Characterization and Comparative Genomic Analysis of Three Virulent E. coli Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment
Abstract
1. Introduction
2. Results and Discussion
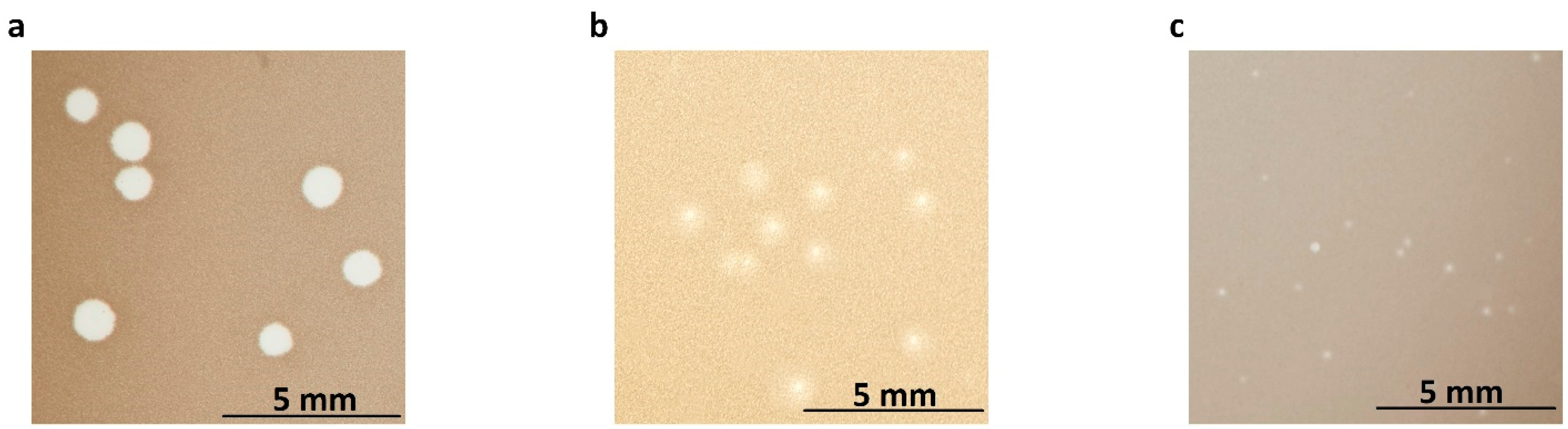
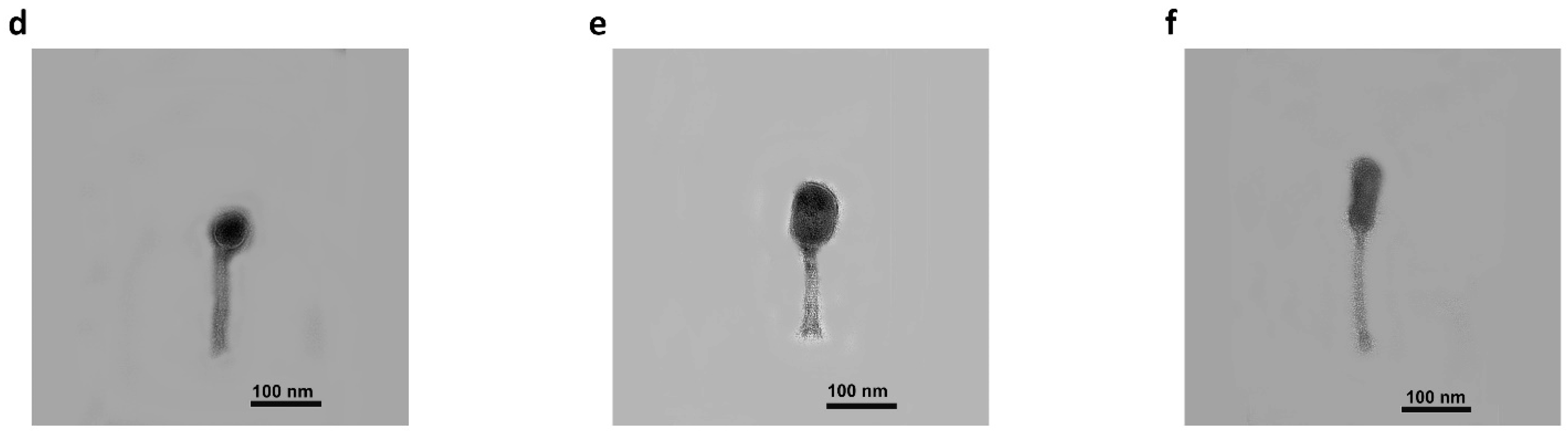
2.1. Morphology and Properties of Phage Virions and Plaques
2.2. General Features of Genomes
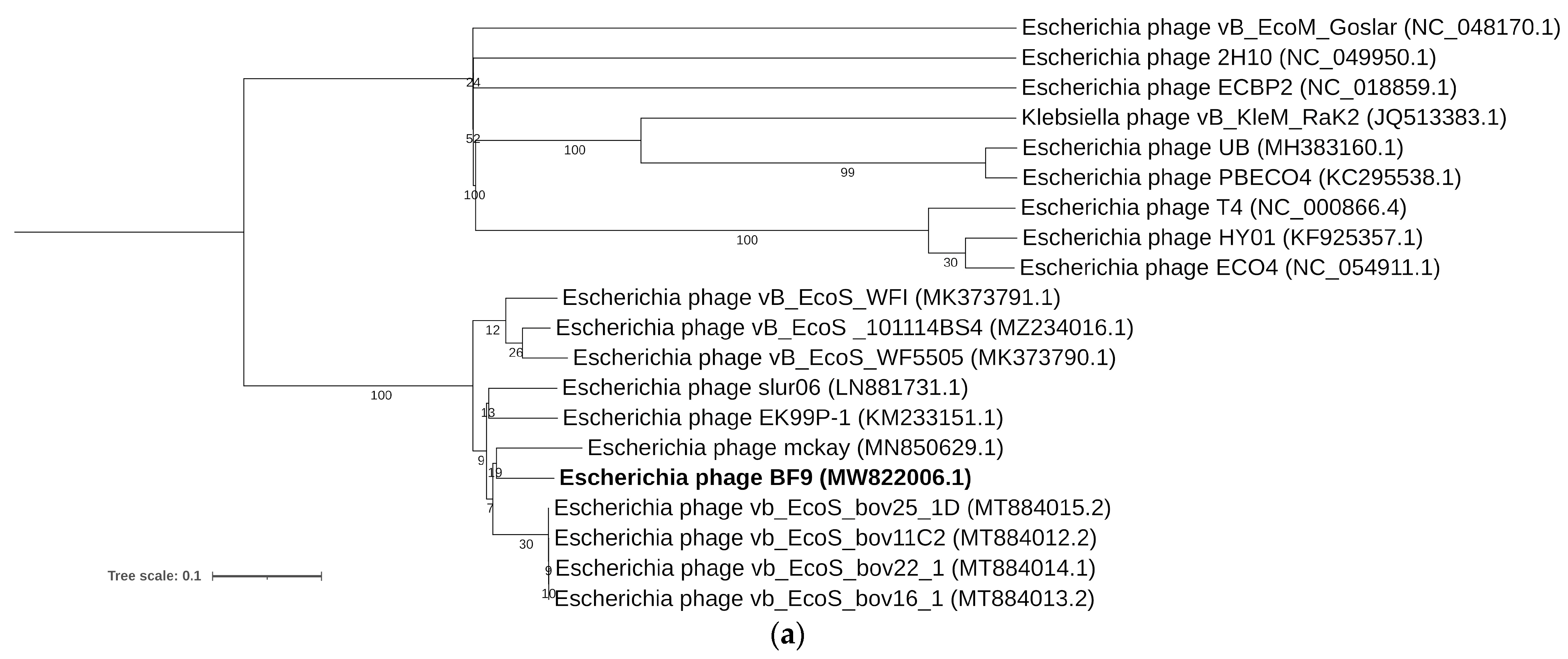
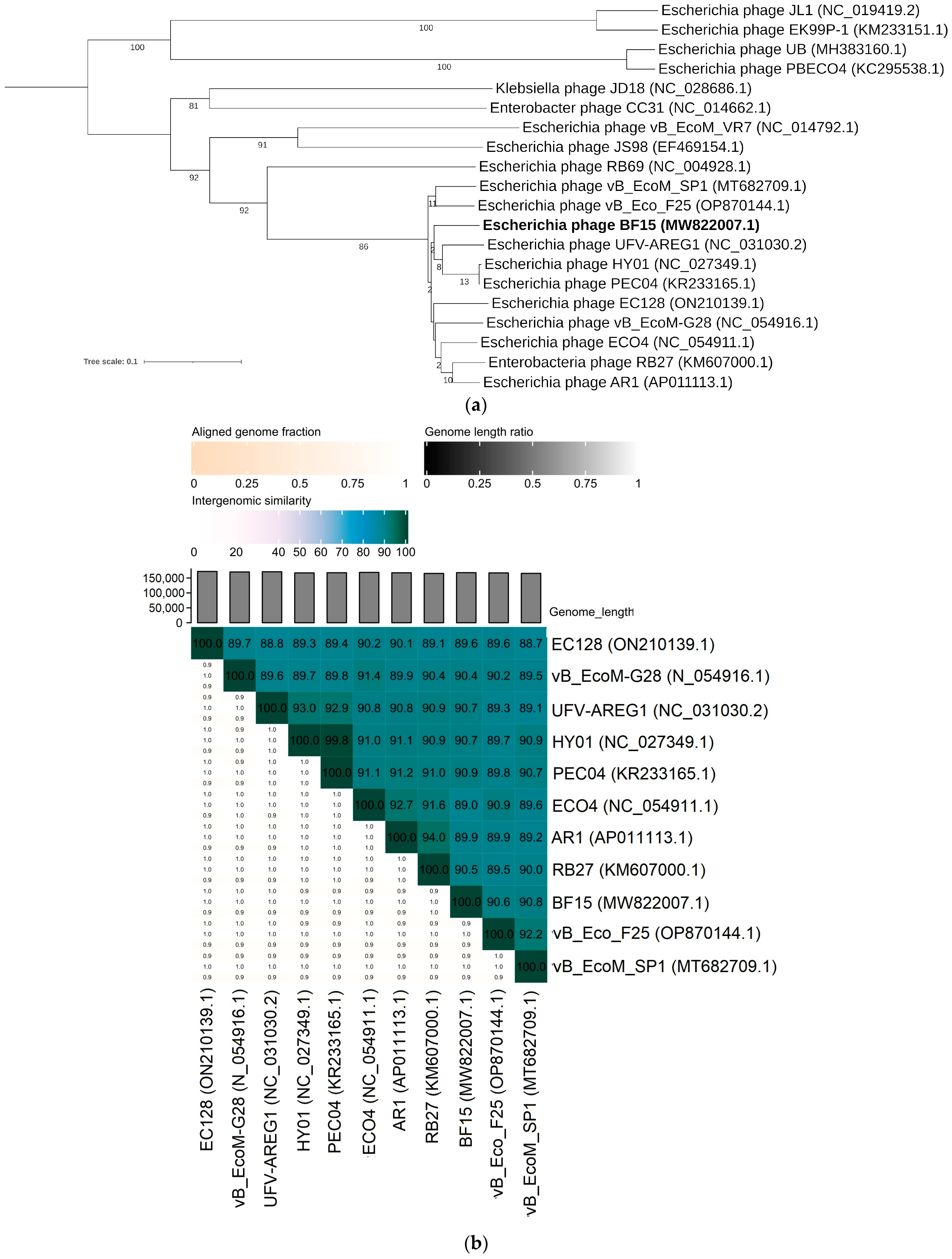
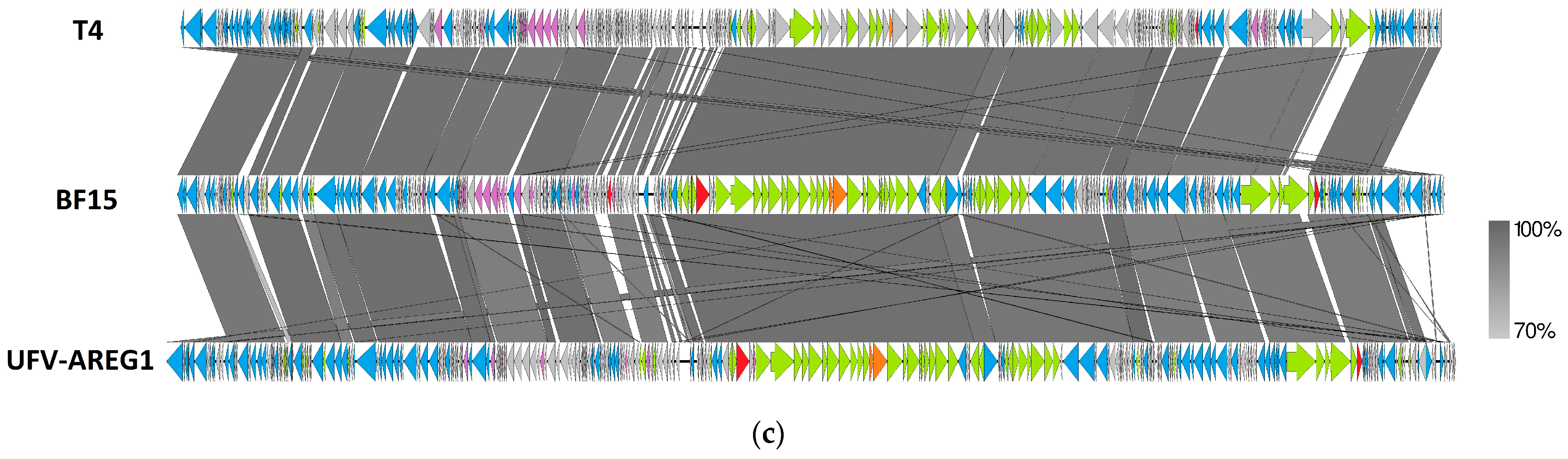
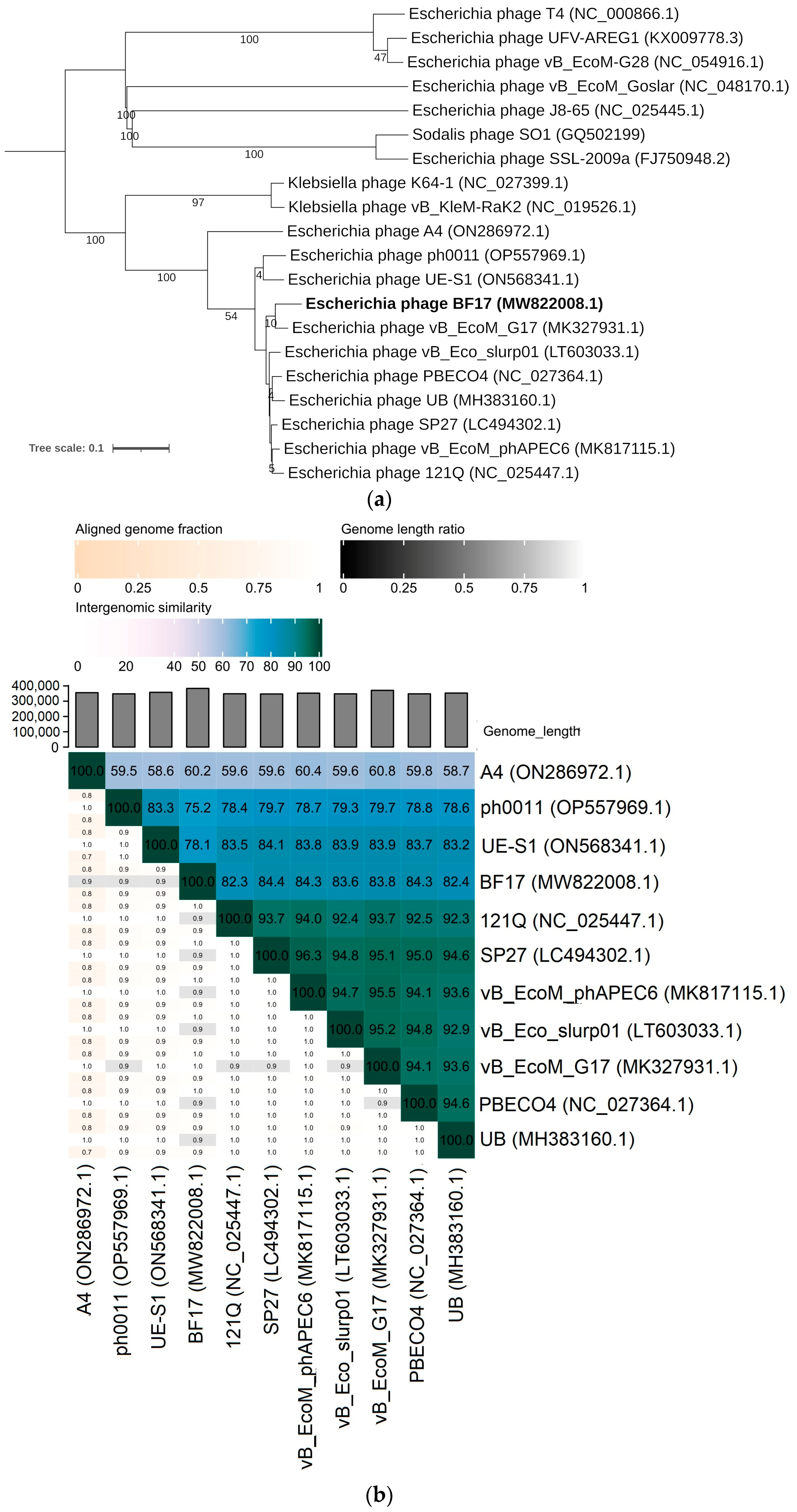
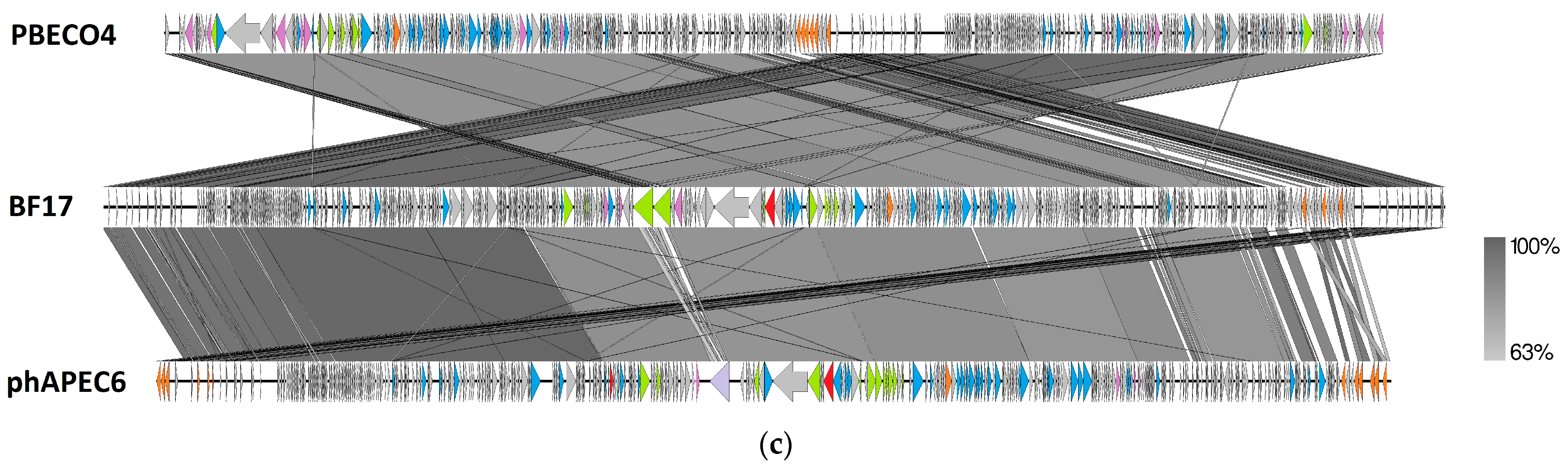
2.3. Comparative Genomics of Phages BF9, BF15, and BF17
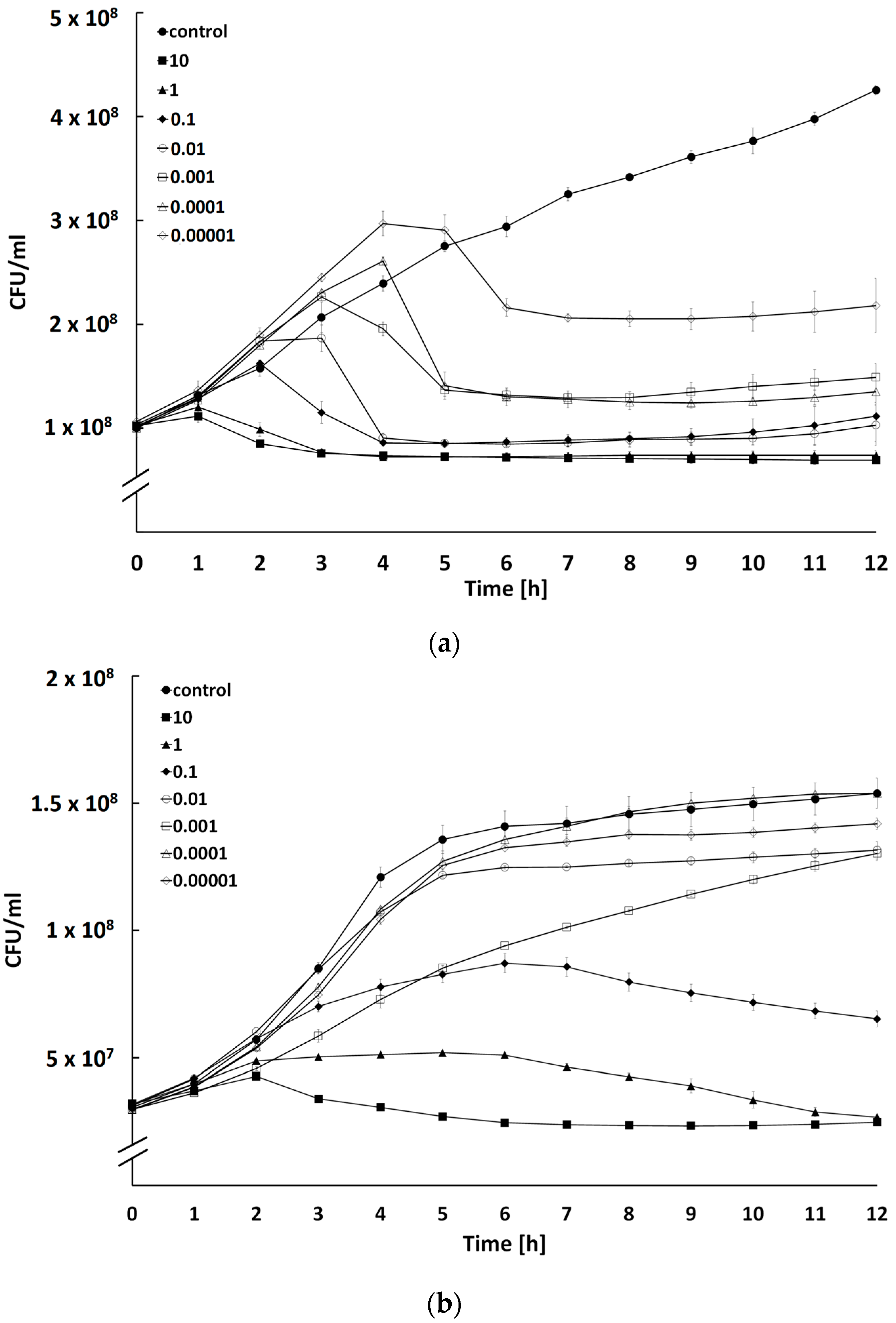
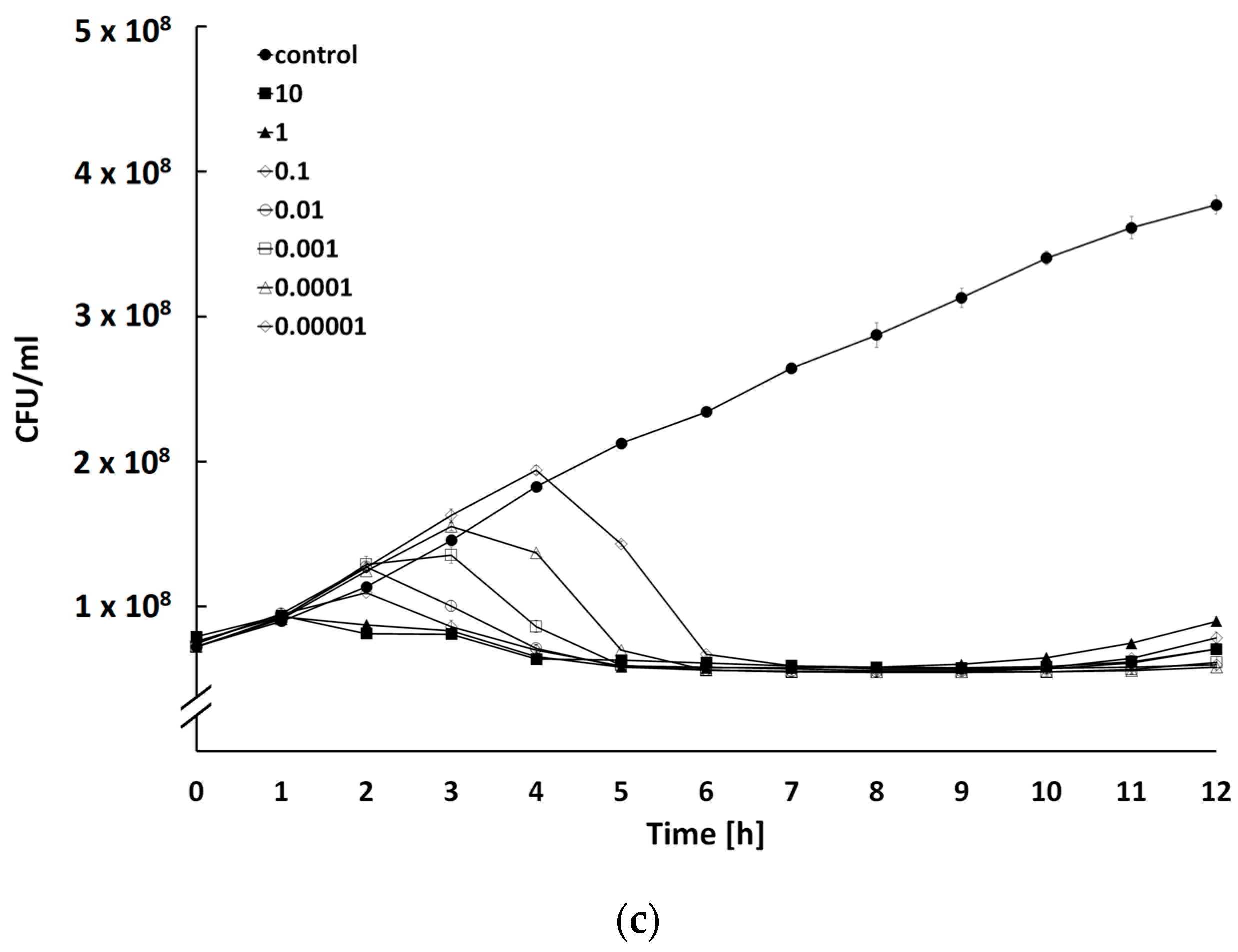
2.4. Determination of Bacteriolytic Activity at Different Multiplicities of Infection
2.5. Influence of Temperature and pH on Activity of Bacteriophages
3. Materials and Methods
3.1. Bacteriophages and Bacteria
3.2. Bacteriophage Propagation
3.3. Morphology of Phage Virions and Plaques
3.4. Bacteriophage DNA Extraction, Sequencing, and Bioinformatic Analysis
3.5. Bacterial Host Reduction at Different MOIs
3.6. Influence of Temperature on Activity of Phages
3.7. Influence of pH on Activity of Phages
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECDC European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2019. Vet. Rec. 2020, 174, 341. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure–Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, A.; Hammerl, J.A.; Falgenhauer, L.; Guiral, E.; Schmoger, S.; Imirzalioglu, C.; Fischer, J.; Guerra, B.; Chakraborty, T.; Käsbohrer, A. Diversity of CTX-M-1-Producing E. Coli from German Food Samples and Genetic Diversity of the BlaCTX-M-1 Region on IncI1 ST3 Plasmids. Vet. Microbiol. 2018, 221, 98–104. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, H.; Seo, Y.H.; Park, G.E.; Lee, H.; Lee, K. Prevalence and Molecular Epidemiology of Extended-Spectrum-β-Lactamase (ESBL)-Producing Escherichia Coli from Multiple Sectors of the Swine Industry in Korea: A Korean Nationwide Monitoring Program for a One Health Approach to Combat Antimicrobial Resista. Ann. Lab. Med. 2021, 41, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Von Wintersdorff, C.J.H.