ARID1A, NOTCH and WNT Signature in Gynaecological Tumours
Abstract
1. Introduction
2. Results
2.1. ARID1A, NOTCH/WNT Pathway Component mRNA Expression in Ovarian Cancer Cell Cultures
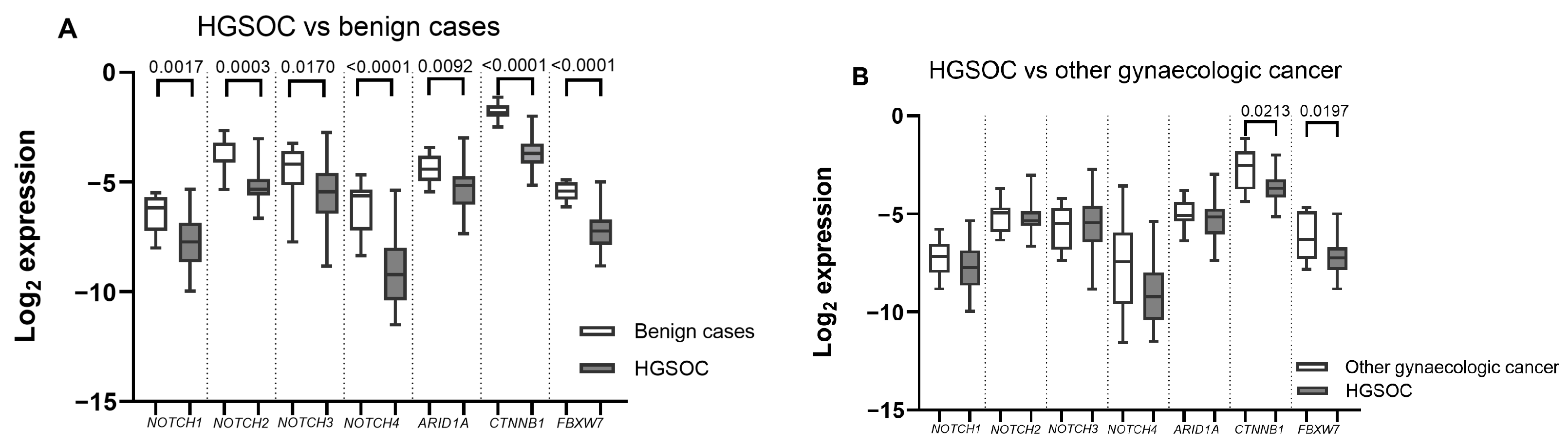
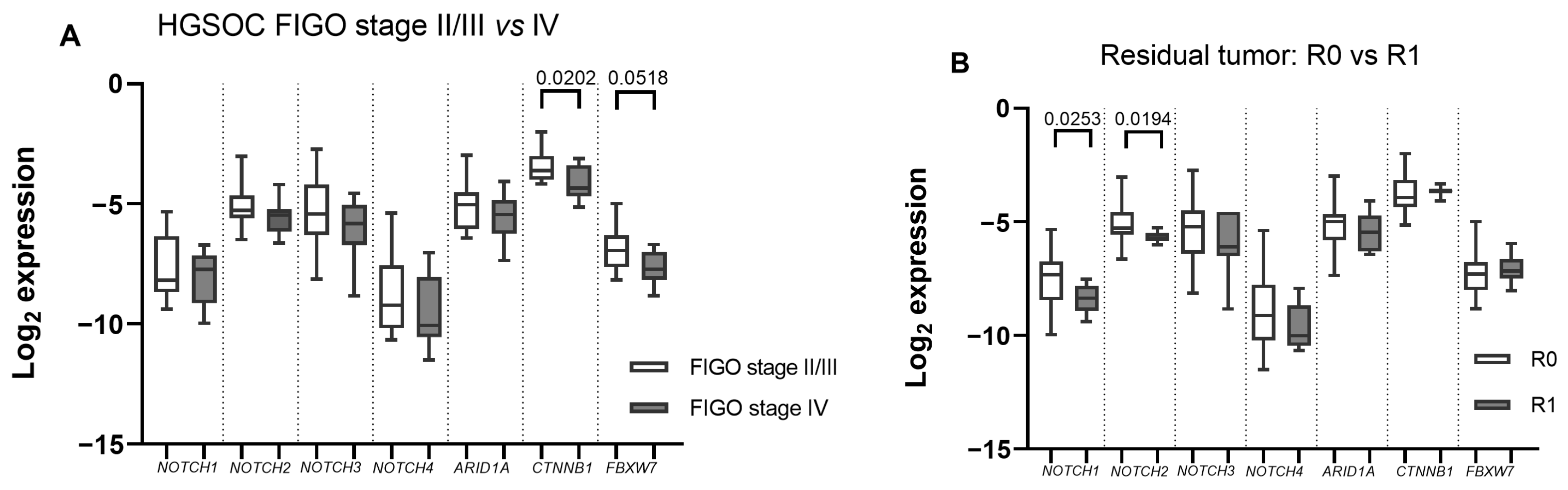
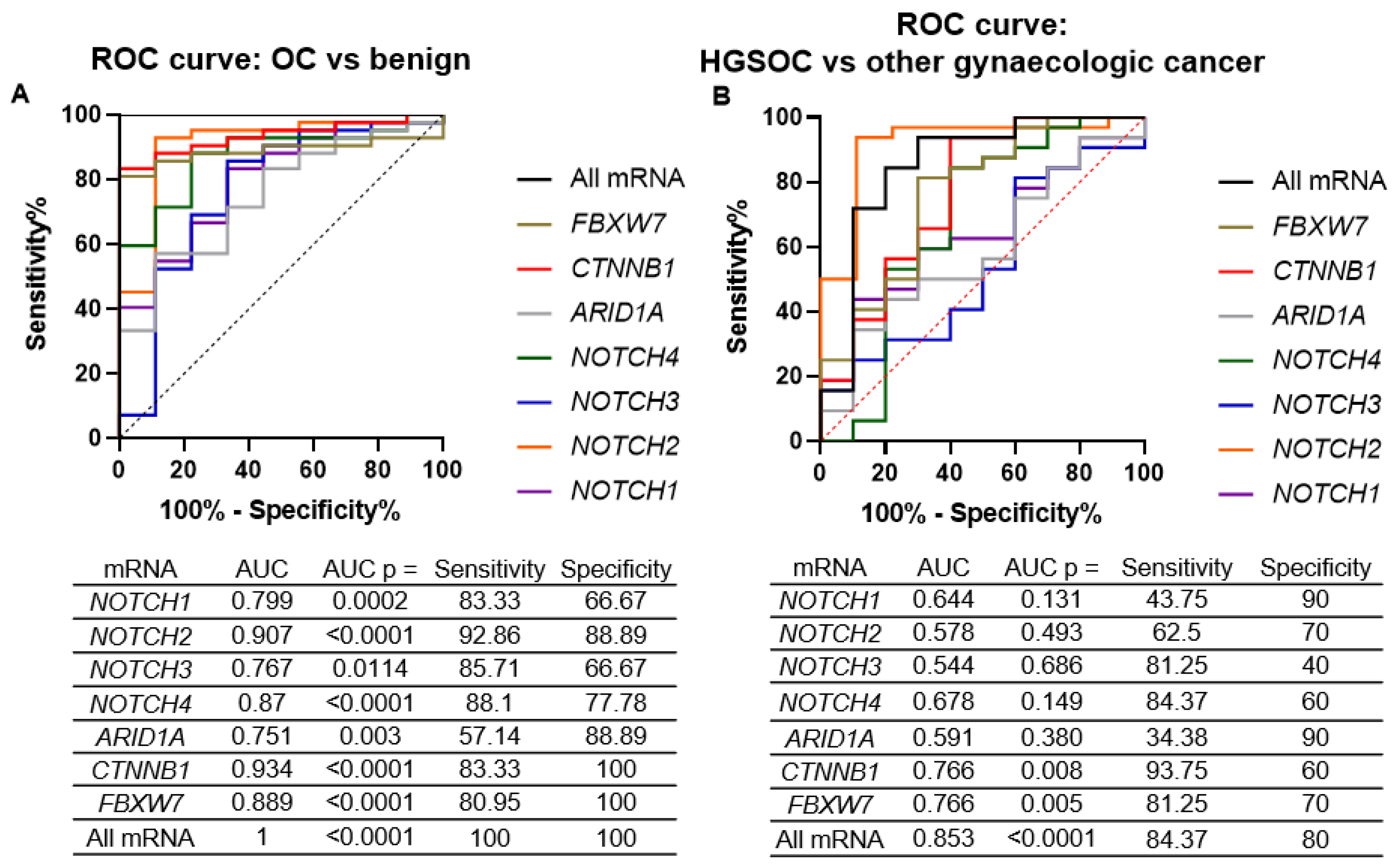
2.2. ARID1A, NOTCH/WNT Pathway Component mRNA Expression in Gynaecological Cancer Tissues
2.3. ARID1A and WNT Pathway Gene Mutations in Gynaecologic Cancer Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Patient Cohort and Sample Collection
4.3. Nucleic Acid Extraction
4.4. cDNA Synthesis and Quantitative PCR
4.5. Targeted Next-Generation Sequencing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OC | Ovarian Cancer |
| EC | Endometrial Cancer |
| HGSOC | High-grade serous ovarian cancer |
| RSS | Risk reducing surgery |
Appendix A
| Gene | Reference Sequence, NCBI | Primer | Primer Sequence |
|---|---|---|---|
| FBXW7 | NM_001013415.2 | Forward 3→5 | GTGATAGAACCCCAGTTTCA |
| Reverse 3→5 | CTTCAGCCAAAATTCTCCAG | ||
| ARID1A | NM_006015.6 | Forward 3→5 | CAGTAAGGGAGGGCAAGAAG |
| Reverse 3→5 | GAGGAGAGAAAGGAGACTGA | ||
| CTNNB1 | NM_001904.4 | Forward 3→5 | TCTGAGGACAAGCCACAAGATTACA |
| Reverse 3→5 | TGGGCACCAATATCAAGTCCAA | ||
| NOTCH1 | NM_017617.5 | Forward 3→5 | CAGCCTCAACATCCCCTACAAG |
| Reverse 3→5 | GCAGCCCACGAAGAACAGAA | ||
| NOTCH2 | NM_024408.4 | Forward 3→5 | GTGGATGGGGTCAACACTTACA |
| Reverse 3→5 | CACTCCAGCCGTTGACACATAC | ||
| NOTCH3 | NM_000435.3 | Forward 3→5 | CGTGGCTTCTTTCTACTGTGC |
| Reverse 3→5 | CGTTCACCGGATTTGTGTCAC | ||
| NOTCH4 | NM_004557.4 | Forward 3→5 | AACTCCTCCCCAGGAATCTG |
| Reverse 3→5 | CCTCCATCCAGCAGAGGTT | ||
| GAPDH | NC_000012.12 | Forward 3→5 | GAAGGTCGGAGTCAACGGATTT |
