Mechanochemical Synergism of Reactive Oxygen Species Influences on RBC Membrane
Abstract
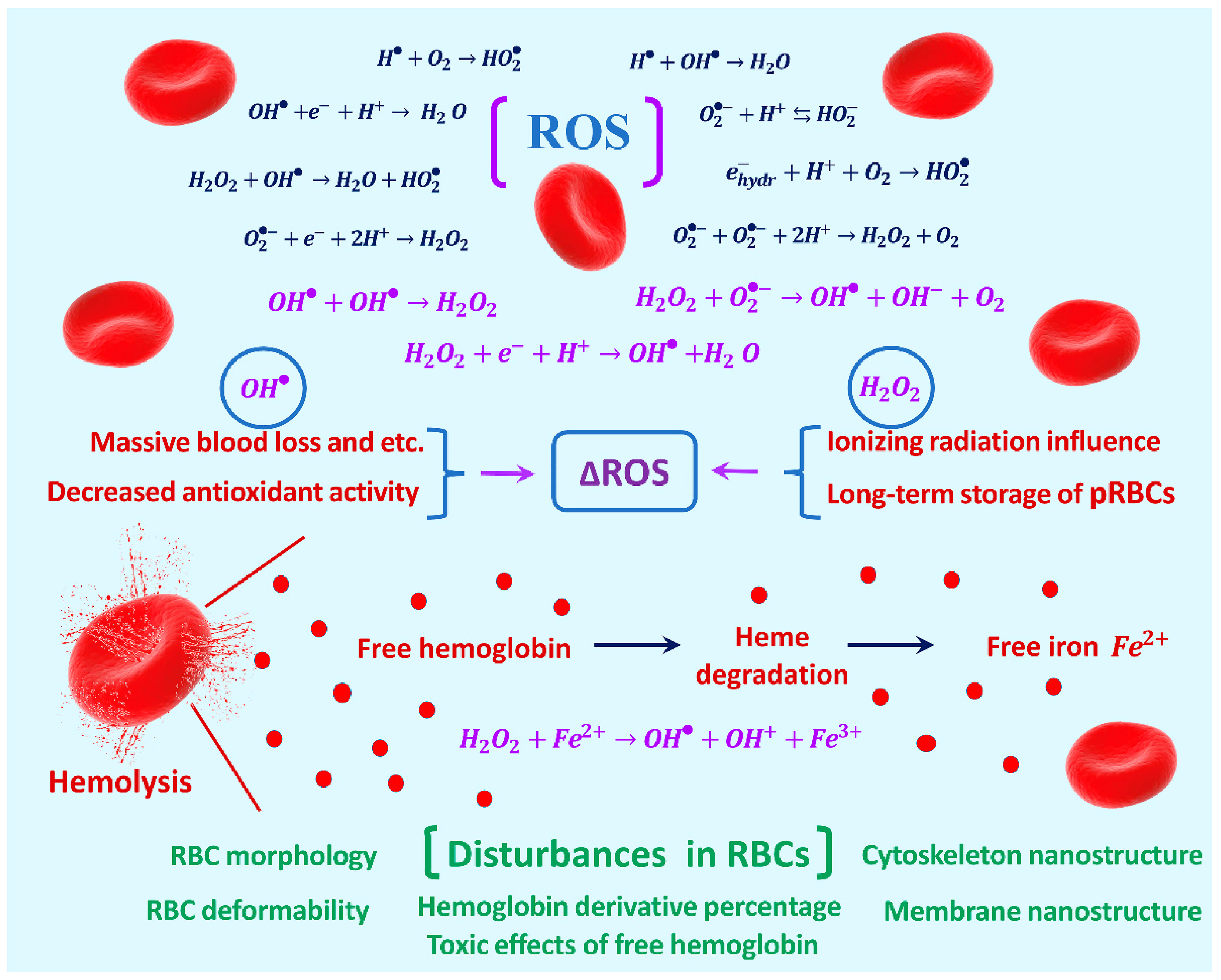
1. Introduction
2. Results and Discussion
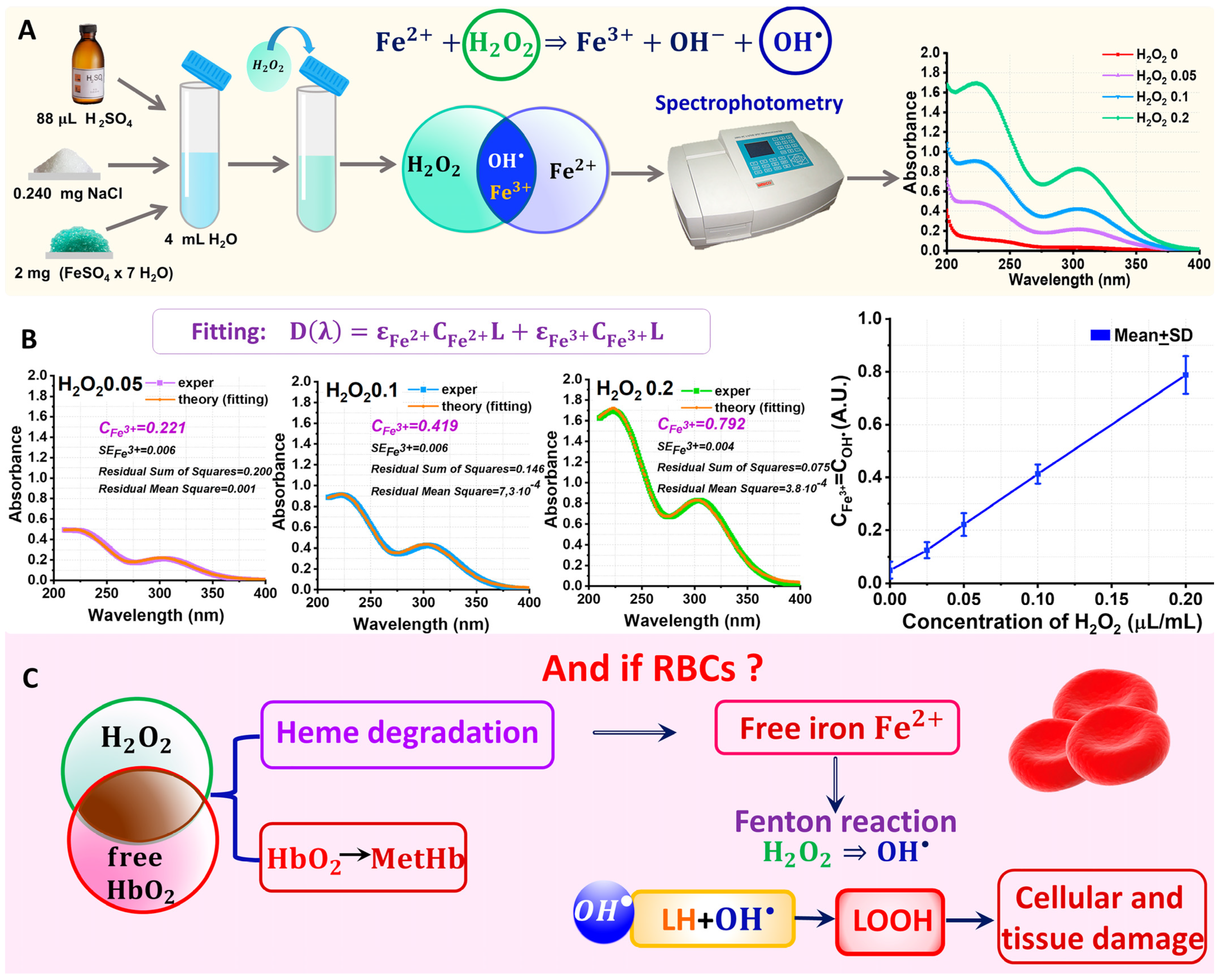
2.1. ROS in Blood Quantitative Study of to Conversion Due to the Fenton Reaction in In Vitro Experiment