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia Coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Ewers, C.; de Jong, A.; Prenger-Berninghoff, E.; El Garch, F.; Leidner, U.; Tiwari, S.K.; Semmler, T. Genomic Diversity and Virulence Potential of ESBL- and AmpC-β-Lactamase-Producing Escherichia Coli Strains From Healthy Food Animals Across Europe. Front. Microbiol. 2021, 12, 626774. [Google Scholar] [CrossRef]
- Projahn, M.; Daehre, K.; Roesler, U.; Friese, A. Extended-Spectrum-Beta-Lactamase- and Plasmid-Encoded Cephamycinase- Producing Enterobacteria in the Broiler Hatchery as a Potential Mode of Pseudo- Vertical Transmission. Appl. Environ. Microbiol. 2017, 83, e02364-16. [Google Scholar] [CrossRef]
- Lambrecht, E.; Van Meervenne, E.; Boon, N.; Van De Wiele, T.; Wattiau, P.; Herman, L.; Heyndrickx, M.; Van Coillie, E. Characterization of Cefotaxime- and Ciprofloxacin-Resistant Commensal Escherichia Coli Originating from Belgian Farm Animals Indicates High Antibiotic Resistance Transfer Rates. Microb. Drug Resist. 2018, 24, 707–717. [Google Scholar] [CrossRef]
- Bernasconi, O.J.; Donà, V.; Tinguely, R.; Endimiani, A. In Vitro Activity of Three Commercial Bacteriophage Cocktails against Multidrug-Resistant Escherichia Coli and Proteus Spp. Strains of Human and Non-Human Origin. J. Glob. Antimicrob. Resist. 2017, 8, 179–185. [Google Scholar] [CrossRef]
- Kittler, S.; Mengden, R.; Korf, I.H.E.; Bierbrodt, A.; Wittmann, J.; Plötz, M.; Jung, A.; Lehnherr, T.; Rohde, C.; Lehnherr, H.; et al. Impact of Bacteriophage-Supplemented Drinking Water on the E. Coli Population in the Chicken Gut. Pathogens 2020, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Korf, I.H.E.; Kittler, S.; Bierbrodt, A.; Mengden, R.; Rohde, C.; Rohde, M.; Kroj, A.; Lehnherr, T.; Fruth, A.; Flieger, A.; et al. In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia Coli. Viruses 2020, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Honjoh, K.; Miyamoto, T. Isolation and Bio-Control of Extended Spectrum Beta-Lactamase (ESBL)-Producing Escherichia Coli Contamination in Raw Chicken Meat by Using Lytic Bacteriophages. LWT-Food Sci. Technol. 