| Reverse 3→5 | ATGGGTGGAATCATATTGGAAC |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Subtypes of Ovarian Cancer and Ovarian Cancer Screening. Diagnostics 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; Bois, A.D.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Caumanns, J.J.; Wisman, G.B.A.; Berns, K.; van der Zee, A.G.; de Jong, S. ARID1A mutant ovarian clear cell carcinoma: A clear target for synthetic lethal strategies. Biochim. Biophys. Acta-Rev. Cancer 2018, 1870, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chen, X.; Su, C.; Ren, S.; Zhou, C. Pan-cancer analysis of ARID1A Alterations as Biomarkers for Immunotherapy Outcomes. J. Cancer 2020, 11, 776–780. [Google Scholar] [CrossRef]
- Reske, J.J.; Wilson, M.R.; Holladay, J.; Siwicki, R.A.; Skalski, H.; Harkins, S.; Adams, M.; Risinger, J.I.; Hostetter, G.; Lin, K.; et al. Co-existing TP53 and ARID1A mutations promote aggressive endometrial tumorigenesis. PLoS Genet. 2021, 17, e1009986. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, M.; Anusewicz, D.; Bednarek, A.K. Functional Gene Expression Differentiation of the Notch Signaling Pathway in Female Reproductive Tract Tissues—A Comprehensive Review With Analysis. Front. Cell Dev. Biol. 2020, 8, 592616. [Google Scholar] [CrossRef]
- Chen, X.; Stoeck, A.; Lee, S.J.; Shih, I.M.; Wang, M.M.; Wang, T.L. Jagged1 expression regulated by Notch3 and Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget 2010, 1, 210–218. [Google Scholar] [CrossRef]
- Thompson, J.J.; Williams, C.S. Protein Phosphatase 2A in the Regulation of Wnt Signaling, Stem Cells, and Cancer. Genes 2018, 9, 121. [Google Scholar] [CrossRef]
- Spaans, V.M.; Trietsch, M.D.; Crobach, S.; Stelloo, E.; Kremer, D.; Osse, E.M.; Haar, N.T.T.; Eijk, R.V.; Muller, S.; Wezel, T.V.; et al. Designing a high-throughput somatic mutation profiling panel specifically for gynaecological cancers. PLoS ONE 2014, 9, e93451. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.E.; Miele, L.; Fazleabas, A.T. Notch signaling in reproduction. Trends Endocrinol. Metab. TEM 2021, 32, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Hallberg, D.; Konecny, G.E.; Bruhm, D.C.; Adleff, V.; Noë, M.; Kagiampakis, I.; Palsgrove, D.; Conklin, D.; Kinose, Y.; et al. Integrated Genomic, Epigenomic, and Expression Analyses of Ovarian Cancer Cell Lines. Cell Rep. 2018, 25, 2617–2633. