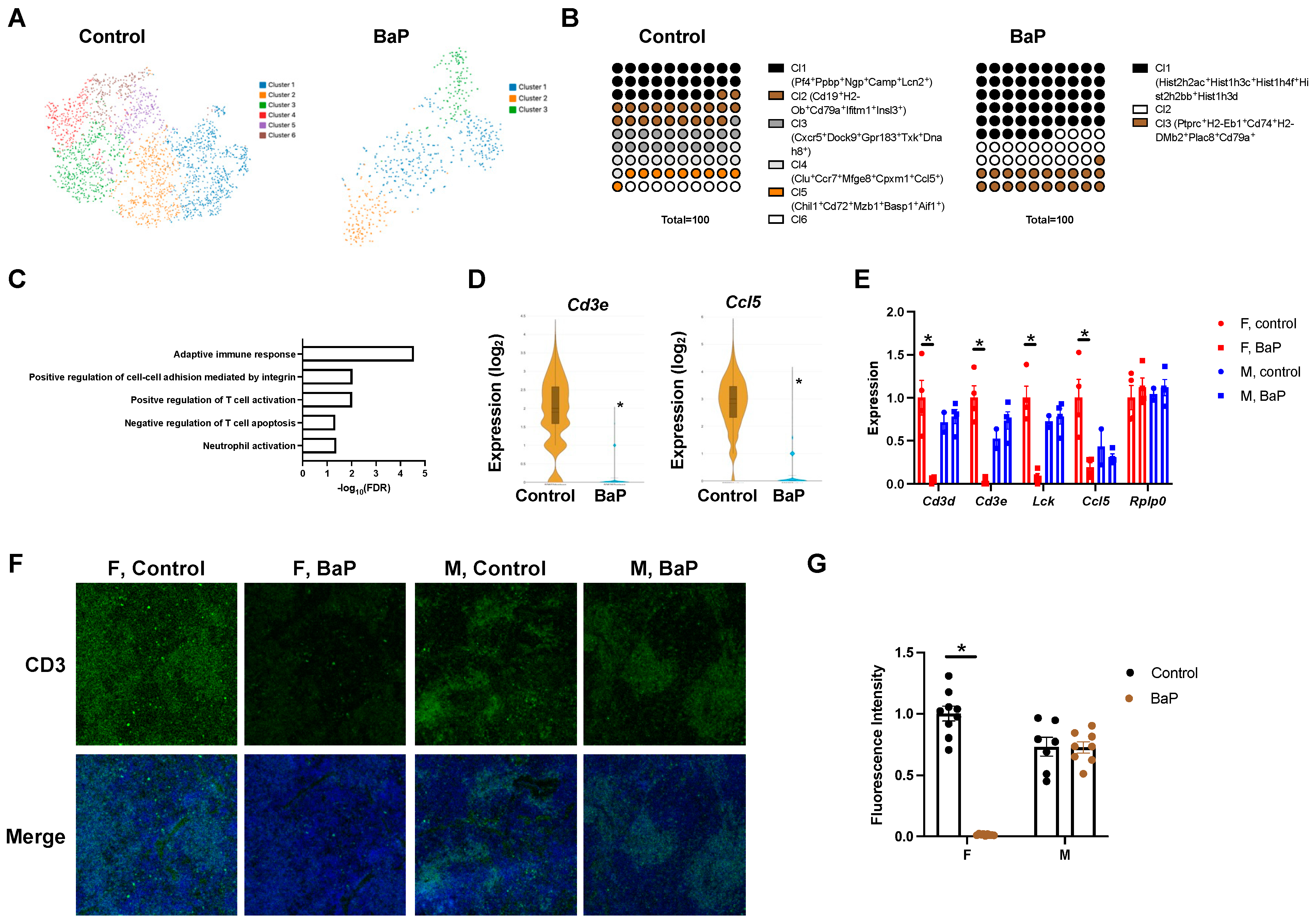
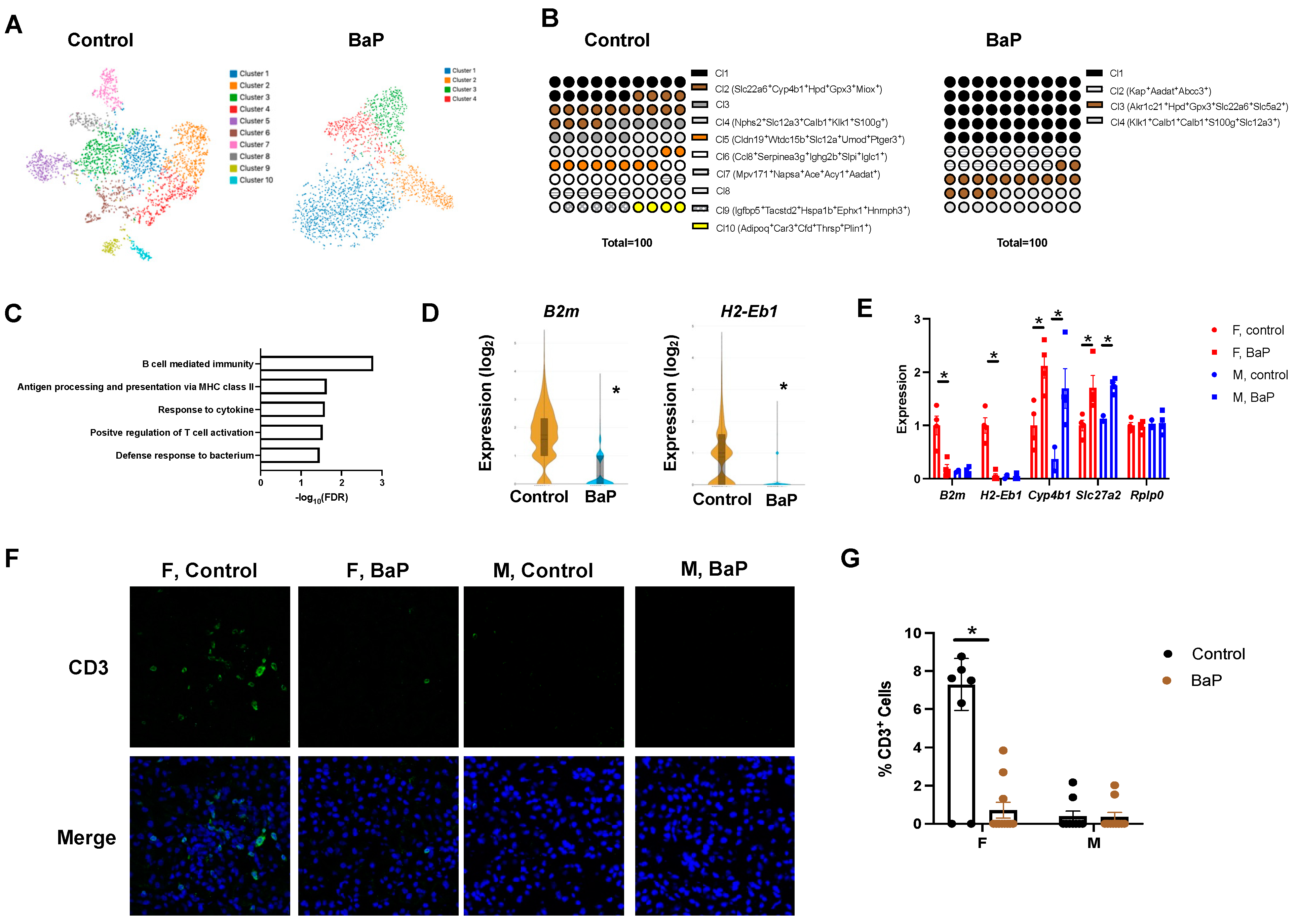
2.3. BaP Treatment Disrupted the T Cell Compartment in the Spleen of Female MRL Mice
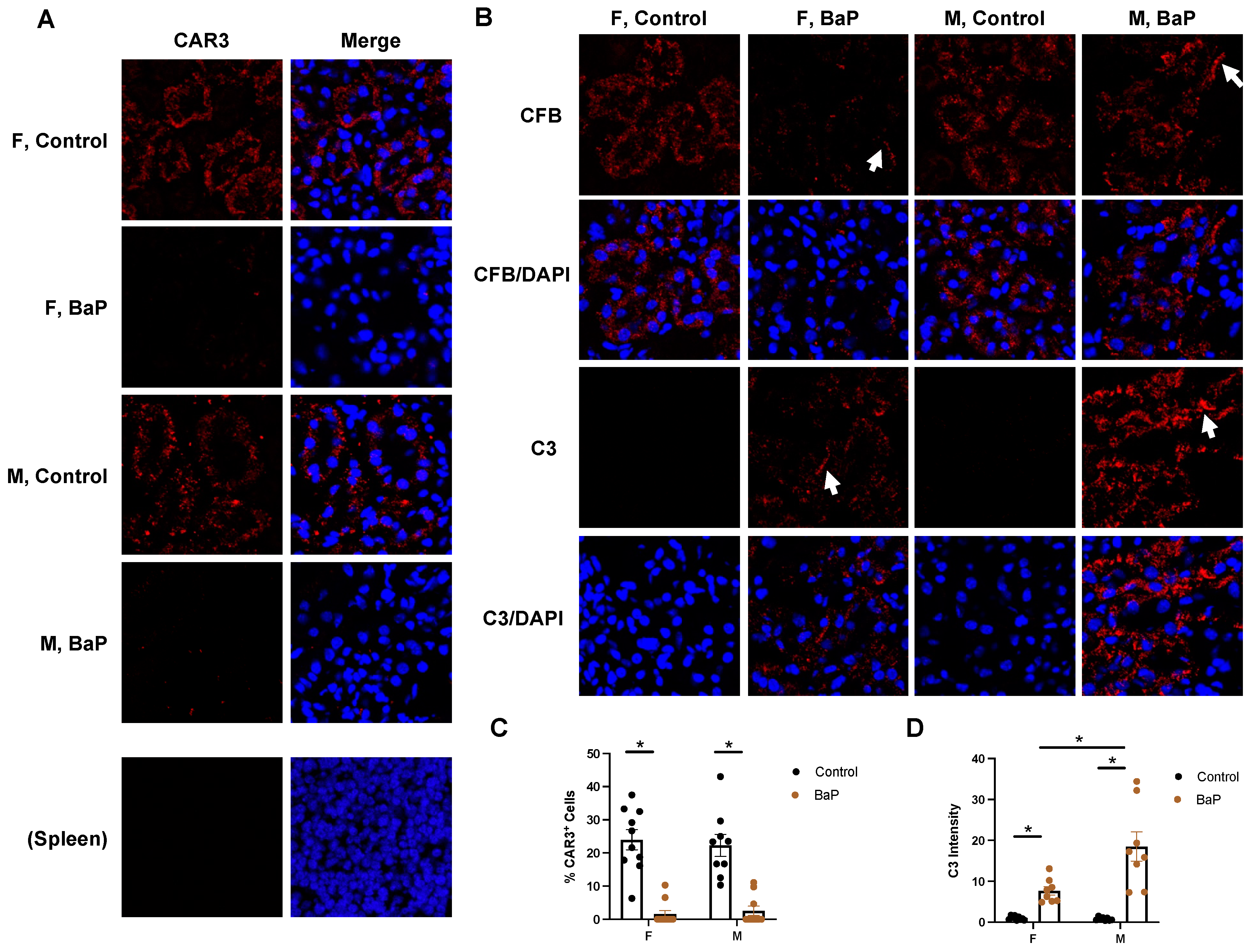
2.4. BaP Treatment Reduced Renal Cell-Type Diversity and Caused Male-Biased C3 Deposition in the Kidney of MRL Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. ELISA
4.3. CLIFT
4.4. Spatial Transcriptomics
4.5. Quantitative Polymerase Chain Reaction (qPCR)
4.6. Immunofluorescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costenbader, K.H.; Gay, S.; Alarcon-Riquelme, M.E.; Iaccarino, L.; Doria, A. Genes, epigenetic regulation and environmental factors: Which is the most relevant in developing autoimmune diseases? Autoimmun. Rev. 2012, 11, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Smyk, D.S.; Rigopoulou, E.I.; Mytilinaiou, M.G.; Heneghan, M.A.; Selmi, C.; Gershwin, M.E. Twin studies in autoimmune disease: Genetics, gender and environment. J. Autoimmun. 2012, 38, J156–J169. [Google Scholar] [CrossRef] [PubMed]
- Marson, A.; Housley, W.J.; Hafler, D.A. Genetic basis of autoimmunity. J. Clin. Investig. 2015, 125, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Pollard, K.M.; Campbell, A.W. Environmental triggers and autoimmunity. Autoimmune Dis. 2014, 2014, 798029. [Google Scholar] [CrossRef]
- Crowe, W.; Allsopp, P.J.; Watson, G.E.; Magee, P.J.; Strain, J.J.; Armstrong, D.J.; Ball, E.; McSorley, E.M. Mercury as an environmental stimulus in the development of autoimmunity—A systematic review. Autoimmun. Rev. 2017, 16, 72–80. [Google Scholar] [CrossRef]
- Wu, P.; Miura, Y.; Hyodoh, F.; Nishimura, Y.; Hatayama, T.; Hatada, S.; Sakaguchi, H.; Kusaka, M.; Katsuyama, H.; Tomita, M.; et al. Reduced function of CD4+25+ regulatory T cell fraction in silicosis patients. Int. J. Immunopathol. Pharmacol. 2006, 19, 357–368. [Google Scholar] [CrossRef]
- Takata-Tomokuni, A.; Ueki, A.; Shiwa, M.; Isozaki, Y.; Hatayama, T.; Katsuyama, H.; Hyodoh, F.; Fujimoto, W.; Ueki, H.; Kusaka, M.; et al. Detection, epitope-mapping and function of anti-Fas autoantibody in patients with silicosis. Immunology 2005, 116, 21–29. [Google Scholar] [CrossRef]
