Analogues of Anticancer Natural Products: Chiral Aspects
Abstract
1. Introduction
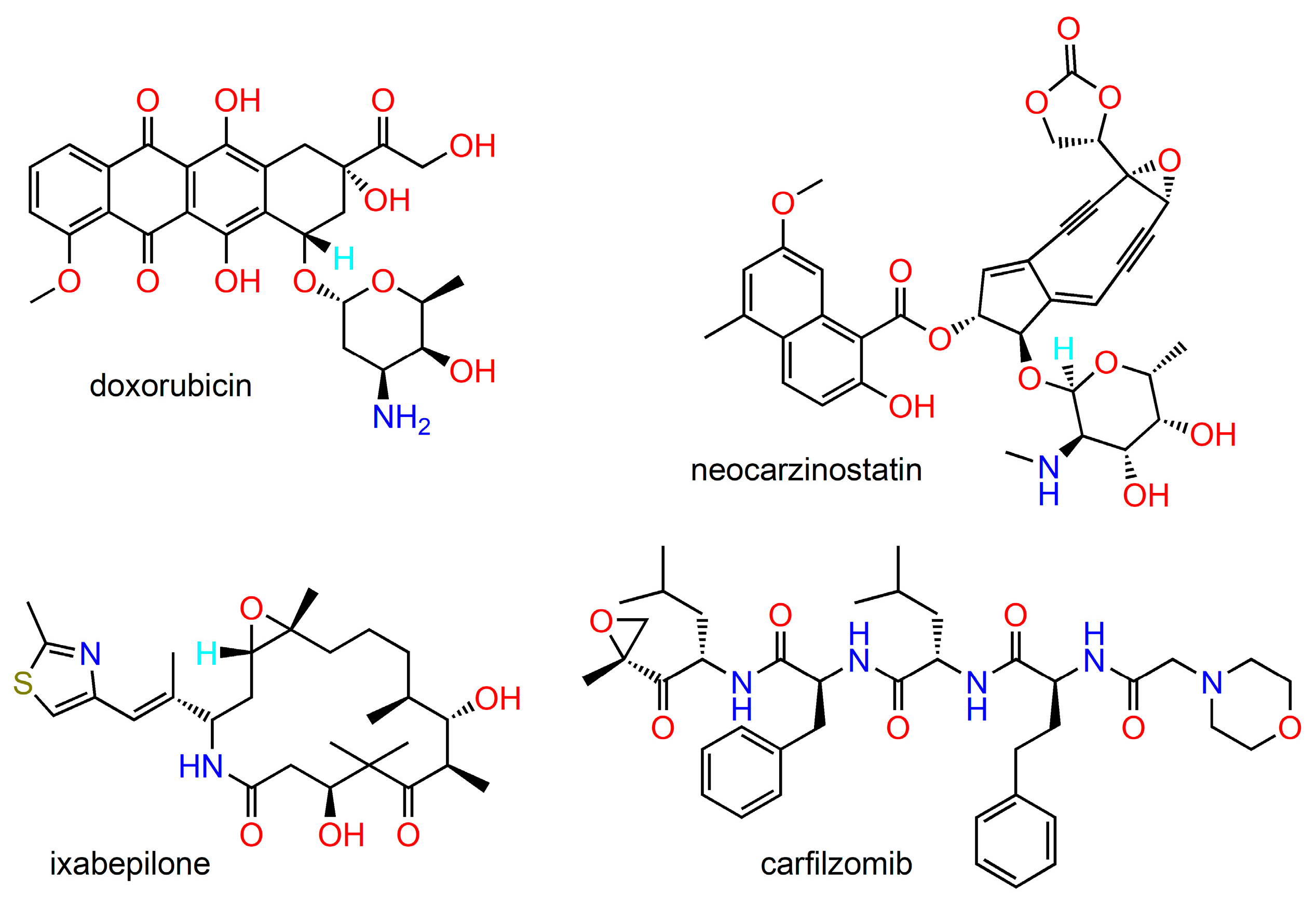
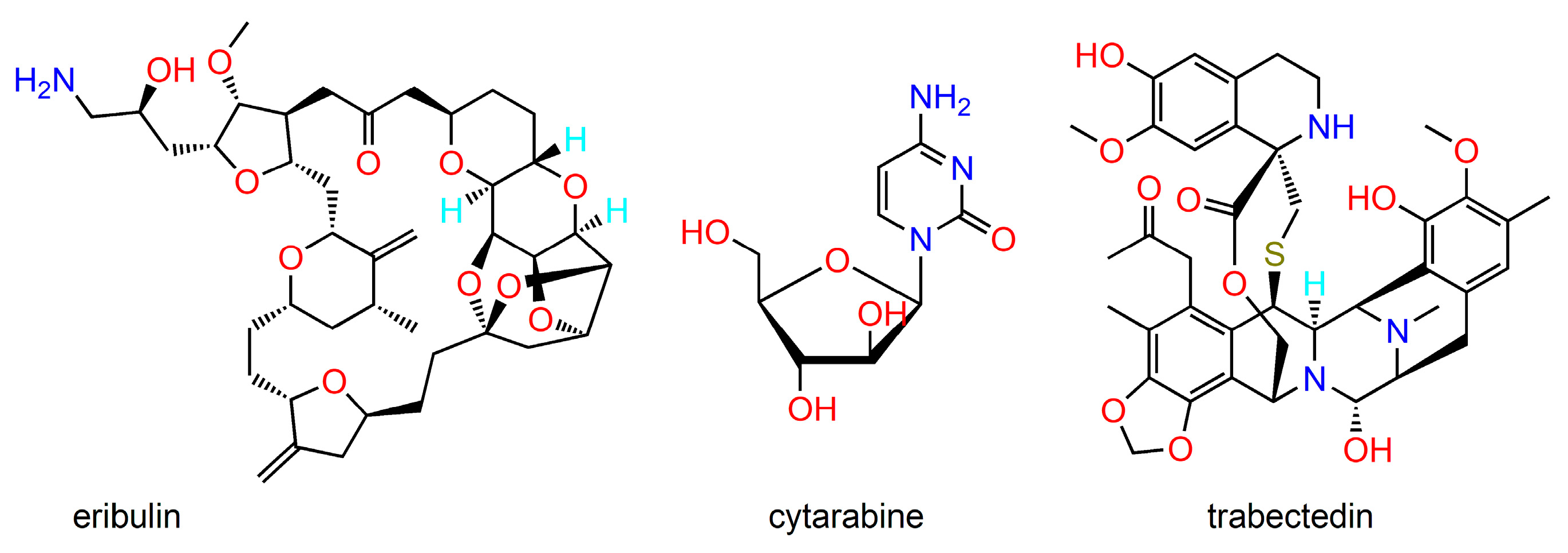
2. Anticancer Natural Products and Their