Genome-Wide Identification and Functional Analysis of the Roles of SAUR Gene Family Members in the Promotion of Cucumber Root Expansion
Abstract
1. Introduction
2. Results
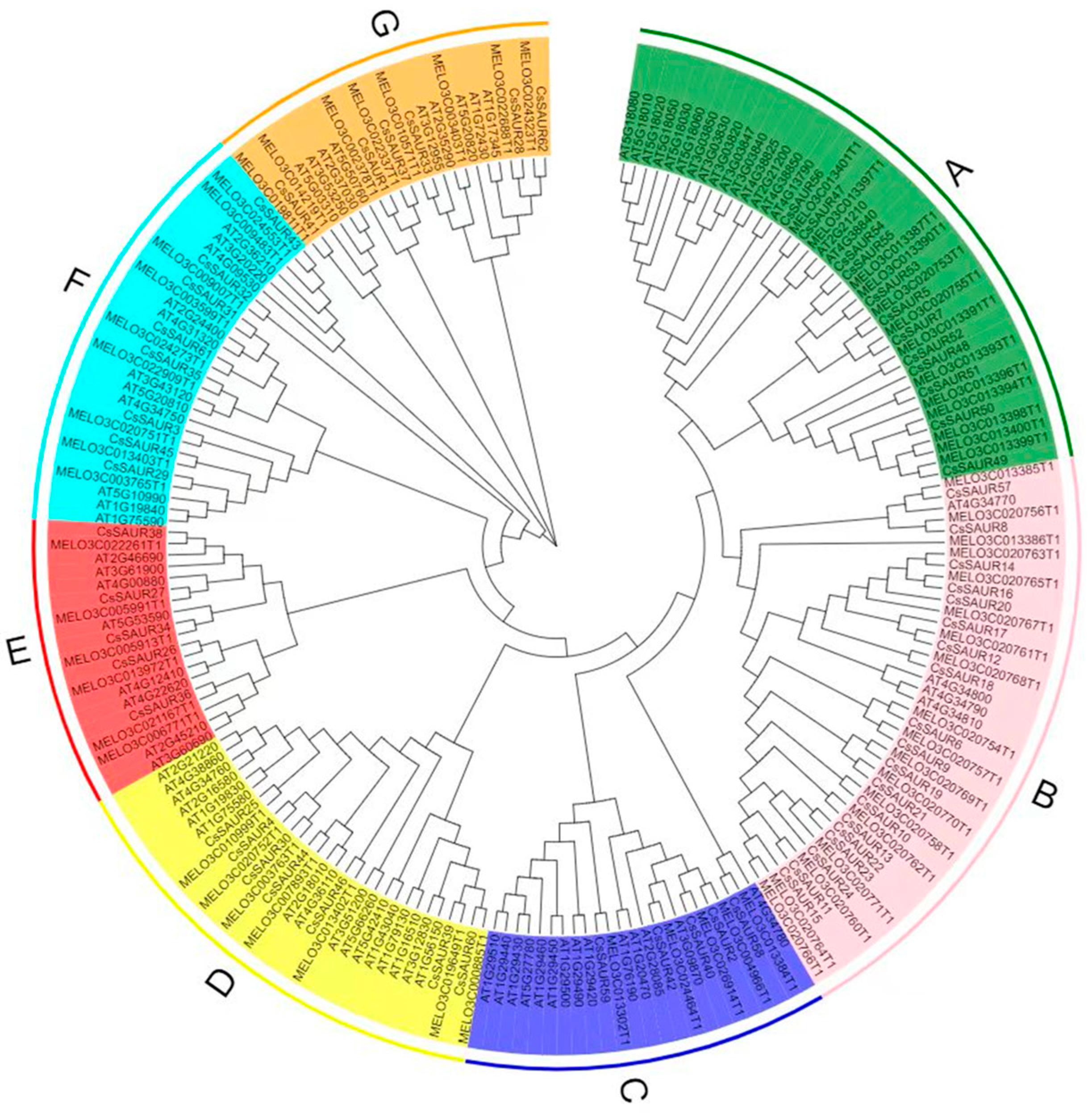
2.1. CsSAUR Gene Identification and Phylogenetic Analysis
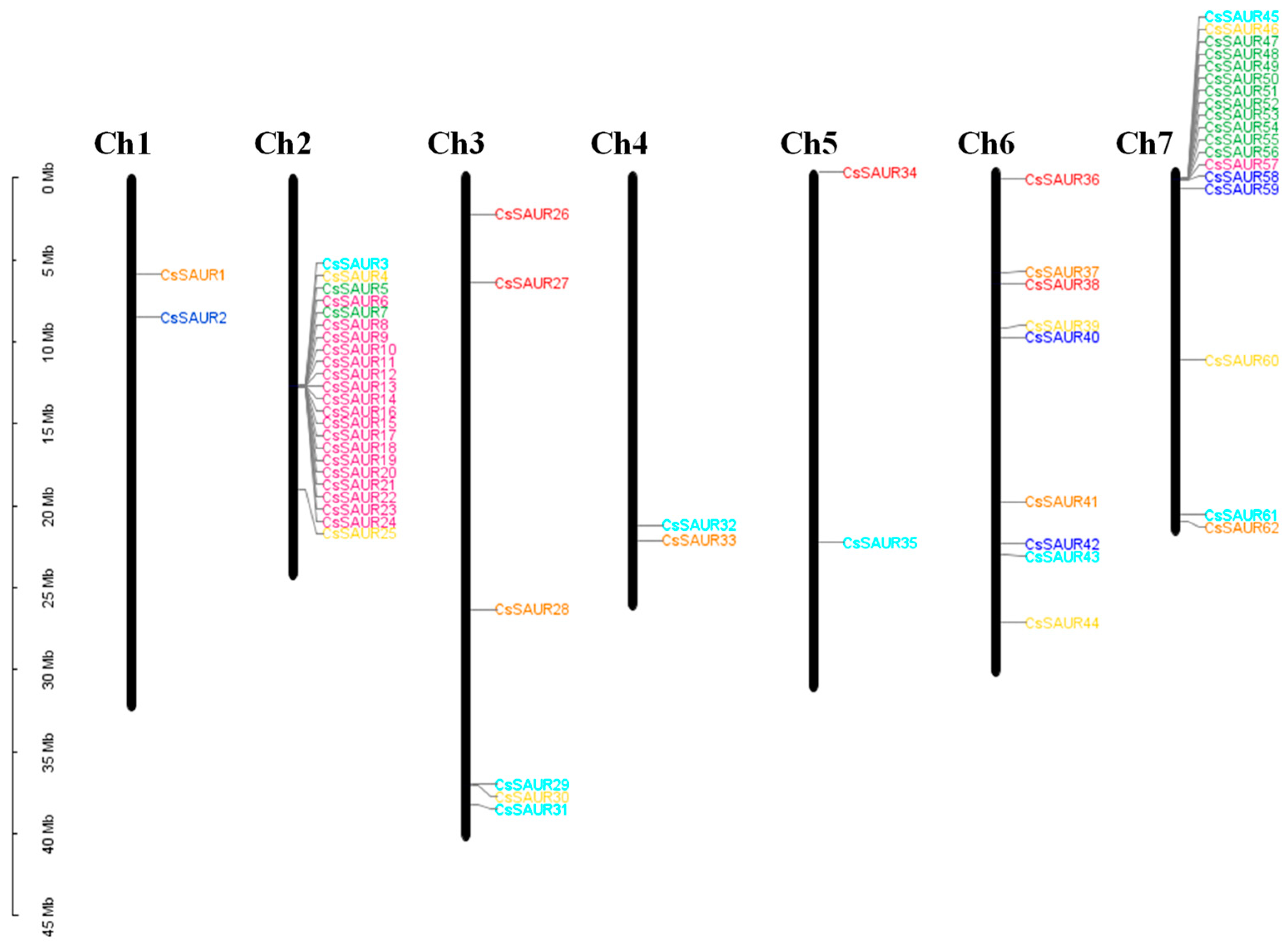
2.2. CsSAUR Gene Chromosomal Distributions
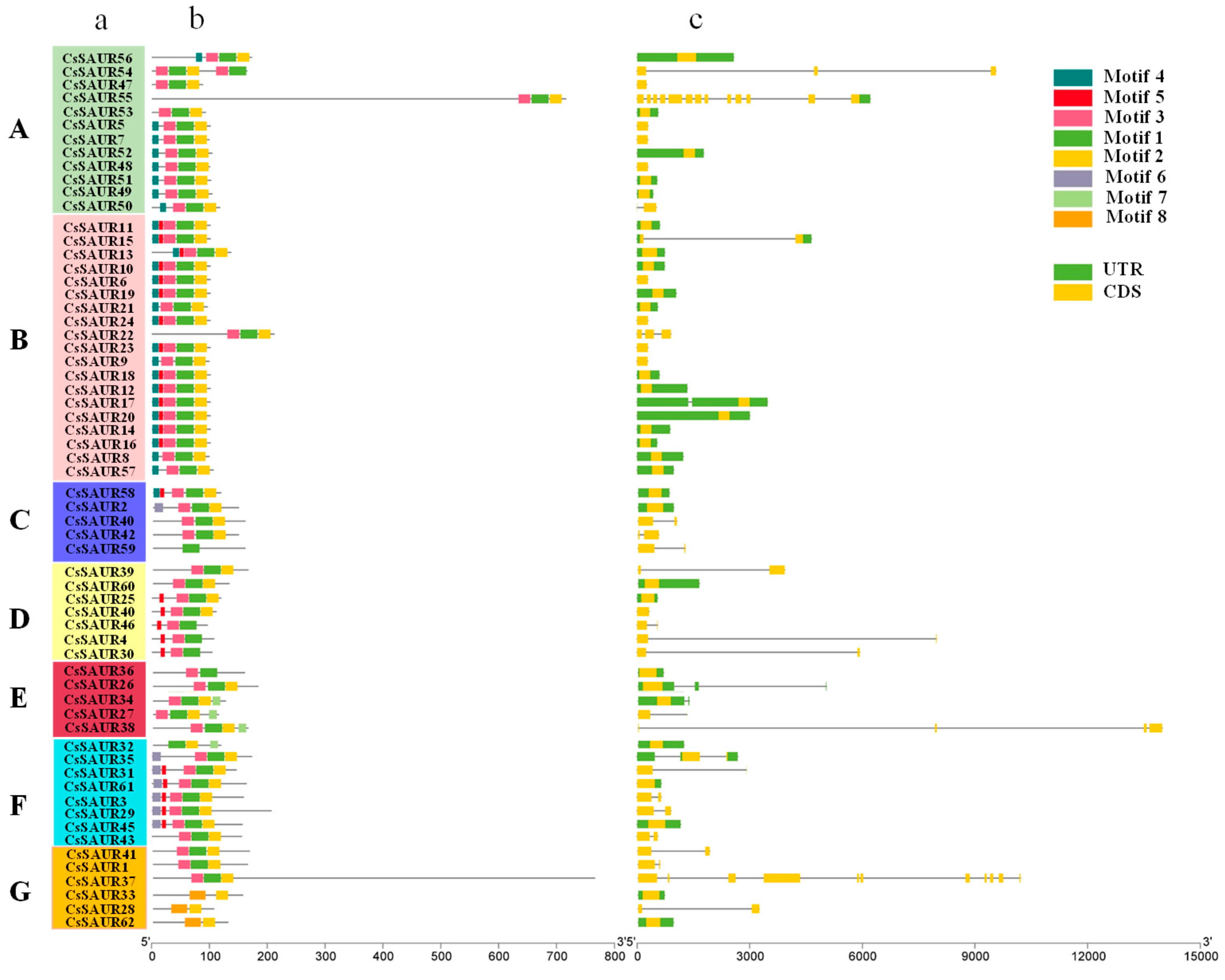
2.3. Analyses of CsSAUR Gene Structures and Conserved Motifs
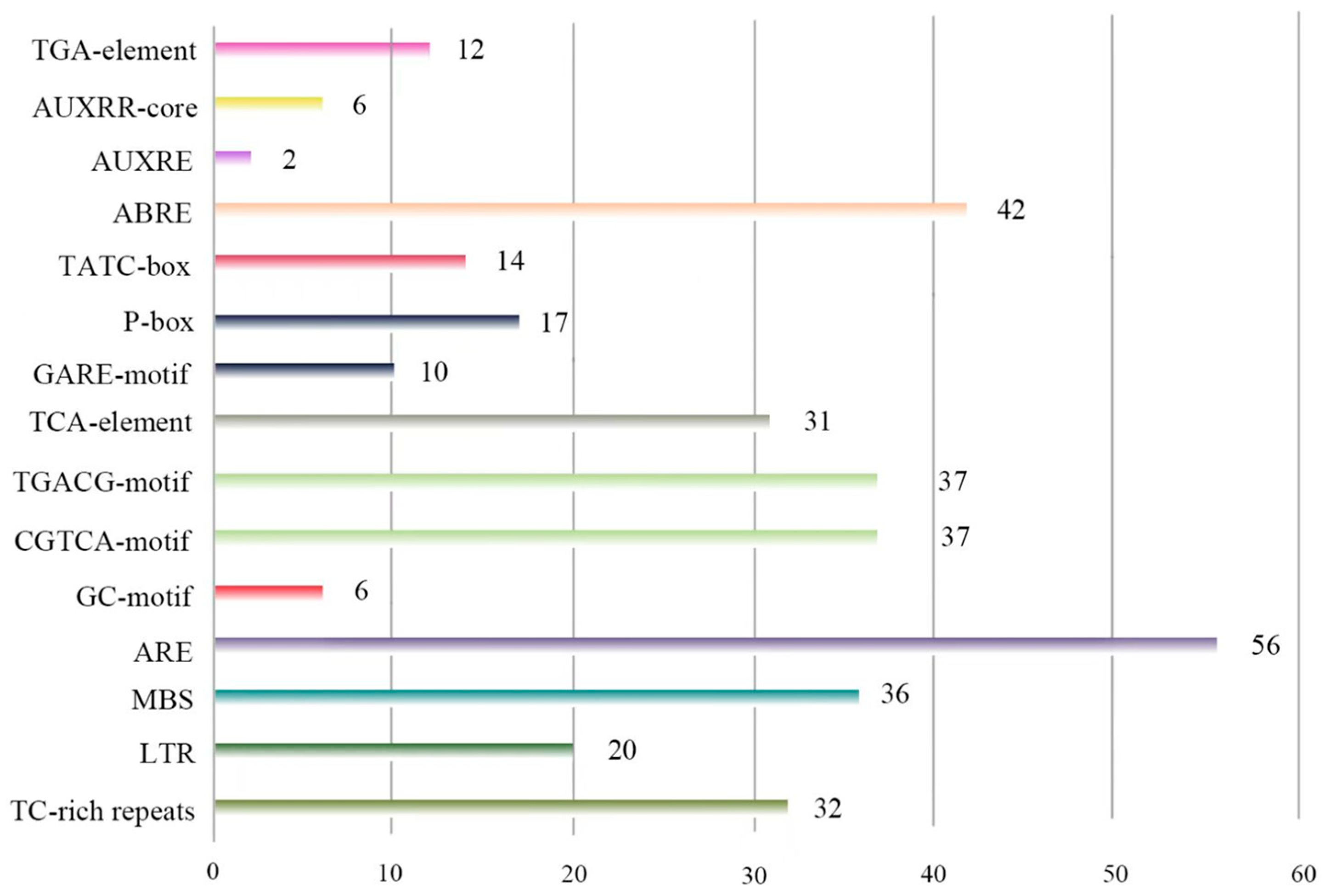
2.4. Identification of Cis-Acting Elements
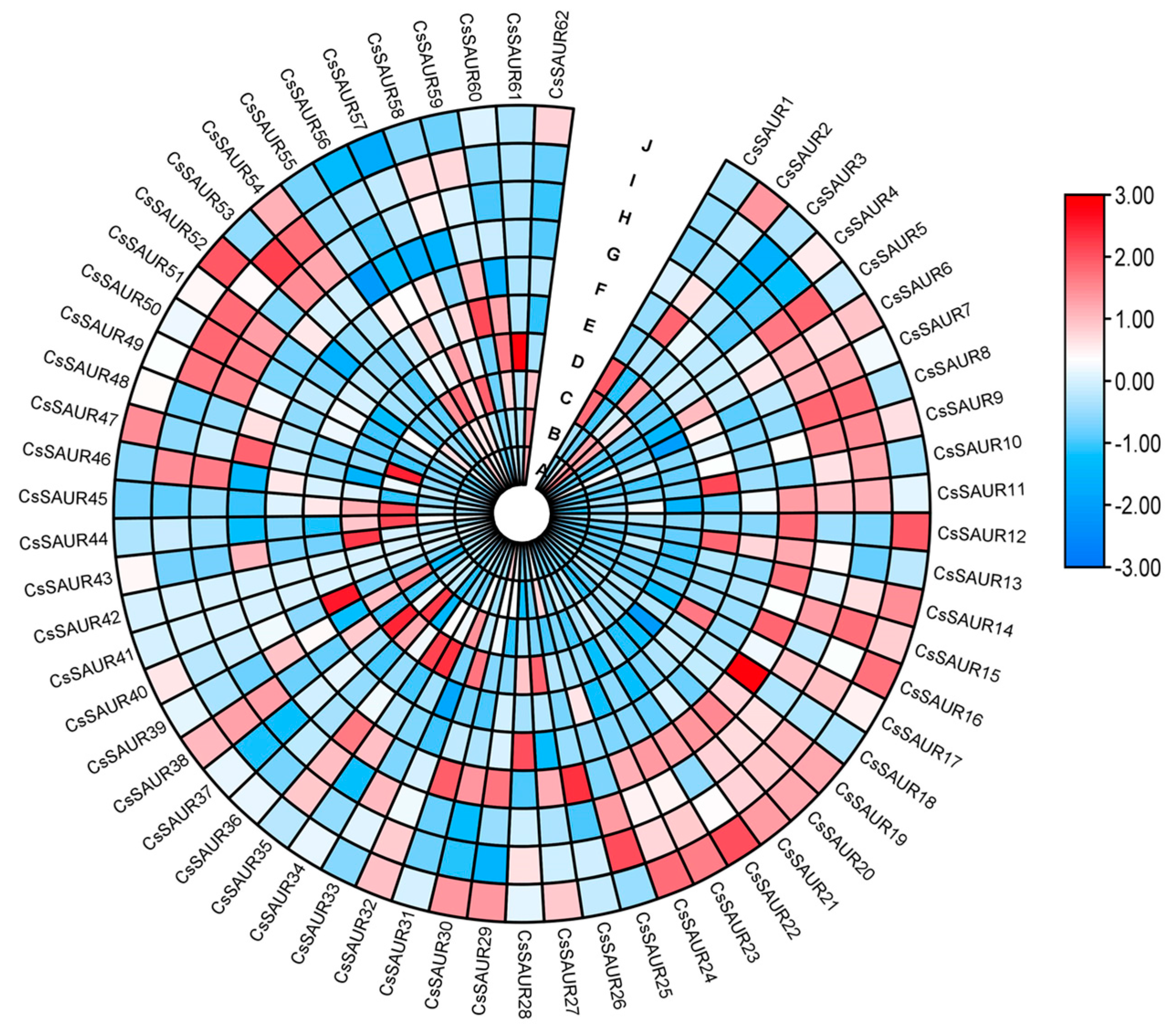
2.5. Analyses of CsSAUR Expression Patterns
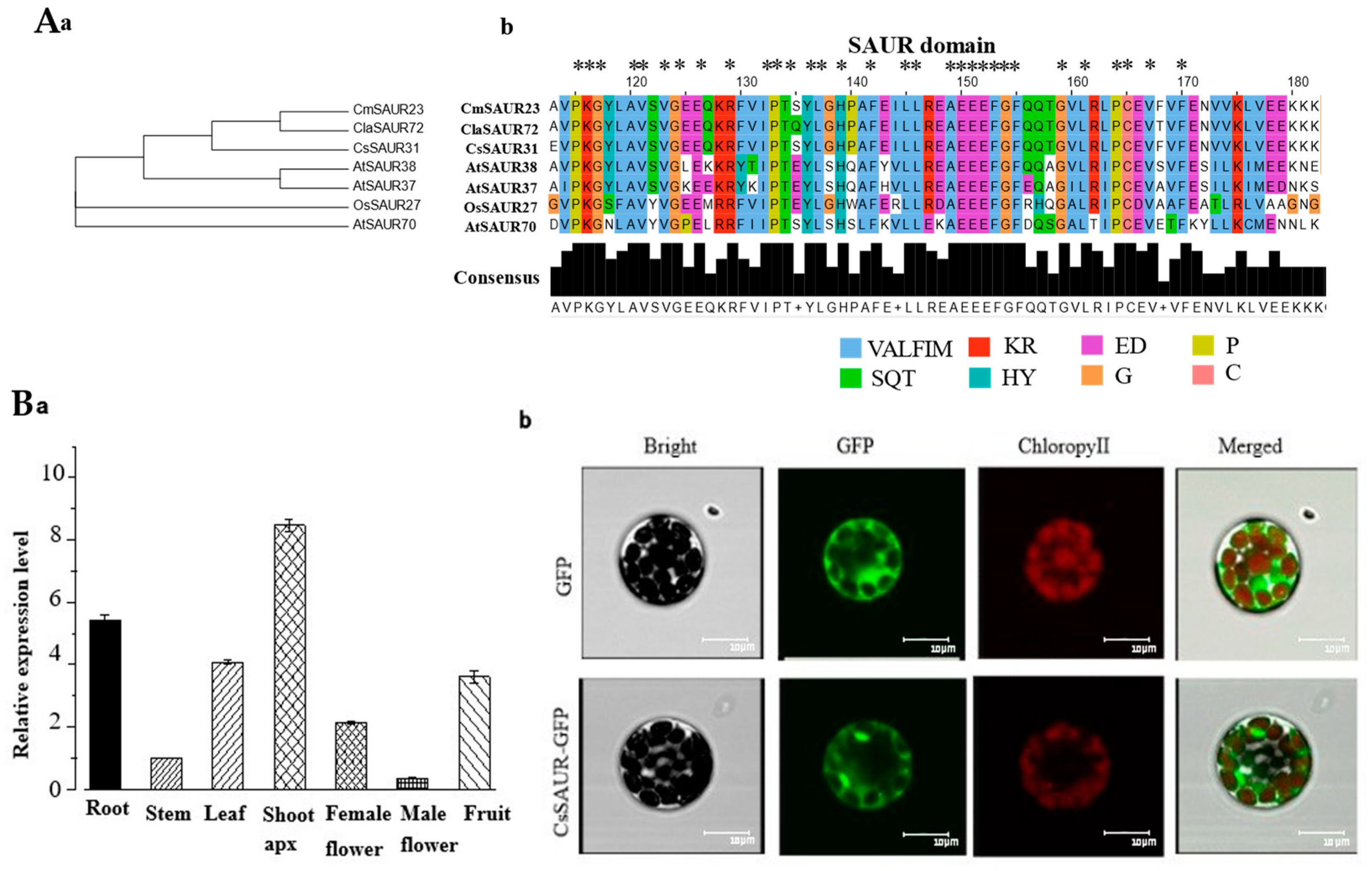
2.6. Phylogenetic and Sequence Alignment Analyses of CsSAUR31
2.7. Expression and Subcellular Localization of CsSAUR31
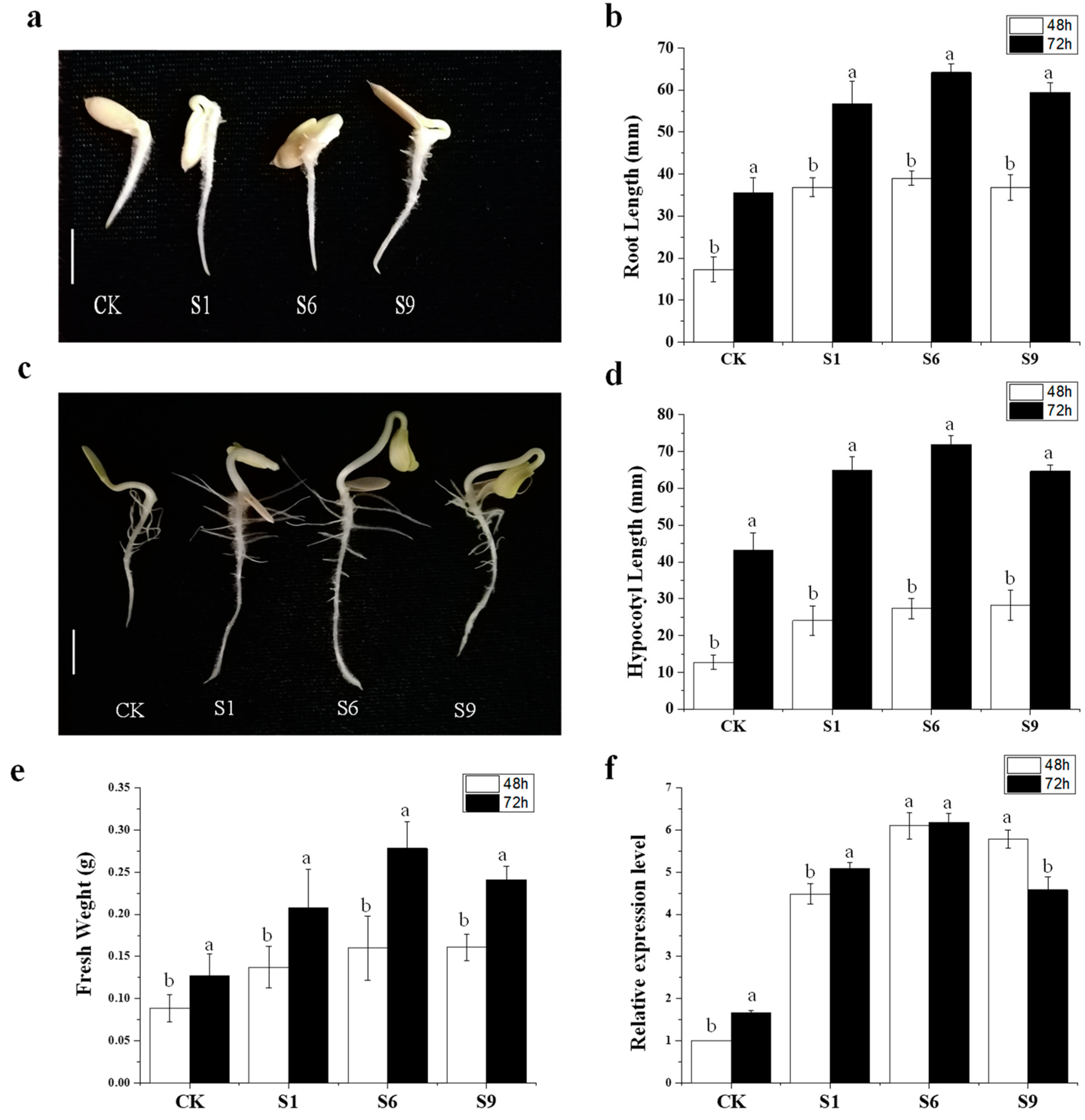
2.8. CsSAUR31 Functions as a Promoter of Hypocotyl and Root Elongation
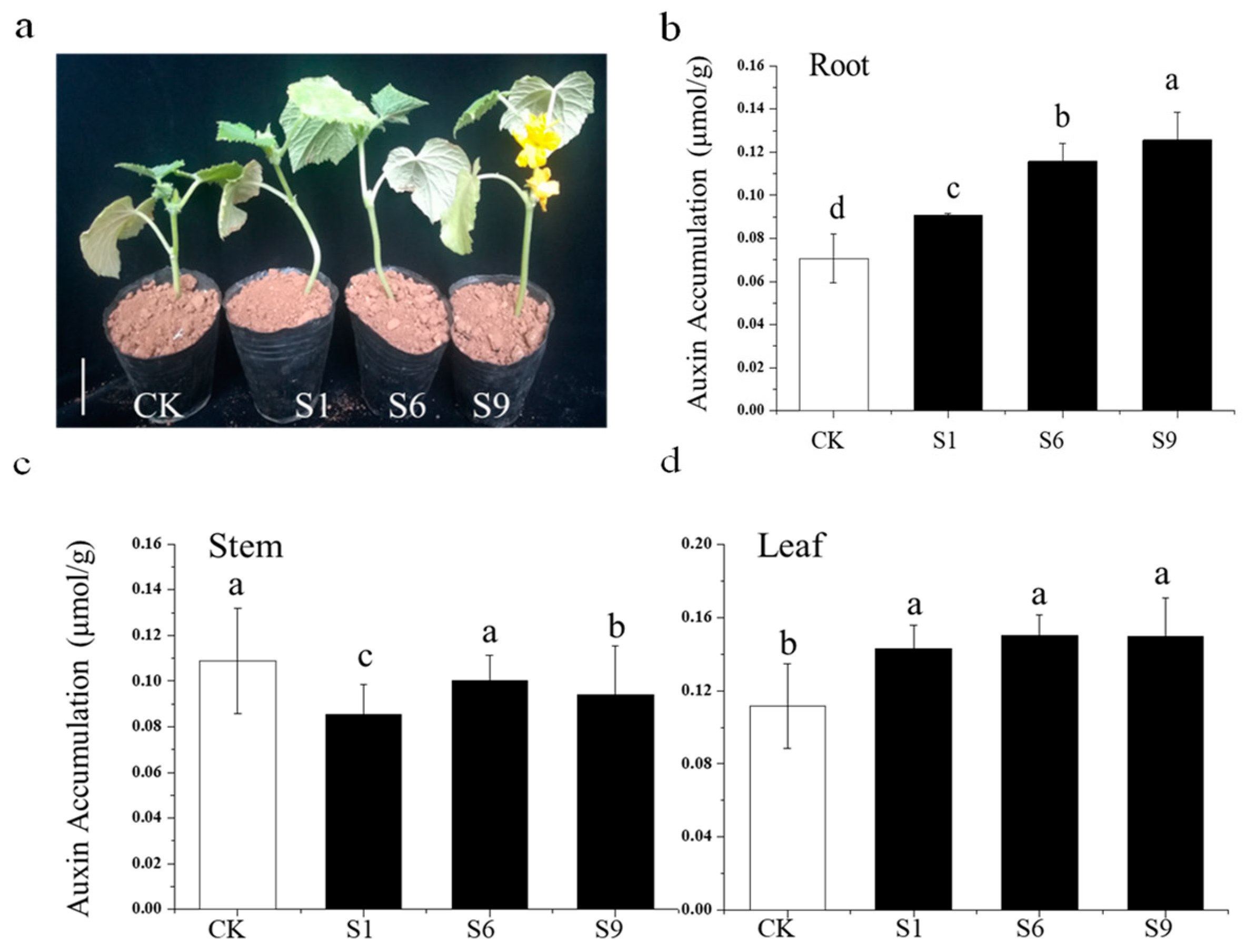
2.9. Auxin Accumulation in the Context of CsSAUR31 Overexpression
3. Discussion
4. Materials and Methods
4.1. SAUR Gene Identification and Phylogenetic Analysis
4.2. Chromosomal Location Analyses
4.3. Exon/Intron Structural Analyses and Conserved Motif Identification
4.4. Cis-Acting Element Analyses
4.5. Analysis of the Expression Patterns of the Cucumber SAUR Genes Family
4.6. Plant Materials
4.7. CsSAUR31 Cloning and Bioinformatic Analysis
4.8. RT-qPCR
4.9. Subcellular Localization Analyses
