Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease
Abstract
1. Introduction
2. Environmental Factors That Cause Skin Diseases
2.1. UV Irradiation in Skin Diseases
2.1.1. Skin Inflammation
2.1.2. Skin Aging
2.1.3. Skin Carcinogenesis
2.2. Particulate Matter in Skin Diseases
2.2.1. Air Pollution
2.2.2. Cohort Analysis
3. The Role of SFKs in Skin Disease
3.1. Structural Characteristics of SFKs
3.2. The Role of SFKs in Skin Diseases
3.2.1. Upstream and Downstream Regulators for SFK Activity
3.2.2. Importance of SFK Activities in Skin Diseases
3.2.3. Molecular Mechanism of Src in Skin Diseases
4. Molecular Mechanism of AhR in Skin Diseases
4.1. AhR Ligands
4.2. Structure and Molecular Mechanism of AhR
5. Prevention of Skin Diseases Using SFKs and/or AhR-Targeted Phytochemicals
5.1. Functions of SFK-Targeted Phytochemicals in Skin Diseases
5.2. Phytochemicals Targeting AhR for the Regulation of Skin Diseases
6. Limitations of Animal Model for AhR Targeting Phytochemicals
7. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Lyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J.; et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.; Breathnach, S.M.; Cox, N.; Griffiths, C. Rook’s Textbook of Dermatology, 7th ed.; Blackwell Science: Hoboken, NJ, USA, 2004; Volume 2. [Google Scholar]
- Fujiwara, H.; Tsutsui, K.; Morita, R. Multi-tasking epidermal stem cells: Beyond epidermal maintenance. Dev. Growth Differ. 2018, 60, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- So, B.R.; Yeo, H.J.; Lee, J.J.; Jung, Y.H.; Jung, S.K. Cellulose nanocrystal preparation from Gelidium amansii and analysis of its anti-inflammatory effect on the skin in vitro and in vivo. Carbohydr. Polym. 2021, 254, 117315. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; So, B.R.; Yeo, H.J.; Kang, H.J.; Kim, M.J.; Lee, J.J.; Jung, S.K.; Jung, Y.H. Preparation of cellulose microfibril (CMF) from Gelidium amansii and feasibility of CMF as a cosmetic ingredient. Carbohydr. Polym. 2021, 257, 117569. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.J.; Park, J.; Lee, J.; Song, K.M.; Um, M.Y.; Cho, S.; Jung, S.K. Rice bran supplement prevents UVB-induced skin photoaging in vivo. Biosci. Biotechnol. Biochem. 2018, 82, 320–328. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Byun, S.; Kang, N.J.; Lim, S.H.; Heo, Y.S.; Bode, A.M.; Bowden, G.T.; Lee, H.J.; Dong, Z. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008, 68, 6021–6029. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.M.; Chang, P.S.; Jeong, C.H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, W.; Wan, X.; Zhuang, P.; Ma, G.; Jiao, J.; Zhang, Y. Advancing metabolic networks and mapping updated urinary metabolic fingerprints after exposure to typical carcinogenic heterocyclic aromatic amines. Environ. Pollut. 2023, 319, 120936. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part Fibre. Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Kim, J.; Goleva, E.; Berdyshev, E.; Lee, J.; Vang, K.A.; Lee, U.H.; Han, S.; Leung, S.; Hall, C.F.; et al. Particulate matter causes skin barrier dysfunction. JCI Insight 2021, 6, e145185. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef]