Semisynthetic Congeners
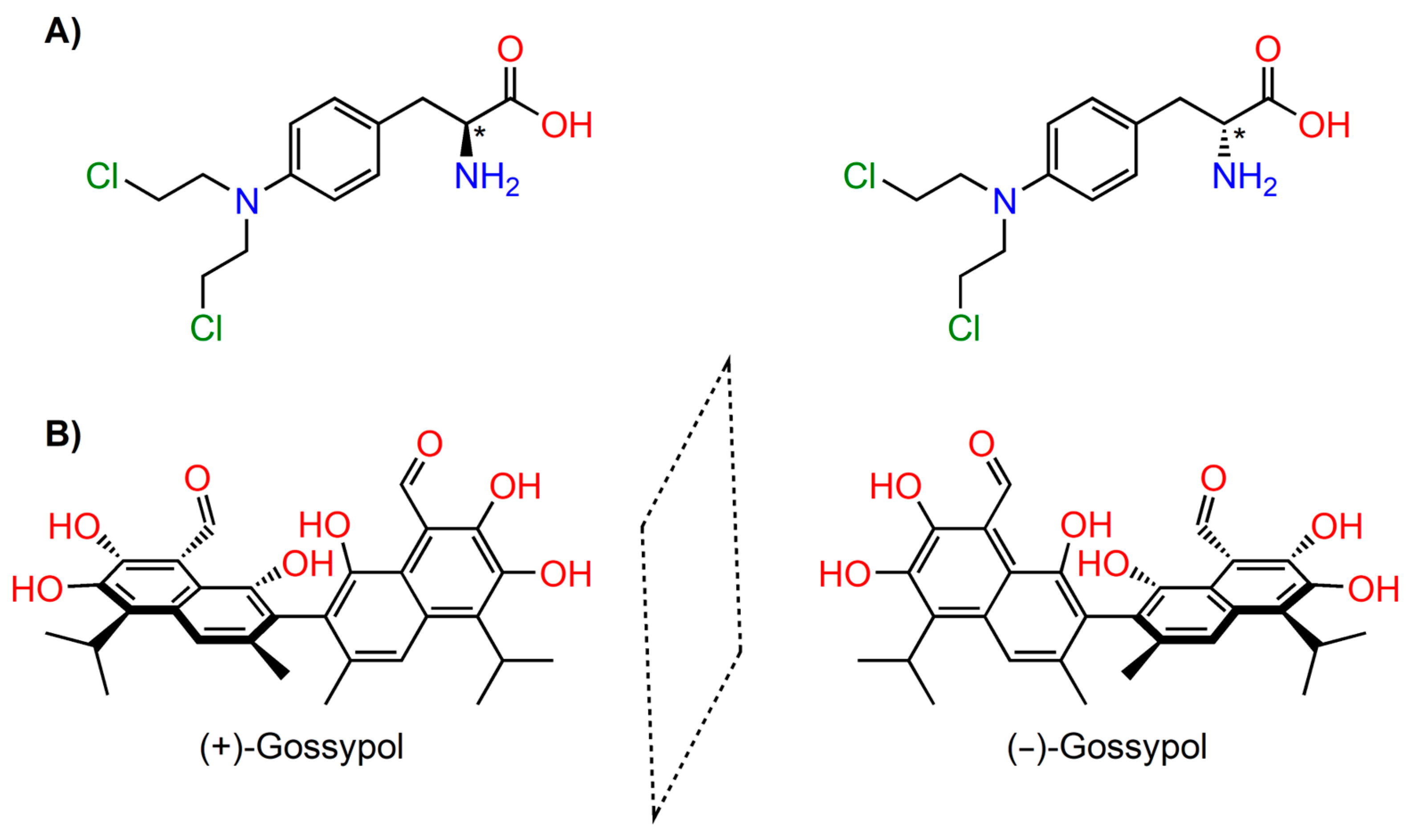
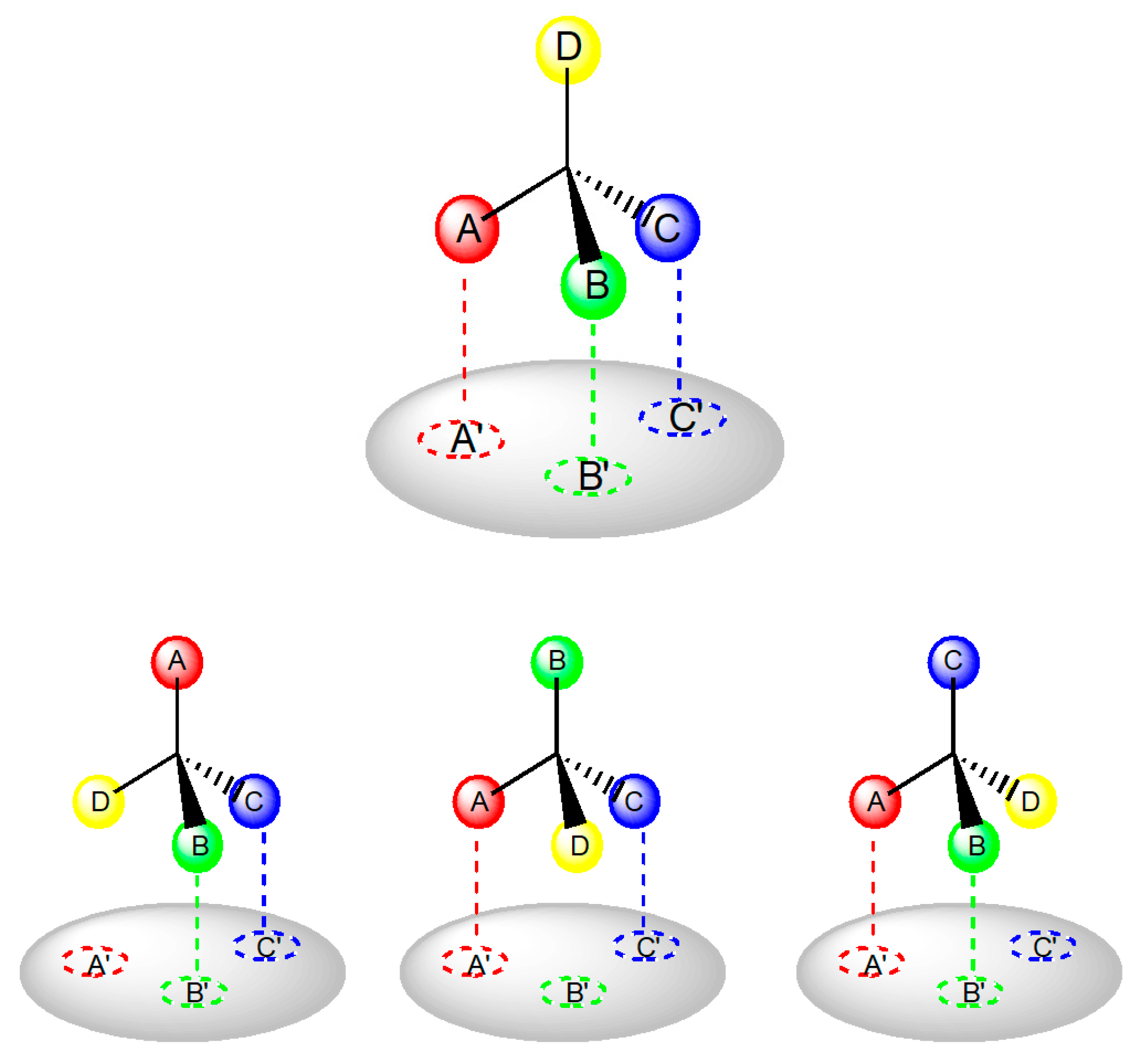