- Krzyzanowski, M. WHO Air Quality Guidelines for Europe. J. Toxicol. Environ. Health A 2008, 71, 47–50. [Google Scholar] [CrossRef]
- Reizer, E.; Csizmadia, I.G.; Palotas, A.B.; Viskolcz, B.; Fiser, B. Formation Mechanism of Benzo(a)pyrene: One of the Most Carcinogenic Polycyclic Aromatic Hydrocarbons (PAH). Molecules 2019, 24, 1040. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Jin, H.; Lu, Q. Effect of polycyclic aromatic hydrocarbons on immunity. J. Transl. Autoimmun. 2022, 5, 100177. [Google Scholar] [CrossRef]
- White, K.L., Jr.; Holsapple, M.P. Direct suppression of in vitro antibody production by mouse spleen cells by the carcinogen benzo(a)pyrene but not by the noncarcinogenic congener benzo(e)pyrene. Cancer Res. 1984, 44, 3388–3393. [Google Scholar] [PubMed]
- Dean, J.H.; Luster, M.I.; Boorman, G.A.; Lauer, L.D.; Leubke, R.W.; Lawson, L. Selective immunosuppression resulting from exposure to the carcinogenic congener of benzopyrene in B6C3F1 mice. Clin. Exp. Immunol. 1983, 52, 199–206. [Google Scholar] [PubMed]
- De Jong, W.H.; Kroese, E.D.; Vos, J.G.; Van Loveren, H. Detection of immunotoxicity of benzo[a]pyrene in a subacute toxicity study after oral exposure in rats. Toxicol. Sci. 1999, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.D.; White, K.L., Jr. Benzo(a)pyrene-induced anemia and splenomegaly in NZB/WF1 mice. Food Chem. Toxicol. 2005, 43, 1423–1431. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Luster, M.I. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001, 98, 2–10. [Google Scholar] [CrossRef]
- Sun, L.L.; Ye, Z.M.; Ling, Y.X.; Cai, S.F.; Xu, J.L.; Fan, C.H.; Zhong, Y.H.; Shen, Q.; Li, Y.J. Relationship between polycyclic aromatic hydrocarbons and rheumatoid arthritis in US general population, NHANES 2003–2012. Sci. Total Environ. 2020, 704, 135294. [Google Scholar] [CrossRef]
- Seise, I.; Pilz, Z.A.; Kusi, M.Y.; Bogan, B.; McHale, B.J.; Gato, W.E. Dietary ingestion of 2-aminoanthracene (2AA) and the risk for type-1 diabetes (T1D). J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2020, 55, 1638–1645. [Google Scholar] [CrossRef]