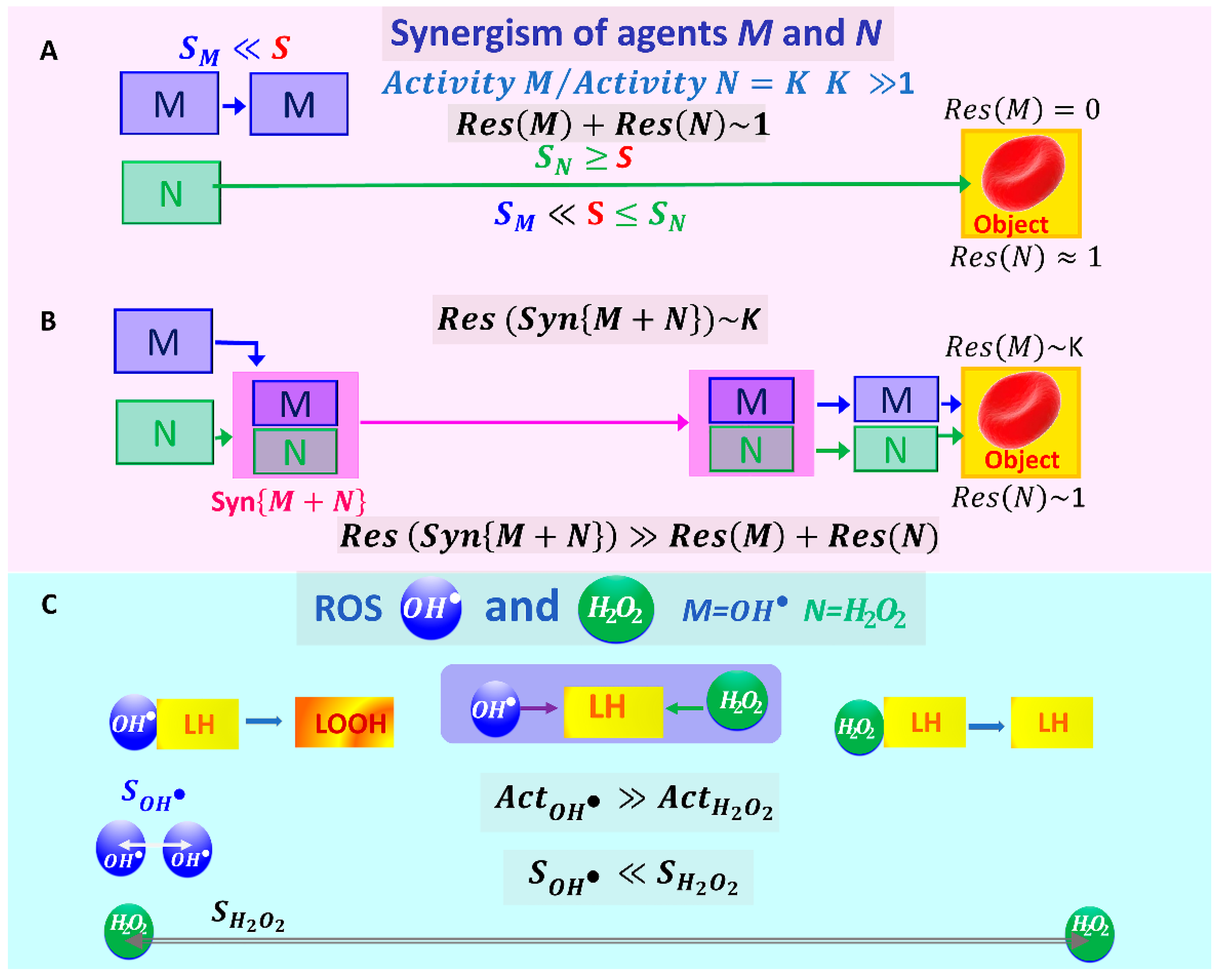
2.2. Mechanochemical Synergism of Two Factors in Their Effect on RBCs
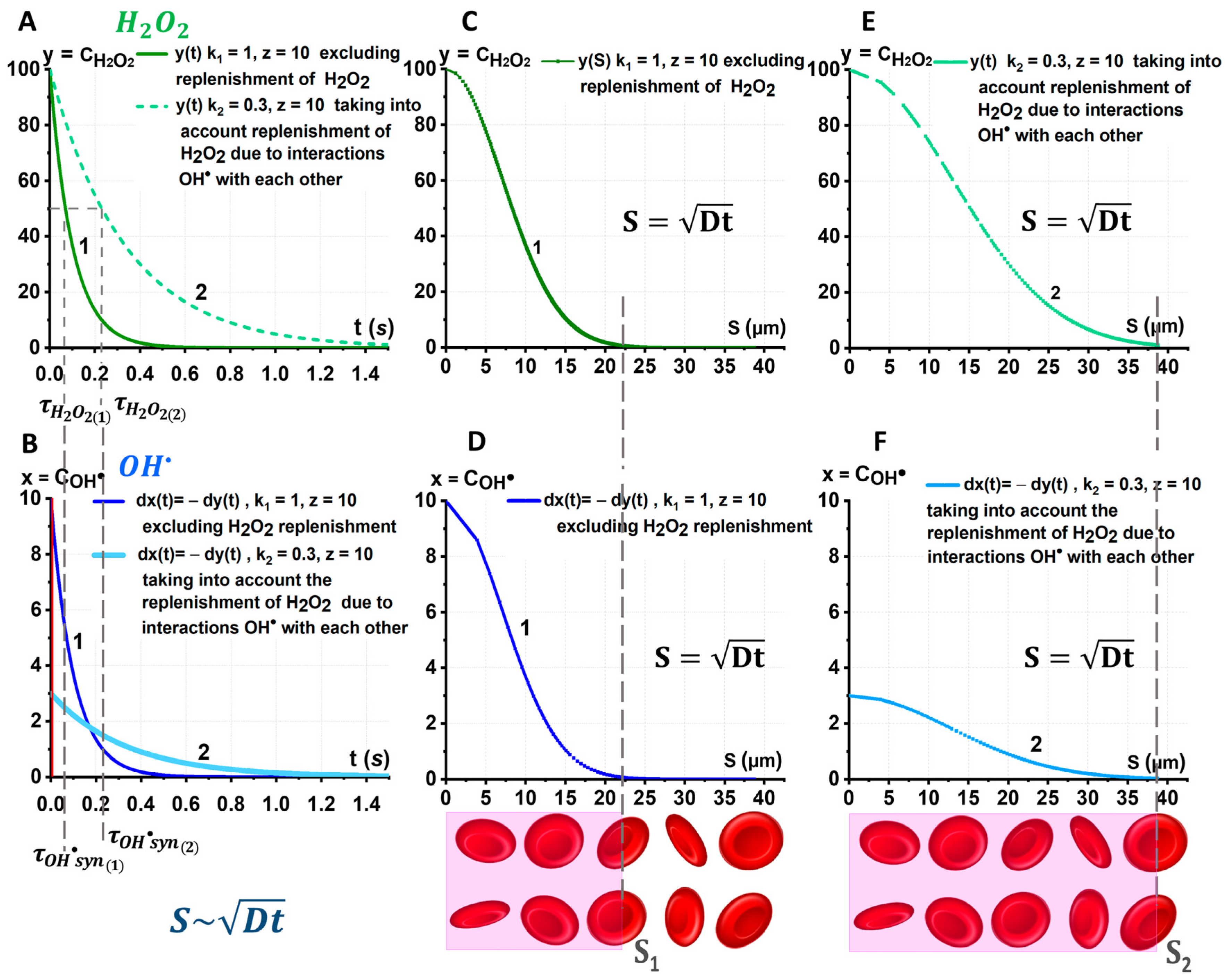
2.3. Biophysical Basis for Identification of a Pair of ROS That May Be Involved in the Synergistic Process in Blood