2016, 71, 339–346. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Application of Bacteriophages in Simultaneously Controlling Escherichia Coli O157:H7 and Extended-Spectrum Beta-Lactamase Producing Escherichia Coli. Appl. Microbiol. Biotechnol. 2018, 102, 10259–10271. [Google Scholar] [CrossRef] [PubMed]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under “One Health Approach”. Indian J. Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef]
- Skaradzińska, A.; Śliwka, P.; Kuźmińska-Bajor, M.; Skaradziński, G.; Rzasa, A.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U.H. The Efficacy of Isolated Bacteriophages from Pig Farms against ESBL/AmpC-Producing Escherichia Coli from Pig and Turkey Farms. Front. Microbiol. 2017, 8, 530. [Google Scholar] [CrossRef]
- Roschanski, N.; Fischer, J.; Guerra, B.; Roesler, U. Development of a Multiplex Real-Time PCR for the Rapid Detection of the Predominant Beta-Lactamase Genes CTX-M, SHV, TEM and CIT-Type Ampcs in Enterobacteriaceae. PLoS ONE 2014, 9, e100956. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, M. Jumbo Bacteriophages: An Overview. Front. Microbiol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A Tool for Fast and Accurate Determination of Phage Termini and Packaging Mechanism Using next-Generation Sequencing Data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Buttimer, C.; Hendrix, H.; Oliveira, H.; Casey, A.; Neve, H.; McAuliffe, O.; Paul Ross, R.; Hill, C.; Noben, J.P.; O’Mahony, J.; et al. Things Are Getting Hairy: Enterobacteria Bacteriophage VB_PcaM_CBB. Front. Microbiol. 2017, 8, 44. [Google Scholar] [CrossRef]
- Lood, C.; Danis-Wlodarczyk, K.; Blasdel, B.G.; Jang, H.B.; Vandenheuvel, D.; Briers, Y.; Noben, J.P.; van Noort, V.; Drulis-Kawa, Z.; Lavigne, R. Integrative Omics Analysis of Pseudomonas Aeruginosa Virus PA5oct Highlights the Molecular Complexity of Jumbo Phages. Environ. Microbiol. 2020, 22, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Askora, A.; Blanc-Mathieu, R.; Kawasaki, T.; Li, Y.; Nakano, M.; Ogata, H.; Yamada, T. Xanthomonas Citri Jumbo Phage XacN1 Exhibits a Wide Host Range and High Complement of TRNA Genes. Sci. Rep. 2018, 8, 4486. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef]