3. Chirality in Drug Design
4. Chiral Analogues of Natural Compounds with Stereospecific Cytotoxic Activity
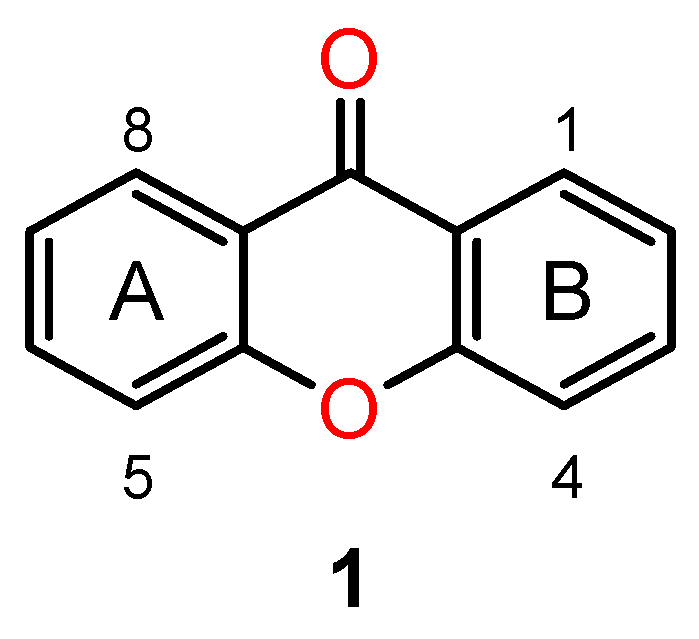
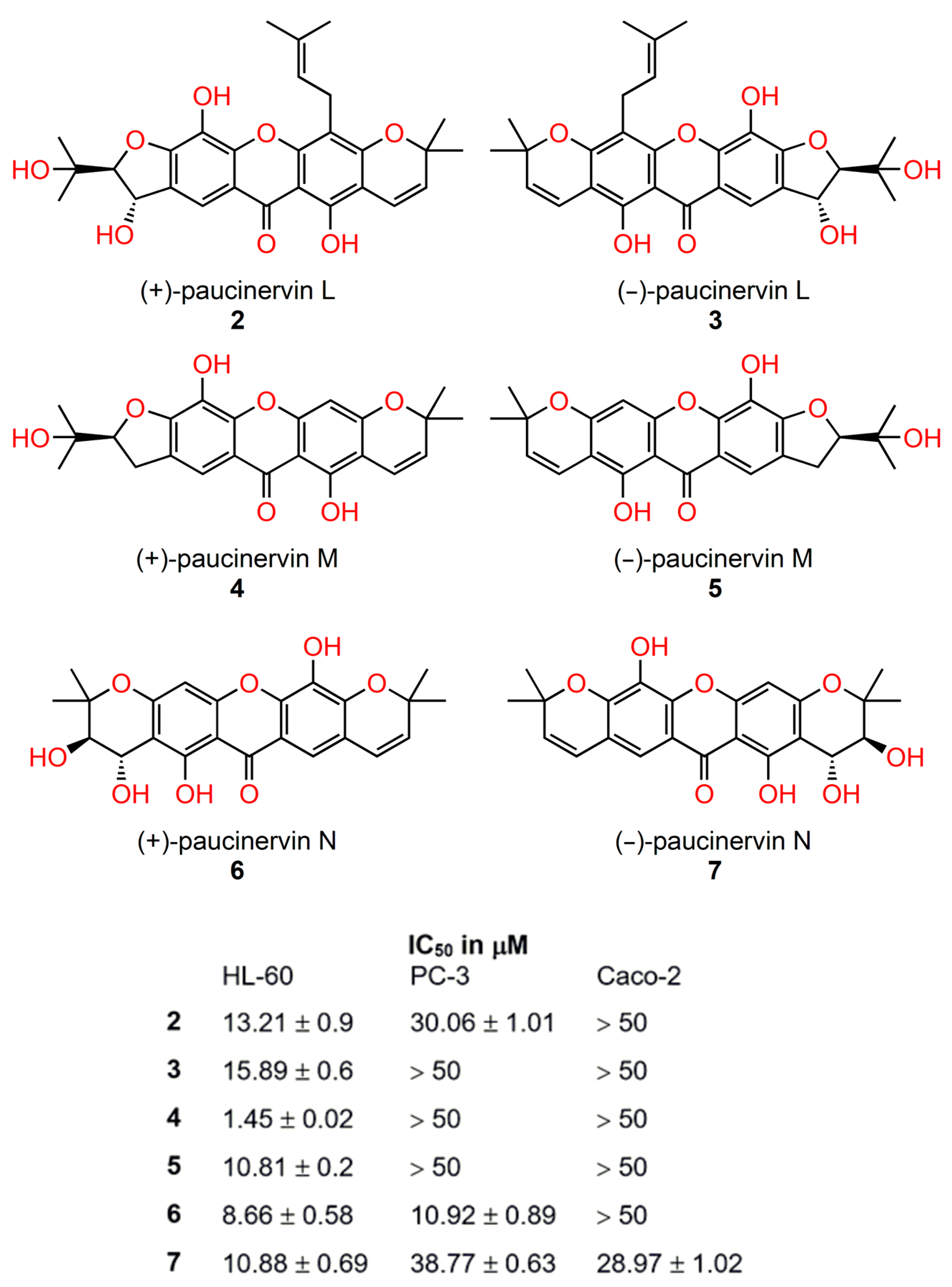
