Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Population—Blood Cell Biomarkers of Inflammation
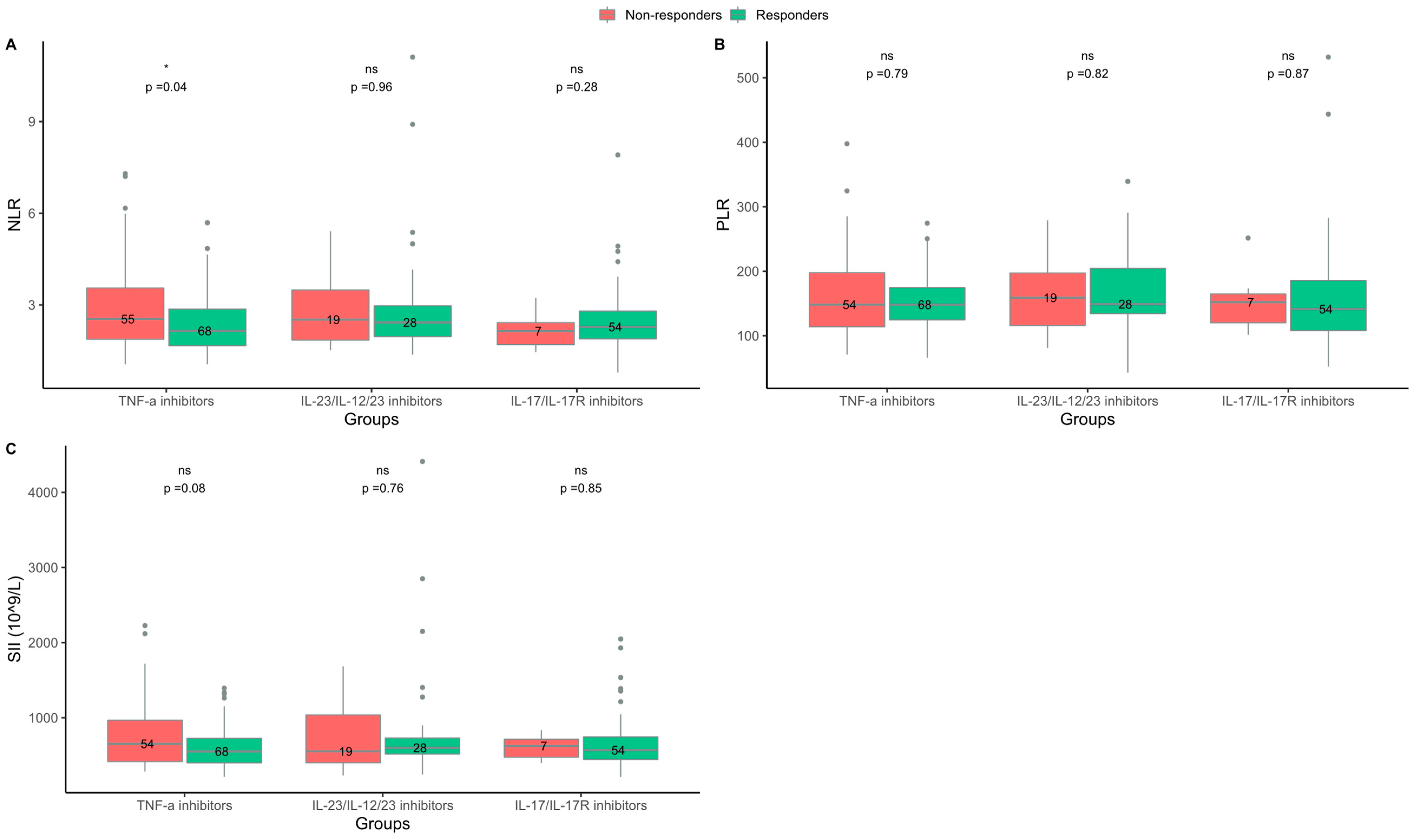
2.2. Responders to TNF-α Inhibitors Had Lower NLR at Baseline Than Non-Responders
2.3. Characteristics of the Study Population—Blood Cytokines
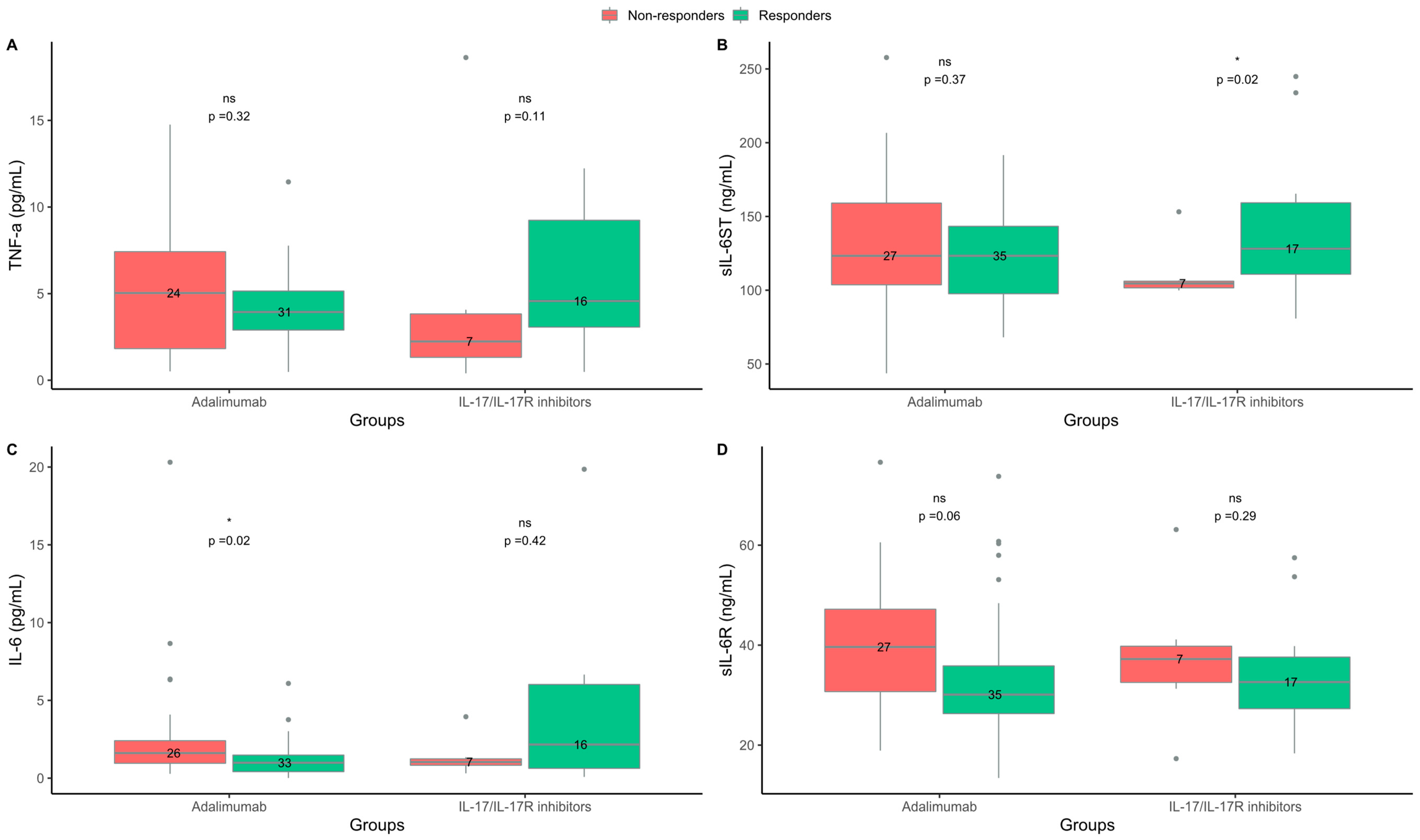