- Korf, I.H.E.; Meier-Kolthoff, J.P.; Adriaenssens, E.M.; Kropinski, A.M.; Nimtz, M.; Rohde, M.; van Raaij, M.J.; Wittmann, J. Still Something to Discover: Novel Insights into Escherichia Coli Phage Diversity and Taxonomy. Viruses 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.S.; Forero-Junco, L.; Kot, W.; Hansen, L.H. Exploring the Remarkable Diversity of Culturable Escherichia Coli Phages in the Danish Wastewater Environment. Viruses 2020, 12, 986. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Huang, H.; Tan, L.; Ni, Q.; Shang, W.; Yang, Y.; Hu, Z.; Zhu, J.; Li, M.; et al. Comparative Analysis and Characterization of Enterobacteria Phage SSL-2009a and ‘HK578likevirus’ Bacteriophages. Virus Res. 2019, 259, 77–84. [Google Scholar] [CrossRef]
- Pham-Khanh, N.H.; Sunahara, H.; Yamadeya, H.; Sakai, M.; Nakayama, T.; Yamamoto, H.; Truong Thi Bich, V.; Miyanaga, K.; Kamei, K. Isolation, Characterisation and Complete Genome Sequence of a Tequatrovirus Phage, Escherichia Phage KIT03, Which Simultaneously Infects Escherichia Coli O157:H7 and Salmonella Enterica. Curr. Microbiol. 2019, 76, 1130–1137. [Google Scholar] [CrossRef]
- Slobodníková, L.; Markusková, B.; Kajsík, M.; Andrezál, M.; Straka, M.; Liptáková, A.; Drahovská, H. Characterization of Anti-Bacterial Effect of the Two New Phages against Uropathogenic Escherichia Coli. Viruses 2021, 13, 1348. [Google Scholar] [CrossRef]
- Comeau, A.M.; Bertrand, C.; Letarov, A.; Tétart, F.; Krisch, H.M. Modular Architecture of the T4 Phage Superfamily: A Conserved Core Genome and a Plastic Periphery. Virology 2007, 362, 384–396. [Google Scholar] [CrossRef]
- Iyer, L.M.; Anantharaman, V.; Krishnan, A.; Burroughs, A.M.; Aravind, L. Jumbo Phages: A Comparative Genomic Overview of Core Functions and Adaptions for Biological Conflicts. Viruses 2021, 13, 63. [Google Scholar] [CrossRef]
