Faecal Microbiota Transplantation, Paving the Way to Treat Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
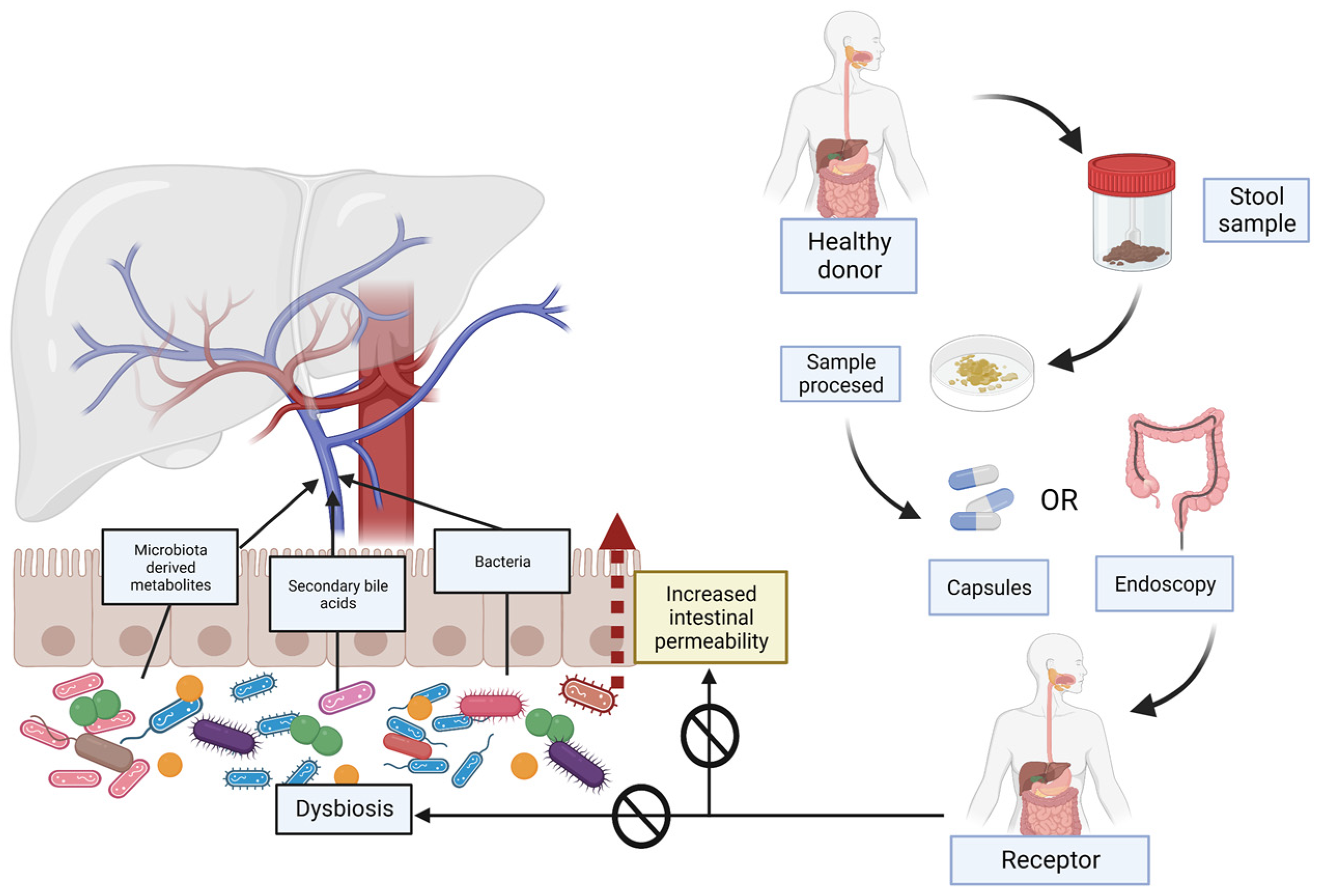
2. Gut Microbiome, Dysbiosis and Increased Intestinal Permeability Associated with NAFLD
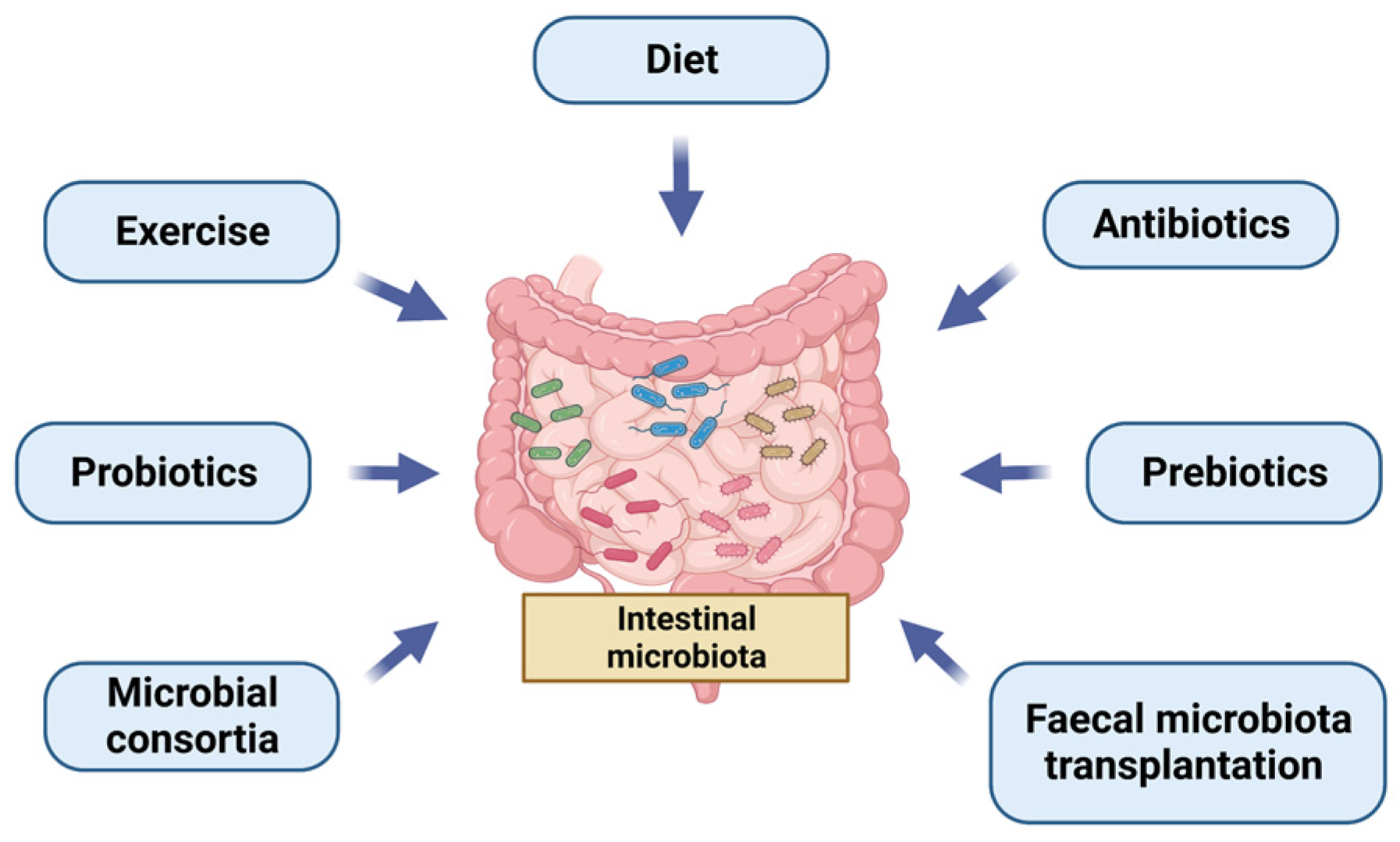
3. Different Ways of Managing the Microbiota, Focus on Faecal Microbiota Transplantation (FMT)
4. Current Indications for FMT
5. FMT in Chronic Liver Diseases (CLD) Other Than NAFLD
6. Possible Treatments for NAFLD Modulating GM
7. FMT and NAFLD
8. Limitations of FMT and Future
9. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- El-Kassas, M.; Cabezas, J.; Coz, P.I.; Zheng, M.-H.; Arab, J.P.; Awad, A. Nonalcoholic Fatty Liver Disease: Current Global Burden. Semin. Liver Dis. 2022, 42, 401–412. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.-S.; Castellanos, M.; La Fuente, R.A.-D.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martín, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e417. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Al Shabeeb, R.; Golabi, P.; Younossi, I.; Henry, L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology 2022, 76, 1423–1437. [Google Scholar] [CrossRef]
- Allen, A.M.; Van Houten, H.K.; Sangaralingham, L.R.; Talwalkar, J.A.; McCoy, R.G. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology 2018, 68, 2230–2238. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Santos-Laso, A.; Gutiérrez-Larrañaga, M.; Alonso-Peña, M.; Medina, J.M.; Iruzubieta, P.; Arias-Loste, M.T.; López-Hoyos, M.; Crespo, J. Pathophysiological Mechanisms in Non-Alcoholic Fatty Liver Disease: From Drivers to Targets. Biomedicines 2022, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Choudhary, N.S.; Mishra, S.K. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1875–1887. [Google Scholar] [CrossRef]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Guarner, F.; Manichanh, C. Structure and functions of the gut microbiome. Endocr. Metab. Immune Disord.