HPLC Analysis of Polyphenols Derived from Hungarian Aszú from Tokaj Wine Region and Its Effect on Inflammation in an In Vitro Model System of Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. Analytical Studies
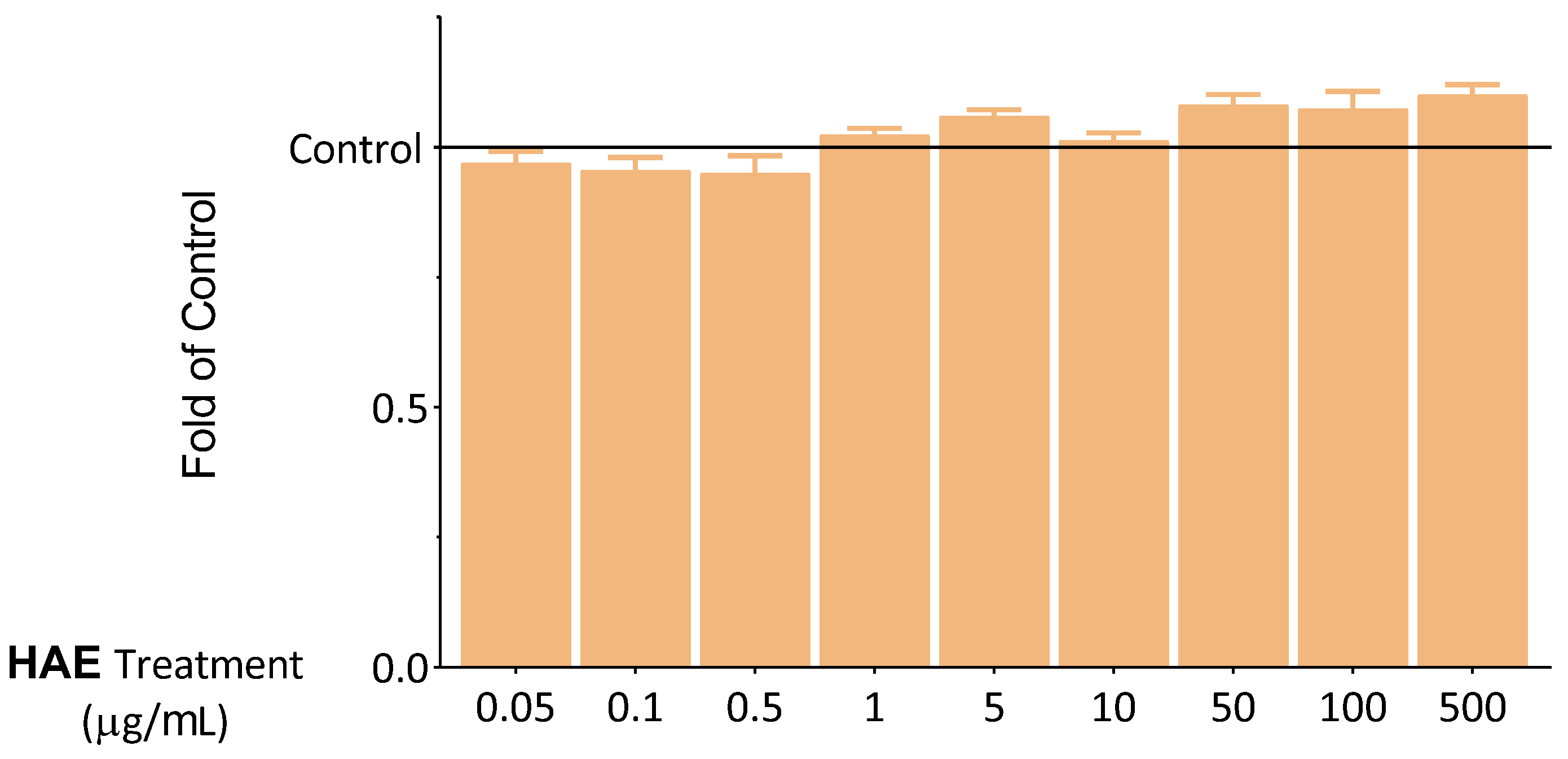
2.2. Seeking Optimal HAE Concentration
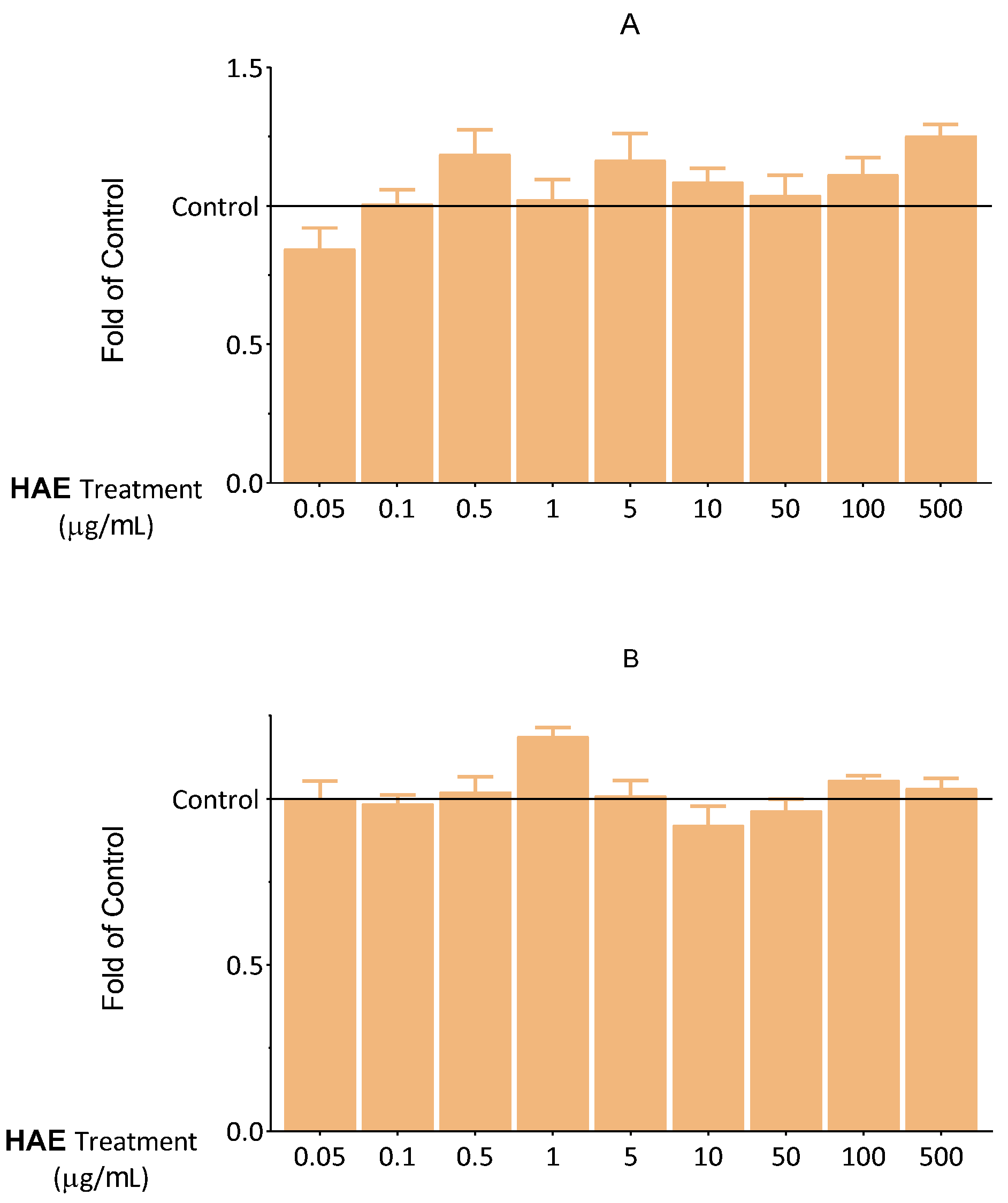
2.2.1. Results of Viability Tests
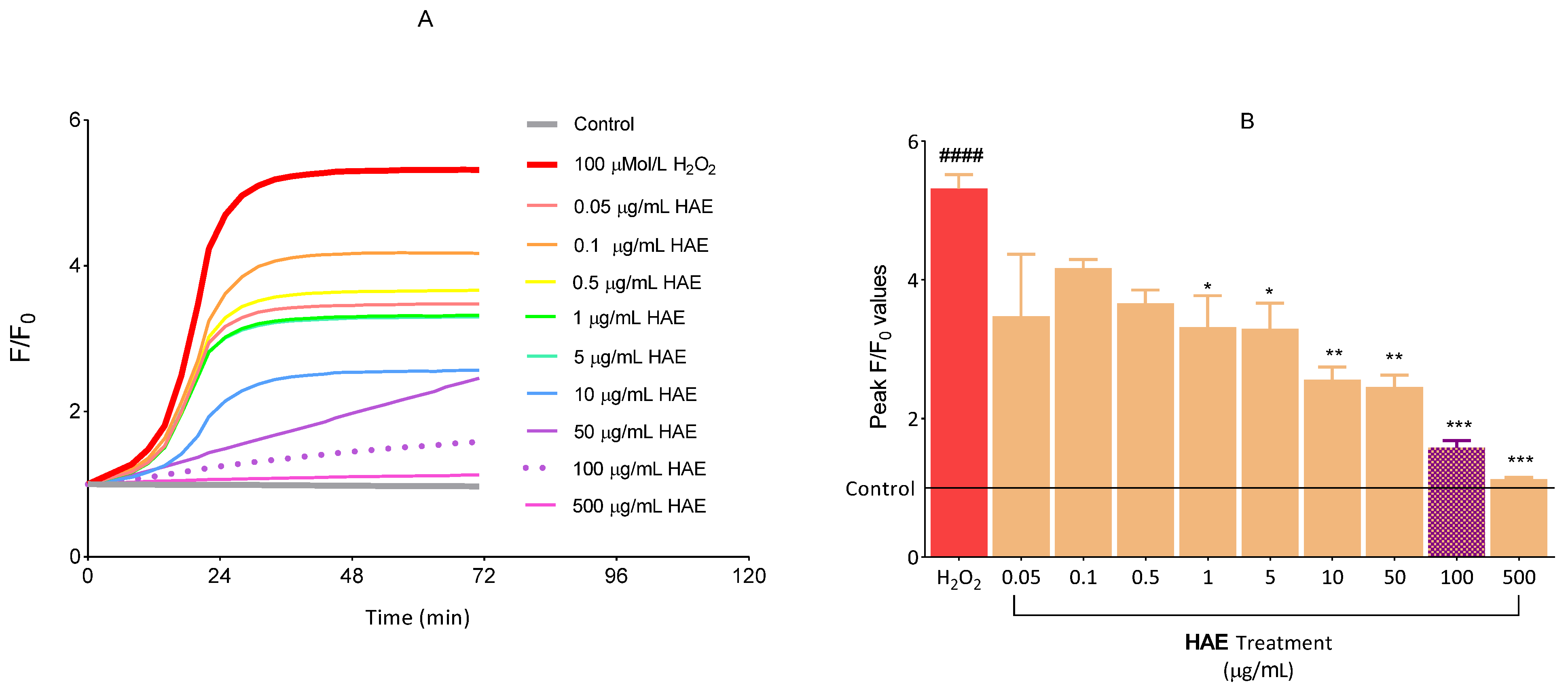
2.2.2. The Antioxidant Effect of HAE
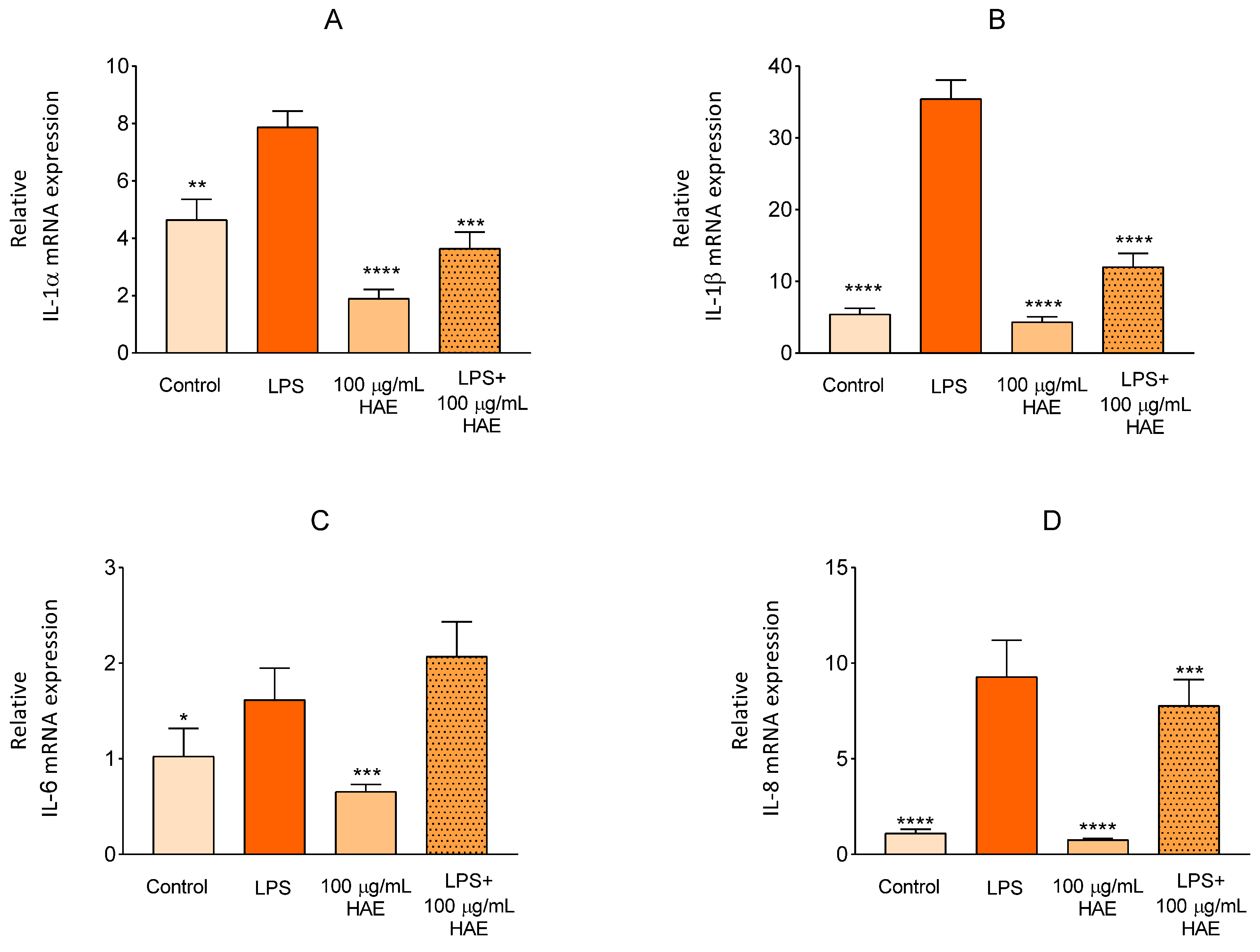
2.2.3. The Effect of HAE on Inflammatory Response in HUVEC
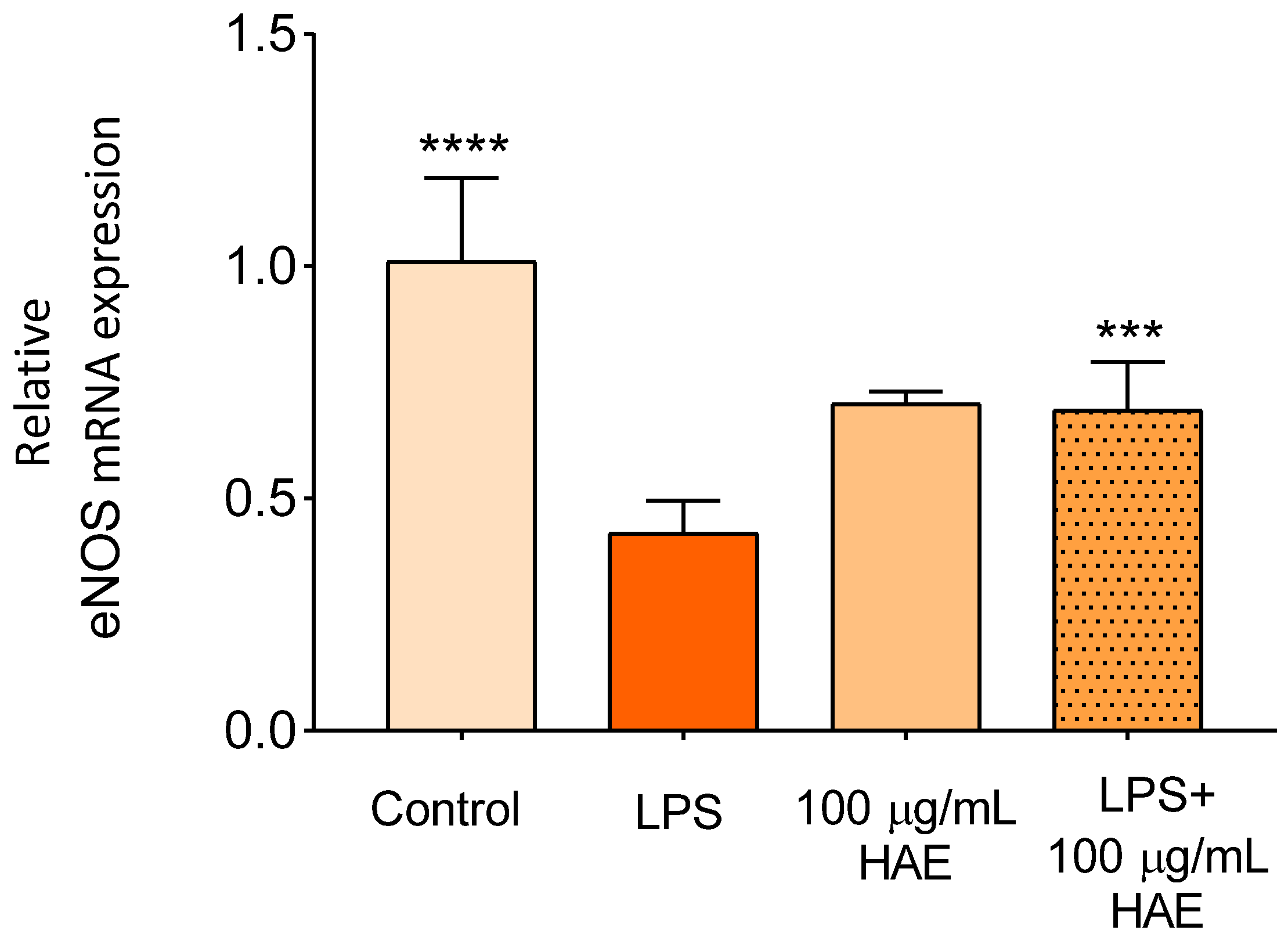
2.2.4. The Effect of HAE on The Expression of eNOS
3. Discussion
4. Material and Methods
4.1. Materials
Chemicals
4.2. Methods
4.2.1. Preparation of Hungarian Aszú Extract (HAE)
4.2.2. High-Performance Liquid Chromatography
4.2.3. Cell Culture
4.2.4. Determination of Cellular Viability
4.2.5. Determination of Intracellular Lipids
4.2.6. Determination of Apoptosis
4.2.7. Determination of Necrosis
4.2.8. Evaluation of ROS Production
4.2.9. Quantitative Real-Time PCR (qPCR)
4.2.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, J.; Kumric, M.; Ticinovic Kurir, T.; Males, I.; Martinovic, D.; Vilovic, M.; Bozic, J. Effects of Wine Components in Inflammatory Bowel Diseases. Molecules 2021, 26, 5891. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Carluccio, M.A.; Grieco, F. Wine Polyphenols and Health. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1135–1155. [Google Scholar] [CrossRef]