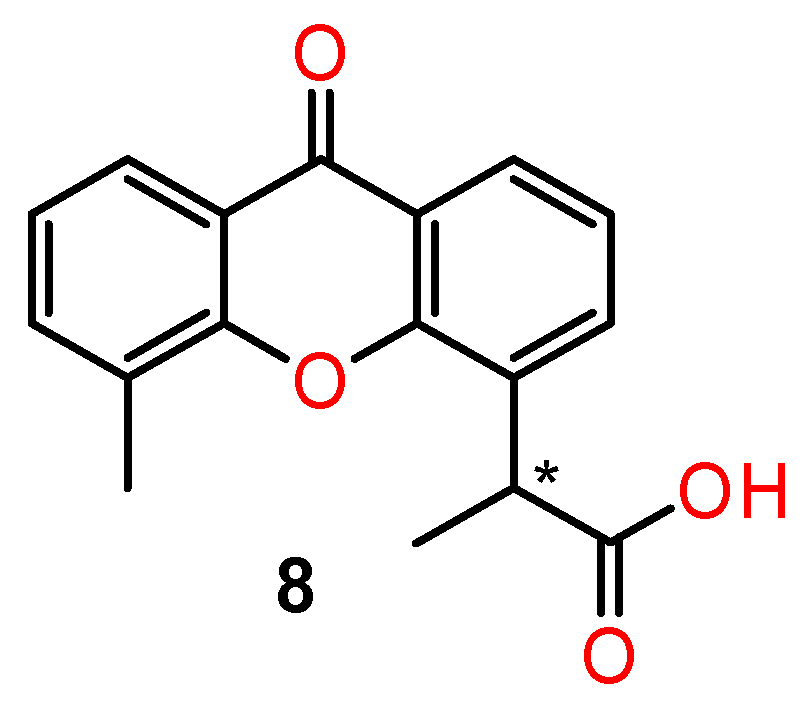
4.1. Chiral Xanthones
4.2. Chiral Baicalin
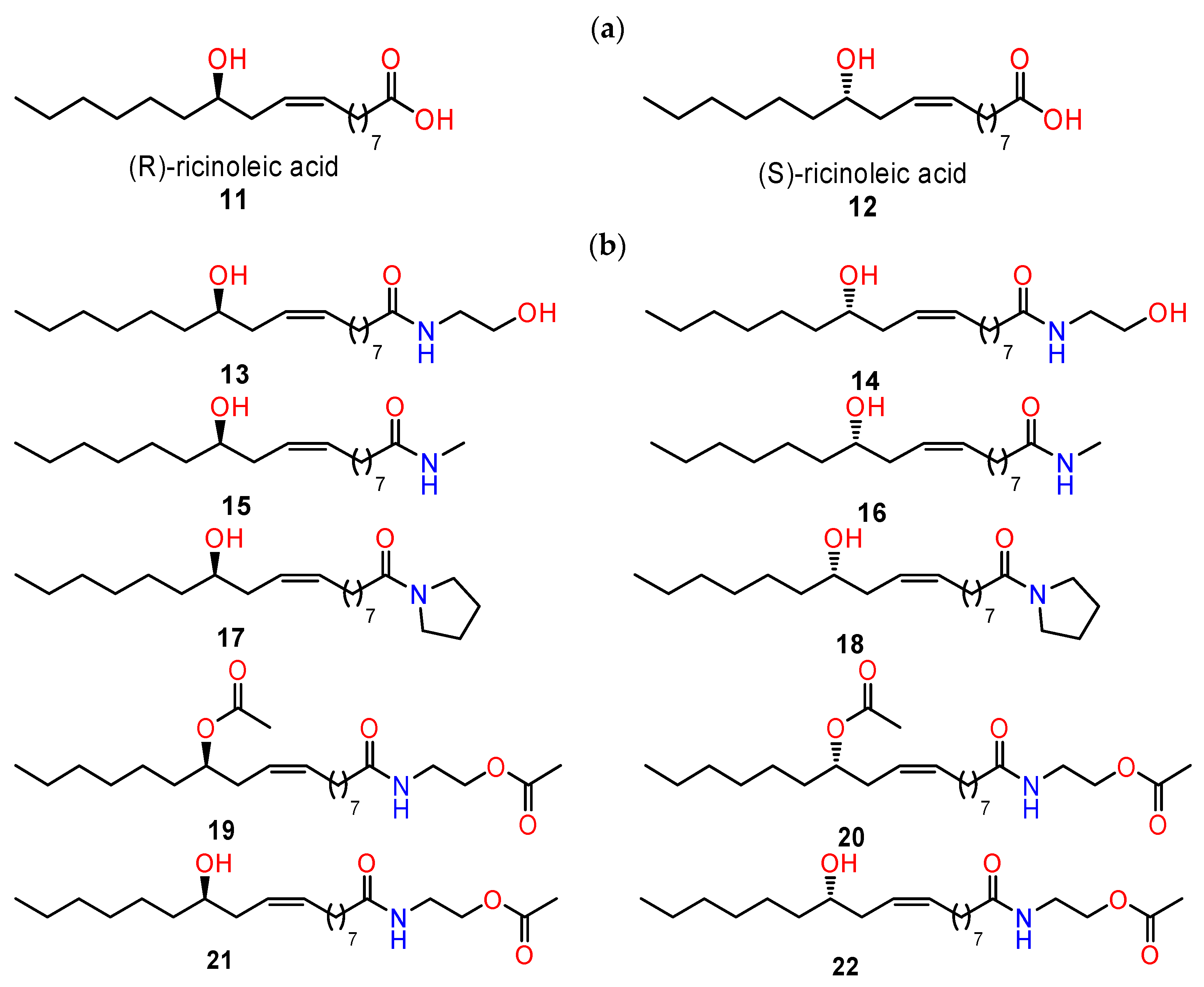
