Investigating Vitamin D Receptor Genetic Markers in a Cluster Headache Meta-Analysis
Abstract
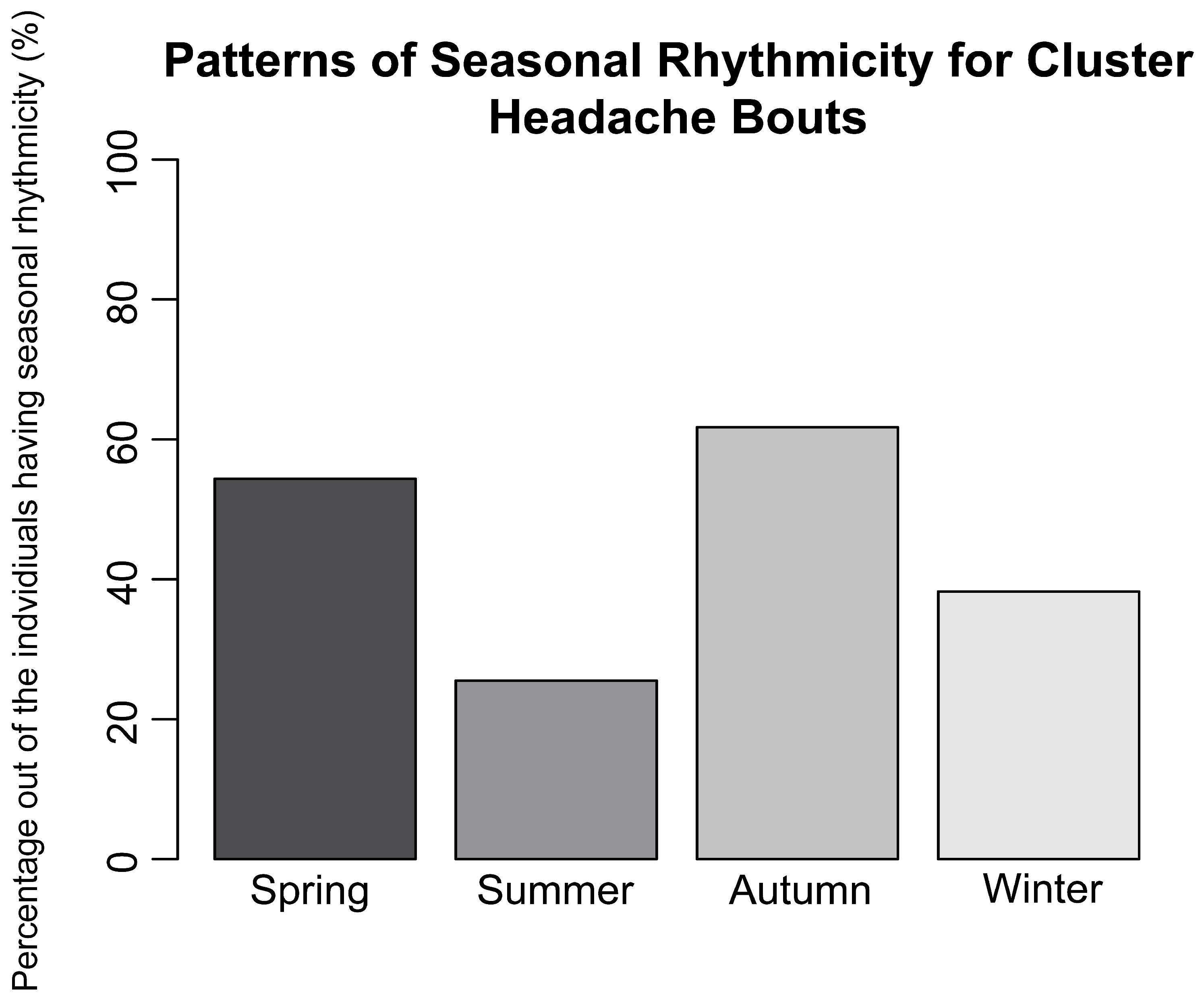
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Participant Information and DNA Isolation
4.2. qPCR of rs2228570
4.3. Meta-Analysis
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Rozen, T.D.; Fishman, R.S. Cluster Headache in the United States of America: Demographics, Clinical Characteristics, Triggers, Suicidality, and Personal Burden. Headache J. Head Face Pain 2012, 52, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Naber, W.C.; Fronczek, R.; Haan, J.; Doesborg, P.; Colwell, C.S.; Ferrari, M.D.; Meijer, J.H. The Biological Clock in Cluster Headache: A Review and Hypothesis. Cephalalgia 2019, 39, 1855–1866. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chen, Y.T.; Ou, S.M.; Li, S.Y.; Yang, A.C.; Tang, C.H.; Wang, S.J. Temperature Variation and the Incidence of Cluster Headache Periods: A Nationwide Population Study. Cephalalgia 2014, 34, 656–663. [Google Scholar] [CrossRef]
- Fischera, M.; Marziniak, M.; Gralow, I.; Evers, S. The Incidence and Prevalence of Cluster Headache: A Meta-Analysis of Population-Based Studies. Cephalalgia 2008, 6, 614–618. [Google Scholar] [CrossRef]
- Donnet, A.; Lanteri-Minet, M.; Guegan-Massardier, E.; Mick, G.; Fabre, N.; Géraud, G.; Lucas, C.; Navez, M.; Valade, D. Chronic Cluster Headache: A French Clinical Descriptive Study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1354. [Google Scholar] [CrossRef]