- Kim, J.G.; Kang, H.Y.; Kim, M.J.; Lim, S.; Lee, C.J.; Kim, K.M.; Jung, S.K. 4-phenylpyridine suppresses UVB-induced skin inflammation by targeting c-Src in vitro and in vivo. J. Cell Mol. Med. 2022, 26, 3891–3901. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, F.; Wu, L.; Kuang, H.; Wang, Q.; Cheng, G. Total withanolides ameliorates imiquimod-induced psoriasis-like skin inflammation. J. Ethnopharmacol. 2022, 285, 114895. [Google Scholar] [CrossRef]
- Jia, J.; Mo, X.; Liu, J.; Yan, F.; Wang, N.; Lin, Y.; Li, H.; Zheng, Y.; Chen, D. Mechanism of danshensu-induced inhibition of abnormal epidermal proliferation in psoriasis. Eur. J. Pharmacol. 2020, 868, 172881. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine 2021, 81, 153418. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef]
- Shi, Y.; Zeng, Z.; Liu, J.; Pi, Z.; Zou, P.; Deng, Q.; Ma, X.; Qiao, F.; Xiong, W.; Zhou, C.; et al. Particulate matter promotes hyperpigmentation via AhR/MAPK signaling activation and by increasing alpha-MSH paracrine levels in keratinocytes. Environ. Pollut. 2021, 278, 116850. [Google Scholar] [CrossRef]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Vogeley, C.; Rolfes, K.M.; Krutmann, J.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor in the pathogenesis of environmentally-induced squamous cell carcinomas of the skin. Front. Oncol. 2022, 12, 841721. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, S.K. Nutraceuticals for prevention of atherosclerosis: Targeting monocyte infiltration to the vascular endothelium. J. Food Biochem. 2020, 44, e13200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Niu, Y.; Jiang, Y.; Chen, B.; Peng, L.; Mi, T.; Huang, N.; Li, W.; Xu, D.; Chen, R.; et al. Protective effects of dietary fish-oil supplementation on skin inflammatory and oxidative stress biomarkers induced by fine particulate air pollution: A pilot randomized, double-blind, placebo-controlled trial. Br. J. Dermatol. 2021, 184, 261–269. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, K.W.; Byun, S.; Jung, S.K.; Seo, S.K.; Heo, Y.S.; Bode, A.M.; Lee, H.J.; Dong, Z. 5-deoxykaempferol plays a potential therapeutic role by targeting multiple signaling pathways in skin cancer. Cancer Prev. Res. 2010, 3, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015, 234, 74–80. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Brenner, D.J. Exposure of human skin models to KrCl excimer lamps: The impact of optical filtering(dagger). Photochem. Photobiol. 2021, 97, 517–523. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Skin cancer: Role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health 2014, 29, 265–273. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Jung, S.K.; Ha, S.J.; Kim, Y.A.; Lee, J.H.; Lim, T.G.; Kim, Y.T.; Lee, N.H.; Park, J.S.; Yeom, M.H.; Lee, H.J.; et al. MLK3 is a novel target of dehydroglyasperin D for the reduction in UVB-induced COX-2 expression in vitro and in vivo. J. Cell Mol. Med. 2015, 19, 135–142. [Google Scholar] [CrossRef]
