Chondrocyte Isolation from Loose Bodies—An Option for Reducing Donor Site Morbidity for Autologous Chondrocyte Implantation
Abstract
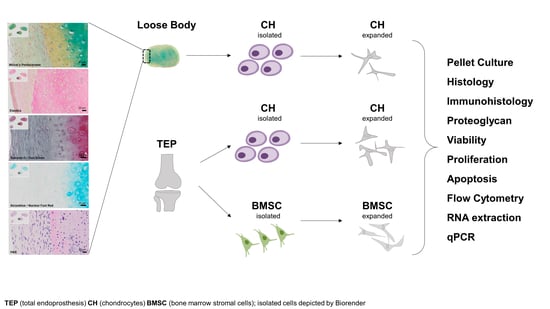
1. Introduction
2. Results
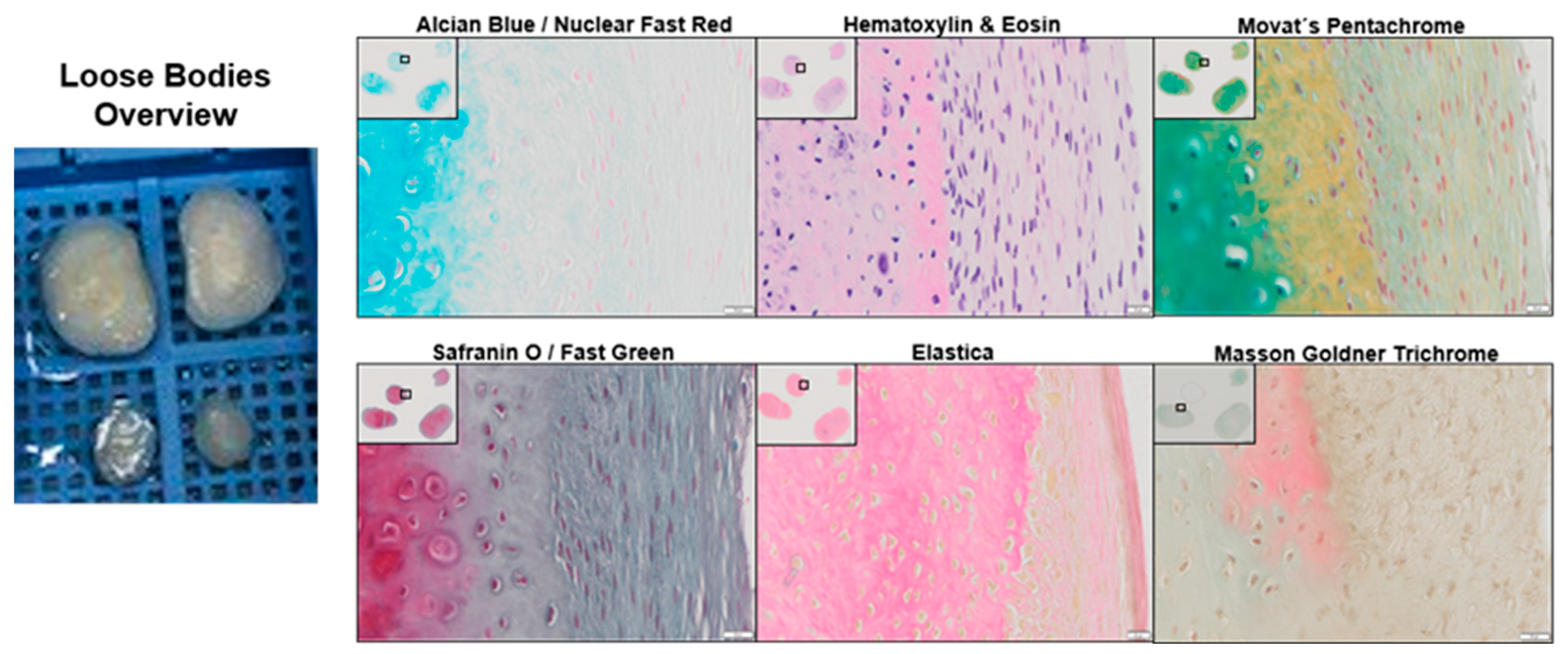
2.1. Histological Analysis of a Loose Body
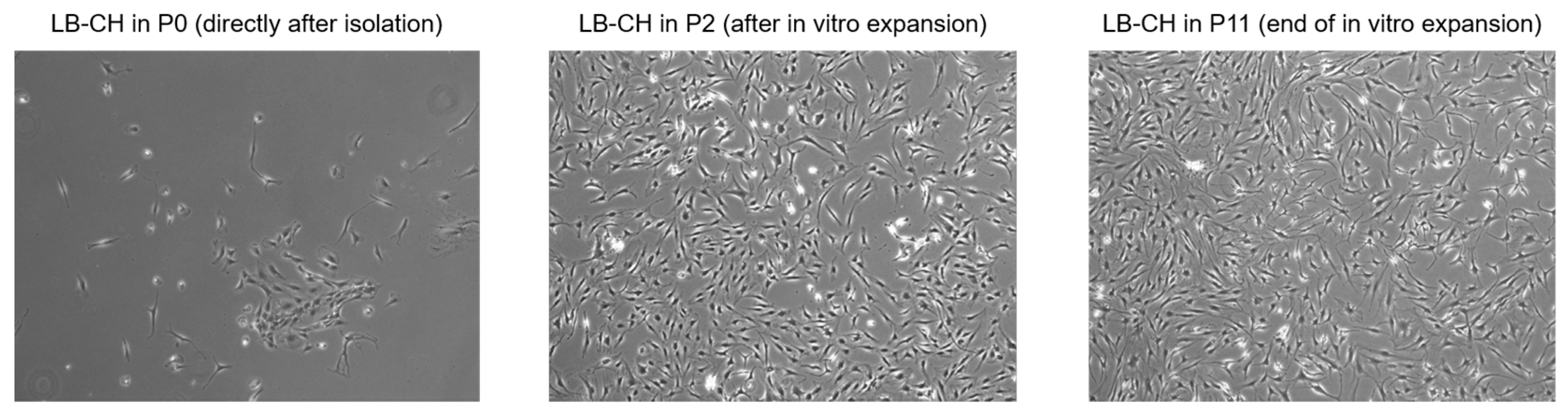
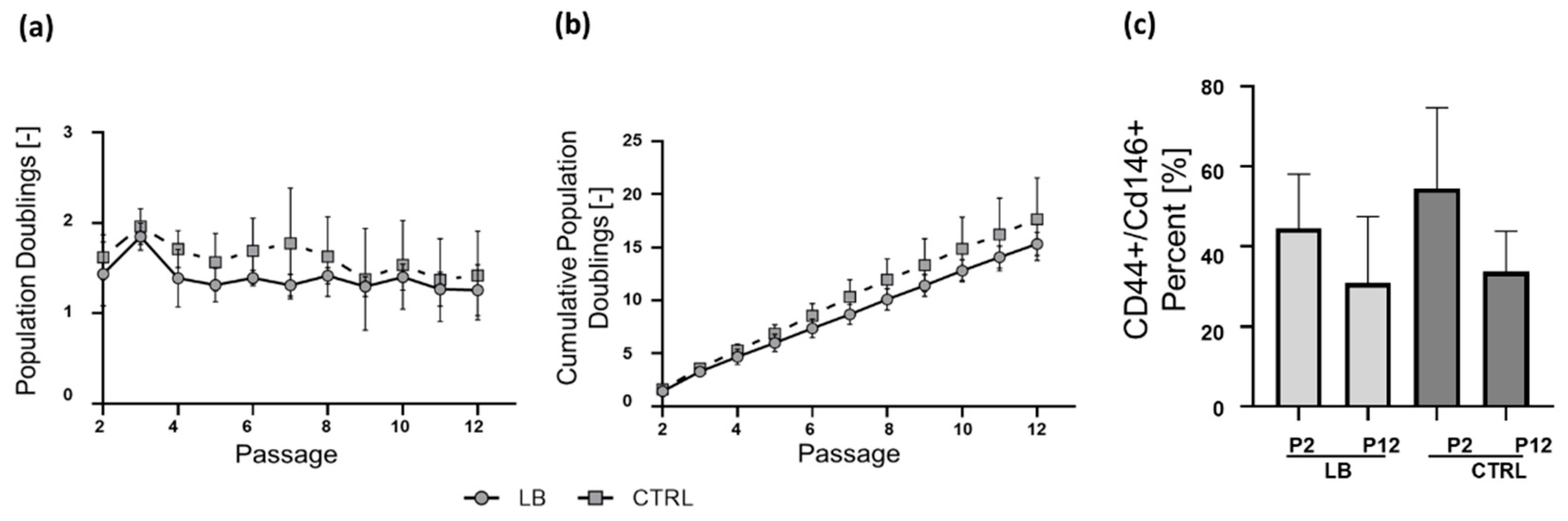
2.2. Isolation and Long-Term Proliferation
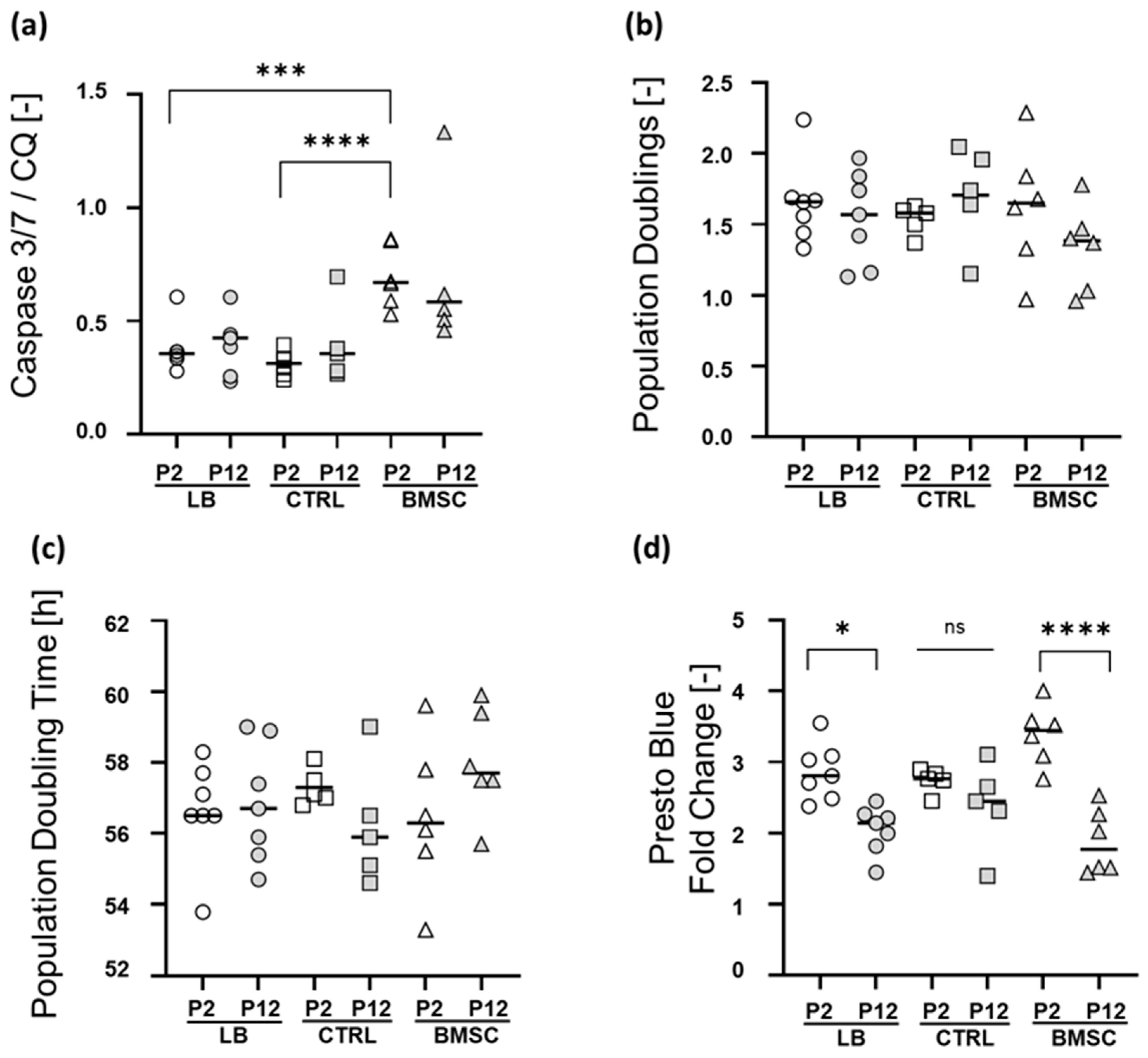
2.3. Apoptosis Rate, Short-Term Proliferation, and Metabolic Activity at Early and Late Passage
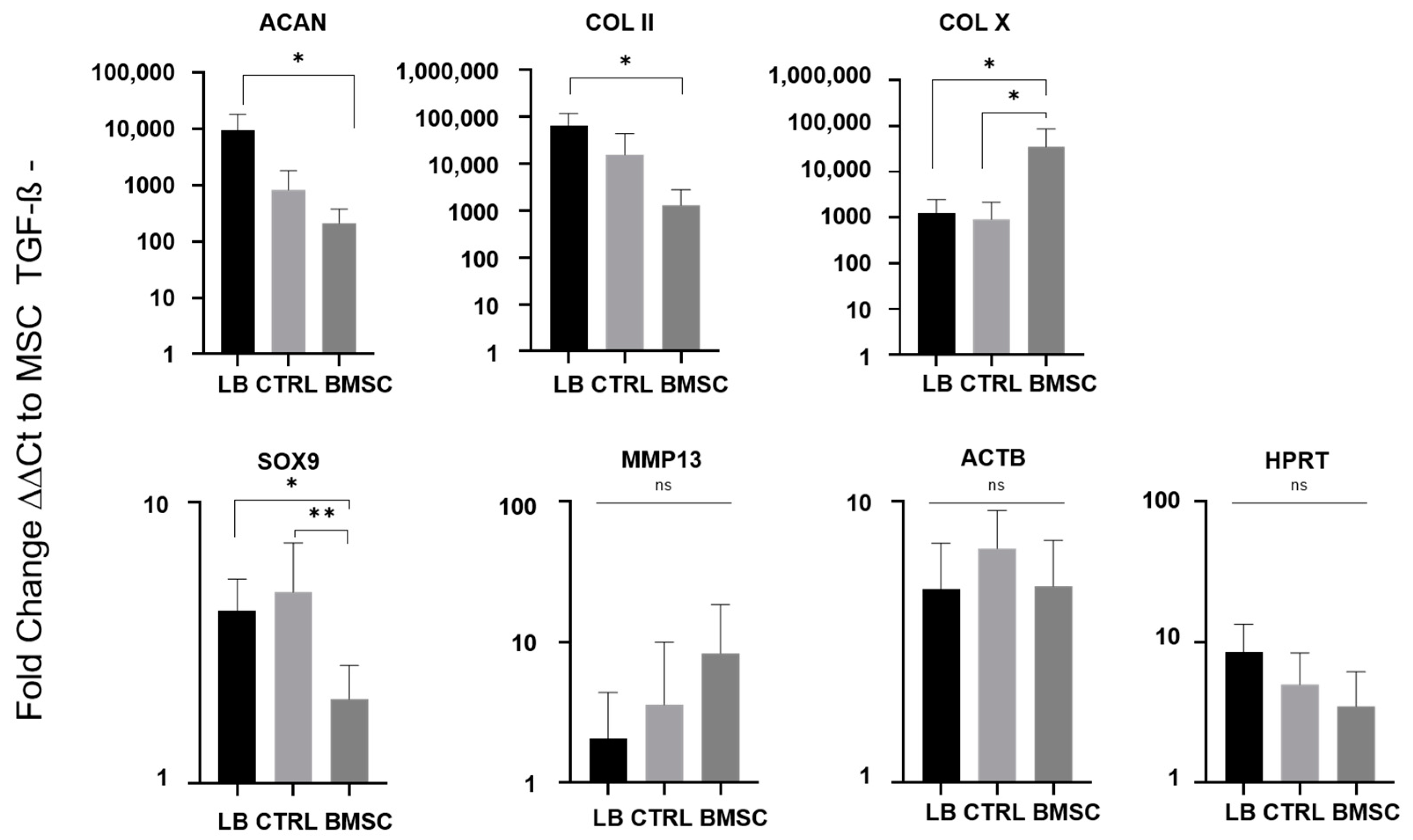
2.4. Chondrogenic Re-Differentiation
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Chondrocyte Isolation and Culture
4.3. BMSC Isolation and Culture
4.4. Chondrogenic Differentiation
4.5. Viability and Proliferation