- Lee, Y.; Son, B.; Cha, Y.; Ryu, S. Characterization and Genomic Analysis of PALS2, a Novel Staphylococcus Jumbo Bacteriophage. Front. Microbiol. 2021, 12, 622755. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.; Alrashid, B.; Patel, F.; Dowah, A.S.A.; Brown, N.; Millard, A.; Clokie, M.R.J.; Galyov, E.E. VB_PaeM_MIJ3, a Novel Jumbo Phage Infecting Pseudomonas Aeruginosa, Possesses Unusual Genomic Features. Front. Microbiol. 2019, 10, 2772. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.G.; Kim, H.J.; Giri, S.S.; Kim, S.W.; Lee, S.B.; Park, S.C. Isolation and Characterization of Salmonella Jumbo-Phage Psal-Snuabm-04. Viruses 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Wagemans, J.; Tsonos, J.; Holtappels, D.; Fortuna, K.; Hernalsteens, J.P.; Greve, H.D.; Estrozi, L.F.; Bacia-Verloop, M.; Moriscot, C.; Noben, J.P.; et al. Structural Analysis of Jumbo Coliphage PhAPEC6. Int. J. Mol. Sci. 2020, 21, 3119. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.D.; Hong, S.S.; Park, K.; Ko, K.S.; Myung, H. Phage-Encoded Colanic Acid-Degrading Enzyme Permits Lytic Phage: Infection of a Capsule-Forming Resistant Mutant Escherichia Coli Strain. Appl. Environ. Microbiol. 2015, 81, 900–909. [Google Scholar] [CrossRef]
- Hua, Y.; An, X.; Pei, G.; Li, S.; Wang, W.; Xu, X.; Fan, H.; Huang, Y.; Zhang, Z.; Mi, Z.; et al. Characterization of the Morphology and Genome of an Escherichia Coli Podovirus. Arch. Virol. 2014, 159, 3249–3256. [Google Scholar] [CrossRef]
- Fernández-Gómez, P.; Trigal, E.; Alegría, Á.; Santos, J.A.; López, M.; Prieto, M.; Alvarez-Ordóñez, A. Biofilm Formation Ability and Tolerance to Food-Associated Stresses among ESBL-Producing Escherichia Coli Strains from Foods of Animal Origin and Human Patients. LWT 2022, 168, 113961. [Google Scholar] [CrossRef]
- Korniienko, N.; Kharina, A.; Zrelovs, N.; Jindřichová, B.; Moravec, T.; Budzanivska, I.; Burketová, L.; Kalachova, T. Isolation and Characterization of Two Lytic Phages Efficient Against Phytopathogenic Bacteria From Pseudomonas and Xanthomonas Genera. Front. Microbiol. 2022, 13, 853593. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, Y.T.; Salvador, A.; Lavenburg, V.M.; Wu, V.C.H. Characterization of Two New Shiga Toxin-Producing Escherichia Coli O103-Infecting Phages Isolated from an Organic Farm. Microorganisms 2021, 9, 1527. [Google Scholar] [CrossRef]