—Drug Targets 2014, 14, 290–299. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and Meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Johnson, J.S.; Angeles, J.E.; Behling, C.; Belt, P.H.; Borecki, I.; Bross, C.; Durelle, J.; Goyal, N.P.; Hamilton, G.; et al. Microbiome Signatures Associated with Steatohepatitis and Moderate to Severe Fibrosis in Children with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157, 1109–1122. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.M.B.; Stefano, J.T.; Miele, L.; Ponziani, F.R.; Souza-Basqueira, M.; Okada, L.S.R.R.; de Barros Costa, F.G.; Toda, K.; Mazo, D.F.C.; Sabino, E.C.; et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Mehal, W.Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 637–644. [Google Scholar] [CrossRef]
- Iruzubieta, P.; Medina, J.M.; Fernández-López, R.; Crespo, J.; De La Cruz, F. A Role for Gut Microbiome Fermentative Pathways in Fatty Liver Disease Progression. J. Clin. Med. 2020, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.C.; Littlejohn, P.T.; Ayala, V.; Creus-Cuadros, A.; Finlay, B.B. Nonalcoholic Fatty Liver Disease and the Gut-Liver Axis: Exploring an Undernutrition Perspective. Gastroenterology 2022, 162, 1858–1875.e2. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Clark, S.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018, 9, 4462. [Google Scholar] [CrossRef] [PubMed]
- Armet, A.M.; Deehan, E.C.; O’Sullivan, A.F.; Mota, J.F.; Field, C.J.; Prado, C.M.; Lucey, A.J.; Walter, J. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe 2022, 30, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Losno, E.; Sieferle, K.; Perez-Cueto, F.