- Jung, S.K.; Ha, S.J.; Jung, C.H.; Kim, Y.T.; Lee, H.K.; Kim, M.O.; Lee, M.H.; Mottamal, M.; Bode, A.M.; Lee, K.W.; et al. Naringenin targets ERK2 and suppresses UVB-induced photoaging. J. Cell. Mol. Med. 2016, 20, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Budden, T.; Bowden, N.A. The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis. Int. J. Mol. Sci. 2013, 14, 1132–1151. [Google Scholar] [CrossRef] [PubMed]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Hecker, E. Three stage carcinogenesis in mouse skin--recent results and present status of an advanced model system of chemical carcinogenesis. Toxicol. Pathol. 1987, 15, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.Y.; Lin, B.; Chen, Y.T.; Huang, Y.P.; Feng, W.P.; Wu, Y.; Long, G.H.; Zou, Y.N.; Liu, Y.; Lin, B.Q.; et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ. Toxicol. Pharmacol. 2021, 81, 103512. [Google Scholar] [CrossRef]
- Zhang, W.; Hanks, A.N.; Boucher, K.; Florell, S.R.; Allen, S.M.; Alexander, A.; Brash, D.E.; Grossman, D. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis 2005, 26, 249–257. [Google Scholar] [CrossRef]
- Einspahr, J.G.; Bowden, G.T.; Alberts, D.S. Skin cancer chemoprevention: Strategies to save our skin. Recent Results Cancer Res. 2003, 163, 151–164, discussion 264–266. [Google Scholar]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Hüls, A.; Vierkötter, A.; Gao, W.; Krämer, U.; Yang, Y.; Ding, A.; Stolz, S.; Matsui, M.; Kan, H.; Wang, S.; et al. Traffic-related air pollution contributes to development of facial lentigines: Further epidemiological evidence from Caucasians and Asians. J. Investig. Dermatol. 2016, 136, 1053–1056. [Google Scholar] [CrossRef]
- Fuks, K.B.; Hüls, A.; Sugiri, D.; Altug, H.; Vierkötter, A.; Abramson, M.J.; Goebel, J.; Wagner, G.G.; Demuth, I.; Krutmann, J.; et al. Tropospheric ozone and skin aging: Results from two German cohort studies. Environ. Int. 2019, 124, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Bae, Y.J.; Park, K.Y.; Han, H.S.; Kim, Y.S.; Hong, J.Y.; Han, T.Y.; Seo, S.J. Effects of particulate matter in a mouse model of oxazolone-induced atopic dermatitis. Ann. Dermatol. 2020, 32, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol inhibits particulate matter-induced inflammatory responses in human keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.K.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox. Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999, discussion 1000. [Google Scholar] [CrossRef]
- Yi, O.; Kwon, H.J.; Kim, H.; Ha, M.; Hong, S.J.; Hong, Y.C.; Leem, J.H.; Sakong, J.; Lee, C.G.; Kim, S.Y.; et al. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ. Res. 2012, 113, 40–45. [Google Scholar] [CrossRef]
- Kim, L.C.; Song, L.X.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef]
- Min, J.K.; Park, H.S.; Lee, Y.B.; Kim, J.G.; Kim, J.I.; Park, J.B. Cross-talk between Wnt signaling and Src tyrosine kinase. Biomedicines 2022, 10, 1112. [Google Scholar] [CrossRef]
- Linder, M.E.; Deschenes, R.J. Palmitoylation: Policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Leopizzi, M.; Urciuoli, E.; Peruzzi, B. Nuclear functions of the tyrosine kinase Src. Int. J. Mol. Sci. 2020, 21, 2675. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.E.; Oppermann, H.; Czernilofsky, A.P.; Purchio, A.F.; Erikson, R.L.; Bishop, J.M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc. Natl. Acad. Sci. USA 1981, 78, 6013–6017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Song, Z.H.; Ying, M.D.; Zhu, H.; He, Q.J.; Yang, B.; Cao, J. N-myristoylation: From cell biology to translational medicine. Acta Pharmacol. Sin. 2020, 41, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.J.; Baltimore, D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993, 3, 8–13. [Google Scholar] [CrossRef]