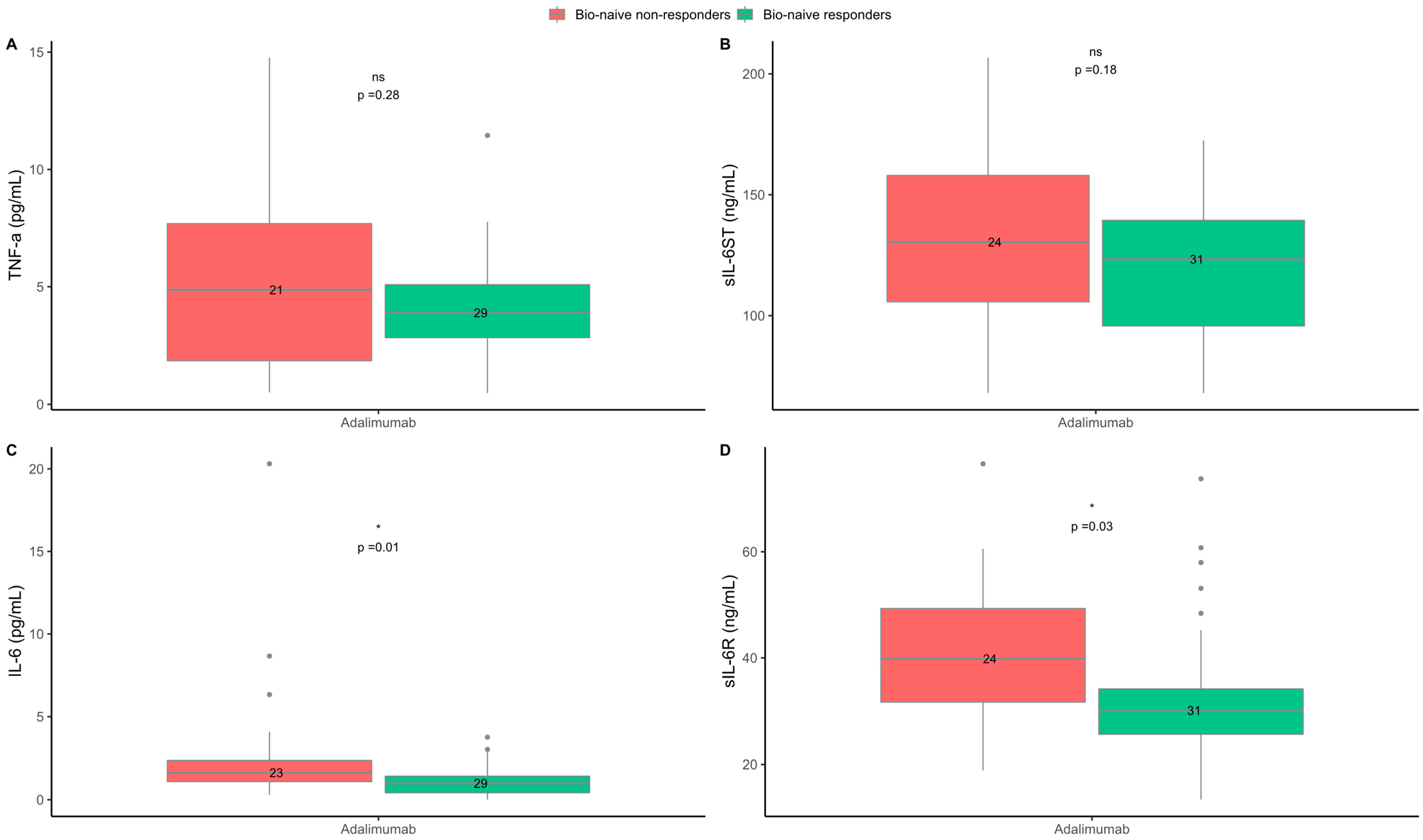
2.4. Levels of IL-6 and sIL-6R Were Higher in Non-Responders to the TNF-α Inhibitor Adalimumab
3. Discussion
4. Materials and Methods
4.1. Patients and Data Sources
4.2. Analyses of Blood Cell Biomarkers and Cytokines at Baseline
4.3. Definition of Treatment Response
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Selskab, D.D. Guideline DDS 2. Generations Immunmodulatorisk Behandling Opdateret December 2021. Available online: https://dds.nu/wp-content/uploads/2022/03/Guideline-DDS-2.-generations-immunomodulatorisk-behandling-opdateret-december-2021-opdatering-1.pdf (accessed on 13 January 2023).