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Álvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Ojima, M.; Shimizu, K.; Motooka, D.; Ishihara, T.; Nakamura, S.; Shintani, A.; Ogura, H.; Iida, T.; Yoshiya, K.; Shimazu, T. Gut Dysbiosis Associated with Antibiotics and Disease Severity and Its Relation to Mortality in Critically Ill Patients. Dig. Dis. Sci. 2022, 67, 2420–2432. [Google Scholar] [CrossRef]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1870402. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J.; Michel, C. How to Manipulate the Microbiota: Prebiotics. Adv. Exp. Med. Biol. 2016, 902, 119–142. [Google Scholar]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.J.S. Revisión del papel de los probióticos en la patología gastrointestinal del adulto. Gastroenterol. Hepatol. 2017, 40, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Castellanos, J.F.V.; Biclot, A.; Vrancken, G.; Huys, G.R.; Raes, J. Design of synthetic microbial consortia for gut microbiota modulation. Curr. Opin. Pharmacol. 2019, 49, 52–59. [Google Scholar] [CrossRef]
- El Hage, R.; Hernandez-Sanabria, E.; Arroyo, M.C.; Props, R.; Van De Wiele, T. Propionate-Producing Consortium Restores Antibiotic-Induced Dysbiosis in a Dynamic in vitro Model of the Human Intestinal Microbial Ecosystem. Front. Microbiol. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Högenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Mégraud, F.; et al. A standardised model for stool banking for faecal microbiota transplantation: A consensus report from a multidisciplinary UEG working group. United Eur. Gastroenterol. J. 2021, 9, 229–247. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.A.; Bytzer, P.; Pedersen, O.B.; Engberg, J. Establishing a donor stool bank for faecal microbiota transplantation: Methods and feasibility. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Ad-verse Reactions Due to Transmission of Multi-Drug Resistant Organisms|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse (accessed on 8 October 2022).