- Lee, C.; Choi, I.Y.; Park, D.H.; Park, M.K. Isolation and Characterization of a Novel Escherichia Coli O157:H7-Specific Phage as a Biocontrol Agent 06 Biological Sciences 0605 Microbiology 11 Medical and Health Sciences 1108 Medical Microbiology 06 Biological Sciences 0604 Genetics. J. Environ. Heal. Sci. Eng. 2020, 18, 189–199. [Google Scholar] [CrossRef]
- Peng, Q.; Yuan, Y. Characterization of a Newly Isolated Phage Infecting Pathogenic Escherichia Coli and Analysis of Its Mosaic Structural Genes. Sci. Rep. 2018, 8, 8086. [Google Scholar] [CrossRef] [PubMed]
- Litt, P.K.; Jaroni, D. Isolation and Physiomorphological Characterization of Escherichia Coli O157:H7-Infecting Bacteriophages Recovered from Beef Cattle Operations. Int. J. Microbiol. 2017, 2017, 7013236. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Sakin, T.; Akçelik, M.; Akçelik, N. Identification and Characterization of Lytic Bacteriophages Specific to Foodborne Pathogenic Escherichia Coli O157:H7. Food Sci. Technol. Int. 2020, 27, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Carrigy, N.B.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.E.; Connerton, I.F.; Vehring, R. Spray-Dried Anti-Campylobacter Bacteriophage CP30A Powder Suitable for Global Distribution without Cold Chain Infrastructure. Int. J. Pharm. 2019, 569, 118601. [Google Scholar] [CrossRef]
- Zurabov, F.; Zhilenkov, E. Characterization of Four Virulent Klebsiella Pneumoniae Bacteriophages, and Evaluation of Their Potential Use in Complex Phage Preparation. Virol. J. 2021, 18, 9. [Google Scholar] [CrossRef]
- Li, M.; Guo, M.; Chen, L.; Zhu, C.; Xiao, Y.; Li, P.; Guo, H.; Chen, L.; Zhang, W.; Du, H. Isolation and Characterization of Novel Lytic Bacteriophages Infecting Epidemic Carbapenem-Resistant Klebsiella Pneumoniae Strains. Front. Microbiol. 2020, 11, 1554. [Google Scholar] [CrossRef]