- Corbett, M.; Ramessur, R.; Marshall, D.; Acencio, M.L.; Ostaszewski, M.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Haddad, S.; Jensen, A.H.M.; et al. Biomarkers of systemic treatment response in people with psoriasis: A scoping review. Br. J. Dermatol. 2022, 187, 494–506. [Google Scholar] [CrossRef]
- Talamonti, M.; Galluzzo, M.; van den Reek, J.M.; de Jong, E.M.; Lambert, J.L.W.; Malagoli, P.; Bianchi, L.; Costanzo, A. Role of the HLA-C*06 allele in clinical response to ustekinumab: Evidence from real life in a large cohort of European patients. Br. J. Dermatol. 2017, 177, 489–496. [Google Scholar] [CrossRef]
- Schwarz, C.W.; Loft, N.; Rasmussen, M.K.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Egeberg, A.; Skov, L. Predictors of Response to Biologics in Patients with Moderate-to-severe Psoriasis: A Danish Nationwide Cohort Study. Acta Derm. Venereol. 2021, 101, adv00579. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.K.; Braue, A.; Martyres, R.; Varigos, G. Baseline patients’ characteristics as predictors for therapeutic survival and response in patients with psoriasis on biological treatments. Australas. J. Dermatol. 2018, 59, e247–e252. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.B.; Marsden, A.; Tomenson, B.; Mason, K.J.; Soliman, M.M.; Burden, A.D.; Reynolds, N.J.; Stocken, D.; Emsley, R.; Griffiths, C.E.M.; et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: A multicentre longitudinal cohort study. Br. J. Dermatol. 2019, 180, 1069–1076. [Google Scholar] [CrossRef]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef]
- Khandpur, S.; Gupta, V.; Das, D.; Sharma, A. Is there a correlation of serum and tissue T helper-1 and -2 cytokine profiles with psoriasis activity and severity? A cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 414–418. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Satta, R.; Deligia, G.; Farina, G.; Bassu, S.; Mangoni, A.A.; Carru, C.; Zinellu, A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: A systematic review and meta-analysis. Clin. Exp. Med. 2018, 19, 37–45. [Google Scholar] [CrossRef]
- Yorulmaz, A.; Hayran, Y.; Akpinar, U.; Yalcin, B. Systemic Immune-Inflammation Index (SII) Predicts Increased Severity in Psoriasis and Psoriatic Arthritis. Curr. Health Sci. J. 2020, 46, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kvist-Hansen, A.; Kaiser, H.; Wang, X.; Krakauer, M.; Gørtz, P.M.; McCauley, B.D.; Zachariae, C.; Becker, C.; Hansen, P.R.; Skov, L. Neutrophil Pathways of Inflammation Characterize the Blood Transcriptomic Signature of Patients with Psoriasis and Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 10818. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Teague, H.L.; Adamstein, N.H.; Rodante, J.A.; Playford, M.P.; Chen, M.Y.; Bluemke, D.A.; Gelfand, J.M.; Ridker, P.M.; Mehta, N.N. Association of neutrophil-to-lymphocyte ratio with non-calcified coronary artery burden in psoriasis: Findings from an observational cohort study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.; Wang, X.; Kvist-Hansen, A.; Krakauer, M.; Gørtz, P.M.; McCauley, B.D.; Skov, L.; Becker, C.; Hansen, P.R. Biomarkers of subclinical atherosclerosis in patients with psoriasis. Sci. Rep. 2021, 11, 21438. [Google Scholar] [CrossRef]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil-lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef]