- Ianiro, G.; Mullish, B.H.; Kelly, C.R.; Sokol, H.; Kassam, Z.; Ng, S.C.; Fischer, M.; Allegretti, J.R.; Masucci, L.; Zhang, F.; et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: Suggestions for urgent updates from an international expert panel. Lancet Gastroenterol. Hepatol. 2020, 5, 430–432. [Google Scholar] [CrossRef]
- Green, C.A.; Quraishi, M.N.; Shabir, S.; Sharma, N.; Hansen, R.; Gaya, D.R.; Hart, A.L.; Loman, N.J.; Iqbal, T.H. Screening faecal microbiota transplant donors for SARS-CoV-2 by molecular testing of stool is the safest way forward. Lancet Gastroenterol. Hepatol. 2020, 5, 531. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Borody, T.; Lin, E.; Finlayson, S.; Walsh, A.J.; Samuel, D.; Bogaerde, J.V.D.; Leong, R.; Connor, S.; Ng, W.; et al. Donor Recruitment for Fecal Microbiota Transplantation. Inflamm. Bowel Dis. 2015, 21, 1600–1606. [Google Scholar] [CrossRef]
- Ianiro, G.; Porcari, S.; Bibbò, S.; Giambò, F.; Quaranta, G.; Masucci, L.; Sanguinetti, M.; Gasbarrini, A.; Cammarota, G. Donor program for fecal microbiota transplantation: A 3-year experience of a large-volume Italian stool bank. Dig. Liver Dis. 2021, 53, 1428–1432. [Google Scholar] [CrossRef]
- He, J.; He, X.; Ma, Y.; Yang, L.; Fang, H.; Shang, S.; Xia, H.; Lian, G.; Tang, H.; Wang, Q.; et al. A comprehensive approach to stool donor screening for faecal microbiota transplantation in China. Microb. Cell Factories 2021, 20, 216. [Google Scholar] [CrossRef]
- Wilson, B.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, 755–757. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kelly, C.R.; Grinspan, A.; Mullish, B.; Kassam, Z.; Fischer, M. Outcomes of Fecal Microbiota Transplantation in Patients with Inflammatory Bowel Diseases and Recurrent Clostridioides difficile Infection. Gastroenterology 2020, 159, 1982–1984. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbò, S.; Porcari, S.; Settanni, C.R.; Giambò, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: Experience of a large-volume European FMT center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef]
- Stojek, M.; Jabłońska, A.; Adrych, K. The Role of Fecal Microbiota Transplantation in the Treatment of Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 4055. [Google Scholar] [CrossRef]
- Caldeira, L.D.F.; Borba, H.H.; Tonin, F.S.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Fecal microbiota transplantation in inflammatory bowel disease patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238910. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Z.; Ding, L.; Fu, Y.; Fan, J.; Mei, Q.; Lou, L.; Wang, J.; Yin, N.; Lu, Y.; et al. Fecal microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J. Transl. Med. 2022, 20, 354. [Google Scholar] [CrossRef]
- Fang, H.; Fu, L.; Li, X.; Lu, C.; Su, Y.; Xiong, K.; Zhang, L. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: A prospective randomized pilot study. Microb. Cell Factories 2021, 20, 18. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; Moura, D.; Neto, A.M.P.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.; de Moura, E.G. Fecal microbiota transplantation improves metabolic syndrome parameters: Systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Soto, M.T.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Ooijevaar, R.; Terveer, E.; Verspaget, H.; Kuijper, E.; Keller, J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Salzman, N.H.; Acharya, C.; Sterling, R.K.; White, M.B.; Gavis, E.A.; Fagan, A.; Hayward, M.; Holtz, M.L.; Matherly, S.; et al. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology 2019, 70, 1690–1703. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Salzman, N.; Acharya, C.; Takei, H.; Kakiyama, G.; Fagan, A.; White, M.B.; Gavis, E.A.; Holtz, M.L.; Hayward, M.; et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight 2019, 4, e133410. [Google Scholar] [CrossRef]