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.; Wang, L.; Xin, X. Down-regulation of Notch1 by gamma-secretase inhibition contributes to cell growth inhibition and apoptosis in ovarian cancer cells A2780. Biochem. Biophys. Res. Commun. 2010, 393, 144–149. [Google Scholar] [CrossRef]
- McAuliffe, S.M.; Morgan, S.L.; Wyant, G.A.; Tran, L.T.; Muto, K.W.; Chen, Y.S.; Chin, K.T.; Partridge, J.C.; Poole, B.B.; Cheng, K.H.; et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E2939–E2948. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, T.; Ivan, C.; Sun, Y.; Huang, J.; Mangala, L.S.; Miyake, T.; Dalton, H.J.; Pradeep, S.; Rupaimoole, R.; et al. Notch3 pathway alterations in ovarian cancer. Cancer Res. 2014, 74, 3282–3293. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhuang, L.; Wang, X.; Li, Q.; Sang, Y.; Xu, J. FBXW7γ is a tumor-suppressive and prognosis-related FBXW7 transcript isoform in ovarian serous cystadenocarcinoma. Future Oncol. 2020, 16, 1921–1930. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Kitade, S.; Onoyama, I.; Kobayashi, H.; Yagi, H.; Yoshida, S.; Kato, M.; Tsunematsu, R.; Asanoma, K.; Sonoda, K.; Wake, N.; et al. FBXW7 is involved in the acquisition of the malignant phenotype in epithelial ovarian tumors. Cancer Sci. 2016, 107, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Langer, R.; Becker, K.; Hapfelmeier, A.; Ott, K.; Novotny, A.; Höfler, H.; Keller, G. Expression profiling of stem cell-related genes in neoadjuvant-treated gastric cancer: A NOTCH2, GSK3B and β-catenin gene signature predicts survival. PLoS ONE 2012, 7, e44566. [Google Scholar] [CrossRef]
- Abravanel, D.L.; Belka, G.K.; Pan, T.C.; Pant, D.K.; Collins, M.A.; Sterner, C.J.; Chodosh, L.A. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J. Clin. Investig. 2015, 125, 2484–2496. [Google Scholar] [CrossRef]