- Alonso Borbalán, Á.M.; Zorro, L.; Guillén, D.A.; García Barroso, C. Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. ESPEN 2015, 10, e224–e233. [Google Scholar] [CrossRef]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; Skala, T. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Bratisl. Lek. Listy 2017, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Bulló, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Gea, A.; Gómez-Gracia, E.; Lapetra, J.; et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br. J. Nutr. 2015, 113 (Suppl. 2), S121–S130. [Google Scholar] [CrossRef]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An Aspiring Agent in Promoting Longevity and Preventing Chronic Diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Magyar, I. Botrytized Wines. Adv. Food Nutr. Res. 2011, 63, 147–206. [Google Scholar] [CrossRef]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef]
- Migliori, M.; Panichi, V.; de la Torre, R.; Fitó, M.; Covas, M.; Bertelli, A.; Muñoz-Aguayo, D.; Scatena, A.; Paoletti, S.; Ronco, C. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015, 39, 218–223. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Remenyik, J.; Biró, A.; Klusóczki, Á.; Juhász, K.Z.; Szendi-Szatmári, T.; Kenesei, Á.; Szőllősi, E.; Vasvári, G.; Stündl, L.; Fenyvesi, F.; et al. Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures. Int. J. Mol. Sci. 2022, 23, 9036. [Google Scholar] [CrossRef] [PubMed]
- Biro, A.; Markovich, A.; Homoki, J.R.; Szőllősi, E.; Hegedűs, C.; Tarapcsák, S.; Lukács, J.; Stündl, L.; Remenyik, J. Anthocyanin-Rich Sour Cherry Extract Attenuates the Lipopolysaccharide-Induced Endothelial Inflammatory Response. Molecules 2019, 24, 3427. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Markovics, A.; Tóth, K.F.; Sós, K.E.; Magi, J.; Gyöngyösi, A.; Benyó, Z.; Zouboulis, C.C.; Bíró, T.; Oláh, A. Nicotinic acid suppresses sebaceous lipogenesis of human sebocytes via activating hydroxycarboxylic acid receptor 2 (HCA2). J. Cell. Mol. Med. 2019, 23, 6203–6214. [Google Scholar] [CrossRef] [PubMed]
- Magyar, I.; Soós, J. Botrytized wines—Current perspectives. Int. J. Wine Res. 2016, 8, 29–39. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 21—Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–271. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; Lopez de Dicastillo, C. Evaluation of Polyphenol Content and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxid. Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Fiers, W.; Pober, J.S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J. Immunol. 