- Fernández, J.; Piano, S.; Bartoletti, M.; Wey, E.Q. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J. Hepatol. 2021, 75, S101–S117. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Shamsaddini, A.; Fagan, A.; Sterling, R.K.; Gavis, E.; Khoruts, A.; Fuchs, M.; Lee, H.; Sikaroodi, M.; Gillevet, P.M. Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials. Hepatol. Commun. 2020, 5, 258–271. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Carrellas, M.; Mullish, B.; Marchesi, J.R.; Pechlivanis, A.; Smith, M.; Gerardin, Y.; Timberlake, S.; Pratt, D.S.; et al. Fecal Microbiota Transplantation in Patients with Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am. J. Gastroenterol. 2019, 114, 1071–1079. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Gavis, E.A.; Fagan, A.; Wade, J.B.; Thacker, L.R.; Fuchs, M.; Patel, S.; Davis, B.; Meador, J.; Puri, P.; et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology 2021, 73, 1688–1700. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, Z.; Yang, L.; Jin, L.; Wei, W.; Deng, Y.; Chen, X.; Xiao, C.; Yu, X.; Xu, H.; et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017, 65, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Kumar, R.; Sharma, S.; Mahanta, M.; Vayuuru, S.K.; Nayak, B.; Kumar, S. Shalimar Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig. Dis. Sci. 2021, 66, 873–880. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Fagan, A.; Gavis, E.A.; Kassam, Z.; Sikaroodi, M.; Gillevet, P.M. Long-term Outcomes of Fecal Microbiota Transplantation in Patients with Cirrhosis. Gastroenterology 2019, 156, 1921–1923.e3. [Google Scholar] [CrossRef]
- Aller, R.; Fernández-Rodríguez, C.; Iacono, O.L.; Bañares, R.; Abad, J.; Carrión, J.A.; García-Monzón, C.; Caballería, J.; Berenguer, M.; Rodríguez-Perálvarez, M.; et al. Documento de consenso. Manejo de la enfermedad hepática grasa no alcohólica (EHGNA). Guía de práctica clínica. Gastroenterol. Hepatol. 2018, 41, 328–349. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Behrouz, V.; Aryaeian, N.; Zahedi, M.J.; Jazayeri, S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J. Food Sci. 2020, 85, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointest. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nor, M.H.M.; Ayob, N.; Mokhtar, N.M.; Ali, R.A.R.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; AlShathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef]
- Pinheiro, I.; Barberá, A.; Raurell, I.; Estrella, F.; de Leeuw, M.; Bolca, S.; Gottardi, D.; Horscroft, N.; Possemiers, S.; Salcedo, M.T.; et al. A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo. Biomedicines 2022, 10, 1191. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Shen, F.; Cao, H.-X.; Ding, W.-J.; Chen, Y.-W.; Fan, J.-G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017, 7, 1529. [Google Scholar] [CrossRef]
- Mitsinikos, F.T.; Chac, D.; Schillingford, N.; DePaolo, R.W. Modifying macronutrients is superior to microbiome transplantation in treating nonalcoholic fatty liver disease. Gut Microbes 2020, 12, 1792256. [Google Scholar] [CrossRef] [PubMed]
- García-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genescà, J.; Martell, M.; et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology 2017, 67, 1485–1498. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals with Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef]
- Intestinal Microbiota Transplantation for Nonalcoholic Fatty Liver Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03648086?term=microbiota+transplantation&cond=nafld&draw=2&rank=1 (accessed on 26 October 2022).
- The Effect of Consecutive Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease (NAFLD)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04465032?term=microbiota+transplantation&cond=nafld&draw=2&rank=2 (accessed on 26 October 2022).
- Effects of Fecal Microbiota Transplantation on Weight in Obese Patients with Non-Alcoholic Fatty Liver Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04594954?term=microbiota+transplantation&cond=nafld&draw=2&rank=3 (accessed on 26 October 2022).
- Fecal Microbiota Transplantation (FMT) in Nonalcoholic Steatohepatitis(NASH). A Pilot Study—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02469272?term=microbiota+transplantation&cond=nafld&draw=2&rank=4 (accessed on 26 October 2022).
- Fecal Microbiota Transplantation for the Treatment of Non-Alcoholic Steatohepatitis—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03803540?term=microbiota+transplantation&cond=nafld&draw=2&rank=5 (accessed on 26 October 2022).
- A Prospective, Randomized, Placebo-Controlled Pilot Study to Characterize the Intestinal Microbiome and to Evaluate the Safety and Fecal Microbiome Changes Following Administration of Lyophilized PRIM-DJ2727 or Placebo Given Orally in Subjects with Nonalcoholic Fatty Liver Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04371653?term=microbiota+transplantation&cond=nafld&draw=2&rank=6 (accessed on 26 October 2022).
