NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol
Abstract
:1. Introduction
2. Results
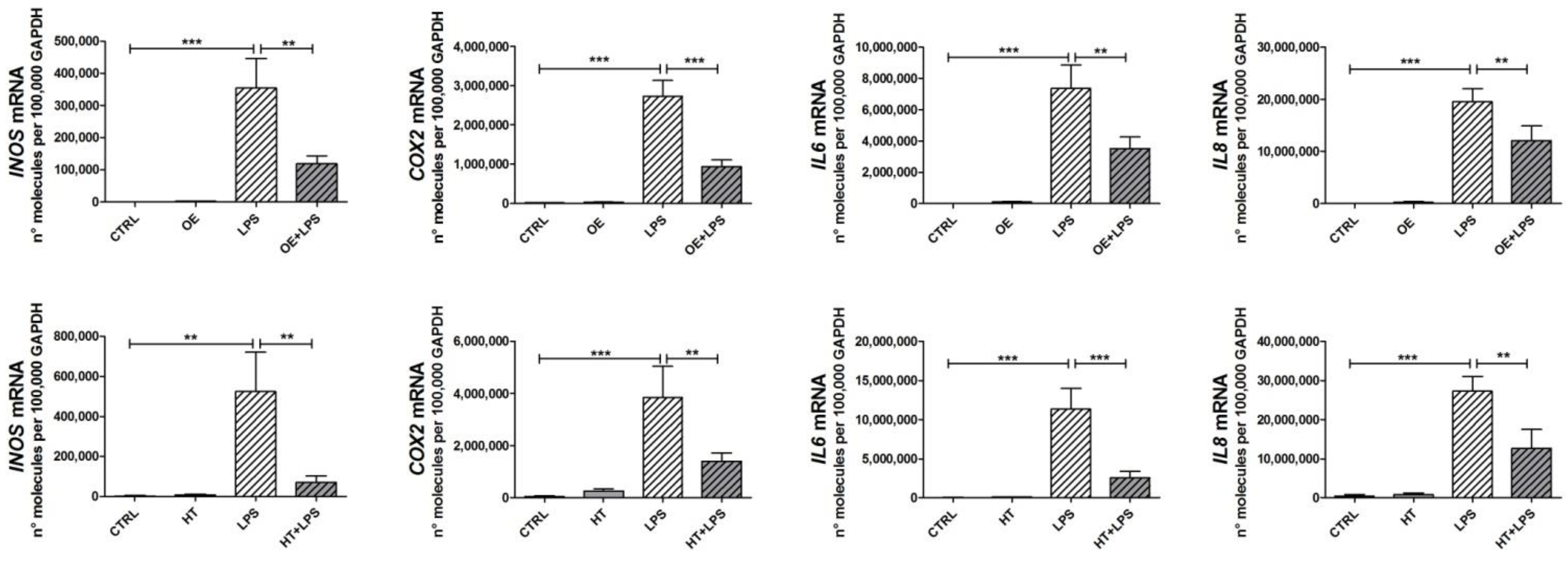
2.1. LPS Induces the Transcription of Inflammatory Genes in Both Primary Chondrocytes and C-28/I2 Cells
2.2. OE and HT Show Antioxidant Activities against LPS-Induced ROS Production in C28/I2 Cells
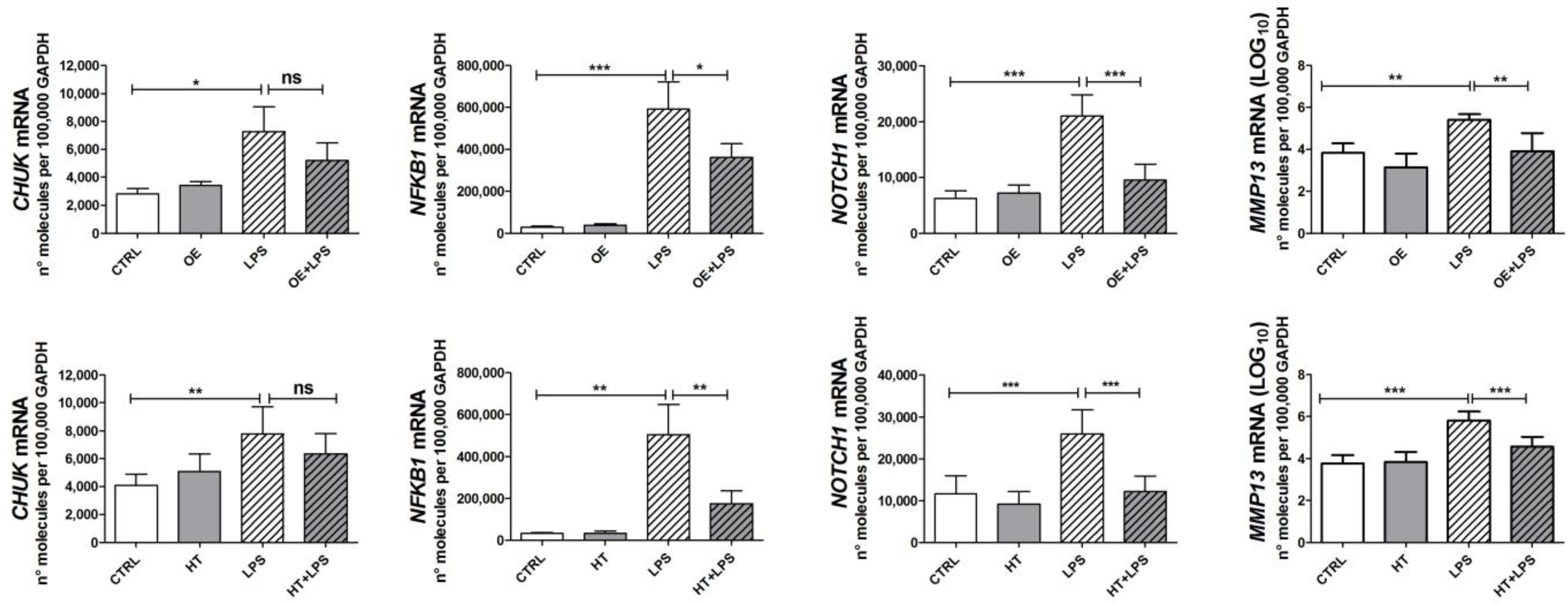
2.3. OE and HT Prevent LPS-Mediated Induction of Inflammatory and Catabolic Genes following LPS Exposure in Chondrocytes
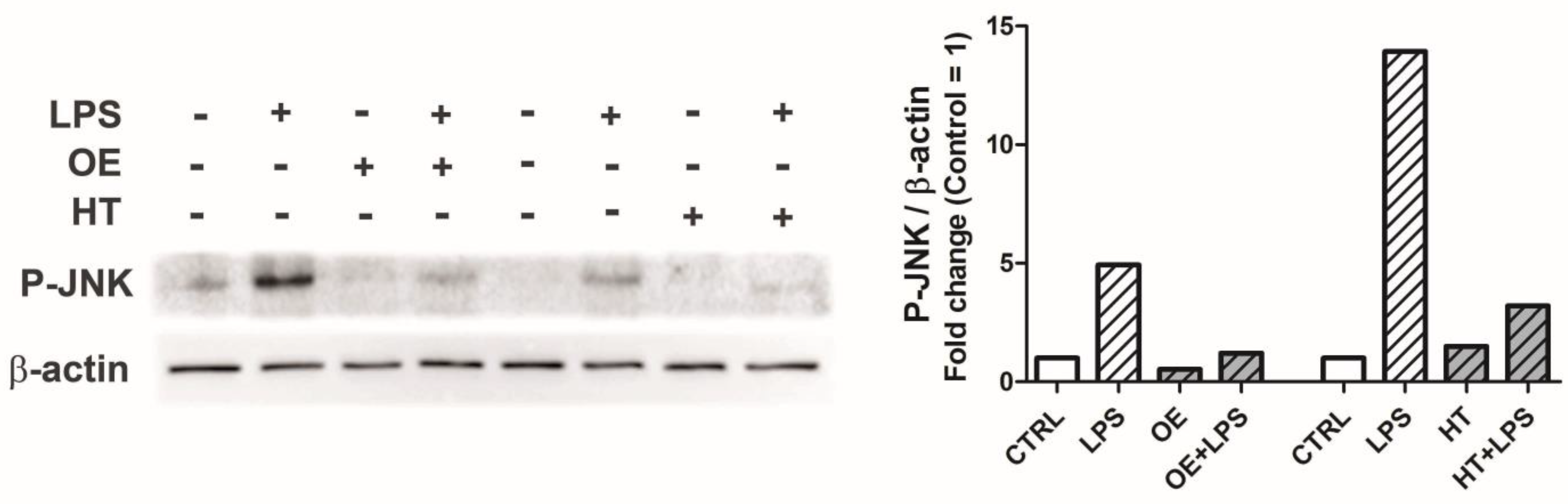
2.4. OE and HT Prevent JNK Activation following LPS Exposure in Chondrocytes
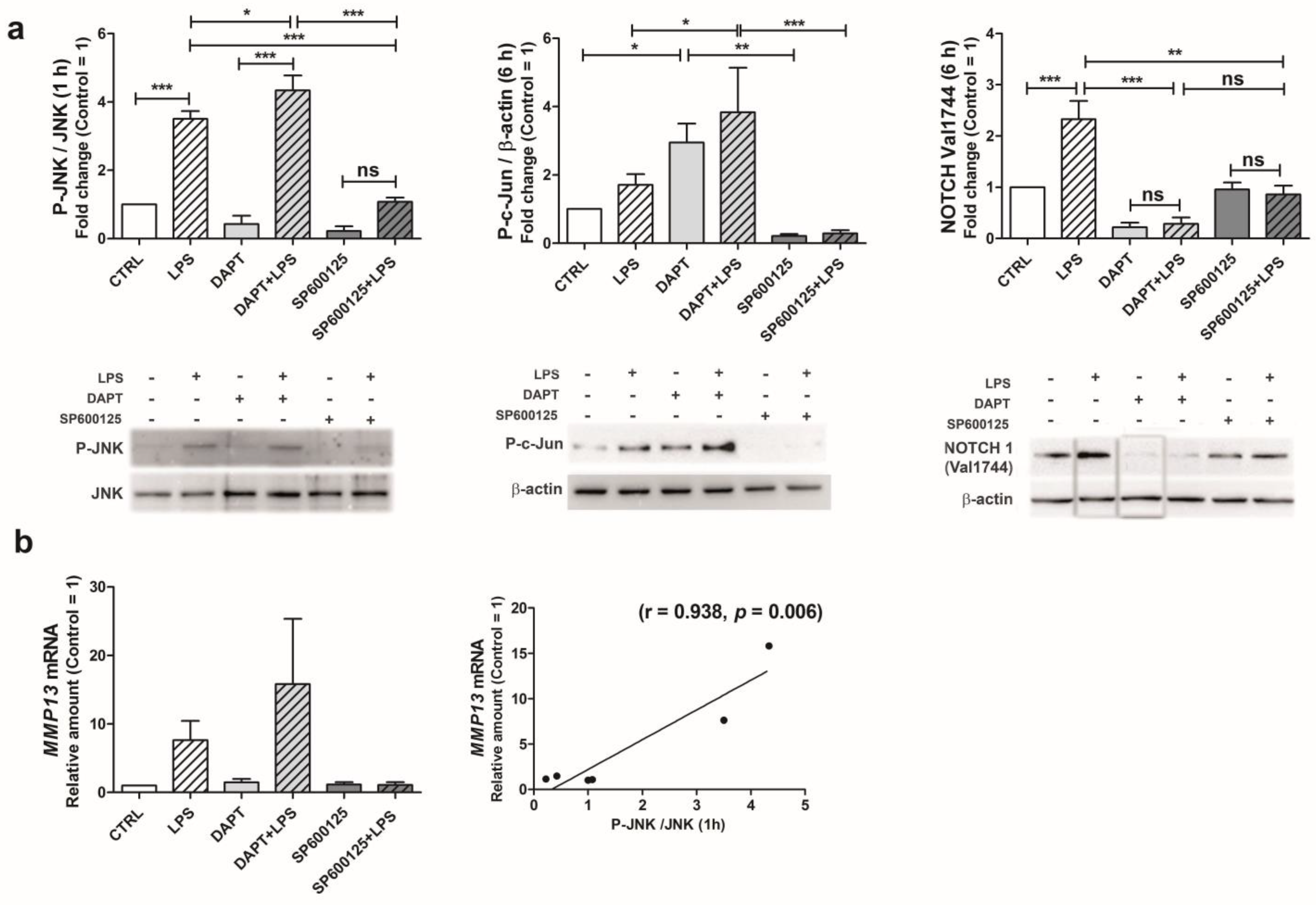
2.5. Following LPS Exposure, JNK Activation Triggers NOTCH1 Pathway Leading to Increased MMP-13 Gene Transcription
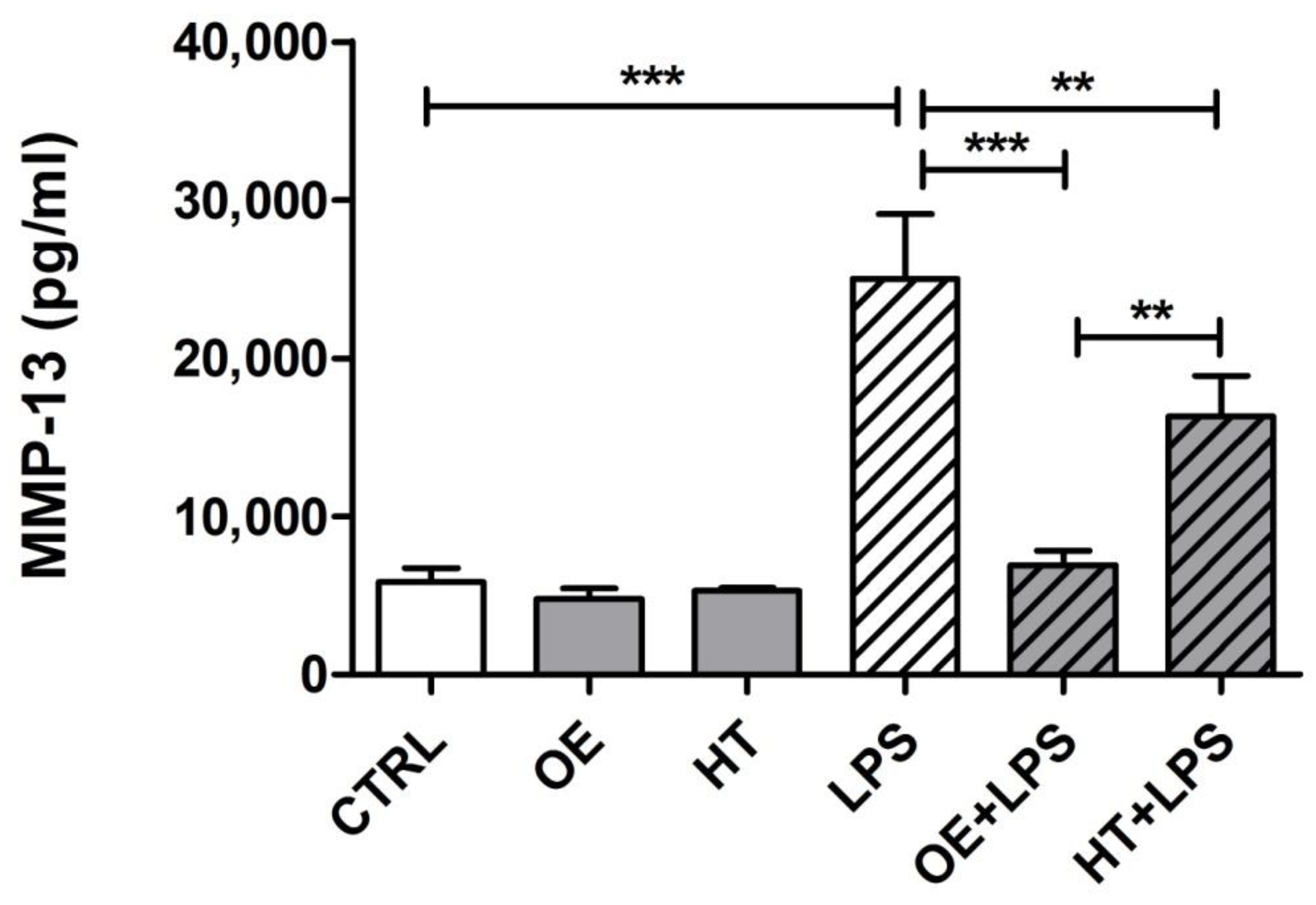
2.6. OE/HT Pretreatment of Both C28/I2 and Primary Chondrocytes Inhibits MMP-13 Synthesis and Release following LPS Exposure
3. Discussion
4. Materials and Methods
4.1. Cells Isolation and Treatment
4.2. Western Blot
4.3. DCHF-DA Cellular ROS Assay
4.4. Real-Time RT-PCR Analysis
4.5. ELISA Assay for MMP-13 Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Kraan, P.M.; van den Berg, W.B. Osteoarthritis in the context of ageing and evolution. Loss of chondrocyte differentiation block during ageing. Ageing Res. Rev. 2008, 7, 106–113. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzi, R.M. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free. Radic. Biol. Med. 2020, 153, 159–172. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzi, R.M. Nutraceutical activity in osteoarthritis biology: A focus on the nutrigenomic role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A lancet commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzi, R.M. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxid. Med. Cell. Longev. 2018, 2018, 3075293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Mobasheri, A.; Saarakkala, S.; Finnila, M.; Karsdal, M.A.; Bay-Jensen, A.C.; van Spil, W.E. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 2019, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the nu-age project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Pang, K.L. Therapeutic effects of olive and its derivatives on osteoarthritis: From bench to bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the healthy brand of olive oil: Insights and perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Finger, F.; Schorle, C.; Zien, A.; Gebhard, P.; Goldring, M.B.; Aigner, T. Molecular phenotyping of human chondrocyte cell lines t/c-28a2, t/c-28a4, and c-28/i2. Arthritis Rheum. 2003, 48, 3395–3403. [Google Scholar] [CrossRef]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in oa? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [Green Version]
- Barreto, G.; Manninen, M.; Eklund, K.K. Osteoarthritis and toll-like receptors: When innate immunity meets chondrocyte apoptosis. Biology 2020, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Gomez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. Tlr4 signalling in osteoarthritis--finding targets for candidate dmoads. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mapk signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [Green Version]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (mmp-1, mmp-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon mmp-13 regulation in osteoarthritis. Eur. Cell. Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Borzi, R.M.; Olivotto, E.; Pagani, S.; Vitellozzi, R.; Neri, S.; Battistelli, M.; Falcieri, E.; Facchini, A.; Flamigni, F.; Penzo, M.; et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010, 62, 2370–2381. [Google Scholar] [CrossRef] [Green Version]