- Fu, P.; Zhao, Q.; Shi, L.; Xiong, Q.; Ren, Z.; Xu, H.; Chai, S.; Xu, Q.; Sun, X.; Sang, M. Identification and Characterization of Two Bacteriophages with Lytic Activity against Multidrug-Resistant Escherichia Coli. Virus Res. 2021, 291, 198196. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Santos, J.F.; Siliakus, M.F.; van Lent, J.W.M.; Kengen, S.W.M.; Azeredo, J.; Kluskens, L.D. Genetically Manipulated Phages with Improved PH Resistance for Oral Administration in Veterinary Medicine. Sci. Rep. 2016, 6, 39235. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with Alginate/CaCO3: A Strategy for Improved Phage Therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef]
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajor, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage Amplification–a Comparison of Selected Methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef]
- Adams, M. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959; 620p. [Google Scholar]
- Ackermann, H.W. Phage Classification and Characterization. Methods Mol. Biol. 2009, 501, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Kȩsik-Szeloch, A.; Drulis-Kawa, Z.; Weber-Dabrowska, B.; Kassner, J.; Majkowska-Skrobek, G.; Augustyniak, D.; Łusiak-Szelachowska, M.; Zaczek, M.; Górski, A.; Kropinski, A.M. Characterising the Biology of Novel Lytic Bacteriophages Infecting Multidrug Resistant Klebsiella Pneumoniae. Virol. J. 2013, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A Self-Training Method for Prediction of Gene Starts in Microbial Genomes. Implications for Finding Sequence Motifs in Regulatory Regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative Pathogenomic Platform with an Interactive Web Interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Laslett, D.; Canback, B. ARAGORN, a Program to Detect TRNA Genes and TmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-Based Phylogeny and Classification of Prokaryotic Viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC—A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.; Seto, D.; Mahadevan, P. CoreGenes5.0: An Updated User-Friendly Webserver for the Determination of Core Genes from Sets of Viral and Bacterial Genomes. Viruses 2022, 14, 2534. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Rezk, N.; Fayez, M.S.; Abdelmoteleb, M.; Atteya, R.; Elhadidy, M.; El-Shibiny, A. Isolation, Characterization, and Genomic Analysis of Three Novel E. Coli Bacteriophages That Effectively Infect E. Coli O18. Microorganisms 2022, 10, 589. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Costa, P.; Almeida, A. Bacteriophages with Potential to Inactivate Aeromonas Hydrophila in Cockles: In Vitro and in Vivo Preliminary Studies. Antibiotics 2021, 10, 710. [Google Scholar] [CrossRef]




| Bacteriophage | Bacterial Isolates | Bacterial Sources | Reference |
|---|---|---|---|
| BF9 | Escherichia coli B19 | pig manure | [16] |
| BF15 | Escherichia coli B39 | pig manure | |
| BF17 | Escherichia coli B73 | pig manure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwka, P.; Weber-Dąbrowska, B.; Żaczek, M.; Kuźmińska-Bajor, M.; Dusza, I.; Skaradzińska, A. Characterization and Comparative Genomic Analysis of Three Virulent E. coli Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment. Int. J. Mol. Sci. 2023, 24, 5696. https://doi.org/10.3390/ijms24065696
Śliwka P, Weber-Dąbrowska B, Żaczek M, Kuźmińska-Bajor M, Dusza I, Skaradzińska A. Characterization and Comparative Genomic Analysis of Three Virulent E. coli Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment. International Journal of Molecular Sciences. 2023; 24(6):5696. https://doi.org/10.3390/ijms24065696
Chicago/Turabian StyleŚliwka, Paulina, Beata Weber-Dąbrowska, Maciej Żaczek, Marta Kuźmińska-Bajor, Izabela Dusza, and Aneta Skaradzińska. 2023. "Characterization and Comparative Genomic Analysis of Three Virulent E. coli Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment" International Journal of Molecular Sciences 24, no. 6: 5696. https://doi.org/10.3390/ijms24065696
APA StyleŚliwka, P., Weber-Dąbrowska, B., Żaczek, M., Kuźmińska-Bajor, M., Dusza, I., & Skaradzińska, A. (2023). Characterization and Comparative Genomic Analysis of Three Virulent E. coli Bacteriophages with the Potential to Reduce Antibiotic-Resistant Bacteria in the Environment. International Journal of Molecular Sciences, 24(6), 5696. https://doi.org/10.3390/ijms24065696