1987, 139, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Kalló, G.; Kunkli, B.; Győri, Z.; Szilvássy, Z.; Csősz, É.; Tőzsér, J. Compounds with Antiviral, Anti-Inflammatory and Anticancer Activity Identified in Wine from Hungary's Tokaj Region via High Resolution Mass Spectrometry and Bioinformatics Analyses. Int. J. Mol. Sci. 2020, 21, 9547. [Google Scholar] [CrossRef]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Kim, Y.M.; Deutsch, J.; Katrich, E.; Gorinstein, S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules 2021, 26, 6686. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, Y.; Bao, Y.-r.; Wang, S.; Li, T.-j.; Leng, J.-p.; Meng, X.-s. Potential effects of fructus aurantii ethanol extracts against colitis-associated carcinogenesis through coordination of Notch/NF-κB/IL-1 signaling pathways. Biomed. Pharmacother. 2022, 152, 113278. [Google Scholar] [CrossRef]
- Blonska, M.; Czuba, Z.P.; Krol, W. Effect of flavone derivatives on interleukin-1beta (IL-1beta) mRNA expression and IL-1beta protein synthesis in stimulated RAW 264.7 macrophages. Scand. J. Immunol. 2003, 57, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.A.; Szendzielorz, K.; Mazur, B.; Król, W. The inhibitory effect of flavonoids on interleukin-8 release by human gastric adenocarcinoma (AGS) cells infected with cag PAI (+) Helicobacter pylori. Cent. Eur. J. Immunol. 2016, 41, 229–235. [Google Scholar] [CrossRef]