4.10. Cucumber Transformation
4.11. Measurement of Auxin Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Song, Y.; Yang, H.; Liu, Z.; Zhu, G.; Yang, Y. An auxin responsive endogenous peptide regulates root development in Arabidopsis. J. Integr. Plant Biol. 2014, 56, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Tyagi, A.K.; Khurana, J.P. Genome-wide analysis, evolutionary expansion, and expression of early auxin-responsive SAUR gene family in rice (Oryza sativa). Genomics 2006, 88, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Bai, Y.H.; Shen, C.J.; Wu, Y.R.; Zhang, S.N.; Jiang, D.A.; Guilfoyle, T.J.; Chen, M.; Qi, Y.H. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; He, Y.; Guan, X.; Zhu, X.; Cheng, L.; Wang, J.; Lu, G. Genome-wide analysis of SAUR gene family in Solanaceae species. Gene 2012, 509, 38–50. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, X.; Bao, Y.N.; Wang, B.; Zeng, H.X.; Cheng, W.S.; Tang, M.; Li, Y.H.; Ren, J.; Sun, Y.H. Genome-wide identification of SAUR genes in watermelon (Citrullus lanatus). Physiol. Mol. Biol. Plants 2017, 23, 619–628. [Google Scholar] [CrossRef]
- Tian, Z.; Han, J.; Che, G.; Hasi, A. Genome-wide characterization and expression analysis of SAUR gene family in Melon (Cucumis melo L.). Planta 2022, 255, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Dai, S.; Qin, N.; Zhu, C.; Qin, J.; Li, J. Genome-wide identification and expression analysis of the SAUR gene family in foxtail millet (Setaria italica L.). BMC Plant Biol. 2023, 23, 31. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, X.; Xue, X.; Liu, M.; Zhang, X.; Xiao, X.; Lin, Y. Genome-wide analysis of the SAUR gene family and function exploration of DlSAUR32 during early longan somatic embryogenesis. Plant Physiol. Biochem. 2023, 195, 362–374. [Google Scholar] [CrossRef]
- Knauss, S.; Rohrmeier, T.; Lehle, L. The Auxin-induced maize gene ZmSAUR2 encodes a short-lived nuclear protein expressed in elongating tissues. J. Biol. Chem. 2003, 278, 23936. [Google Scholar] [CrossRef]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012, 71, 684–697. [Google Scholar] [CrossRef]
- Nagpal, P.; Reeves, P.H.; Wong, J.H.; Armengot, L.; Chae, K.; Rieveschl, N.B.; Reed, J.W. SAUR63 stimulates cell growth at the plasma membrane. PLoS Genet. 2022, 18, e1010375. [Google Scholar] [CrossRef]
- Gastaldi, V.; Lucero, L.E.; Ferrero, L.V.; Ariel, F.D.; Gonzalez, D.H. Class-I TCP Transcription Factors Activate the SAUR63 Gene Subfamily in Gibberellin-Dependent Stamen Filament Elongation. Plant Physiol. 2020, 182, 2096–2110. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inze, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Wu, D.; Zhang, N.; Hua, J. Polymorphisms in cis-elements confer SAUR26 gene expression difference for thermo-response natural variation in Arabidopsis. New Phytol. 2020, 229, 2751–2764. [Google Scholar] [CrossRef]