- Zyla, R.E.; Olkhov-Mitsel, E.; Amemiya, Y.; Bassiouny, D.; Seth, A.; Djordjevic, B.; Nofech-Mozes, S.; Parra-Herran, C. CTNNB1 Mutations and Aberrant β-Catenin Expression in Ovarian Endometrioid Carcinoma: Correlation With Patient Outcome. Am. J. Surg. Pathol. 2021, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kurnit, K.C.; Djordjevic, B.; Singh, C.; Munsell, M.F.; Wang, W.L.; Lazar, A.J.; Zhang, W.; Broaddus, R. Nuclear β-catenin localization and mutation of the CTNNB1 gene: A context-dependent association. Mod. Pathol. 2018, 31, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Sallum, L.F.; Andrade, L.; Costa, L.B.E.D.; Ramalho, S.; Ferracini, A.C.; Natal, R.D.A.; Brito, A.B.C.; Sarian, L.O.; Derchain, S. BRCA1, Ki67, and β-Catenin Immunoexpression Is Not Related to Differentiation, Platinum Response, or Prognosis in Women With Low- and High-Grade Serous Ovarian Carcinoma. Int. J. Gynecol. Cancer 2018, 28, 437–447. [Google Scholar] [CrossRef]
- Taylor, S.E.; O’Connor, C.M.; Wang, Z.; Shen, G.; Song, H.; Leonard, D.; Sangodkar, J.; LaVasseur, C.; Avril, S.; Waggoner, S.; et al. The highly recurrent PP2A Aa-subunit mutation P179R alters protein structure and impairs PP2A enzyme function to promote endometrial tumorigenesis. Cancer Res. 2019, 79, 4242–4257. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.D.; Ravegnini, G.; Musiani, F.; Maloberti, T.; Visani, M.; Sanza, V.; Angelini, S.; Perrone, A.M.; Iaco, P.D.; Corradini, A.G.; et al. Relevance of ARID1A Mutations in Endometrial Carcinomas. Diagnostics 2022, 12, 592. [Google Scholar] [CrossRef]
- Lapke, N.; Chen, C.H.; Chang, T.C.; Chao, A.; Lu, Y.J.; Lai, C.H.; Tan, K.T.; Chen, H.C.; Lu, H.Y.; Chen, S.J. Genetic alterations and their therapeutic implications in epithelial ovarian cancer. BMC Cancer 2021, 21, 499. [Google Scholar] [CrossRef]