- Oikonen, M.; Laaksonen, M.; Laippala, P.; Oksaranta, O.; Lilius, E.M.; Lindgren, S.; Rantio-Lehtimaki, A.; Anttinen, A.; Koski, K.; Eralinna, J.P. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology 2003, 22, 95–99. [Google Scholar] [CrossRef]
- O’Driscoll, C.A.; Owens, L.A.; Gallo, M.E.; Hoffmann, E.J.; Afrazi, A.; Han, M.; Fechner, J.H.; Schauer, J.J.; Bradfield, C.A.; Mezrich, J.D. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part Fibre Toxicol. 2018, 15, 35. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Barbhaiya, M.; Castro-Webb, N.; Conte, C.; Tedeschi, S.K.; Leatherwood, C.; Costenbader, K.H.; Rosenberg, L. Relationship of Cigarette Smoking and Alcohol Consumption to Incidence of Systemic Lupus Erythematosus in a Prospective Cohort Study of Black Women. Arthritis Care Res. 2019, 71, 671–677. [Google Scholar] [CrossRef]
- Jung, C.R.; Chung, W.T.; Chen, W.T.; Lee, R.Y.; Hwang, B.F. Long-term exposure to traffic-related air pollution and systemic lupus erythematosus in Taiwan: A cohort study. Sci. Total Environ. 2019, 668, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M.; Studenski, S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch. Intern. Med. 1992, 152, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Ghaussy, N.O.; Sibbitt, W., Jr.; Bankhurst, A.D.; Qualls, C.R. Cigarette smoking and disease activity in systemic lupus erythematosus. J. Rheumatol. 2003, 30, 1215–1221. [Google Scholar] [PubMed]
- Theofilopoulos, A.N.; Dixon, F.J. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985, 37, 269–390. [Google Scholar] [PubMed]
- Richard, M.L.; Gilkeson, G. Mouse models of lupus: What they tell us and what they don’t. Lupus Sci. Med. 2018, 5, e000199. [Google Scholar] [CrossRef]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef]
- Isenberg, D.A.; Manson, J.J.; Ehrenstein, M.R.; Rahman, A. Fifty years of anti-ds DNA antibodies: Are we approaching journey’s end? Rheumatology 2007, 46, 1052–1056. [Google Scholar] [CrossRef]
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 1526–1533. [Google Scholar] [CrossRef]