- An, I.; Ucmak, D.; Ozturk, M. The effect of biological agent treatment on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, mean platelet volume, and C-reactive protein in psoriasis patients. Adv. Dermatol. Allergol. Dermatol. I Alergol. 2020, 37, 202. [Google Scholar] [CrossRef]
- Asahina, A.; Kubo, N.; Umezawa, Y.; Honda, H.; Yanaba, K.; Nakagawa, H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: Response to therapy with biologics. J. Dermatol. 2017, 44, 1112–1121. [Google Scholar] [CrossRef]
- Karabay, E.A.; Çerman, A.A.; Demir, D.; Altunay, I.K. The Effects of Systemic Psoriasis Therapies on the C-Reactive Protein and the Neutrophil-Lymphocyte Ratio. Ann. Dermatol. 2019, 31, 601. [Google Scholar] [CrossRef]
- Hoffmann, J.H.O.; Knoop, C.; Enk, A.; Hadaschik, E.N. Detailed Long-term Dynamics of Neutrophil-to-Lymphocyte Ratio under Biologic Treatment Reveals Differential Effects of Tumour Necrosis Factor-alpha and Interleukin 12/23 Antagonists. Acta Derm. Venereol. 2021, 101, adv00568. [Google Scholar] [CrossRef]
- Lee, H.-N.; Kim, Y.-K.; Kim, G.-T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.-G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol. Int. 2019, 39, 859–868. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris-Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Iversen, L.; Skov, L. Response to Biologics During the First Six Months of Therapy in Biologic-naïve Patients with Psoriasis Predicts Risk of Disease Flares: A Danish Nationwide Study. Acta Derm. Venereol. 2021, 101, adv00357. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.; Bregnhøj, A.; Fage, S.; Nielsen, C.H.; Enevold, C.; Zachariae, C.; Iversen, L.; Skov, L. Effectiveness of brodalumab after previous treatment failure of interleukin-17A inhibitors in patients with psoriasis. Dermatol. Ther. 2021, 34, e15106. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Saha, B. Protumor vs antitumor functions of IL-17. J. Immunol. 2009, 183, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, S.; Bianchi, E.; Rogge, L. Anti-TNF Therapy in Spondyloarthritis and Related Diseases, Impact on the Immune System and Prediction of Treatment Responses. Front. Immunol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Girolomoni, G.; Griffiths, C.E.M.; Krueger, J.; Nestle, F.O.; Nicolas, J.F.; Prinz, J.C.; Puig, L.; Ståhle, M.; Van De Kerkhof, P.C.M.; Allez, M.; et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: A hypothesis paper. J. Dermatolog. Treat. 2015, 26, 103–112. [Google Scholar] [CrossRef]