- Qiu, T.; Chen, Y.; Li, M.; Kong, Y.; Zhu, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. The tissue-specific and developmentally regulated expression patterns of the SAUR41 subfamily of SMALL AUXIN UP RNA genes. Plant Signal. Behav. 2014, 8, e25283. [Google Scholar] [CrossRef]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Lu, G. Citation: The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Qiu, T.; Wang, J. The SAUR41 subfamily of cell expansion-promoting genes modulates abscisic acid sensitivity and root touch response: A possible connection to ion homeostasis regulation. Plant Signal. Behav. 2020, 15, 1702239. [Google Scholar] [CrossRef]
- Wang, J.; Sun, N.; Zhang, F.; Yu, R.; Chen, H.; Deng, X.W.; Wei, N. Differential regulation of arabidopsis PP2C-D1 by SAUR17 and SAUR50 in apical hook development and cotyledon opening. Plant Cell 2020, 32, 3792–3811. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Hepworth, S.R.; Ma, C.; Li, H.; Li, J.; Wang, S.M.; Yin, H. SAUR15 interaction with BRI1 activates plasma membrane H+-ATPase to promote organ development of Arabidopsis. Plant Physiol. 2022, 189, 2454–2466. [Google Scholar] [CrossRef]
- Kathare, K.P.; Dharmasiri, S.; Dharmasiri, N. SAUR53 regulates organ elongation and apical hook development in Arabidopsis. Plant Signal. Behav. 2018, 13, e1514896. [Google Scholar] [CrossRef]