- Fabrizio, C.; Fulvia, C.; Carlo, P.; Laura, M.; Elisa, M.; Francesca, M.; Romana, S.F.; Simona, T.; Cristiano, A.; Guido, V. Systemic Lupus Erythematosus with and without Anti-dsDNA Antibodies: Analysis from a Large Monocentric Cohort. Mediat. Inflamm. 2015, 2015, 328078. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, P.; Ferri, S.; Pappas, G.; Quarneti, C.; Lenzi, M.; Bianchi, F.B.; Muratori, L. Diagnosis and therapy of autoimmune hepatitis. Mini Rev. Med. Chem. 2009, 9, 847–860. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Tovoli, F.; Muratori, P. Diagnostic role of anti-dsDNA antibodies: Do not forget autoimmune hepatitis. Nat. Rev. Rheumatol. 2021, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001, 2, 777–780. [Google Scholar] [CrossRef]
- Manuel, R.S.J.; Liang, Y. Sexual dimorphism in immunometabolism and autoimmunity: Impact on personalized medicine. Autoimmun. Rev. 2021, 20, 102775. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Ramsey-Goldman, R.; Urowitz, M.B.; Hanly, J.G.; Gordon, C.; Petri, M.A.; Ginzler, E.M.; Wallace, D.J.; Bae, S.C.; Romero-Diaz, J.; et al. Cancer Risk in a Large Inception Systemic Lupus Erythematosus Cohort: Effects of Demographic Characteristics, Smoking, and Medications. Arthritis Care Res. 2021, 73, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman-Morris, J.; Putterman, C. Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin. Dev. Immunol. 2012, 2012, 604892. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Lau, C.S.; Chan, T.M.; Wong, R.W. Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus 1999, 8, 188–196. [Google Scholar] [CrossRef]
- Klein, J.A.; Singh, S.K.; Goswami, D.G.; Braucher, L.N.; Wright, H.N.; Noland, E.L.; Agarwal, R.; Tewari-Singh, N. Exposure to Environmental Pollutant Benzo[a]pyrene Exacerbates Skin Inflammation in a Mouse Psoriatic Model. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Huang, L.; Duan, S.; Shao, H.; Zhang, A.; Chen, S.; Zhang, P.; Wang, N.; Wang, W.; Wu, Y.; Wang, J.; et al. NLRP3 deletion inhibits inflammation-driven mouse lung tumorigenesis induced by benzo(a)pyrene and lipopolysaccharide. Respir. Res. 2019, 20, 20. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Penninger, J.M.; Irie-Sasaki, J.; Sasaki, T.; Oliveira-dos-Santos, A.J. CD45: New jobs for an old acquaintance. Nat. Immunol. 