- Fecal Microbiota Therapy versus Standard Therapy in NASH Related Cirrhosis—Full Text View—Clini-calTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02721264?term=microbiota+transplantation&cond=nafld&draw=2&rank=7 (accessed on 26 October 2022).
- Transplantation of Microbes for Treatment of Metabolic Syndrome & NAFLD—Full Text View—Clinical-Trials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02496390?term=microbiota+transplantation&cond=nafld&draw=2&rank=8 (accessed on 26 October 2022).
- Ensayos Clínicos Sobre Pacientes Con Esteatohepatitis No Alcohólica: Grupo 1 o Grupo Experimental, Grupo 2 o Grupo de Control—Registro de Ensayos Clínicos—ICH GCP. Available online: https://ichgcp.net/es/clinical-trials-registry/NCT05622526 (accessed on 5 February 2023).
- Lu, Q.; Stappenbeck, T.S. Local barriers configure systemic communications between the host and microbiota. Science 2022, 376, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; FMT-Standardization Study Group. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Danne, C.; Rolhion, N.; Sokol, H. Recipient factors in faecal microbiota transplantation: One stool does not fit all. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 503–513. [Google Scholar] [CrossRef] [PubMed]
| Study | Population | Number | Liver Biopsy | Sample | Results |
|---|---|---|---|---|---|
| Li F. 2021 [18] | NAFLD patients | 15 studies (1265) | 9 studies | Stool | Increase in Escherichia, Prevotella and Streptococcus Decrease in Coprococcus, Faecalibacterium and Ruminococcus No differences in Bifidobacterium, Blautia, Clostridium, Dorea, Lactobacillus, Parabacteroides or Roseburia |
| Wang B. 2016 [19] | Non obese adult patients with or without NAFLD | 126 | - | Stool | Reduction in diversity. Increase in gram negative and decrease in gram positive Increase in Bacteroidetes (Bacteroidia) Decrease in Firmicutes (Lachnospiraceae, Ruminococcaceae, Lactobacillaceae and Peptostreptococcaceae) |
| Schwimmer J. 2019 [20] | Children with NAFLD vs. overweight or obese | 124 | Yes | Stool | Increase in Bacteroidetes and Proteobacteriae Decrease in Firmicutes and Lower α-diversity; No difference in β-diversity NASH individuals: more Proteobacteriae and lower α-diversity F1: increase in Verrucomicrobia and Firmicutes; F ≥ 2: Bacteroidetes, Proteobacteria and TM7 |
| Zhu L. 2013 [22] | Children and adolescents with NASH or obesity vs healthy controls | 63 | Yes | Stool | Low species abundance Increase in Bacteroidetes (Prevotellaceae) and increase in Proteobacteriae (Enterobacteriae: E: coli; 90% OTU #20341) Decrease in Firmicutes (Lachanospiraceae and Ruminococcaceae) and decrease in Actinobacteriae (Bifidobacterium) |
| Boursier J. 2016 [21] | NAFLD patients | 57 | Yes | Stool | Increase in Bacteroides and decrease in Prevotella in NASH patients Increase in Bacteroides and Ruminococcus in patients with significant fibrosis (F ≥ 2); decrease in Prevotella in patients with significant fibrosis (F ≥ 2) |
| Duarte S. 2018 [23] | Individuals with NASH vs lean healthy controls | 23 | Yes | Stool | Lean NASH: lower abundance of Faecalibacterium and Ruminococcus Overweight NASH: enriched in Bifidobacterium Obese NASH: enriched in Lactobacilli |
| Loomba R. 2017 [24] | NAFLD patients (F0–2 vs. F3–4) | 87 | Yes | Stool | F0–2: more abundance of Firmicutes; F3–4: more abundance of Proteobacteria |
| Study | Aetiology | Sample Size | Patients Characteristics | FMT Type | Donors | Main Objective | Secondary Aim |
|---|---|---|---|---|---|---|---|
| Allegretti JR. 2019 [81] | PSC | 10 | No cirrhosis, 9 UC and 1 CD, only with mesalamine or azathioprine, and 4-week washout period for UDCA | By colonoscopy 90 mL bowel preparation with polyethylene glycol on the day before. | A single healthy donor | Safety: no adverse events related to FMT. | 30% experienced a decrease in ALP ≥ 50% during the 24 weeks. Early changes in diversity as from first week that were maintained to 24 weeks. Increase in short-chain fatty acid producing genera. Correlation between the abundance of engrafter OTUs and a decrease in ALP Levels. No changes in stool bile acid profile clustering. |