- Katagiri, A.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Katagiri, H.; Nakayama, N.; Ishikawa, M.; Ishibashi, T.; Iida, K.; Kobayashi, H.; et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod. Pathol. 2012, 25, 282–288. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsushita, Y.; Shigeto, T.; Futagami, M.; Mizunuma, H. Decreased ARID1A expression is correlated with chemoresistance in epithelial ovarian cancer. J. Gynecol. Oncol. 2014, 25, 58–63. [Google Scholar] [CrossRef]
- Guan, B.; Rahmanto, Y.S.; Wu, R.C.; Wang, Y.; Wang, Z.; Wang, T.L.; Shih, I.M. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J. Natl. Cancer Inst. 2014, 106, dju146. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Hennessy, B.T.; Leung, S.; Wang, Y.; Ju, Z.; McGahren, M.; Kalloger, S.E.; Finlayson, S.; Stemke-Hale, K.; Lu, Y.; et al. A functional proteogenomic analysis of endometrioid and clear cell carcinomas using reverse phase protein array and mutation analysis: Protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation. BMC Cancer 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.M.; Nelson, L.; Tighe, A.; Burghel, G.J.; Lin, I.H.; Desai, S.; McGrail, J.C.; Morgan, R.D.; Taylor, S.S. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2018, 35, 1978–1980. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.; Zhang, K.; Zhang, Y.; Hu, S.; Zhao, T.; Teng, H.; Li, X.; Jiang, Y.; Ji, L.; et al. VarCards: An integrated genetic and clinical database for coding variants in the human genome. Nucleic Acids Res. 2018, 46, D1039–D1048. [Google Scholar] [CrossRef]

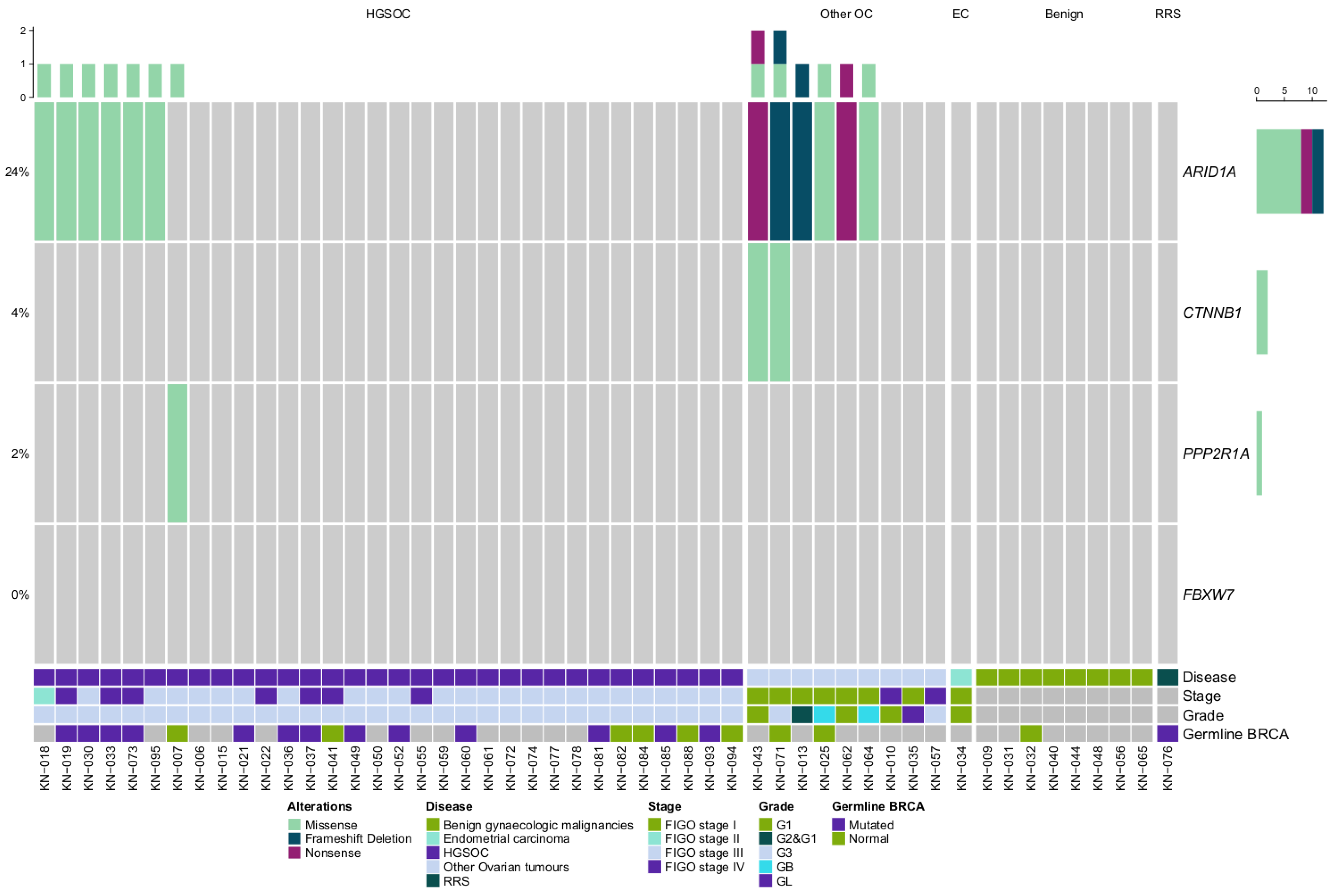
| No. | Samples | Histology/Disease Type | Locus | Gene | Coding Sequence | Amino Acid Change | Variant Effect | ClinVar | dbSNP | VarSome Verdict | VarCards “Damaging Score” |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | KN-007 | HGSOC | chr19:52715971 | PPP2R1A | c.536C>G | p.Pro179Arg | missense | Likely pathogenic | rs786205228 | Likely pathogenic | 0.83 |