2001, 2, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Langlois, B.; Koczorowska, M.M.; Radwanska, A.; Sun, Z.; Hussenet, T.; Lefebvre, O.; Murdamoothoo, D.; Arnold, C.; Klein, A.; et al. Tenascin-C Orchestrates Glioblastoma Angiogenesis by Modulation of Pro- and Anti-angiogenic Signaling. Cell Rep. 2016, 17, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Oweida, A.; Bhatia, S.; Hirsch, K.; Calame, D.; Griego, A.; Keysar, S.; Pitts, T.; Sharma, J.; Eckhardt, G.; Jimeno, A.; et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol. Carcinog. 2017, 56, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.; zum Buttel, H.; Bogurcu, N.; Cuesta, A.M.; Aburto, M.R.; Seidel, S.; Finkelmeier, F.; Foss, F.; Hofmann, J.; Kaulich, K.; et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat. Commun. 2016, 7, 12329. [Google Scholar] [CrossRef]
- Volpert, O.V.; Pili, R.; Sikder, H.A.; Nelius, T.; Zaichuk, T.; Morris, C.; Shiflett, C.B.; Devlin, M.K.; Conant, K.; Alani, R.M. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell 2002, 2, 473–483. [Google Scholar] [CrossRef]
- Mura, M.; Swain, R.K.; Zhuang, X.; Vorschmitt, H.; Reynolds, G.; Durant, S.; Beesley, J.F.; Herbert, J.M.; Sheldon, H.; Andre, M.; et al. Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene 2012, 31, 293–305. [Google Scholar] [CrossRef]
- Yoshitomi, Y.; Ikeda, T.; Saito-Takatsuji, H.; Yonekura, H. Emerging Role of AP-1 Transcription Factor JunB in Angiogenesis and Vascular Development. Int. J. Mol. Sci. 2021, 22, 2804. [Google Scholar] [CrossRef]
- Perez-Benavente, B.; Fathinajafabadi, A.; de la Fuente, L.; Gandia, C.; Martinez-Ferriz, A.; Pardo-Sanchez, J.M.; Milian, L.; Conesa, A.; Romero, O.A.; Carretero, J.; et al. New roles for AP-1/JUNB in cell cycle control and tumorigenic cell invasion via regulation of cyclin E1 and TGF-beta 2. Genome Biol. 2022, 23, 252. [Google Scholar] [CrossRef]
- Lambert, M.P.; Meng, R.; Xiao, L.; Harper, D.C.; Marks, M.S.; Kowalska, M.A.; Poncz, M. Intramedullary megakaryocytes internalize released platelet factor 4 and store it in alpha granules. J. Thromb. Haemost. 2015, 13, 1888–1899. [Google Scholar] [CrossRef]