- Mengshol, J.A.; Vincenti, M.P.; Brinckerhoff, C.E. Il-1 induces collagenase-3 (mmp-13) promoter activity in stably transfected chondrocytic cells: Requirement for runx-2 and activation by p38 mapk and jnk pathways. Nucleic Acids Res. 2001, 29, 4361–4372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minguzzi, M.; Panichi, V.; D’Adamo, S.; Cetrullo, S.; Cattini, L.; Flamigni, F.; Mariani, E.; Borzi, R.M. Pleiotropic roles of notch1 signaling in the loss of maturational arrest of human osteoarthritic chondrocytes. Int. J. Mol. Sci. 2021, 22, 12012. [Google Scholar] [CrossRef]
- Xiao, D.; Bi, R.; Liu, X.; Mei, J.; Jiang, N.; Zhu, S. Notch signaling regulates mmp-13 expression via runx2 in chondrocytes. Sci. Rep. 2019, 9, 15596. [Google Scholar] [CrossRef] [Green Version]
- Tsao, P.N.; Wei, S.C.; Huang, M.T.; Lee, M.C.; Chou, H.C.; Chen, C.Y.; Hsieh, W.S. Lipopolysaccharide-induced notch signaling activation through jnk-dependent pathway regulates inflammatory response. J. Biomed. Sci. 2011, 18, 56. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of inos in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 2003, 33, 155–167. [Google Scholar] [CrossRef]
- Tiku, M.L.; Gupta, S.; Deshmukh, D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free. Radic. Res. 1999, 30, 395–405. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The nf-kappab family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ros activate mapk pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Tanaka, S. Molecular mechanisms underlying osteoarthritis development: Notch and nf-kappab. Arthritis Res. Ther. 2017, 19, 94. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Madonna, R.; Melino, G.; Caruso, C. The emerging role of notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res. Rev. 2016, 29, 50–65. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. Nf-kappab signaling: Multiple angles to target oa. Curr. Drug. Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, M.J.; Kim, K.J.; Yun, H.J.; Chae, J.S.; Hwang, S.G.; Chang, T.S.; Park, H.S.; Lee, K.W.; Han, P.L.; et al. Notch interferes with the scaffold function of jnk-interacting protein 1 to inhibit the jnk signaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14308–14313. [Google Scholar] [CrossRef] [Green Version]
- Ulivi, V.; Tutolo, G.; Mallein-Gerin, F.; Daga, A.; Cancedda, R.; Cancedda, F.D. A common pathway in differentiation and inflammation: P38 mediates expression of the acute phase sip24 iron binding lipocalin in chondrocytes. J. Cell. Physiol. 2006, 206, 728–737. [Google Scholar] [CrossRef]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Platano, D.; Olivotto, E.; Filardo, G.; Trisolino, G.; Facchini, A.; Borzi, R.M.; Meliconi, R. Human osteoarthritic cartilage shows reduced in vivo expression of il-4, a chondroprotective cytokine that differentially modulates il-1beta-stimulated production of chemokines and matrix-degrading enzymes in vitro. PLoS ONE 2014, 9, e96925. [Google Scholar] [CrossRef] [Green Version]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free. Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Attur, M.G.; Dave, M.; Akamatsu, M.; Katoh, M.; Amin, A.R. Osteoarthritis or osteoarthrosis: The definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthr. Cartil. 2002, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Barthwal, M.K. Il-1 beta genesis: The art of regulating the regulator. Cell. Mol. Immunol. 2018, 15, 998–1000. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, V.; Tiwari, R.L.; Chandra, T.; Kumar, A.; Dikshit, M.; Barthwal, M.K. The irak-erk-p67phox-nox-2 axis mediates tlr4, 2-induced ros production for il-1beta transcription and processing in monocytes. Cell. Mol. Immunol. 2016, 13, 745–763. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, X.; Liu-Bryan, R. Role of tlr2 and tlr4 in regulation of articular chondrocyte homeostasis. Osteoarthr. Cartil. 2020, 28, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell. Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Monsalve, E.; Perez, M.A.; Rubio, A.; Ruiz-Hidalgo, M.J.; Baladron, V.; Garcia-Ramirez, J.J.; Gomez, J.C.; Laborda, J.; Diaz-Guerra, M.J. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J. Immunol. 2006, 176, 5362–5373. [Google Scholar] [CrossRef] [Green Version]
- Yugawa, T.; Handa, K.; Narisawa-Saito, M.; Ohno, S.; Fujita, M.; Kiyono, T. Regulation of notch1 gene expression by p53 in epithelial cells. Mol. Cell. Biol. 2007, 27, 3732–3742. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.J.; Li, J.; Yang, X.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence that mir-146a attenuates aging- and trauma-induced osteoarthritis by inhibiting notch1, il-6, and il-1 mediated catabolism. Aging Cell 2018, 17, e12752. [Google Scholar] [CrossRef]
- Tardif, G.; Pelletier, J.P.; Dupuis, M.; Geng, C.; Cloutier, J.M.; Martel-Pelletier, J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999, 42, 1147–1158. [Google Scholar] [CrossRef]
- Facchini, A.; Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Minguzzi, M.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene alpha. PLoS ONE 2014, 9, e109724. [Google Scholar] [CrossRef]