4.6. RNA Extraction and qPCR
4.7. Proteoglycan Determination
4.8. Apoptosis
4.9. Histology and Immunohistology
4.10. FACS Analysis for CD146 and CD44
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Chondrogenic Genes | |||||||
| Protein | Name | Primer | Sequence | Lenght (bp) | MW | Exon-Exon | Product lenght (bp) |
| Aggrecan core protein | ACAN | Left Primer | GGGTTTTCGTGACTCTGAGG | 20 | 6195 | 2–3 | 154 |
| Right Primer | ATGGGGTCGATGAAATAGCA | 20 | 6230 | ||||
| Collagenase 3 | MMP13 | Left Primer | TTGAGCTGGACTCATTGTCG | 20 | 6159 | 1–2 | 176 |
| Right Primer | TCTCGGAGCCTCTCAGTCAT | 20 | 6022 | ||||
| Transcription factor SOX-9 | SOX9 | Left Primer | GAGGAAGTCGGTGAAGAACG | 20 | 6139 | 2–3 | 276 |
| Right Primer | AAGTCGATAGGGGGCTGTCT | 21 | 6044 | ||||
| Collagen alpha-1(X) chain | COL X | Left Primer | CCCAACACCAAGACACAGTTC | 20 | 6280 | 1–2 | 159 |