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.Y.; Cordell, J.L.; Tse, A.G.; van Dongen, J.J.; van Noesel, C.J.; Micklem, K.; Pulford, K.A.; Valensi, F.; Comans-Bitter, W.M.; Borst, J.; et al. The IgM-associated protein mb-1 as a marker of normal and neoplastic B cells. J. Immunol. 1991, 147, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- van Noesel, C.J.; van Lier, R.A.; Cordell, J.L.; Tse, A.G.; van Schijndel, G.M.; de Vries, E.F.; Mason, D.Y.; Borst, J. The membrane IgM-associated heterodimer on human B cells is a newly defined B cell antigen that contains the protein product of the mb-1 gene. J. Immunol. 1991, 146, 3881–3888. [Google Scholar] [CrossRef]
- Kashiwakura, J.; Suzuki, N.; Nagafuchi, H.; Takeno, M.; Takeba, Y.; Shimoyama, Y.; Sakane, T. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J. Exp. Med. 1999, 190, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Takeba, Y.; Nagafuchi, H.; Takeno, M.; Kashiwakura, J.; Suzuki, N. Txk, a member of nonreceptor tyrosine kinase of Tec family, acts as a Th1 cell-specific transcription factor and regulates IFN-gamma gene transcription. J. Immunol. 2002, 168, 2365–2370. [Google Scholar] [CrossRef]
- Dong, D.; Zheng, L.; Lin, J.; Zhang, B.; Zhu, Y.; Li, N.; Xie, S.; Wang, Y.; Gao, N.; Huang, Z. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature 2019, 573, 546–552. [Google Scholar] [CrossRef]
- Moser, B. CXCR5, the Defining Marker for Follicular B Helper T (TFH) Cells. Front. Immunol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Barington, L.; Wanke, F.; Niss Arfelt, K.; Holst, P.J.; Kurschus, F.C.; Rosenkilde, M.M. EBI2 in splenic and local immune responses and in autoimmunity. J. Leukoc. Biol. 2018, 104, 313–322. [Google Scholar] [CrossRef]
- Probst, C.M.; Silva, R.A.; Menezes, J.P.; Almeida, T.F.; Gomes, I.N.; Dallabona, A.C.; Ozaki, L.S.; Buck, G.A.; Pavoni, D.P.; Krieger, M.A.; et al. A comparison of two distinct murine macrophage gene expression profiles in response to Leishmania amazonensis infection. BMC Microbiol. 2012, 12, 22. [Google Scholar] [CrossRef]
- Kishikawa, S.; Sato, S.; Kaneto, S.; Uchino, S.; Kohsaka, S.; Nakamura, S.; Kiyono, H. Allograft inflammatory factor 1 is a regulator of transcytosis in M cells. Nat. Commun. 2017, 8, 14509. [Google Scholar] [CrossRef]