- (1)
- (2)
- (3)
- Correspondingly, the diffusion distances for these particles also vary considerably: .
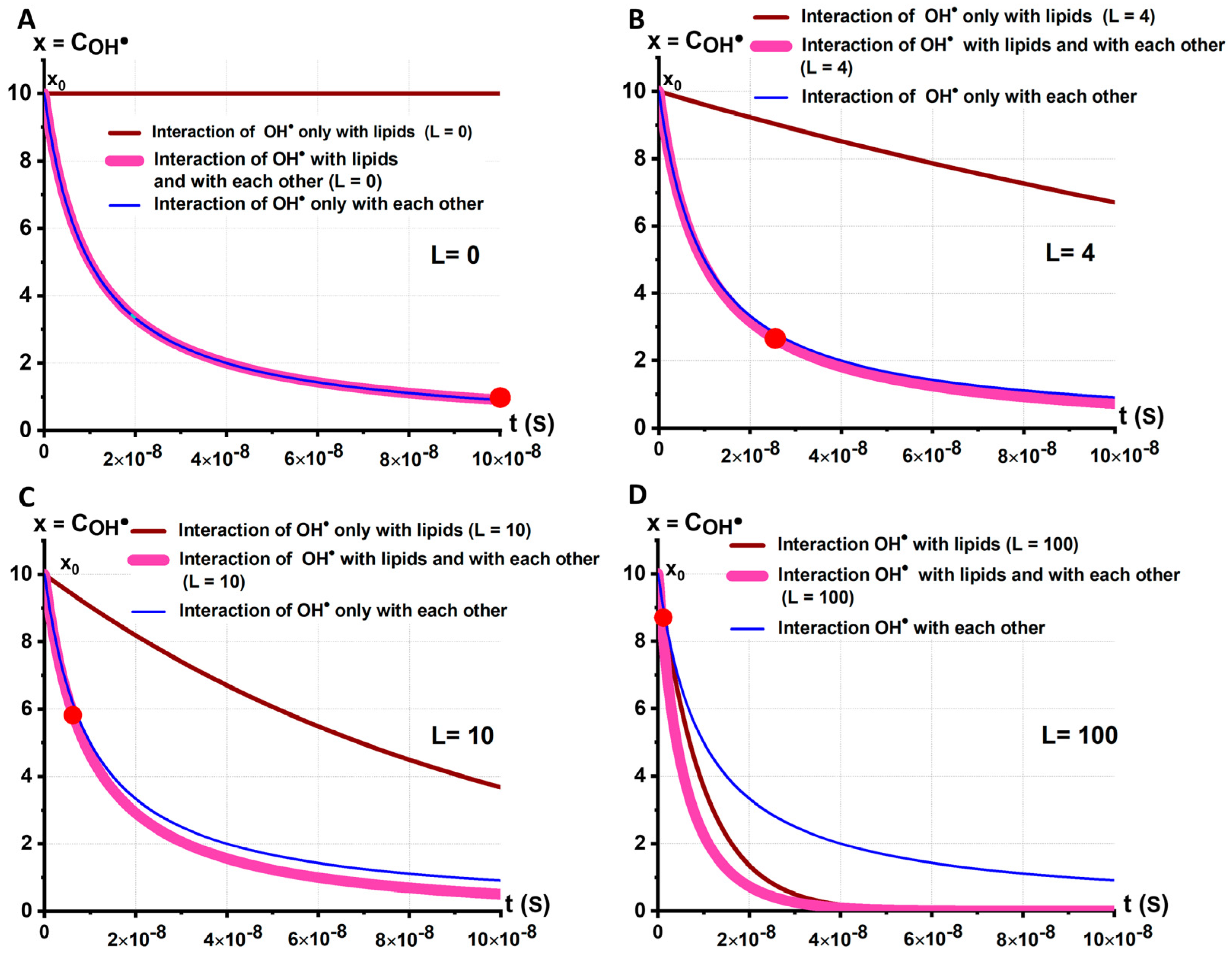
2.4. Synergism of and When Acting on Biological RBC Membranes
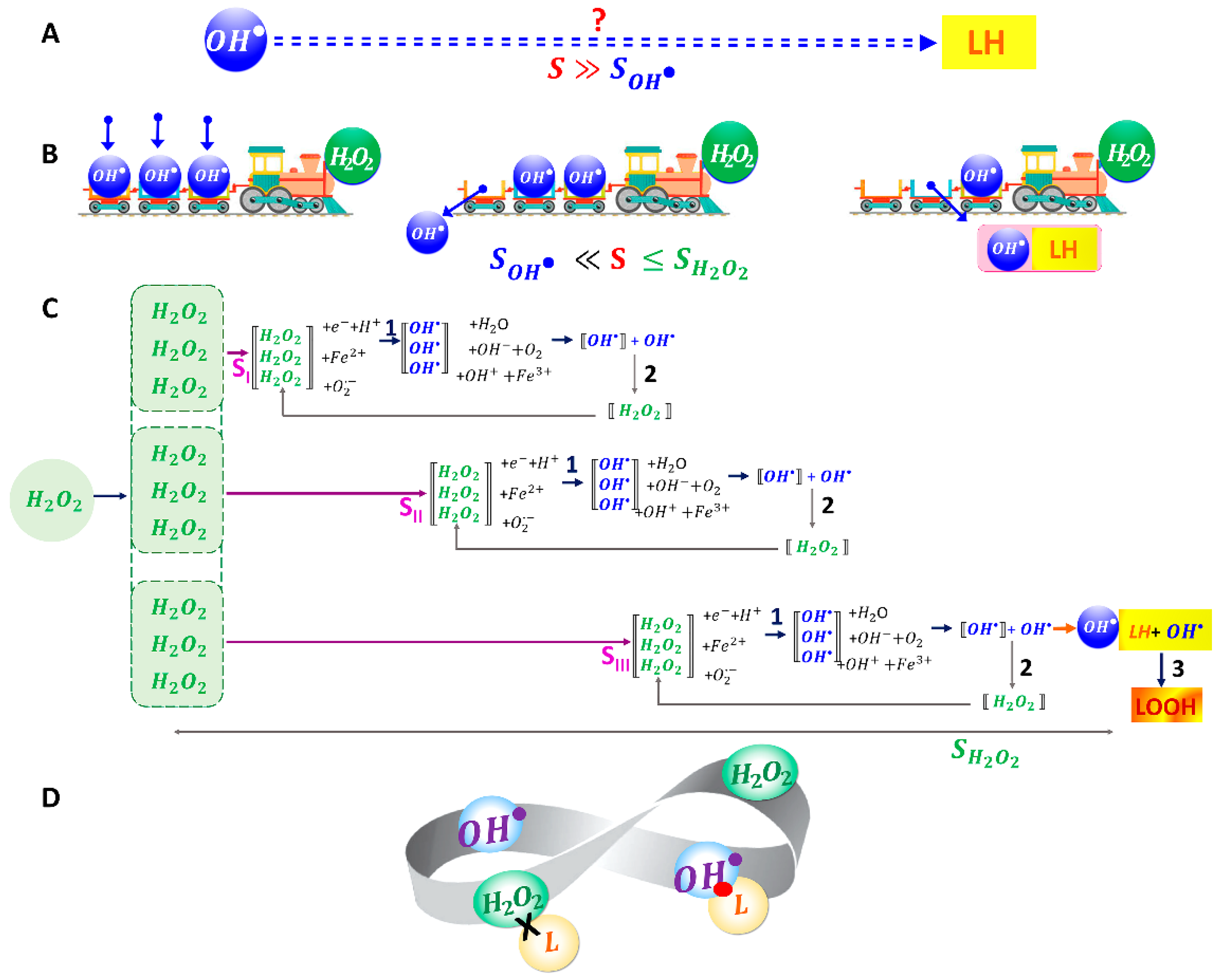
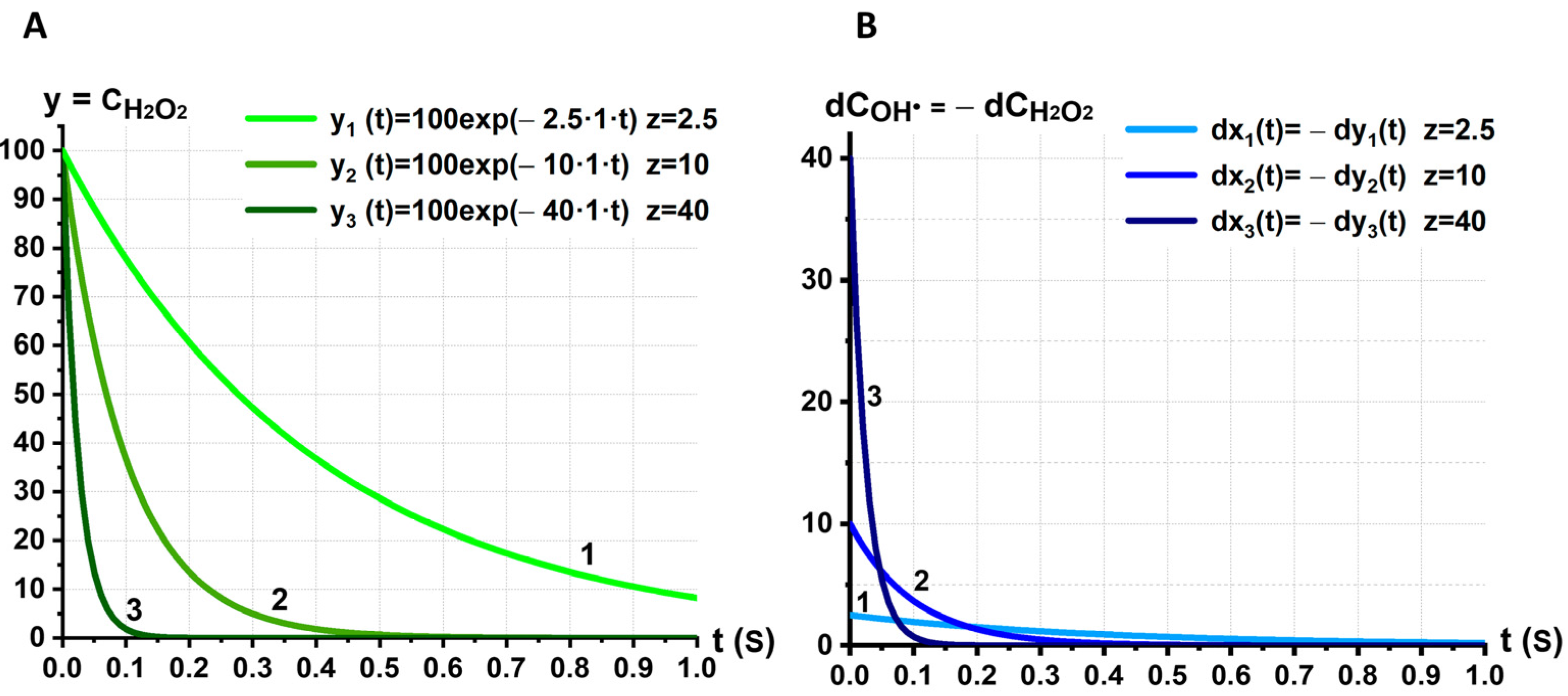
2.5. Kinetic Model of the Mechanochemical Synergism of and Effects on RBC Membrane: The Role of Synergism in the Initiation of RBC Membrane LPO
- (1)
- Interaction of with each other, stage 2 (Figure 4C):
- (2)
- Interaction with membrane lipids LH, stage :
3. Materials and Methods
3.1. Fricke System as a Model for Quantitative Study of to Conversion Due to the Fenton Reaction in In Vitro Experiment
3.1.1. Preparation of the Working Solution
3.1.2. Spectrophotometry for Quantitative Analysis of Fenton Reaction Results
3.1.3. Nonlinear Curve Fitting of Optical Spectra for Determination of the Unknown Concentration
3.2. Statistical Analysis
3.3. Kinetic Model of the Synergistic Interaction between and
- (1)
- We consider chemical processes of the conversion of into by interaction with other molecules and vice versa the conversion of into .