| Right Primer | AGGACTTCCGTAGCCTGGTT | 20 | 6213 | ||||
| Collagen alpha-1(II) chain | COL II | Left Primer | CTGGAAAAGATGGTCCCAAA | 20 | 6313 | 42–43 | 219 |
| Right Primer | CAGGGAATCCTCTCTCACCA | 20 | 6124 | ||||
| Housekeeping Genes | |||||||
| Protein | Name | Primer | Sequence | Lenght (bp) | MW | Exon-Exon | Product lenght (bp) |
| Beta-2-microglobulin | B2M | Left Primer | GGTTTCATCCATCCGACATT | 20 | 6043 | 2 | 157 |
| Right Primer | CGGCAGGCATACTCATCTTT | 20 | 6068 | ||||
| Hypoxanthine-guanine phosphoribosyltransferase | HPRT | Left Primer | TATGGACAGGACTGAACGTC | 20 | 6166 | 3–4 | 122 |
| Right Primer | TGATGTAATCCAGCAGGTCA | 20 | 6141 | ||||
| Actin, cytoplasmic 1 | ACTB | Left Primer | GGACTTCGAGCAAGAGATGG | 20 | 6231 | 4–5 | 234 |
| Right Primer | AGCACTGTGTTGGCGTACAG | 20 | 6173 | ||||

References
- Bruns, J.; Werner, M.; Habermann, C. Osteochondritis Dissecans: Etiology, Pathology, and Imaging with a Special Focus on the Knee Joint. Cartilage 2018, 9, 346–362. [Google Scholar] [CrossRef]
- Grimm, N.L.; Weiss, J.M.; Kessler, J.I.; Aoki, S.K. Osteochondritis dissecans of the knee: Pathoanatomy, epidemiology, and diagnosis. Clin. Sports Med. 2014, 33, 181–188. [Google Scholar] [CrossRef]
- Keenan, O.J.; Turner, P.G.; Yeates, D.; Goldacre, M.J. Epidemiology of hospitalised osteochondritis dissecans in young people: Incidence, geographical variation and trends over time in England from 2002 to 2010. Knee 2014, 21, 497–500. [Google Scholar] [CrossRef]
- Kessler, J.I.; Nikizad, H.; Shea, K.G.; Jacobs, J.C., Jr.; Bebchuk, J.D.; Weiss, J.M. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am. J. Sports Med. 2014, 42, 320–326. [Google Scholar] [CrossRef]
- Weiss, J.M.; Nikizad, H.; Shea, K.G.; Gyurdzhyan, S.; Jacobs, J.C.; Cannamela, P.C.; Kessler, J.I. The Incidence of Surgery in Osteochondritis Dissecans in Children and Adolescents. Orthop. J. Sports Med. 2016, 4, 2325967116635515. [Google Scholar] [CrossRef]
- Koenig, F. Über freie Körper in den Gelenken. Dtsch. Z. f. Chir. 1887, 27, 90–109. [Google Scholar] [CrossRef]
- Available online: http://kneeocd.org/patient-education/ocd-knee/ (accessed on 8 March 2022).
- Cahill, B.R. Osteochondritis Dissecans of the Knee: Treatment of Juvenile and Adult Forms. J. Am. Acad. Orthop. Surg. 1995, 3, 237–247. [Google Scholar] [CrossRef]
- Available online: http://cartilage.org/society/publications/icrs-score/ (accessed on 8 March 2022).