- May, A.; Bahra, A.; Büchel, C.; Frackowiak, R.S.J.; Goadsby, P.J. PET and MRA Findings in Cluster Headache and MRA in Experimental Pain. Neurology 2000, 55, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Bahra, A.; Büchel, C.; Frackowiak, R.S.J.; Goadsby, P.J. Hypothalamic Activation in Cluster Headache Attacks. Lancet 1998, 352, 275–278. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Dell’isola, G.B.; Tulli, E.; Sica, R.; Vinti, V.; Mencaroni, E.; di Cara, G.; Striano, P.; Verrotti, A. The Vitamin D Role in Preventing Primary Headache in Adult and Pediatric Population. J. Clin. Med. 2021, 10, 10. [Google Scholar] [CrossRef]
- Bartley, J. Vitamin D: Emerging Roles in Infection and Immunity. Expert Rev. Anti-Infect. Ther. 2014, 8, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, L.H.; Cai, H.L.; Li, H.D.; Liu, Y.P.; Tang, M.M.; Dang, R.L.; Zhu, W.Y.; Xue, Y.; He, X. Neurochemical Effects of Chronic Administration of Calcitriol in Rats. Nutrients 2014, 6, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, M.; Wiciński, M.; Osiński, S.; Kázmierczak, H. The Role of Vitamin D in Primary Headache—From Potential Mechanism to Treatment. Nutrients 2020, 12, 243. [Google Scholar] [CrossRef]
- Prakash, S.; Mehta, N.C.; Dabhi, A.S.; Lakhani, O.; Khilari, M.; Shah, N.D. The Prevalence of Headache May Be Related with the Latitude: A Possible Role of Vitamin D Insufficiency? J. Headache Pain 2010, 11, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Brotis, A.; Dardiotis, E. Vitamin D Serum Levels in Patients with Migraine: A Meta-Analysis. Rev. Neurol. 2020, 176, 560–570. [Google Scholar] [CrossRef]
- Sohn, J.H.; Chu, M.K.; Park, K.Y.; Ahn, H.Y.; Cho, S.J. Vitamin D Deficiency in Patients with Cluster Headache: A Preliminary Study. J. Headache Pain 2018, 19, 54. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. High Dose Vitamin D Plus Multivitamin in the Prevention of Cluster Headache—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT04570475 (accessed on 3 March 2023).