- (2)
- The second assumption is based on the significant difference in the lifetimes of the free radical and the molecule . This allows the processes of decreasing/increasing concentrations of these ROS to be separated in time and described by individual differential equations.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Voeikov, V. Reactive oxygen species, water, photons and life. Riv. Biol. 2001, 94, 237–258. [Google Scholar]
- Kudryashov, Y.B. Radiation Biophysics Ionizing Radiations; Nova Science Pub. Inc.: Hauppauge, NY, USA, 2006; p. 327. [Google Scholar]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Ping, Z.; Peng, Y.; Lang, H.; Xinyong, C.; Zhiyi, Z.; Xiaocheng, W.; Hong, Z.; Liang, S. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 3579143. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Gudkova, O.; Sergunova, V.; Kuzovlev, A. Transformation of membrane nanosurface of red blood cells under hemin action. Sci. Rep. 2014, 4, 6033. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Misiti, F. Erythrocytes as Potential Link between Diabetes and Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 276. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Dinarelli, S.; Sampaolese, B.; Misiti, F.; Girasole, M. Morphological changes induced in erythrocyte by amyloid beta peptide and glucose depletion: A combined atomic force microscopy and biochemical study. Biochim. Biophys. Acta Biomembr. 2019, 1861, 236–244. [Google Scholar] [CrossRef]
- Ficarra, S.; Tellone, E.; Giardina, B.; Scatena, R.; Russo, A.; Misiti, F. Derangement of Erythrocytic AE1 in Beta-Thalassemia by Caspase 3: Pathogenic Mechanisms and Implications in Red Blood Cell Senescence. J. Membr. Biol. 2009, 228, 43–49. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Lemoff, A.; Wang, G.; Zarek, C.; Lowe, A.; Yan, N.; Reese, T.A. Reactive oxygen species oxidize STING and suppress interferon production. eLife 2020, 9, e57837. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Saleh, J.; Peyssonnaux, C.; Singh, K.K.; Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-S.; Liu, Y.; Li, Z.-Z.; Wang, H.; Zhou, X.-F.; Wang, X.-X.; Zhang, Z.-W.; Kang, Y.-Q.; Su, Y.J.; Guo, J.-R. The effect of different storage times on the oxy-gen-carrying capacity of the exosomes of red blood cells. Adv. Clin. Exp. Med. 2021, 30, 387–394. [Google Scholar] [CrossRef]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Kozlov, A.; Sergunova, V.; Sherstyukova, E.; Gudkova, O. Two-step process of cytoskeletal structural damage during long-term storage of packed red blood cells. Blood Transfus. 2020, 19, 124–134. [Google Scholar]
- Jank, H.; Salzer, U. Vesicles Generated during Storage of Red Blood Cells Enhance the Generation of Radical Oxygen Speciesin Activated Neutrophils. Sci. World J. 2011, 11, 173–185. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxi-dative reactions. Front. Physiol. 2014, 55, 500. [Google Scholar]
- Sadrzadeh, S.M.; Graf, E.; Panter, S.S.; Hallaway, P.E.; Eaton, J.W. Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 1984, 259, 14354–14356. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, L.; Dansen, T.B. Cross-talk between redox signalling and protein aggregation. Biochem. Soc. Trans. 2020, 48, 379–397. [Google Scholar]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Kozlova, E.; Chernysh, A.; Sergunova, V.; Gudkova, O.; Manchenko, E.; Kozlov, A. Atomic force microscopy study of red blood cell membrane nanostructure during oxidation-reduction processes. J. Mol. Recognit. 2018, 31, e2724. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; De la Fuente, M. Immune cells: Free radicals and antioxidants in sepsis. Int. Immunopharmacol. 2004, 4, 327–347. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, S.; Mandal, A.; Ghosh, N.; Sil, P.C. ROS-associated immune response and metabolism: A mechanistic approach with implication of various diseases. Arch. Toxicol. 2020, 94, 2293–2317. [Google Scholar] [CrossRef]
- Kopf, E.W.; Lotka, A.J. Elements of Physical Biology; Williams and Wilkins: Philadelphia, PA, USA, 1925. [Google Scholar]