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Bosch, U.; Bruns, J.; Erggelet, C.; Esenwein, S.A.; Gaissmaier, C.; Krackhardt, T.; Lohnert, J.; Marlovits, S.; Meenen, N.M.; et al. Indications and implementation of recommendations of the working group “Tissue Regeneration and Tissue Substitutes” for autologous chondrocyte transplantation (ACT). Zeitschrift fur Orthopadie und ihre Grenzgebiete 2004, 142, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Matricali, G.A.; Dereymaeker, G.P.; Luyten, F.P. Donor site morbidity after articular cartilage repair procedures: A review. Acta Orthop. Belg. 2010, 76, 669–674. [Google Scholar] [PubMed]
- Rosa, D.; Balato, G.; Ciaramella, G.; Soscia, E.; Improta, G.; Triassi, M. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J. Orthopaed. Traumatol. 2016, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.T.; Sadr, K.N.; Pulido, P.A.; Bugbee, W.D. The Use of Osteochondral Allograft Transplantation for Primary Treatment of Cartilage Lesions in the Knee. Cartilage 2015, 6, 203–207. [Google Scholar] [CrossRef]
- Melrose, J. The knee joint loose body as a source of viable autologous human chondrocytes. Eur. J. Histochem. 2016, 60, 2645. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Garrido, C.; Tanoira, I.; Muscolo, D.L.; Ayerza, M.A.; Makino, A. Viability of loose body fragments in osteochondritis dissecans of the knee. A series of cases. Int. Orthop. 2010, 34, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Furumatsu, T.; Miyazawa, S.; Okada, Y.; Fujii, M.; Ozaki, T. Comparison between normal and loose fragment chondrocytes in proliferation and redifferentiation potential. Int. Orthop. 2013, 37, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.; Anders, J.; Trommer, T.; Liesaus, E.; Seifert, M.; Schomburg, J.; Rolauffs, B.; Wagner, A.; Mollenhauer, J. Histological and cell biological characterization of dissected cartilage fragments in human osteochondritis dissecans of the femoral condyle. Arch. Orthop. Trauma Surg. 2006, 126, 606–614. [Google Scholar] [CrossRef]
- Aurich, M.; Hofmann, G.O.; Muckley, T.; Mollenhauer, J.; Rolauffs, B. In vitro phenotypic modulation of chondrocytes from knees of patients with osteochondritis dissecans: Implications for chondrocyte implantation procedures. J. Bone Jt. Surg. 2012, 94, 62–67. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/home (accessed on 8 March 2022).
- Reissis, D.; Tang, Q.O.; Cooper, N.C.; Carasco, C.F.; Gamie, Z.; Mantalaris, A.; Tsiridis, E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin. Biol. Ther. 2016, 16, 535–557. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Urlic, I.; Ivkovic, A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Otero, M.; Favero, M.; Dragomir, C.; Hachem, K.E.; Hashimoto, K.; Plumb, D.A.; Goldring, M.B. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 2012, 806, 301–336. [Google Scholar] [CrossRef]
- Fickert, S.; Fiedler, J.; Brenner, R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res. 2004, 6, R422–R432. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zuo, W.; Wu, Z.; Chen, J.; Wu, N.; Ma, P.; Xia, Z.; Jiang, C.; Ye, Z.; Liu, S.; et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015, 33, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Lee, D.A.; Salih, V.; Stockton, E.F.; Stanton, J.S.; Bentley, G. Effect of normal synovial fluid on the metabolism of articular chondrocytes in vitro. Clin. Orthop. Relat. Res. 1997, 342, 228–238. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Zeng, L.; He, D.; Wei, X. Nutrition and degeneration of articular cartilage. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1751–1762. [Google Scholar] [CrossRef]
- Malinin, T.; Ouellette, E.A. Articular cartilage nutrition is mediated by subchondral bone: A long-term autograft study in baboons. Osteoarthr. Cartil. 2000, 8, 483–491. [Google Scholar] [CrossRef]