| 2 | KN-043 | OC and EC | chr3:41266104 | CTNNB1 | c.101G>A | p.Gly34Glu | missense | Pathogenic/ Likely pathogenic | rs28931589 | Pathogenic | 0.83 |
| 3 | KN-043 | OC and EC | chr1:27106364 | ARID1A | c.5975C>A | p.Ser1992Ter | nonsense | Pathogenic | 1 | ||
| 4 | KN-071 | Clear-cell OC | chr3:41266113 | CTNNB1 | c.110C>G | p.Ser37Cys | missense | Pathogenic/ Likely pathogenic | rs121913403 | Pathogenic | 0.83 |
| 5 | KN-071 | Clear-cell OC | chr1:27057961 | ARID1A | c.1670_1674delAGTCT | p.Gln557ProfsTer64 | frameshift deletion | Likely pathogenic | - | ||
| 6 | KN-013 | OC and EC | chr1:27099367 | ARID1A | c.3606delG | p.Asn1203IlefsTer3 | frameshift deletion | Pathogenic | - | ||
| 7 | KN-018 | HGSOC | chr1:27099478 | ARID1A | c.3715G>A | p.Ala1239Thr | missense | Likely pathogenic | 0.64 | ||
| 8 | KN-073 | HGSOC | chr1:27023961 | ARID1A | c.1067G>A | p.Arg356Lys | missense | Likely benign | 0.52 | ||
| 9 | KN-019 | HGSOC | chr1:27106228 | ARID1A | c.5839C>G | p.Gln1947Glu | missense | Likely benign | 0.3 | ||
| 10 | KN-033 | HGSOC | chr1:27101532 | ARID1A | c.4814C>T | p.Pro1605Leu | missense | rs375431469 | VUS | 0.61 | |
| 11 | KN-064 | Serous borderline | |||||||||
| 12 | KN-025 | Mucinous borderline | chr1:27089762 | ARID1A | c.2718C>G | p.Asn906Lys | missense | Uncertain significance | rs201864573 | Benign | 0.65 |
| 13 | KN-095 | HGSOC | |||||||||
| 14 | KN-062 | Mucinous borderline | chr1:27101504 | ARID1A | c.4786G>T | p.Glu1596Ter | nonsense | Pathogenic | 1 | ||
| 15 | KN-030 | HGSOC | chr1:27105686 | ARID1A | c.5297A>T | p.Glu1766Val | missense | rs1363371199 | Likely benign | 0.39 |
| Clinical/Pathological Characteristics | Number of Patients (%) | |||
|---|---|---|---|---|
| Disease Group | HGSOC | Other Gynaecologic Cancers | Benign Gynaecologic Tumour | Overall |
| n = | 32 | 10 | 9 | 51 |
| Average Age, years (min–max) | 57.8 (41–82) | 63.7(49–77) | 53.6 (43–72) | 58.3 (41–82) |
| Average CA125 pre op. concentration U/mL (N/A) | 888.5 (1 N/A) | 139.4 (3 N/A) | 51.35 (2 N/A) | 641.8 (6 N/A) |