- Shirsat, N.; Avesh, M.; English, N.J.; Glennon, B.; Al-Rubeai, M. Verhulst and stochastic models for comparing mechanisms of MAb productivity in six CHO cell lines. Cytotechnology 2015, 68, 1499–1511. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Wang, H. The survival analysis of a stochastic Lotka-Volterra competition model with a coexistence equilibrium. Math. Biosci. Eng. 2019, 16, 2717–2737. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Kazmierska-Grebowska, P.; Cichon, N.; Piotrowski, P.; Litwinienko, G. The Effect of Fullerenol C60(OH)36 on the Antioxidant Defense System in Erythrocytes. Int. J. Mol. Sci. 2021, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Kazmierska-Grebowska, P.; Cichon, N.; Konarska, A.; Wolszczak, M.; Litwinienko, G. Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons. Int. J. Mol. Sci. 2022, 23, 10939. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Salvador, A. Synergism analysis of biochemical systems. I. Conceptual framework. Math. Biosci. 2000, 163, 105–129. [Google Scholar] [CrossRef]
- Meyer, K.J.; Nodwell, J.R. Biology and applications of co-produced, synergistic antimicrobials from environmental bacteria. Nat. Microbiol. 2021, 6, 1118–1128. [Google Scholar] [CrossRef]
- Sadhu, A.; Moriyasu, Y.; Acharya, K.; Bandyopadhyay, M. Nitric oxide and ROS mediate autophagy and regulate Alternaria alternata toxin-induced cell death in tobacco BY-2 cells. Sci. Rep. 2019, 9, 8973. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Alves, A.V.S.; de Almeida, W.S.; Sussuchi, E.M.; Lazzeri, L.; D’Errico, F.; de Souza, S.O. Investigation of chelating agents/ligands for Fricke gel dosimeters. Radiat. Phys. Chem. 2018, 150, 151–156. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Lenzen, S.; Lushchak, V.I.; Scholz, F. The pro-radical hydrogen peroxide as a stable hydroxyl radical distributor: Lessons from pancreatic beta cells. Arch. Toxicol. 2022, 96, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.; Thangaraj, P.R.; Sen, A.K. Direct and rapid measurement of hydrogen peroxide in human blood using a microfluidic device. Sci. Rep. 2021, 11, 2960. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Day, C.A.; Kenworthy, A.K. A novel computational framework for D (t) from Fluorescence Recovery after Photobleaching data reveals various anomalous diffusion types in live cell membranes. Traffic 2019, 20, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Nagy, G. Determination of diffusion coefficient in gel and in aqueous solutions using scanning electrochemical microscopy. J. Biochem. Biophys. Methods 2004, 61, 57–67. [Google Scholar] [CrossRef]
- Kozlova, E.; Sherstyukova, E.; Sergunova, V.; Kozlov, A.; Gudkova, O.; Inozemtsev, V.; Chernysh, A. The Toxic Influence of Excess Free Iron on Red Blood Cells in the Biophysical Experiment: An In Vitro Study. J. Toxicol. 2022, 2022, 7113958. [Google Scholar] [CrossRef]
- Spiess, A.-N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlova, E.; Sergunova, V.; Sherstyukova, E.; Grechko, A.; Lyapunova, S.; Inozemtsev, V.; Kozlov, A.; Gudkova, O.; Chernysh, A. Mechanochemical Synergism of Reactive Oxygen Species Influences on RBC Membrane. Int. J. Mol. Sci. 2023, 24, 5952. https://doi.org/10.3390/ijms24065952
Kozlova E, Sergunova V, Sherstyukova E, Grechko A, Lyapunova S, Inozemtsev V, Kozlov A, Gudkova O, Chernysh A. Mechanochemical Synergism of Reactive Oxygen Species Influences on RBC Membrane. International Journal of Molecular Sciences. 2023; 24(6):5952. https://doi.org/10.3390/ijms24065952
Chicago/Turabian StyleKozlova, Elena, Viktoria Sergunova, Ekaterina Sherstyukova, Andrey Grechko, Snezhanna Lyapunova, Vladimir Inozemtsev, Aleksandr Kozlov, Olga Gudkova, and Aleksandr Chernysh. 2023. "Mechanochemical Synergism of Reactive Oxygen Species Influences on RBC Membrane" International Journal of Molecular Sciences 24, no. 6: 5952. https://doi.org/10.3390/ijms24065952
APA StyleKozlova, E., Sergunova, V., Sherstyukova, E., Grechko, A., Lyapunova, S., Inozemtsev, V., Kozlov, A., Gudkova, O., & Chernysh, A. (2023). Mechanochemical Synergism of Reactive Oxygen Species Influences on RBC Membrane. International Journal of Molecular Sciences, 24(6), 5952. https://doi.org/10.3390/ijms24065952