- Guillen-Garcia, P.; Rodriguez-Inigo, E.; Guillen-Vicente, I.; Guillen-Vicente, M.; Fernandez-Jaen, T.; Concejero, V.; Val, D.; Maestro, A.; Abelow, S.; Lopez-Alcorocho, J.M. Viability of Pathologic Cartilage Fragments as a Source for Autologous Chondrocyte Cultures. Cartilage 2016, 7, 149–156. [Google Scholar] [CrossRef]
- Hoburg, A.; Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuzma, T.; Widuchowski, W.; et al. Sustained superiority in KOOS subscores after matrix-associated chondrocyte implantation using spheroids compared to microfracture. Knee Surg. Sports Traumatol. Arthrosc. 2022. [Google Scholar] [CrossRef]
- do Amaral, R.J.; Pedrosa Cda, S.; Kochem, M.C.; Silva, K.R.; Aniceto, M.; Claudio-da-Silva, C.; Borojevic, R.; Baptista, L.S. Isolation of human nasoseptal chondrogenic cells: A promise for cartilage engineering. Stem Cell Res. 2012, 8, 292–299. [Google Scholar] [CrossRef]
- Grogan, S.P.; Barbero, A.; Diaz-Romero, J.; Cleton-Jansen, A.M.; Soeder, S.; Whiteside, R.; Hogendoorn, P.C.; Farhadi, J.; Aigner, T.; Martin, I.; et al. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007, 56, 586–595. [Google Scholar] [CrossRef]
- Geissler, S.; Textor, M.; Kuhnisch, J.; Konnig, D.; Klein, O.; Ode, A.; Pfitzner, T.; Adjaye, J.; Kasper, G.; Duda, G.N. Functional comparison of chronological and in vitro aging: Differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS ONE 2012, 7, e52700. [Google Scholar] [CrossRef]
- Pestka, J.M.; Schmal, H.; Salzmann, G.; Hecky, J.; Sudkamp, N.P.; Niemeyer, P. In vitro cell quality of articular chondrocytes assigned for autologous implantation in dependence of specific patient characteristics. Arch. Orthop. Trauma Surg. 2011, 131, 779–789. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Jahangir, S.; Eglin, D.; Potter, N.; Khozaei Ravari, M.; Stoddart, M.J.; Samadikuchaksaraei, A.; Alini, M.; Baghaban Eslaminejad, M.; Safa, M. Inhibition of hypertrophy and improving chondrocyte differentiation by MMP-13 inhibitor small molecule encapsulated in alginate-chondroitin sulfate-platelet lysate hydrogel. Stem Cell Res. Ther. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Geissler, S.; Ode, A.; Schipp, C.J.; Matziolis, G.; Taylor, W.R.; Knaus, P.; Perka, C.; Duda, G.N.; Kasper, G. Modulation of matrix metalloprotease-2 levels by mechanical loading of three-dimensional mesenchymal stem cell constructs: Impact on in vitro tube formation. Tissue Eng. Part A 2010, 16, 3139–3148. [Google Scholar] [CrossRef]
- Davis, L.A.; Dienelt, A.; zur Nieden, N.I. Absorption-based assays for the analysis of osteogenic and chondrogenic yield. Methods Mol. Biol. 2011, 690, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Geissler, S.; Textor, M.; Schmidt-Bleek, K.; Klein, O.; Thiele, M.; Ellinghaus, A.; Jacobi, D.; Ode, A.; Perka, C.; Dienelt, A.; et al. In serum veritas-in serum sanitas? Cell non-autonomous aging compromises differentiation and survival of mesenchymal stromal cells via the oxidative stress pathway. Cell Death Dis. 2013, 4, e970. [Google Scholar] [CrossRef]
- Hochmann, S.; Mittermeir, M.; Santic, R.; Koszik, F.; Griessner, L.; Sonderegger, A.S.; Hoffmann, T.; Russe, E.; Scheiblhofer, S.; Weiss, R.; et al. Evaluation of modified Interferon alpha mRNA constructs for the treatment of non-melanoma skin cancer. Sci Rep. 2018, 8, 12954. [Google Scholar] [CrossRef]
- Brachtl, G.; Poupardin, R.; Hochmann, S.; Raninger, A.; Jurchott, K.; Streitz, M.; Schlickeiser, S.; Oeller, M.; Wolf, M.; Schallmoser, K.; et al. Batch Effects during Human Bone Marrow Stromal Cell Propagation Prevail Donor Variation and Culture Duration: Impact on Genotype, Phenotype and Function. Cells 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Peking, P.; Krisch, L.; Wolf, M.; Hochmann, S.; Hoog, A.; Vari, B.; Muigg, K.; Poupardin, R.; Scharler, C.; Schmidhuber, S.; et al. Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration. Theranostics 2021, 11, 8430–8447. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Schmitt, J.F.; Lee, E.H. Immunohistochemical analysis of human mesenchymal stem cells differentiating into chondrogenic, osteogenic, and adipogenic lineages. Methods Mol. Biol. 2011, 698, 353–366. [Google Scholar] [CrossRef]