- Teyra, J.; Huang, H.; Jain, S.; Guan, X.; Dong, A.; Liu, Y.; Tempel, W.; Min, J.; Tong, Y.; Kim, P.M.; et al. Comprehensive analysis of the human SH3 domain family reveals a wide variety of non-canonical specificities. Structure 2017, 25, 1598–1610.e3. [Google Scholar] [CrossRef]
- Playford, M.P.; Schaller, M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004, 23, 7928–7946. [Google Scholar] [CrossRef]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef]
- Indovina, P.; Forte, I.M.; Pentimalli, F.; Giordano, A. Targeting SRC family kinases in mesothelioma: Time to upgrade. Cancers 2020, 12, 1866. [Google Scholar] [CrossRef]
- Ma, Y.C.; Huang, X.Y. Novel regulation and function of Src tyrosine kinase. Cell Mol. Life Sci. 2002, 59, 456–462. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Regulation of the SRC family kinases by CSK. Int. J. Biol. Sci. 2012, 8, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn tyrosine kinase as harmonizing factor in neuronal functions and dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Jiang, J.; Kiguchi, K.; Ruffino, L.; Carbajal, S.; Beltrán, L.; Bol, D.K.; Rosenberg, M.P.; DiGiovanni, J. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 2003, 63, 4819–4828. [Google Scholar]
- Lee, J.H.; Pyon, J.K.; Kim, D.W.; Lee, S.H.; Nam, H.S.; Kim, C.H.; Kang, S.G.; Lee, Y.J.; Park, M.Y.; Jeong, D.J.; et al. Elevated c-Src and c-yes expression in malignant skin cancers. J. Exp. Clin. Cancer Res. 2010, 29, 116. [Google Scholar] [CrossRef]
- Cattaneo, F.; De Marino, S.; Parisi, M.; Festa, C.; Castaldo, M.; Finamore, C.; Duraturo, F.; Zollo, C.; Ammendola, R.; Zollo, F.; et al. Wound healing activity and phytochemical screening of purified fractions of Sempervivum tectorum L. leaves on HCT 116. Phytochem. Anal. 2019, 30, 524–534. [Google Scholar] [CrossRef]
- Yagi, R.; Waguri, S.; Sumikawa, Y.; Nada, S.; Oneyama, C.; Itami, S.; Schmedt, C.; Uchiyama, Y.; Okada, M. C-terminal Src kinase controls development and maintenance of mouse squamous epithelia. EMBO J. 2007, 26, 1234–1244. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Oh, J.; Yu, T.; Choi, S.J.; Yang, Y.; Baek, H.S.; An, S.A.; Kwon, L.K.; Kim, J.; Rho, H.S.; Shin, S.S.; et al. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediat. Inflamm. 2012, 2012, 781375. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, M.; Song, E.; Jiang, X.; Song, Y. Selenium deficiency sensitizes the skin for UVB-induced oxidative damage and inflammation which involved the activation of p38 MAPK signaling. Food Chem. Toxicol. 2015, 75, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.G.; Kim, M.Y.; Byeon, S.E.; Rhee, M.H.; Park, J.; Katz, D.R.; Chain, B.M.; Cho, J.Y. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem. Pharmacol. 2010, 79, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Oncol. Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; White, A.C.; Borowsky, A.D. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp. Mol. Med. 2020, 52, 538–547. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Snyder, M.; Kenison, J.E.; Yang, K.K.; Lara, B.; Lydell, E.; Bennani, K.; Novikov, O.; Federico, A.; Monti, S.; et al. How the AHR became important in cancer: The role of chronically active AHR in cancer aggression. Int. J. Mol. Sci 2020, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Bradfield, C.A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008, 21, 102–116. [Google Scholar] [CrossRef]