- Thorpe, J.R.; Wilson, R.A.; Mesiano, S.; Malemud, C.J. Tofacitinib inhibits stat phosphorylation and matrix metalloproteinase-3, -9 and -13 production by c28/i2 human juvenile chondrocytes. Open Access Rheumatol. 2022, 14, 195–209. [Google Scholar] [CrossRef]
- Shalom-Barak, T.; Quach, J.; Lotz, M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and nf-kappab. J. Biol. Chem. 1998, 273, 27467–27473. [Google Scholar] [CrossRef] [Green Version]
- Iacono, A.; Gomez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gomez-Reino, J.J.; Smith, A.B., 3rd; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal inhibits catabolic and inflammatory mediators in lps-activated human primary osteoarthritis (oa) chondrocytes through mapks/nf-kappab pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, M.N.; Beaumont, M.; Sauvageot, N.; Poquet, L.; Saboundjian, M.; Costes, B.; Verdonk, P.; Brands, G.; Brasseur, J.; Urbin-Choffray, D.; et al. An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: Findings from a multicentre-rct and post hoc analysis. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X211070205. [Google Scholar] [CrossRef]
- Varela-Eirin, M.; Carpintero-Fernandez, P.; Sanchez-Temprano, A.; Varela-Vazquez, A.; Paino, C.L.; Casado-Diaz, A.; Calanas-Continente, A.; Mato, V.; Fonseca, E.; Kandouz, M.; et al. Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging (Albany NY) 2020, 12, 15882–15905. [Google Scholar] [CrossRef]
- Otero, M.; Favero, M.; Dragomir, C.; Hachem, K.E.; Hashimoto, K.; Plumb, D.A.; Goldring, M.B. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 2012, 806, 301–336. [Google Scholar]
- Deng, H.; Chilufya, M.M.; Liu, J.; Qiao, L.; Xiao, X.; Zhao, Y.; Guo, Z.; Lv, Y.; Wang, W.; Zhang, J.; et al. Effect of low nutrition and t-2 toxin on c28/i2 chondrocytes cell line and chondroitin sulfate-modifying sulfotransferases. Cartilage 2021, 13, 818S–825S. [Google Scholar] [CrossRef]