| Sample | Pat No | Source | Sex | Age | Diagnostics | Localisation | ICRS |
|---|---|---|---|---|---|---|---|
| LB | 1 | Knee | m | 24 | OD, microfracture and cancellous bone arthroplasty | MFC | III |
| 2 | m | 17 | OD, cartilage defect 2 mm deep, width 7 mm | MFC | IV | ||
| 3 | m | 14 | OD, 2.5 cm, MFC osteonecrosis | MFC | IV | ||
| 4 | m | 29 | OD | MFC | III | ||
| 5 | m | 41 | Acute left patellar dislocation with app. 1 × 1.5 cm cartilage flake distal patella | RP | III–IV | ||
| 6 | m | 27 | OD, app. 2 × 4 cm | MFC | III–IV | ||
| 7 | m | 50 | OD | MFC | IV | ||
| 8 | f | 49 | OD, two app. 6 mm and 8 mm LBs ventral to eminentia intercondylaris | MFC | IV | ||
| 9 | m | 50 | OD, app. 2 × 2.5 cm LB before anterior cruciate ligament; 1 × 1.5 cm LB slightly adherent | MFC | IV | ||
| 10 | m | 29 | Trochlea cartilage damage, app. 2.5 × 2.5 cm LB right | LFC | IV a | ||
| 11 | f | 21 | RP cartilage damage with loose body | RP | III a | ||
| 12 | m | 38 | RP arthrosis right with LB, pangonarthrosis with corpora libra (III–IV), synovialitis right knee | RP | III–IV | ||
| 13 | m | 35 | OD, defect app. 2 × 2 cm | MFC | III | ||
| 14 | f | 25 | OD, app. 3 × 3 cm, 0.5 cm deep | MFC | IV b | ||
| 15 | f | 20 | OD, LB app. 1 × 1.5 cm cartilaginous and bony (app. 2 mm layer; completely sclerosed) | MFC | IV | ||
| 16 | f | 20 | OD, app. 2 × 2 cm | LFC | III | ||
| 17 | m | 32 | OD, LB 1.5 × 2 cm, MFC damage, chondromalacia lateral tibial plaetau right | MFC | III–IV | ||
| 18 | m | 42 | OD, app. 3 × 3 cm | MFC | IV | ||
| n = 18 | 72% m/28% f | Mean 31.2 ± 11.2 | |||||
| CTRL | 19 | Knee | f | 75 | Varus gonarthrosis | ||
| 20 | m | 69 | Varus gonarthrosis | ||||
| 21 | m | 53 | Varus gonarthrosis | ||||
| 22 | f | 66 | Posttraumatic gonarthrosis, secondary gonarthrosis | ||||
| 23 | m | 58 | Varus gonarthrosis | ||||
| n = 5 | 60% m/40% f | Mean 64.2 ± 7.8 | |||||
| BMSC | 24 | Femural Head | m | 88 | Coxarthrosis | ||
| 25 | f | 75 | Coxarthrosis | ||||
| 26 | m | 42 | Coxarthrosis | ||||
| 27 | m | 37 | Post-traumatic coxarthrosis | ||||
| 28 | m | 74 | Coxarthrosis | ||||
| 28 | f | 30 | Dysplasia coxarthrosis | ||||
| n = 6 | 67% m/33% f | Mean 57.7 ± 22.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Textor, M.; Hoburg, A.; Lehnigk, R.; Perka, C.; Duda, G.N.; Reinke, S.; Blankenstein, A.; Hochmann, S.; Stockinger, A.; Resch, H.; et al. Chondrocyte Isolation from Loose Bodies—An Option for Reducing Donor Site Morbidity for Autologous Chondrocyte Implantation. Int. J. Mol. Sci. 2023, 24, 1484. https://doi.org/10.3390/ijms24021484
Textor M, Hoburg A, Lehnigk R, Perka C, Duda GN, Reinke S, Blankenstein A, Hochmann S, Stockinger A, Resch H, et al. Chondrocyte Isolation from Loose Bodies—An Option for Reducing Donor Site Morbidity for Autologous Chondrocyte Implantation. International Journal of Molecular Sciences. 2023; 24(2):1484. https://doi.org/10.3390/ijms24021484
Chicago/Turabian StyleTextor, Martin, Arnd Hoburg, Rex Lehnigk, Carsten Perka, Georg N. Duda, Simon Reinke, Antje Blankenstein, Sarah Hochmann, Andreas Stockinger, Herbert Resch, and et al. 2023. "Chondrocyte Isolation from Loose Bodies—An Option for Reducing Donor Site Morbidity for Autologous Chondrocyte Implantation" International Journal of Molecular Sciences 24, no. 2: 1484. https://doi.org/10.3390/ijms24021484
APA StyleTextor, M., Hoburg, A., Lehnigk, R., Perka, C., Duda, G. N., Reinke, S., Blankenstein, A., Hochmann, S., Stockinger, A., Resch, H., Wolf, M., Strunk, D., & Geissler, S. (2023). Chondrocyte Isolation from Loose Bodies—An Option for Reducing Donor Site Morbidity for Autologous Chondrocyte Implantation. International Journal of Molecular Sciences, 24(2), 1484. https://doi.org/10.3390/ijms24021484