- van der Voort, R.; van Lieshout, A.W.T.; Toonen, L.W.J.; Sloetjes, A.W.; van den Berg, W.B.; Figdor, C.G.; Radstake, T.R.D.J.; Adema, G.J. Elevated CXCL16 expression by synovial macrophages recruits memory T cells into rheumatoid joints. Arthritis Rheum. 2005, 52, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Dnyanmote, A.V.; Bush, K.T.; Wu, W.; Nigam, S.K. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J. Biol. Chem. 2011, 286, 26391–26395. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, N.; North, K.E.; Kopp, J.B.; Mckenzie, L.; Winkler, C. NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: A HuGE review. Genet. Med. 2006, 8, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Buchli, R.; Alberati-Giani, D.; Malherbe, P.; Kohler, C.; Broger, C.; Cesura, A.M. Cloning and functional expression of a soluble form of kynurenine/alpha-aminoadipate aminotransferase from rat kidney. J. Biol. Chem. 1995, 270, 29330–29335. [Google Scholar] [CrossRef]
- Hart, T.C.; Gorry, M.C.; Hart, P.S.; Woodard, A.S.; Shihabi, Z.; Sandhu, J.; Shirts, B.; Xu, L.; Zhu, H.; Barmada, M.M.; et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 2002, 39, 882–892. [Google Scholar] [CrossRef]
- Hassouneh, R.; Nasrallah, R.; Zimpelmann, J.; Gutsol, A.; Eckert, D.; Ghossein, J.; Burns, K.D.; Hebert, R.L. PGE2 receptor EP3 inhibits water reabsorption and contributes to polyuria and kidney injury in a streptozotocin-induced mouse model of diabetes. Diabetologia 2016, 59, 1318–1328. [Google Scholar] [CrossRef]
- Song, C.C.; Wang, S.Q.; Fu, Z.N.; Chi, K.; Geng, X.D.; Liu, C.; Cai, G.Y.; Chen, X.M.; Wu, D.; Hong, Q. IGFBP5 promotes diabetic kidney disease progression by enhancing PFKFB3-mediated endothelial glycolysis. Cell Death Dis. 2022, 13, 340. [Google Scholar] [CrossRef]
- Gailly, P.; Jouret, F.; Martin, D.; Debaix, H.; Parreira, K.S.; Nishita, T.; Blanchard, A.; Antignac, C.; Willnow, T.E.; Courtoy, P.J.; et al. A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction. Kidney Int. 2008, 74, 52–61. [Google Scholar] [CrossRef]