- Sun, Y.-w.; Bao, Y.; Yu, H.; Chen, Q.-j.; Lu, F.; Zhai, S.; Zhang, C.-f.; Li, F.; Wang, C.-z.; Yuan, C.-s. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 2020, 83, 106384. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Grebenchikov, O.A. Transcription Factor Nrf2 as a Potential Therapeutic Target for Prevention of Cytokine Storm in COVID-19 Patients. Biochemistry 2020, 85, 833–837. [Google Scholar] [CrossRef]
- Qiao, Y.; He, H.; Jonsson, P.; Sinha, I.; Zhao, C.; Dahlman-Wright, K. AP-1 Is a Key Regulator of Proinflammatory Cytokine TNFα-mediated Triple-negative Breast Cancer Progression. J. Biol. Chem. 2016, 291, 5068–5079. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef] [PubMed]



| Number | Phenolic Compound | Compound Family | λ Detection (nm) | Concentration (mg/L) |
|---|---|---|---|---|
| 1 | Gallic acid | Hydroxybenzoic acid | 280 | 0.843 |
| 2 | (+)-Catechin | Flavan-3-ol | 280 | 9.963 |
| 3 | Caftaric acid | Hydroxycinnamic acid | 320 | 0.268 |
| 4 | Vanillic acid | Hydroxybenzoic acid | 280 | 9.262 |
| 5 | (−)-Epigallocatechin gallate | Flavan-3-ol | 280 | 0.946 |
| 6 | Caffeic acid | Hydroxycinnamic acid | 320 | 1.768 |
| 7 | Syringic acid | Hydroxybenzoic acid | 280 | 0.355 |
| 8 | (−)-Epicatechin gallate | Flavan-3-ol | 280 | 4.426 |