| Gene | Forward Primer | Reverse Primer | Amplicon Size (Annealing T) |
|---|---|---|---|
| GAPDH | CGGAGTCAACGGATTTGG | CCTGGAAGATGGTGATGG | 218 bp (60 °C) |
| CHUK(IKKα) | GCACAGAGATGGTGAAAATCATTG | CAACTTGCTCAAATGACCAAACAG | 86 bp (60 °C) |
| NFKB1 | CAGGAGACGTGAAGATGCTG | AGTTGAGAATGAAGGTGGATGA | 109 bp (60 °C) |
| NOTCH1 | CCTGAAGAACGGGGCTAACA | GATGTCCCGGTTGGCAAAGT | 127 bp (60 °C) |
| MMP13 | TCACGATGGCATTGCT | GCCGGTGTAGGTGTAGA | 277 bp (58 °C) |
| INOS | ACATTGATCAGAAGCTGTCCCAC | AAAGGCTGTGAGTCCTGCAC | 235 bp (58 °C) |
| COX2 | CAGCACTTCACGCATCAGTTT | GCGCAGTTTACGCTGTCTA | 129 bp (58 °C) |
| IL6 | TAGTGAGGAACAAGCCAGAG | GCGCAGAATGAGATGAGTTG | 184 bp (60 °C) |
| IL8 | CCAAACCTTTCCACCC | ACTTCTCCACAACCCT | 153 bp (60 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panichi, V.; Bissoli, I.; D’Adamo, S.; Flamigni, F.; Cetrullo, S.; Borzì, R.M. NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 5830. https://doi.org/10.3390/ijms24065830
Panichi V, Bissoli I, D’Adamo S, Flamigni F, Cetrullo S, Borzì RM. NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol. International Journal of Molecular Sciences. 2023; 24(6):5830. https://doi.org/10.3390/ijms24065830
Chicago/Turabian StylePanichi, Veronica, Irene Bissoli, Stefania D’Adamo, Flavio Flamigni, Silvia Cetrullo, and Rosa Maria Borzì. 2023. "NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol" International Journal of Molecular Sciences 24, no. 6: 5830. https://doi.org/10.3390/ijms24065830
APA StylePanichi, V., Bissoli, I., D’Adamo, S., Flamigni, F., Cetrullo, S., & Borzì, R. M. (2023). NOTCH1: A Novel Player in the Molecular Crosstalk Underlying Articular Chondrocyte Protection by Oleuropein and Hydroxytyrosol. International Journal of Molecular Sciences, 24(6), 5830. https://doi.org/10.3390/ijms24065830