- Perricone, C.; Ceccarelli, F.; Massaro, L.; Alessandri, C.; Spinelli, F.R.; Marocchi, E.; Miranda, F.; Truglia, S.; Valesini, G.; Conti, F. Systemic Lupus Erythematosus with or without Anti-Dsdna Antibodies: Not So Different. Ann. Rheum. Dis. 2015, 74, 1085–1086. [Google Scholar] [CrossRef]
- Battaglia, M.; Garrett-Sinha, L.A. Bacterial infections in lupus: Roles in promoting immune activation and in pathogenesis of the disease. J. Transl. Autoimmun. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Yan, F.; Robert, M.; Li, Y. Statistical methods and common problems in medical or biomedical science research. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 157–163. [Google Scholar] [PubMed]
- Bland, M. An Introduction to Medical Statistics, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2000; p. xvi. 405p. [Google Scholar]
- Klufio, C.A. An Introduction to Medical Statistics and Research Methodology; Woeli Pub. Services: Accra, Ghana, 2003; p. xii. 324p. [Google Scholar]
- Dean, A.; Morris, M.; Stufken, J.; Bingham, D.R. Handbook of Design and Analysis of Experiments; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2015; p. xix. 940p. [Google Scholar]
- Welham, S.J. Statistical Methods in Biology: Design and Analysis of Experiments and Regression; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2015; p. xx. 582p. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Kennicott, K.; Liang, Y. Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice. Int. J. Mol. Sci. 2023, 24, 6163. https://doi.org/10.3390/ijms24076163
Zhu R, Kennicott K, Liang Y. Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice. International Journal of Molecular Sciences. 2023; 24(7):6163. https://doi.org/10.3390/ijms24076163
Chicago/Turabian StyleZhu, Runqi, Kameron Kennicott, and Yun Liang. 2023. "Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice" International Journal of Molecular Sciences 24, no. 7: 6163. https://doi.org/10.3390/ijms24076163
APA StyleZhu, R., Kennicott, K., & Liang, Y. (2023). Benzo[a]pyrene Exposure Reduces Cell-Type Diversity and Stimulates Sex-Biased Damage Pathways in End Organs of Lupus-Prone Mice. International Journal of Molecular Sciences, 24(7), 6163. https://doi.org/10.3390/ijms24076163