- Ma, Q.; Whitlock, J.P. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Biol. Chem. 1997, 272, 8878–8884. [Google Scholar] [CrossRef]
- Reyes, H.; Reisz-Porszasz, S.; Hankinson, O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 1992, 256, 1193–1195. [Google Scholar] [CrossRef]
- Pongratz, I.; Mason, G.G.F.; Poellinger, L. Dual roles of the 90-kDa heat-shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA-binding activity. J. Biol. Chem. 1992, 267, 13728–13734. [Google Scholar]
- Kim, H.B.; Um, J.Y.; Chung, B.Y.; Kim, J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. Aryl hydrocarbon receptors: Evidence of therapeutic targets in chronic inflammatory skin diseases. Biomedicines 2022, 10, 1087. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Ishihara, Y.; Campbell, C.E.; Kado, S.Y.; Nguyen-Chi, A.; Sweeney, C.; Pollet, M.; Haarmann-Stemmann, T.; Tuscano, J.M. A protective role of aryl hydrocarbon receptor repressor in inflammation and tumor growth. Cancers 2019, 11, 589. [Google Scholar] [CrossRef]
- Enan, E.; Matsumura, F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem. Pharmacol. 1996, 52, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Morita, A.; Chung, J.H. Sun exposure: What molecular photodermatology tells us about its good and bad sides. J. Investig. Dermatol. 2012, 132, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Rannug, U.; Rannug, A.; Sjoberg, U.; Li, H.; Westerholm, R.; Bergman, J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem. Biol. 1995, 2, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Haarmann-Stemmann, T.; Vogel, C.F.A.; Grindel, A.; Hübenthal, U.; Brenden, H.; Grether-Beck, S.; Vielhaber, G.; Johncock, W.; Krutmann, J.; et al. The new aryl hydrocarbon receptor antagonist E/Z-2-benzylindene-5,6-dimethoxy-3,3-dimethylindan-1-one protects against UVB-induced signal transduction. J. Investig. Dermatol. 2014, 134, 556–559. [Google Scholar] [CrossRef]
- Castañeda, A.R.; Pinkerton, K.E.; Bein, K.J.; Magaña-Méndez, A.; Yang, H.T.; Ashwood, P.; Vogel, C.F.A. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol. Lett. 2018, 292, 85–96. [Google Scholar] [CrossRef]
- Tremmel, L.; Rho, O.; Slaga, T.J.; DiGiovanni, J. Inhibition of skin tumor promotion by TPA using a combination of topically applied ursolic acid and curcumin. Mol. Carcinog. 2019, 58, 185–195. [Google Scholar] [CrossRef]
- Kang, N.J.; Jung, S.K.; Lee, K.W.; Lee, H.J. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann. N. Y. Acad. Sci. 2011, 1229, 124–132. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Nandakumar, M.P.; Prabhu, D.; Jeyaraman, J.; Arumugam, M. Conformational insights into the inhibitory mechanism of phyto-compounds against Src kinase family members implicated in psoriasis. J. Biomol. Struct. Dyn. 2020, 38, 1398–1414. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef]