- Dincer, A.B.K.; Sezer, S. Systemic Immune Inflammation Index as a Reliable Disease Activity Marker in Psoriatic Arthritis. J. Coll. Physicians Surg. Pak. 2022, 32, 773–778. [Google Scholar] [CrossRef]
- Mahil, S.K.; Wilson, N.; Dand, N.; Reynolds, N.J.; Griffiths, C.E.M.; Emsley, R.; Marsden, A.; Evans, I.; Warren, R.B.; Stocken, D.; et al. Psoriasis treat to target: Defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br. J. Dermatol. 2020, 182, 1158–1166. [Google Scholar] [CrossRef]

| Responders | Non-Responders | p Value | Bio-Naive, Responders | Bio-Naive, Non-Responders | p Value | |
|---|---|---|---|---|---|---|
| TNF-α inhibitors * | ||||||
| n | 73 | 58 | - | 60 | 48 | - |
| Age, years, median (IQR) | 44 (32–52) | 46 (32–60) | - | 44 (33–52) | 49 (32–60) | - |
| Sex, female, n (%) | 21 (29) | 18(31) | - | 18 (30) | 13 (27) | - |
| BMI, median (IQR) | 26.6 (24.0–30.9) a | 29.4 (24.4–34.3) | - | 26.8 (24.0–30.3) | 28.9 (24.4–33.9) | - |
| Ever-smokers, n (%) | 33 (45) a | 34 (39) | - | 30 (50) | 27 (56) | - |
| PsA, n (%) | 13 (18) b | 18 (31) d | - | 11 (18) c | 14 (29) | - |
| Baseline PASI, median (IQR) | 6.9 (4.6–12.0) e | (9.8 (5.6–12.6) a | - | 7.9 (5.5–12.5) a | 10 (5.4–12.7) a | - |
| Concomitant MTX treatment, n (%) | 5 (7) | 5 (9) | - | 5 (8) | 5 (10) | - |
| Lymphocytes, 109/L, median (IQR) | 1.7 (1.3–2.2) f | 1.7 (1.3–2.3) h | - | 1.7 (1.3–2.2) g | 1.7 (1.3–2.1) e | - |
| Neutrophils, 109/L, median (IQR) | 3.7 (3.2–4.6) f | 4.4 (3.5–5.6) h | - | 3.6 (3.1–4.7) g | 4.5 (3.6–5.6) e | - |
| Platelets, 109/L, median (IQR) | 250.0 (209.5–295.8) a | 261.0 (217.0–313.0) a | - | 253.0 (210.0–297.5) a | 261.0 (215.5–321.0) a | - |
| NLR, median (IQR) | 2.1 (1.7–2.9) f | 2.5 (1.9–3.5) h | 0.04 | 2.2 (1.7–2.7) g | 2.5 (2.0–3.8) e | 0.04 |
| PLR, median (IQR) | 148.2 (124.7–174.5) f | 148.2 (113.9–197.7) g | 0.79 | 151.8 (126.7–178.5) g | 151.3 (121.7–201.4) h | 0.55 |
| SII, 109/L, median (IQR) | 552.7 (400.7–726.4) f | 653.6 (418.4–967.5) g | 0.08 | 552.7 (414.8–775.6) g | 689.0 (502.6–1060.0) h | 0.05 |
| IL-23/IL-12/23 inhibitors ** | ||||||
| n | 29 | 21 | - | 25 | 10 | - |
| Age, years, median (IQR) | 38 (23–45) | 50 (33–55) | - | 38 (23–45) | 42 (30–50) | - |
| Sex, female, n (%) | 11 (38) | 5 (24) | - | 10 (40) | 2 (20) | - |
| BMI, median (IQR) | 25.1 (22.0–30.4) | 28.6 (27.3–32.6) | - | 25.3 (22.4–30.4) | 28.4 (28.0–29.9) | - |
| Ever-smokers, n (%) | 16 (55) | 11 (25) | - | 12 (48) | 4 (40) | - |
| PsA, n (%) | 3 (10) d | 5 (24) | - | 2 (8) i | 1 (10) | - |
| Baseline PASI, median (IQR) | 9.9 (7.3–11.0) | 10.2 (6.6–13.6) | - | 9.6 (7.3–10.5) | 8.1 (6.5–11.8) | - |
| Concomitant MTX treatment, n (%) | 4 (14) | 2 (9) | - | 4 (16) | 1 (10) | - |
| Lymphocytes, 109/L, median (IQR) | 1.6 (1.3–2.0) a | 1.6 (1.3–2.0) e | - | 1.5 (1.3–2.0) | 1.6 (1.5–1.7) a | - |
| Neutrophils, 109/L, median (IQR) | 3.9 (3.1–5.6) a | 4.3 (3.2–5.7) e | - | 3.9 (3.0–5.3) | 3.3 (2.5–4.6) a | - |