- Xu, Y.X.; Xiao, M.Z.; Liu, Y.; Fu, J.L.; He, Y.; Jiang, D.A. The small auxin-up RNA OsSAUR45 affects auxin synthesis and transport in rice. Plant Mol. Biol. 2017, 94, 97–107. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Yao, X.; Chen, J.; Chen, X.; Zhou, H.; Lou, Y.; Ming, F.; Jin, Y. Genome-wide identification and characterization of small auxin-up RNA (SAUR) gene family in plants: Evolution and expression profiles during normal growth and stress response. BMC Plant Biol. 2021, 21, 4. [Google Scholar] [CrossRef]
- Shin, J.; Mila, I.; Liu, M.; Rodrigues, M.A.; Vernoux, T.; Pirrello, J.; Bouzayan, M. The RIN regulated Small Auxin Up RNA SAUR69 is involved in the unripe to ripe phase transition of tomato fruit via enhancement of the sensitivity to ethylene. New Phytol. 2019, 222, 820–836. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Q.; Hu, Z.; Sun, X.; Fan, S.; Zhang, H. Function of the auxin-responsive gene TaSAUR75 under salt and drought stress. Crop J. 2018, 6, 181–190. [Google Scholar] [CrossRef]
- McClure, B.A.; Guilfoyle, T. Characterization of a class of small auxin-inducible soybean polyadenylated RNAs. Plant Mol. Biol. 1987, 9, 611–623. [Google Scholar] [CrossRef]
- Boerjan, W.; Cervera, M.T.; Delarue, M.; Beeckman, T.; Dewitte, W.; Bellini, C.; Caboche, M.; Van Onckelen, H.; Van Montagu, M.; Inze, D. Superroot, a recessive mutation in arabidopsis, confers auxin overproduction. Plant Cell 1995, 7, 1405–1419. [Google Scholar]