- Papasavva, M.; Vikelis, M.; Siokas, V.; Katsarou, M.S.; Dermitzakis, E.; Raptis, A.; Dardiotis, E.; Drakoulis, N. VDR Gene Polymorphisms and Cluster Headache Susceptibility: Case–Control Study in a Southeastern European Caucasian Population. J. Mol. Neurosci. 2022, 72, 382–392. [Google Scholar] [CrossRef]
- Kato, S. The Function of Vitamin D Receptor in Vitamin D Action. J. Biochem. 2000, 127, 717–722. [Google Scholar] [CrossRef]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the Vitamin D Receptor Gene by Environment, Genetics and Epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; van Meurs, J.B.J.; D’Alesio, A.; Jhamai, M.; Zhao, H.; Rivadeneira, F.; Hofman, A.; van Leeuwen, J.P.T.; Jehan, F.; Pols, H.A.P.; et al. Promoter and 3′-Untranslated-Region Haplotypes in the Vitamin D Receptor Gene Predispose to Osteoporotic Fracture: The Rotterdam Study. Am. J. Hum. Genet. 2005, 77, 807–823. [Google Scholar] [CrossRef]
- Durrin, L.K.; Haile, R.W.; Ingles, S.A.; Coetzee, G.A. Vitamin D Receptor 3′-Untranslated Region Polymorphisms: Lack of Effect on MRNA Stability. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 1999, 1453, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, L. Association between Vitamin D Receptor Gene Polymorphisms and Breast Cancer Risk: A Meta-Analysis of 39 Studies. PLoS ONE 2014, 9, e96125. [Google Scholar] [CrossRef] [PubMed]
- Dastani, Z.; Li, R.; Richards, B. Genetic Regulation of Vitamin D Levels. Calcif. Tissue Int. 2013, 92, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.J.; Pols, H.A.P.; van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef]
- Jurutka, P.W.; Remus, L.S.; Whitfield, G.K.; Thompson, P.D.; Hsieh, J.C.; Zitzer, H.; Tavakkoli, P.; Galligan, M.A.; Dang, H.T.L.; Haussler, C.A.; et al. The Polymorphic N Terminus in Human Vitamin D Receptor Isoforms Influences Transcriptional Activity by Modulating Interaction with Transcription Factor IIB. Mol. Endocrinol. 2000, 14, 401–420. [Google Scholar] [CrossRef]
- Poon, A.H.; Gong, L.; Brasch-Andersen, C.; Litonjua, A.A.; Raby, B.A.; Hamid, Q.; Laprise, C.; Weiss, S.T.; Altman, R.B.; Klein, T.E. Very Important Pharmacogene Summary for VDR. Pharm. Genom. 2012, 22, 758–763. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Jiang, Q.; Zhang, Y.; Liu, A.; Wang, H.; Zhang, J.; Qin, Q.; Hong, Z.; Li, B. An Do Genetic Polymorphisms of the Vitamin D Receptor Contribute to Breast/Ovarian Cancer? A Systematic Review and Network Meta-Analysis. Gene 2018, 677, 211–227. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, R.; Feng, Y.; Zheng, G.X.; Liang, Y.; Zhang, J.Y.; Qin, X.Q.; Chen, W.; Wu, J.Q.; Zhong, Y.H. Association of Vitamin D Receptor Gene Polymorphism with the Risk of Lung Cancer: A Meta-Analysis. J. Recept. Signal Transduct. 2014, 34, 500–505. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Meloni, M.; Riboldazzi, G.; Zangaglia, R.; Sturchio, A.; Casali, C.; di Lorenzo, C.; et al. The VDR FokI (Rs2228570) Polymorphism Is Involved in Parkinson’s Disease. J. Neurol. Sci. 2021, 428, 117606. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; de Luis-Román, D.A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