| Bajaj J. 2021 [82] | AUD | 20 | Cirrhosis with a MELD score of 8.9 points | Placebo or FMT enema 1:1 (90 mL, 27 g stool, 2.7 × 1012 CFU) | OpenBiome where donor selection was performed to maximize Lachnospiraceae and Ruminococcaceae, which were lacking in the patients | Safety: 2 patients in FMT group had an adverse event but FMT-unrelated | Reduction of craving in 90%, psychosocial QOL improved and reduction in urinary EtG/creatinine. Reduction in systemic inflammation (IL-6) and in intestinal permeability (lower LBP). Microbial diversity increased with higher Ruminococcaceae and other SCFA producing taxa. |
| Bajaj J. 2017 [61] | Several | 20 | Cirrhotic with recurrent HE (at least two overt HE episodes requiring therapy), MELD < 17 and no active alcohol abuse. | 5 days of antibiotics prior to FMT enema (Three frozen-then-thawed FMT units; 90 mL); Lactulose and rifaximin were continued. | 1 donor with the optimal microbiota deficient in HE (Lachnospiraceae and Ruminococcacea) | Safety: at 150 days, 2 patients (20%) in FMT group had an adverse event but FMT-unrelated. | No FMT patients developed further HE in 5 months follow up vs. 50% in SOC. Improvement in PHES total score and EncephalApp Stroop. Increase in diversity and beneficial taxa (Lactobacillaceae, Bifidobacteriaceae, Lachnospiraceaeae and Ruminococcaceae). MELD score transiently worsened post-antibiotics. |
| Bajaj J. 2019 [85] | Several | 20 | Cirrhotic outpatients with recurrent HE | 5 days of pre-FMT antibiotics 90 mL enema containing 2.7 × 1012 CFU | A single donor: rich in Lachnospiraceae and Ruminococcaceae | Well-tolerated. | Reduced need for hospitalization and HE episodes. Increase in diversity and increase in relative abundance of Burkholderiaceae and decreased Acidaminoccocaceae but not in Lachnospiraceae and Ruminococcaceae. |
| Bajaj J. 2019 [77] | Several | 20 | Cirrhotic patients with recurrent HE with MELD < 17. | 15 FMT capsules (4.125 g stool) at once vs. placebo No pre-antibiotic therapy | A single donor rich in Lachnospiraceae and Ruminococcaceae | Safe and well-tolerated. | One patient had an HE (related to TIPS) vs. 3 patients in SOC (1 of them 5 episodes). No differences in stool diversity at day 30. Post-FMT, duodenal mucosal diversity increased with higher Ruminococcaceae, Bifidobacteriaceae and lower Streptococcaceae and Veillonellaceae. Reduction in Veillonellaceae was seen post-FMT in sigmoid and stool. IL-6 and serum LBP reduced post-FMT. |
| Bajaj J. 2021 [80] | Several | 40 (20 + 20) | Cirrhotic outpatients with recurrent HE | FMT 15 capsules vs. SOC Enema (90 mL) vs. SOC | 1 donor rich in Lachnospiraceae and Ruminococcaceae | Less SAEs in antibiotics + FMT Group. | Beta-lactamase and vancomycin-resistance reduction after FMT, regardless of the mode of administration. No difference in infections. |
| Bajaj J. 2019 [78] | Several | 20 | Cirrhotic outpatients with recurrent HE with MELD < 17 | FMT capsules vs. placebo | Not specified | Reduction, at 5 months of number of total HE episodes: 6 vs. 1 and in how many patients (3 vs. 1). | An increase in relative abundance of Lachnospiraceae and Ruminococ caceae. Significant reduction of IL-6. Reduction in total primary BAs and an increase in secondary BAs and secondary/primary BA ratio. No significant changes in MELD. |
| Chauhan A. 2020 [84] | HBV | 29 | HBeAg-positive on oral antivirals ≥1 year irrespective of serum levels of HBV-DNA or AST/ALT | In duodenum; 30 g of fresh stool, diluted in 150 mL of saline ×6 cycles at 4 weeks interval | A single healthy donor | Two patients in FMT arm had HBeAg clearance 16.7% vs. 0%. | No achieved HBsAg clearance. DNA became negative faster (25% negative in 6 months). No differences in ALT 6 patients (42.8%) minor adverse events and 1 serious (abdominal pain requiring hospitalization). |