- Chen, Y.; Xi, H.; Cao, J. Small auxin upregulated RNA (SAUR) gene family in maize: Identification, evolution, and its phylogenetic comparison with Arabidopsis, rice, and sorghum. J. Integr. Plant Biol. 2014, 56, 133–150. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef]
- Vision, T.J.; Brown, D.G.; Tanksley, S.D. The origins of genomic duplications in Arabidopsis. Science 2000, 290, 2114–2117. [Google Scholar] [CrossRef]
- Van Mourik, H.; Van Dijk, A.D.J.; Stortenbeker, N.; Angenent, G.C.; Bemer, M. Divergent regulation of Arabidopsis SAUR genes: A focus on the SAUR10-clade. BMC Plant Biol. 2017, 17, 245. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lor, V.S.; Ren, H.; Olszewski, N.E.; Miller, N.D.; Wu, G.; Spalding, E.P.; Gray, W.M. Constitutive expression of arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol. 2017, 173, 1453–1462. [Google Scholar] [CrossRef]
- Stamm, P.; Kumar, P.P. Auxin and gibberellin responsive Arabidopsis SMALL AUXIN UP RNA36 regulates hypocotyl elongation in the light. Plant Cell Rep. 2013, 32, 759–769. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Gao, C.; She, W.; Lin, W.; Chen, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. Tissue-specific expression of SMALL AUXIN UP RNA41 differentially regulates cell expansion and root meristem patterning in arabidopsis. Plant Cell Physiol. 2013, 54, 609–621. [Google Scholar] [CrossRef]