| Platelets, 109/L, median (IQR) | 262.0 (215.0–341.0) | 255.0 (220.0–285.0) | - | 249.0 (212.0–278.0) | 252.5 (227.0–262.5) | - |
| NLR, median (IQR) | 2.4 (2.0–3.0) a | 2.5 (1.8–3.5) e | 0.96 | 2.4 (1.9–2.6) | 1.9 (1.6–3.5) a | 0.54 |
| PLR, median (IQR) | 149.0 (134.3–204.3) a | 159.0 (116.0–197.3) e | 0.82 | 148.7 (121.4–179.2) | 159.0 (141.9–179.5) a | 0.59 |
| SII, 109/L, median (IQR) | 600.5 (517.8–730.3) a | 554.1 (403.0–1037.4) e | 0.76 | 584.3 (503.9–678.7) | 492.3 (363.8–970.6) a | 0.47 |
| IL-17/IL-17R inhibitors *** | ||||||
| n | 54 | 11 | - | 23 | 1 | - |
| Age, years, median (IQR) | 44 (31–57) | 54 (47–62) | - | 41 (31–49) | - | - |
| Sex, female, n (%) | 21 (39) | 4 (36) | - | 8 (35) | - | - |
| BMI, median (IQR) | 26.1 (22.8–29.5) | 34.7 (26.9–40.7) | - | 23.8 (21.3–28.5) | - | - |
| Ever-smokers, n (%) | 22 (41) | 7 (64) | - | 9 (39) | - | - |
| PsA, n (%) | 19 (35) d | 2 (18) e | - | 6 (26) i | - | - |
| Baseline PASI, median (IQR) | 9.2 (7.0–11.4) | 8.4 (6.2–11.2) | - | 10.5 (7.5–13.8) | - | - |
| Concomitant MTX treatment, n (%) | 3 (5) | 0 (0) | - | 3 (12) | - | - |
| Lymphocytes, 109/L, median (IQR) | 1.8 (1.4–1.9) | 1.8 (1.7–2.2) g | - | 1.8 (1.4–2.0) | - | - |
| Neutrophils, 109/L, median (IQR) | 4.0 (3.1–4.8) | 3.4 (2.8–6.0) g | - | 4.4 (3.3–4.9) | - | - |
| Platelets, 109/L, median (IQR) | 246.8 (208.0–294.0) | 271.5 (269.0–288.0) a | - | 247.0 (215.0–294.0) | - | - |
| NLR, median (IQR) | 2.3 (1.9–2.8) | 2.1 (1.7–2.4) g | 0.28 | 2.5 (2.0–3.2) | - | - |
| PLR, median (IQR) | 141.3 (108.1–185.4) | 152.0 (120.2–164.8) g | 0.87 | 143.3 (121.5–184.9) | - | - |
| SII, 109/L, median (IQR) | 569.4 (446.3–745.7) | 625.6 (476.2–714.2) g | 0.85 | 701.6 (463.8–789.9) | - | - |
| TNF-α Inhibitor Treatment Group | ||||
| Responder OR (95% CI) | Responder OR (95% CI) Adjusted * | Bio-Naive, Responder OR (95% CI) | Bio-Naive, Responder OR (95% CI) Adjusted * | |
| NLR. | 0.690 (0.487–0.943) | 0.687 (0.479–0.952) | 0.663 (0.450–0.931) | 0.650 (0.436–0.924) |
| IL-6 (pg/mL) | 0.720 (0.473–0.961) | 0.716 (0.469–0.964) | 0.578 (0.303–0-904) | 0.528 (0.267–0.857) |
| sIL-6R (ng/mL) | 0.968 (0.928–1.01) | 0.968 (0.928–1.01) | 0.961 (0.917–1.00) | 0.963 (0.919–1.00) |
| IL-17/IL-17R Inhibitor Treatment Group | ||||
| Responder OR (95% CI) | Responder OR (95% CI) Adjusted * | - | - | |
| sIL-6ST (ng/mL) | 1.04 (1.00–1.10) | 1.04 (1.00–1.12) | - | - |
| Responders | Non-Responders | p Value | Bio-Naive, Responders | Bio-Naive, Non-Responders | p Value | |
|---|---|---|---|---|---|---|
| Adalimumab | ||||||
| n | 35 | 27 | - | 31 | 24 | - |
| Age, years, median (IQR) | 43 (34–54) | 54 (33–60) | - | 42 (34–53) | 51 (33–60) | - |
| Sex, female, n (%) | 11 (31) | 7 (26) | - | 10 (32) | 6 (25) | - |
| BMI, median (IQR) | 28.2 (24.6–31.3) | 27.7 (24.4–34) | - | 28.3 (25.2–32.0) | 28.5 (25.3–34.7) | - |
| Ever-smokers, n (%) | 16 (46) | 14 (52) a | - | 14 (45) | 12 (50) | - |