- O’Connor, E.; Fourier, C.; Ran, C.; Sivakumar, P.; Liesecke, F.; Southgate, L.; Harder, A.V.E.; Vijfhuizen, L.S.; Yip, J.; Giffin, N.; et al. Genome-Wide Association Study Identifies Risk Loci for Cluster Headache. Ann. Neurol. 2021, 90, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Y.; Lee, J.T.; Peng, G.S.; Yang, F.C. Sildenafil Can Induce the Onset of a Cluster Headache Bout. Can. Urol. Assoc. J. 2014, 8, e378–e380. [Google Scholar] [CrossRef] [PubMed]
- Sandor, P.S.; Irimia, P.; Jager, H.R.; Goadsby, P.J.; Kaube, H. Onset of cluster headache triggered by emotional effect: A case report. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Fourier, C.; Ran, C.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Sex Differences in Clinical Features, Treatment, and Lifestyle Factors in Patients With Cluster Headache. Neurology 2022, in press. [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, s13742-015-0047-8. [Google Scholar] [CrossRef]
- Dupont, W.D.; Plummer, W.D. Power and Sample Size Calculations. A Review and Computer Program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
| Attack Trigger % (n) | Bout Trigger % (n) | |
|---|---|---|
| Any trigger | 54.3 (454) | 29.8 (89) |
| Warmth or cold | 10.1 (46) | 7.9 (7) |
| Sunlight or darkness | 3.1 (14) | 4.5 (4) |
| Weather changes or weather | 4.2 (19) | 15.7 (14) |
| Any of the above (Warmth, cold, sunlight, darkness, or weather/weather changes) | 15.4 (70) | 23.6 (21) |
| Allele | Allele Frequency | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Cluster Headache | Controls | ||||
| rs2228570 | C % (n) | 60.7 (744) | 60.2 (806) | 0.99 (0.85–1.16) | 0.89 |
| T % (n) | 39.3 (482) | 39.8 (532) | |||
| SNP | Greek Cohort CH/Control [18] | Swedish Cohort CH/Control | Wildtype Allele | Minor Allele | OR | p-Value | Cochrane’s Q Statistic p-Value |
|---|---|---|---|---|---|---|---|
| rs2228570 | 131/281 | 617/672 | C | T | 0.96 | 0.59 | 0.57 |
| rs1544410 | 131/281 | 643/1299 * | G | A | 0.93 | 0.26 | 0.42 |
| rs731236 | 131/281 | 643/1299 * | T | C | 0.94 | 0.36 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jennysdotter Olofsgård, F.; Ran, C.; Qin, Y.; Fourier, C.; Sjöstrand, C.; Waldenlind, E.; Steinberg, A.; Belin, A.C. Investigating Vitamin D Receptor Genetic Markers in a Cluster Headache Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5950. https://doi.org/10.3390/ijms24065950
Jennysdotter Olofsgård F, Ran C, Qin Y, Fourier C, Sjöstrand C, Waldenlind E, Steinberg A, Belin AC. Investigating Vitamin D Receptor Genetic Markers in a Cluster Headache Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(6):5950. https://doi.org/10.3390/ijms24065950
Chicago/Turabian StyleJennysdotter Olofsgård, Felicia, Caroline Ran, Yuyan Qin, Carmen Fourier, Christina Sjöstrand, Elisabet Waldenlind, Anna Steinberg, and Andrea Carmine Belin. 2023. "Investigating Vitamin D Receptor Genetic Markers in a Cluster Headache Meta-Analysis" International Journal of Molecular Sciences 24, no. 6: 5950. https://doi.org/10.3390/ijms24065950
APA StyleJennysdotter Olofsgård, F., Ran, C., Qin, Y., Fourier, C., Sjöstrand, C., Waldenlind, E., Steinberg, A., & Belin, A. C. (2023). Investigating Vitamin D Receptor Genetic Markers in a Cluster Headache Meta-Analysis. International Journal of Molecular Sciences, 24(6), 5950. https://doi.org/10.3390/ijms24065950