- Bemer, M.; van Mourik, H.; Muino, J.M.; Ferrandiz, C.; Kaufmann, K.; Angenent, G.C. FRUITFULL controls SAUR10 expression and regulates Arabidopsis growth and architecture. J. Exp. Bot. 2017, 68, 3391–3403. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Bai, M.Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Plant Physiol. 2014, 3, e03031. [Google Scholar]
- Walcher, C.L.; Nemhauser, J.L. Bipartite promoter element required for auxin response. Plant Physiol. 2012, 158, 273–282. [Google Scholar] [CrossRef]
- Yu, J.; Wu, S.; Sun, H.; Wang, X.; Tang, X.; Guo, S.; Zhang, Z.; Huang, S.; Xu, Y.; Weng, Y.; et al. CuGenDBv2: An updated database for cucurbit genomics. Nucleic Acids Res. 2022, 51, D1457–D1464. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the expasy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Totowa: Clifton, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Koichiro, T.; Glen, S.; Sudhir, K. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar]
- Balakrishnan, S.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nuclc Acids Res. 2019, 47, W270–W275. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative realtime polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yan, Y.; Liu, W.; Zhang, W. Knock-down of CsNRT2.1, a cucumber nitrate transporter, reduces nitrate uptake, root length, and lateral root number at low external nitrate concentration. Front. Plant Sci. 2018, 9, 722. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Chen, S.; Songkumarn, P.; Liu, J.; Wang, G.L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009, 150, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Liu, B.; Wang, W.; Liu, X.; Chen, C.; Liu, X.; Yang, S.; Ren, H. A GAMYB homolog CsGAMYB1 regulates sex expression of cucumber via an ethylene-independent pathway. J. Exp. Bot. 2014, 65, 3201–3213. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Xin, M.; Qin, Z. Genome-Wide Identification and Functional Analysis of the Roles of SAUR Gene Family Members in the Promotion of Cucumber Root Expansion. Int. J. Mol. Sci. 2023, 24, 5940. https://doi.org/10.3390/ijms24065940
Luan J, Xin M, Qin Z. Genome-Wide Identification and Functional Analysis of the Roles of SAUR Gene Family Members in the Promotion of Cucumber Root Expansion. International Journal of Molecular Sciences. 2023; 24(6):5940. https://doi.org/10.3390/ijms24065940
Chicago/Turabian StyleLuan, Jie, Ming Xin, and Zhiwei Qin. 2023. "Genome-Wide Identification and Functional Analysis of the Roles of SAUR Gene Family Members in the Promotion of Cucumber Root Expansion" International Journal of Molecular Sciences 24, no. 6: 5940. https://doi.org/10.3390/ijms24065940
APA StyleLuan, J., Xin, M., & Qin, Z. (2023). Genome-Wide Identification and Functional Analysis of the Roles of SAUR Gene Family Members in the Promotion of Cucumber Root Expansion. International Journal of Molecular Sciences, 24(6), 5940. https://doi.org/10.3390/ijms24065940