| Ren YD, 2017 [83] | HBV | 18 | Persistently positive for HBeAg following >3 years of antiviral; HBV DNA level of <10,000 IU/mL and ALT <80 U/L | FMT to duodenum every 4 weeks until HBeAg clearance was achieved vs. placebo. | Healthy donors. | HBeAg titre declined gradually after each round of FMT; | No HBeAg seroconversion No significant adverse events. |
| Study | Size | NAFLD Criteria | FMT Type | Main Objective | Secondary Aims |
|---|---|---|---|---|---|
| Craven L. 2020 [100] | 21 (15 allogenic and 6 autologous) | ASSLD guideline 2018 | Endoscope to a distal duodenum (2 g of stool), 3:1 allogenic vs. autologous | No significant decrease in the IR measured by HOMA-IR at six months after FMT | No difference in the hepatic PDFF 6 months post-transplant. Improvement in small intestinal permeability assessed using the lactulose: mannitol urine test. Lower concentrations of non-sterified fatty acids and a decrease in the total:HDL cholesterol ratio. No differences in cholesterol, HDL, LDL cholesterol, triglycerides and APoB:ApoA1. |
| Witjes J. 2020 [101] | 21 (11 autologous and 10 allogenic) | NAFLD by ultrasound | FMT from lean vegan vs. autologous 3 FMT at 8 weeks interval: first by gastroduodenoscopy and then by nasoduodenal tube | Improvement in necro-inflammation score. Trend toward no worsening of fibrosis but not significant. Liver genes: increase in ARHGAP18 and serine dehydratase; and decreased RECQL5 and SF3B3 | GGT and ALT decreased. No difference in duodenal microbiota diversity. No significant changes in faecal microbiota diversity, but more Ruminococcus, Eubacterium hallii, Faecalibacterium, and Prevotella copri. Change in plasma metabolites: increase in amino acids isoleucine and phenylacetylglutamine. |
| Xue L. 2022 [102] | 75 (FMT 47 vs. non-FMT 28) | ASSLD guideline 2018 | Oral probiotics vs. FMT colonoscopy (100 g of faeces with 500 mL of 0.9% saline) + 3 enema (a total of 200 mL of fresh bacteria solution) | Balancing gut microbiota (no statistical differences in Chaol Indexes between two groups after FMT); Fat attenuation degrees decreased from 278.3 to 263.9 dB in FMT group and increased in non-FMT | Decreases in Proteobacteria and increase in Bacteroidetes, Firmicutes, Fusobacteria and Actinobacteria. No differences in blood lipid and liver function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Barrio, M.; Lavín, L.; Santos-Laso, Á.; Arias-Loste, M.T.; Odriozola, A.; Rodriguez-Duque, J.C.; Rivas, C.; Iruzubieta, P.; Crespo, J. Faecal Microbiota Transplantation, Paving the Way to Treat Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 6123. https://doi.org/10.3390/ijms24076123
Del Barrio M, Lavín L, Santos-Laso Á, Arias-Loste MT, Odriozola A, Rodriguez-Duque JC, Rivas C, Iruzubieta P, Crespo J. Faecal Microbiota Transplantation, Paving the Way to Treat Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2023; 24(7):6123. https://doi.org/10.3390/ijms24076123
Chicago/Turabian StyleDel Barrio, María, Lucía Lavín, Álvaro Santos-Laso, Maria Teresa Arias-Loste, Aitor Odriozola, Juan Carlos Rodriguez-Duque, Coral Rivas, Paula Iruzubieta, and Javier Crespo. 2023. "Faecal Microbiota Transplantation, Paving the Way to Treat Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 24, no. 7: 6123. https://doi.org/10.3390/ijms24076123
APA StyleDel Barrio, M., Lavín, L., Santos-Laso, Á., Arias-Loste, M. T., Odriozola, A., Rodriguez-Duque, J. C., Rivas, C., Iruzubieta, P., & Crespo, J. (2023). Faecal Microbiota Transplantation, Paving the Way to Treat Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 24(7), 6123. https://doi.org/10.3390/ijms24076123