| FIGO Stage | ||||
| IA | 8 (80.0) | 8 (15.6) | ||
| IIB | 1 (3.1) | 1 (2.0) | ||
| IIIB | 2 (6.3) | 2 (4.0) | ||
| IIIC | 17 (53.1) | 17 (33.3) | ||
| IVB | 12 (37.5) | 2 (20.0) | 14 (27.5) | |
| N/A 1 | 9 (100.0) | 9 (17.6) | ||
| Tumour differentiation grade | ||||
| G1 | 4 (40.0) | 4 (7.8) | ||
| G2 | 1 (10.0) | 1 (2.0) | ||
| G3 | 32 (100.0) | 2 (20.0) | 4 (66.7) | |
| GB/GL 2 | 3 (30.0) | 3 (5.9) | ||
| N/A1 | 9 (100.0) | 9 (17.6) | ||
| Progressed disease | ||||
| Yes | 12 (37.5) | 1 (10.0) | 13 (25.5) | |
| No | 20 (51.4) | 9 (90.0) | 9 (100.0) | 38 (74.5) |
| Radical disease after surgery | ||||
| R0 | 24 (47.1) | 8 (80.0) | 9 (100.0) | 41 (80.3) |
| R1 | 6 (18.8) | 2 (20.0) | 8 (15.7) | |
| R3 | 1 (3.1) | 1 (2.0) | ||
| N/A 3 | 1 (3.1) | 1 (2.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaicekauskaitė, I.; Dabkevičienė, D.; Šimienė, J.; Žilovič, D.; Čiurlienė, R.; Jarmalaitė, S.; Sabaliauskaitė, R. ARID1A, NOTCH and WNT Signature in Gynaecological Tumours. Int. J. Mol. Sci. 2023, 24, 5854. https://doi.org/10.3390/ijms24065854
Vaicekauskaitė I, Dabkevičienė D, Šimienė J, Žilovič D, Čiurlienė R, Jarmalaitė S, Sabaliauskaitė R. ARID1A, NOTCH and WNT Signature in Gynaecological Tumours. International Journal of Molecular Sciences. 2023; 24(6):5854. https://doi.org/10.3390/ijms24065854
Chicago/Turabian StyleVaicekauskaitė, Ieva, Daiva Dabkevičienė, Julija Šimienė, Diana Žilovič, Rūta Čiurlienė, Sonata Jarmalaitė, and Rasa Sabaliauskaitė. 2023. "ARID1A, NOTCH and WNT Signature in Gynaecological Tumours" International Journal of Molecular Sciences 24, no. 6: 5854. https://doi.org/10.3390/ijms24065854
APA StyleVaicekauskaitė, I., Dabkevičienė, D., Šimienė, J., Žilovič, D., Čiurlienė, R., Jarmalaitė, S., & Sabaliauskaitė, R. (2023). ARID1A, NOTCH and WNT Signature in Gynaecological Tumours. International Journal of Molecular Sciences, 24(6), 5854. https://doi.org/10.3390/ijms24065854