- Buyanravjikh, S.; Han, S.; Lee, S.; Jeong, A.L.; Ka, H.I.; Park, J.Y.; Boldbaatar, A.; Lim, J.S.; Lee, M.S.; Yang, Y. Cryptotanshinone inhibits IgE mediated degranulation through inhibition of spleen tyrosine kinase and tyrosine-protein kinase phosphorylation in mast cells. Mol. Med. Rep. 2018, 18, 1095–1103. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ito, T.; Tsuji, G.; Furue, M. Baicalein inhibits benzo[a]pyrene-induced toxic response by downregulating Src phosphorylation and by upregulating NRF2-HMOX1 system. Antioxidants 2020, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Lamar, J.M.; Xiao, Y.X.; Norton, E.; Jiang, Z.G.; Gerhard, G.M.; Kooner, S.; Warren, J.S.A.; Hynes, R.O. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J. Biol. Chem. 2019, 294, 2302–2317. [Google Scholar] [CrossRef]
- Li, W.; Cao, Y.; Xu, J.; Wang, Y.; Li, W.; Wang, Q.; Hu, Z.; Hao, Y.; Hu, L.; Sun, Y.; et al. YAP transcriptionally regulates COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor, overcomes drug resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2017, 36, 144. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Kim, S.; Lee, K.H.; Lee, Y.S.; Hong, I.; Lee, M.O.; Min, D.; Chang, I.; Hwang, J.S.; Park, J.S.; et al. Transcriptional profiling in human HaCaT keratinocytes in response to kaempferol and identification of potential transcription factors for regulating differential gene expression. Exp. Mol. Med. 2008, 40, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.L.; Huang, P.H.; Wang, H.C.; Tseng, C.H.; Yen, F.L. Pterostilbene attenuates particulate matter-induced oxidative stress, inflammation and aging in keratinocytes. Antioxidants 2021, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, J.E.; Jang, H.S.; Hong, S.Y.; Lee, J.B.; Park, S.Y.; Hwang, J.S. Oleanolic acid protects the skin from particulate matter-induced aging. Biomol. Ther. 2021, 29, 220–226. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Cha, H.J.; Choi, E.O.; Kim, C.H.; Kim, G.Y.; Yoo, Y.H.; Hwang, H.J.; Park, H.T.; Yoon, H.M.; Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019, 16, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, L.; Wang, H.; Zhu, P.; Jiang, S.; Qi, R.; Wu, Y.; Gao, X. Activation of aryl hydrocarbon receptor ameliorates rosacea-like eruptions in mice and suppresses the TLR signaling pathway in LL-37-induced HaCaT cells. Toxicol. Appl. Pharmacol. 2022, 451, 116189. [Google Scholar] [CrossRef]
- Liu, J.; Pi, Z.; Xiao, Y.; Zeng, Z.; Yu, J.; Zou, P.; Tang, B.; Qiu, X.; Tang, R.; Shi, Y.; et al. Esomeprazole alleviates fibrosis in systemic sclerosis by modulating AhR/Smad2/3 signaling. Pharmacol. Res. 2022, 176, 106057. [Google Scholar] [CrossRef]
- Haas, K.; Weighardt, H.; Deenen, R.; Köhrer, K.; Clausen, B.E.; Zahner, S.; Boukamp, P.; Bloch, W.; Krutmann, J.; Esser, C. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J. Investig. Dermatol. 2016, 136, 2260–2269. [Google Scholar] [CrossRef]
- Tauchi, M.; Hida, A.; Negishi, T.; Katsuoka, F.; Noda, S.; Mimura, J.; Hosoya, T.; Yanaka, A.; Aburatani, H.; Fujii-Kuriyama, Y.; et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol. Cell Biol. 2005, 25, 9360–9368. [Google Scholar] [CrossRef]
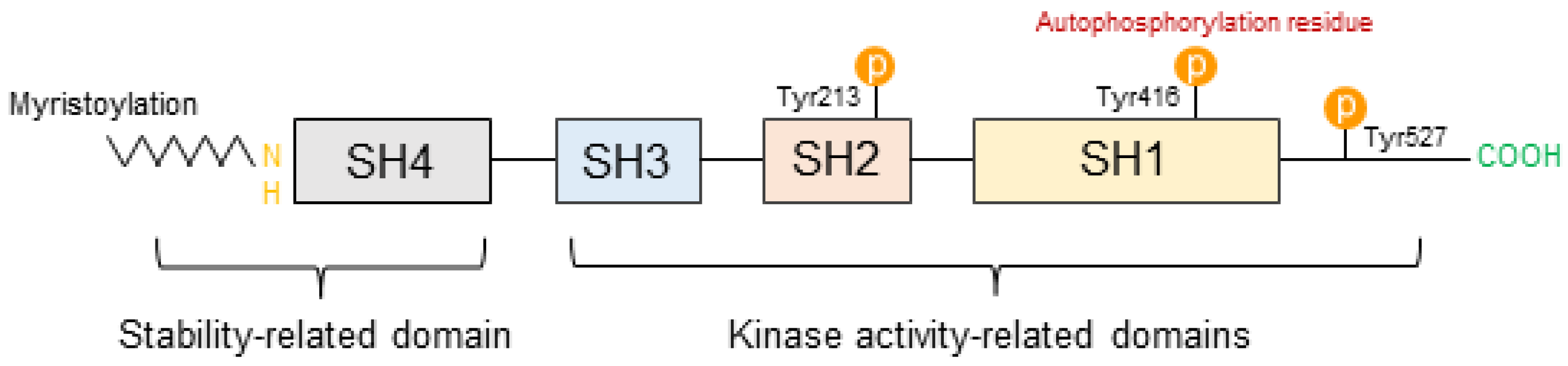
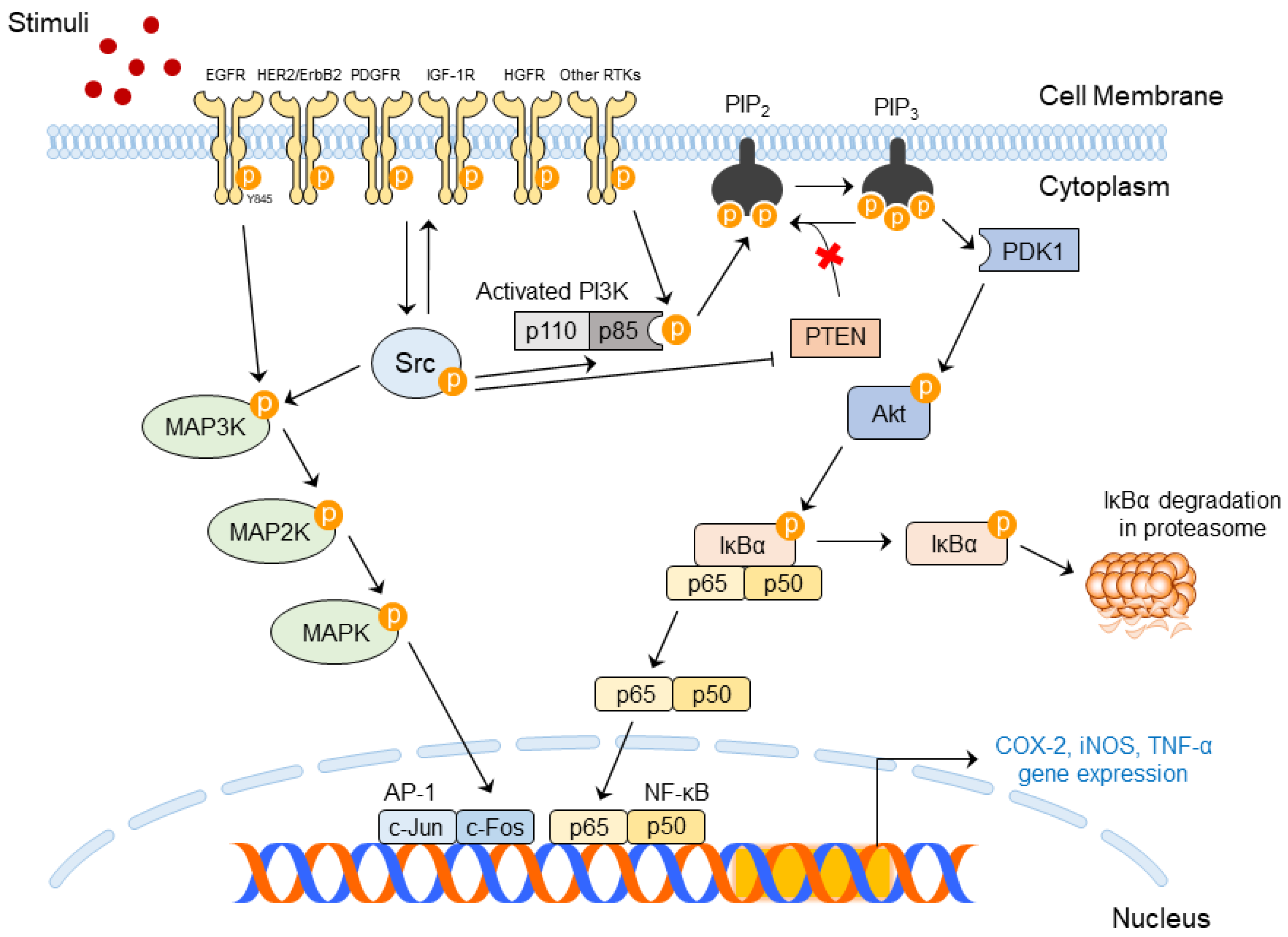
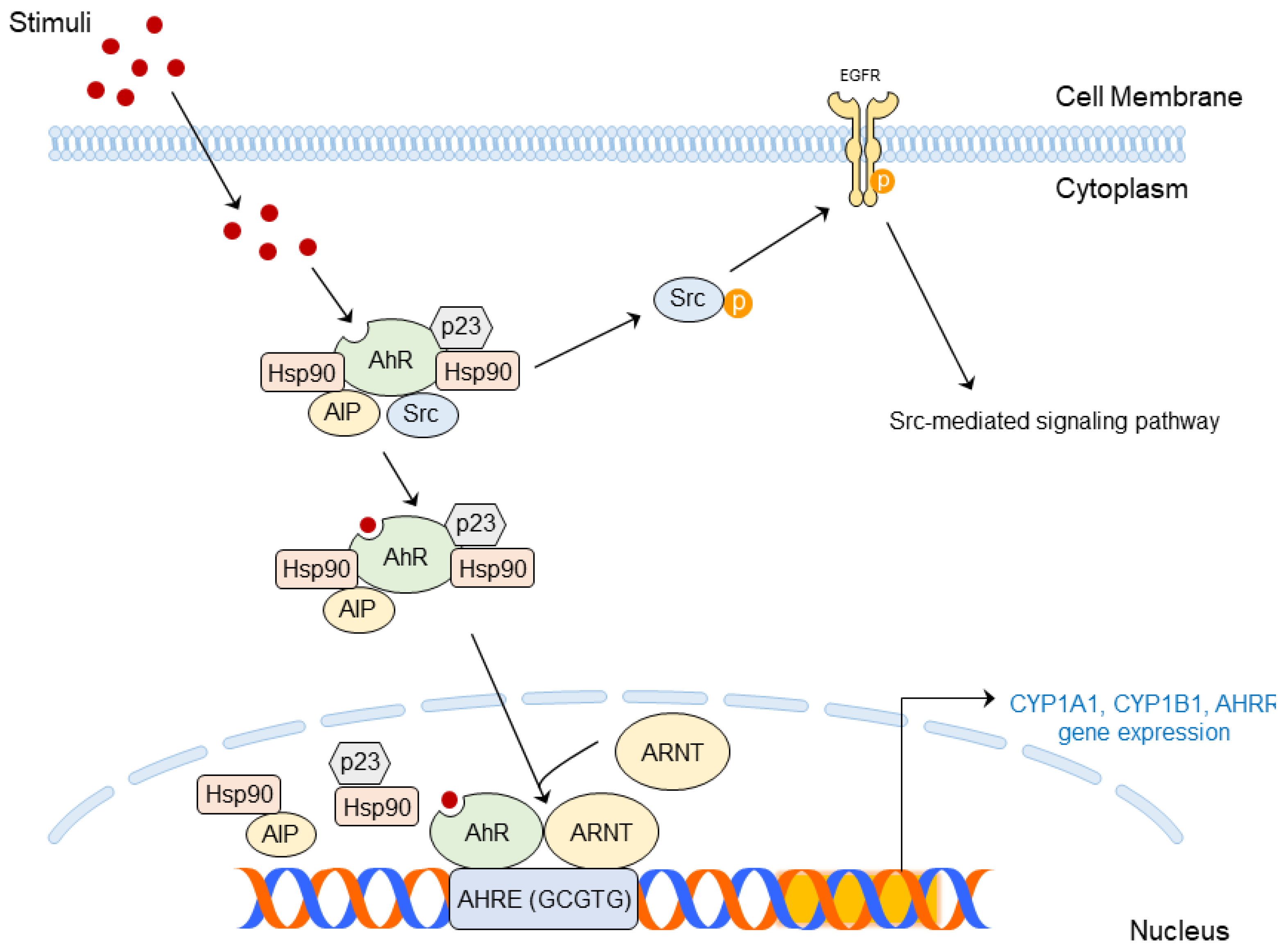