| 9 | p-Coumaric acid | Hydroxybenzoic acid | 320 | 0.638 |
| 10 | Ferulic acid | Hydroxycinnamic acid | 320 | 0.277 |
| 11 | Ellagic acid | Hydroxybenzoic acid | 280 | 0.417 |
| IL-1α | Forward | GCA TTA CAT AAT CTG GAT GAA GCA G |
| Reversed | GGT TTT GGG TAT CTC AGG CAT C | |
| Probe | 6-Fam-TGG TCT TCA TCT TGG GCA GTC ACA-BHQ-1 | |
| IL-1β | Forward | GGC AAT GAG GAT GAC TTG TTC |
| Reversed | CGG AGA TTC GTA GCT GGA TG | |
| Probe | 6-Fam-TGA TGG CCC TAA ACA GAT GAA GTG-BHQ-1 | |
| IL-6 | Forward | AAT TCG GTA CAT CCT CGA CG |
| Reversed | GAT TTT CAC CAG GCA AGT CTC | |
| Probe | 6-Fam-TGT TAC ATG TCT CCT TTC TCA GGG C-BHQ-1 | |
| IL-8 | Forward | TCC TGA TTT CTG CAG CTC TG |
| Reversed | GTC CAC TCT CAA TCA CTC TCA G | |
| Probe | 6-Fam-CAT ACT CCA AAC CTT TCC ACC CC-BHQ-1 | |
| eNOS | Forward | CTT CCT GGA CAT CAC CTC C |
| Reversed | AAC CAC TTC CAC TCC TCG | |
| Probe | 6-Fam-TCC TGC TGT TCC CTG GGC-BHQ-1 | |
| GAPDH | Forward | CCT CCA CCT TTG ACG CTG |
| Reversed | CTC TTC CTC TTG TGC TCT TGC | |
| Probe | Hex-CAT TGC CCT CAA CGA CCA CTT T-BHQ-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovics, A.; Csige, L.; Szőllősi, E.; Matyi, H.; Lukács, A.D.; Perez, N.R.; Bacsó, Z.R.; Stündl, L.; Remenyik, J.; Biró, A. HPLC Analysis of Polyphenols Derived from Hungarian Aszú from Tokaj Wine Region and Its Effect on Inflammation in an In Vitro Model System of Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 6124. https://doi.org/10.3390/ijms24076124