| PsA, n (%) | 5 (14) | 7 (26) | - | 4 (13) | 6 (25) | - |
| Baseline PASI, median (IQR) | 8.4 (4.2–13.8) | 9.6 (6.0–12.0) | - | 10.0 (5.3–14.0) | 9.7 (6.2–11.9) | - |
| Concomitant MTX treatment, n (%) | 1 (3) | 1 (4) | - | 1 (3) | 1 (4) | - |
| TNF-α, pg/mL, median (IQR) | 3.94 (2.90–5.15) b | 5.04 (1.83–7.42) d | 0.32 | 3.89 (2.83–5.09) c | 4.87 (1.85–7-69) d | 0.28 |
| IL-6, pg/mL, median (IQR) | 0.99 (0.42–1.48) c | 1.62 (0.96–2.41) e | 0.02 | 0.99 (0.42–1.42) c | 1.63 (1.10–2.36) e | 0.01 |
| sIL-6ST, ng/mL, median (IQR) | 123.34 (97.67–143.30) | 123.36 (103.72–159.04) | 0.37 | 123.34 (95.74–139.44) | 130.44 (105.73–157.99) | 0.18 |
| sIL-6R, ng/mL, median (IQR) | 30.11 (26.31–35.84) | 39.63 (30.71–47.17) | 0.06 | 30.11 (25.64–34.15) | 39.83 (31.67–49.30) | 0.03 |
| IL-17/IL-17R inhibitors * | ||||||
| n | 17 | 7 | - | 0 | 0 | - |
| Age, years, median (IQR) | 52 (41–59) | 61 (52–69) | - | - | - | - |
| Sex, female, n (%) | 6 (35) | 3 (43) | - | - | - | - |
| BMI, median (IQR) | 28.6 (26.6–33.7) | 31.6 (24.1–39.8) | - | - | - | - |
| Ever-smokers, n (%) | 7 (41) | 1 (14) | - | - | - | - |
| PsA, n (%) | 5 (29) | 1 (14) | - | - | - | - |
| Baseline PASI, median (IQR) | 7.2 (5.3–8.9) | 8.4 (5.0–10.3) | - | - | - | - |
| Concomitant MTX treatment, n (%) | 0 (0) | 0 (0) | - | - | - | - |
| TNF-α, pg/mL, median (IQR) | 4.57 (3.07–9.24) e | 2.24 (1.33–3.83) | 0.11 | - | - | - |
| IL-6, pg/mL, median (IQR) | 2.16 (0.64–6.02) e | 1.04 (0.84–1.24) | 0.42 | - | - | - |
| sIL-6ST, ng/mL, median (IQR) | 128.12 (110.85–159.21) | 104.60 (101.64–106.10) | 0.02 | - | - | - |
| sIL-6R, ng/mL, median (IQR) | 32.62 (27.29–37.61) | 37.22 (32.55–39.77) | 0.29 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, C.S.B.; Kvist-Hansen, A.; Siewertsen, M.; Enevold, C.; Hansen, P.R.; Kaur-Knudsen, D.; Zachariae, C.; Nielsen, C.H.; Loft, N.; Skov, L. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. Int. J. Mol. Sci. 2023, 24, 6111. https://doi.org/10.3390/ijms24076111
Andersen CSB, Kvist-Hansen A, Siewertsen M, Enevold C, Hansen PR, Kaur-Knudsen D, Zachariae C, Nielsen CH, Loft N, Skov L. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. International Journal of Molecular Sciences. 2023; 24(7):6111. https://doi.org/10.3390/ijms24076111
Chicago/Turabian StyleAndersen, Clara Sophie Bramsen, Amanda Kvist-Hansen, Mie Siewertsen, Christian Enevold, Peter Riis Hansen, Diljit Kaur-Knudsen, Claus Zachariae, Claus Henrik Nielsen, Nikolai Loft, and Lone Skov. 2023. "Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis" International Journal of Molecular Sciences 24, no. 7: 6111. https://doi.org/10.3390/ijms24076111
APA StyleAndersen, C. S. B., Kvist-Hansen, A., Siewertsen, M., Enevold, C., Hansen, P. R., Kaur-Knudsen, D., Zachariae, C., Nielsen, C. H., Loft, N., & Skov, L. (2023). Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. International Journal of Molecular Sciences, 24(7), 6111. https://doi.org/10.3390/ijms24076111