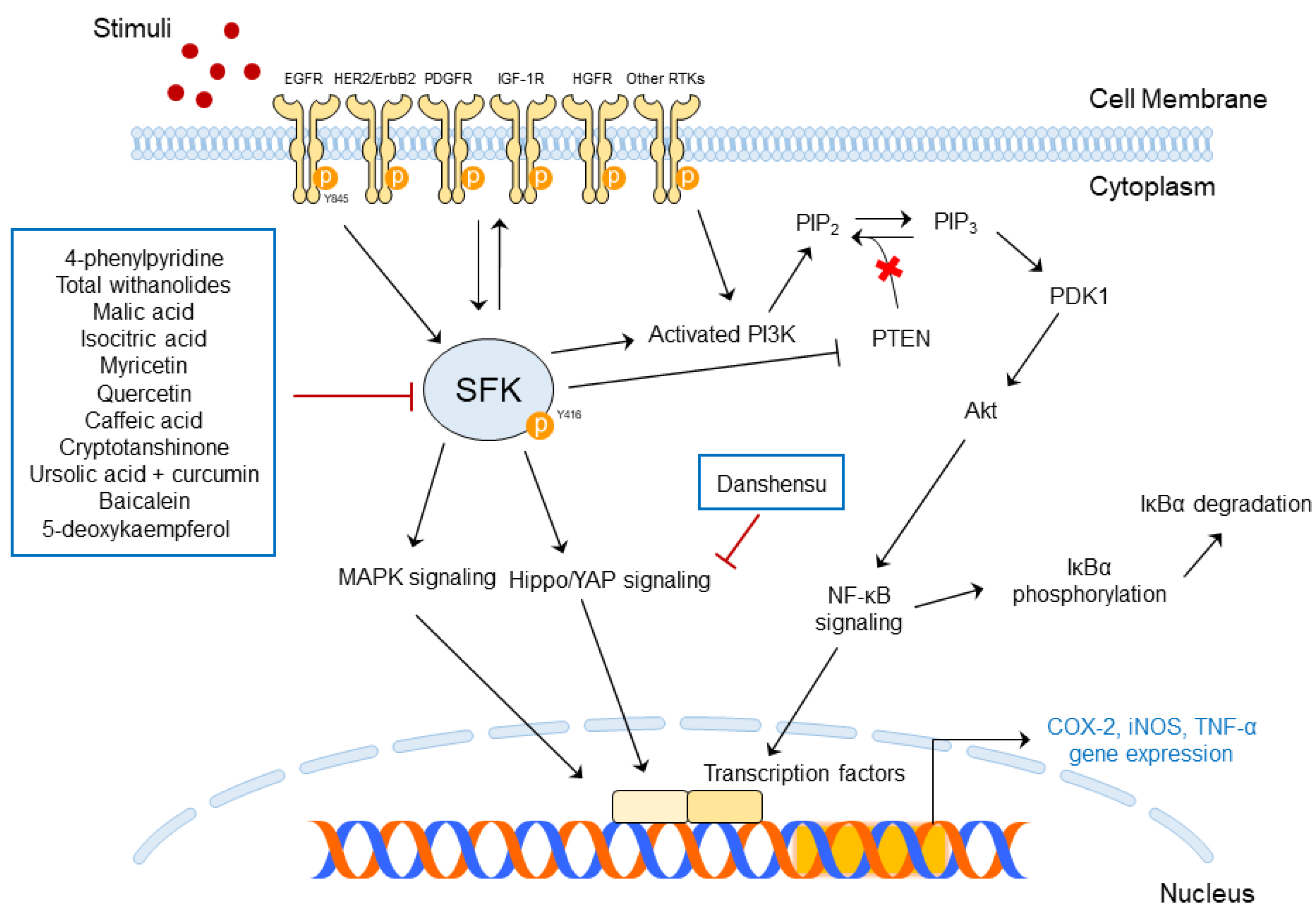

| UV Dose | Term | Rodent Species | Skin Diseases | Abnormal Changes | Ref |
|---|---|---|---|---|---|
| 0.5 J/cm2 | Single | ICR and SKH-1 hairless mice | Inflammation | Increased epidermal thickness | [16,31] |
| 0.045–0.18 J/cm2 | 15 weeks (3 times/week) | SKH-1 hairless mice | Photoaging | Wrinkle formation, increase in epidermal thickness, irregular brown spot | [32] |
| 0.18 J/cm2 | 15 weeks (3 times/week) | SKH-1 hairless mice | Skin cancer | Development of neoplasm, increase in epidermal thickness | [22] |
| Name | Stimuli | Target Disease | Target Signaling | In Vitro Markers | In Vivo Markers | Ref |
|---|---|---|---|---|---|---|
| 4-Phenylpyridine | UVB | Skin inflammation | Src | COX-2 PGE2 Direct binding with c-Src | Epidermal thickness | [16] |
| Total withanolides | Imiquimod (IMQ) | Psoriasis-like skin inflammation | Lyn Lck Src Treg/Th17 axis | Skin damage site pigmentation PASI score Dermatological changes | [17] | |
| Malic acid | Wound healing | Src | EGFR Y845 phosphorylation Src Y416 phosphorylation STAT3 Y705 phosphorylation | [67] | ||
| Isocitric acid | Wound healing | Src | EGFR Y845 phosphorylation Src Y416 phosphorylation STAT3 Y705 phosphorylation | [67] | ||
| Danshensu | M5 (in vitro) IMQ (in vivo) | Psoriasis | YAP | YAP mRNA expression YAP expression | Gross morphology Epidermal thickening YAP expression | [18] |
| Myricetin | UVB | Skin cancer | Fyn | COX-2 AP-1, NF-κB transactivation MAPK phosphorylation P90RSK, MSK phosphorylation MEK, Raf, Fyn phosphorylation Fyn kinase activity Direct binding with Fyn | Fyn kinase activity COX-2 MAPK phosphorylation Direct binding with Fyn External appearance of tumors Number of tumors Tumor volume | [9,98] |
| Quercetin | Skin inflammation (psoriasis) | Src | Src, Lyn, Fyn expressions | [99] | ||
| Caffeic acid | UVB | Skin cancer | Fyn | COX-2 PGE2 generation Fyn kinase activity Direct binding with Fyn | COX-2 Fyn kinase activity Direct binding with Fyn | [100] |
| Cryptotanshinone | IgE, DNCB | Atopic dermatitis | Lyn, Syk, PLC | Lyn, Syk phosphorylation PLCγ, PKCδ, IKKβ phosphorylation | Skin pigmentation Ear thickness Accumulation of CD11b+ cells IgE in serum and ear p65 phosphorylation | [101] |
| Ursolic acid + Curcumin | 12-ο-Tetradecanoylphobol-13-acetate (TPA) | Skin carcinogenesis | Src | EGFR Y1086, Src Y416, p70S6K T389, c-Jun S73 phosphorylation | [97] | |
| Baicalein | Benzo[a]pyrene (BAP) | Oxidative stress | Src | CYP1A1 mRNA Src phosphorylation AhR translocation | [102] | |
| 5-Deoxykaempferol | UVB | Skin carcinogenesis | Src, Rsk | COX-2 Src phosphorylation Direct binding with Src, PI3K, RSK | Skin pigmentation Number of tumors Number of tumor-bearing mice Tumor volume Blood vessel formation | [26] |
| Name | Stimuli | Target Disease | Target Signaling | In Vitro Markers | In Vivo Markers | Ref |
|---|---|---|---|---|---|---|
| Diosmin | AhR ligand (endogenous for kynurenine, exogenous for TCDD) | Atopic dermatitis | AhR | CYP1A1 transactivity↑ FLG, CYP1A1, NQO1, OVOL1, LOR, HRNR, IVL mRNA expression↑ proFLG, LOR, OVOL1, FLG, HRNR, IVL, NQO1 expression↑ Epidermal thickness↑ (3D-skin model) STAT3 phosphorylation↓ AhR nuclear translocation↑ Direct binding with AhR | [19] | |