Markovics A, Csige L, Szőllősi E, Matyi H, Lukács AD, Perez NR, Bacsó ZR, Stündl L, Remenyik J, Biró A. HPLC Analysis of Polyphenols Derived from Hungarian Aszú from Tokaj Wine Region and Its Effect on Inflammation in an In Vitro Model System of Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(7):6124. https://doi.org/10.3390/ijms24076124
Chicago/Turabian StyleMarkovics, Arnold, László Csige, Erzsébet Szőllősi, Hajnalka Matyi, Andrea Diána Lukács, Nóra Réka Perez, Zsófia Réka Bacsó, László Stündl, Judit Remenyik, and Attila Biró. 2023. "HPLC Analysis of Polyphenols Derived from Hungarian Aszú from Tokaj Wine Region and Its Effect on Inflammation in an In Vitro Model System of Endothelial Cells" International Journal of Molecular Sciences 24, no. 7: 6124. https://doi.org/10.3390/ijms24076124
APA StyleMarkovics, A., Csige, L., Szőllősi, E., Matyi, H., Lukács, A. D., Perez, N. R., Bacsó, Z. R., Stündl, L., Remenyik, J., & Biró, A. (2023). HPLC Analysis of Polyphenols Derived from Hungarian Aszú from Tokaj Wine Region and Its Effect on Inflammation in an In Vitro Model System of Endothelial Cells. International Journal of Molecular Sciences, 24(7), 6124. https://doi.org/10.3390/ijms24076124