| Cinnamaldehyde | BaP | Oxidative stress | AhR NRF2/HO-1 pathway | CYP1A1 mRNA expression↓ AhR nuclear translocation X AhR expression↓ NRF2 expression↑ NRF2 nuclear translocation↑ HO-1 mRNA expression↑ ROS production↓ | [20] | |
| Cynaropicrin | UVB | Oxidative stress | AhR-NRF2-Nqo1 pathway | Without UVB radiation (1) AhR nuclear translocation↑ (2) CYP1A1, NRF2, Nqo1 mRNA expressions↑ (3) NRF2 nuclear translocation↑ With UVB radiation (1) ROS production↓ (in a NRF2 dose-dependent manner) (2) IL-6, TNF-α production↓ | [27] | |
| Baicalein | BaP | Oxidative stress | AhR NRF2/HMOX1 axis | CYP1A1 mRNA↓ HMOX1 mRNA↑ NRF2 nuclear translocation↑ ROS production↓ IL-1α, IL-1β↓ Src phosphorylation↓ AhR nuclear translocation↓ | [102] | |
| Kaempferol | UVB | X | AhR | Microarray p65, RelB translocation↓ PPARα, PPARβ transcriptional activity↑ | [109] | |
| Pterostilbene | PM | Skin inflammation Skin aging Decreased moisturizing ability | AhR | ROS generation↓ AhR translocation↓ CYP1A1 expression↓ MAPK phosphorylation↓ MMP-1, MMP-2, MMP-9↓ COX-2↓ AQP-3↑ | [110] | |
| Oleanolic acid | PM | Skin aging | AhR | CYP1A1 mRNA↓ TNF-α mRNA↓ IL-6 secretion↓ MMP-1 expression↓ P62, LC-3 I, II↓ | [111] |
| Stimuli | Method | Target Disease | In Vivo Markers | Ref |
|---|---|---|---|---|
| Calcipotriol (MC903) | AhR-null mice | Atopic dermatitis | Ear thickness Scratching frequency | [113] |
| MC903 | AhR antagonist (CH223191) | Atopic dermatitis | Gross appearance of mice ears Ear thickness Scratching frequency TSLP mRNA expression Serum IgE level | [113] |
| IMQ | AhR+/+, AhR −/− mice | Psoriasis Atopic dermatitis | Clinical score IL-17A, IL-17F secretion | [114] |
| LL-37 | AhR agonist (benvitimod) | Rosacea-like eruptions | Redness score Redness area Dermal inflammatory cell infiltration TLR2 expression Chemokine expression | [115] |
| Bleomycin (BLM) | AhR antagonist (CH223191) | Skin fibrosis | Dermal thickness COL1A1, α-SMA, CTGF, TGF-β1 mRNA level | [116] |
| AhR-KO mice | Skin barrier integrity | Trans-epidermal water loss (TEWL) Structural changes in epidermis Microarray | [117] | |
| Removal of AhR ligands from diet | Skin barrier integrity | Barrier integrity | [117] | |
| Constitutive active form of AhR (AhR-CA) transgenic mice | Skin inflammation | Total duration of grooming behavior Scratching frequency Severity of skin disorder CYP1A1 expression CYP1B1, NQO1, ADH, AhRR, K1, K6, K16, CCL20, IL-18 gene expression IgG1, IgE, IL-4, IL-5 secretion | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, S.J.; Kim, D.J.; Jung, S.K. Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease. Int. J. Mol. Sci. 2023, 24, 5953. https://doi.org/10.3390/ijms24065953
Paik SJ, Kim DJ, Jung SK. Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease. International Journal of Molecular Sciences. 2023; 24(6):5953. https://doi.org/10.3390/ijms24065953
Chicago/Turabian StylePaik, So Jeong, Dong Joon Kim, and Sung Keun Jung. 2023. "Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease" International Journal of Molecular Sciences 24, no. 6: 5953. https://doi.org/10.3390/ijms24065953
APA StylePaik, S. J., Kim, D. J., & Jung, S. K. (2023). Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease. International Journal of Molecular Sciences, 24(6), 5953. https://doi.org/10.3390/ijms24065953