4.3. Chiral Derivatives of Ricinoleic Acid
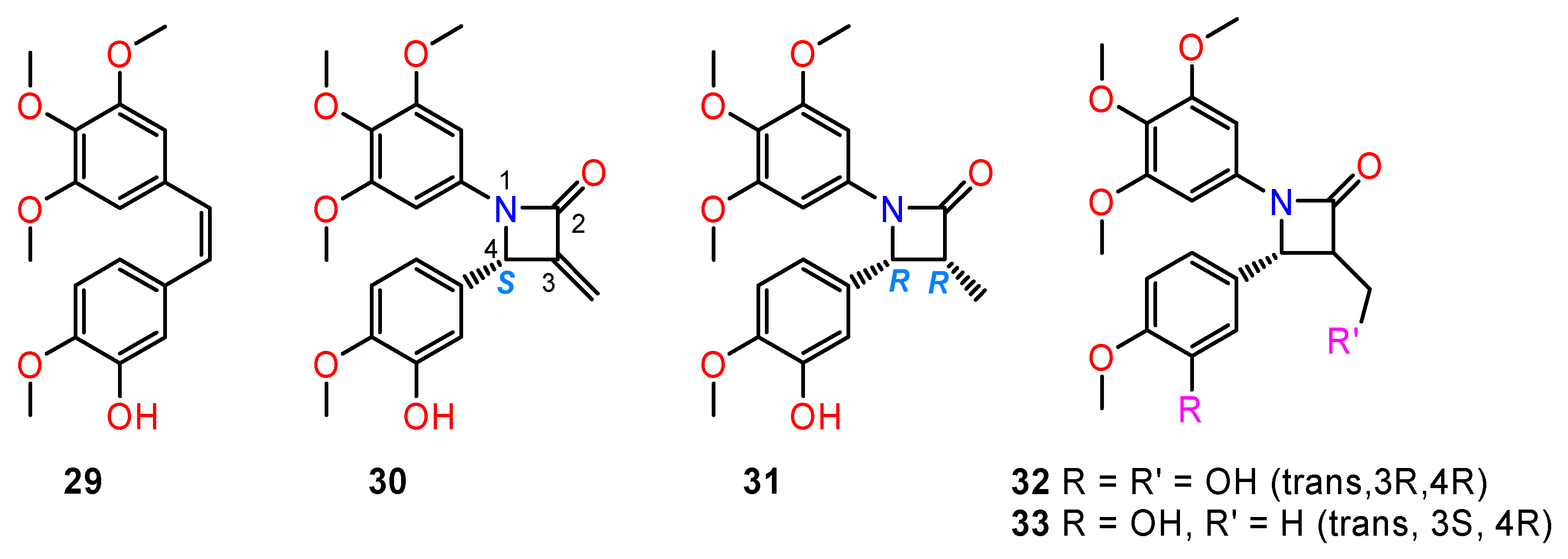
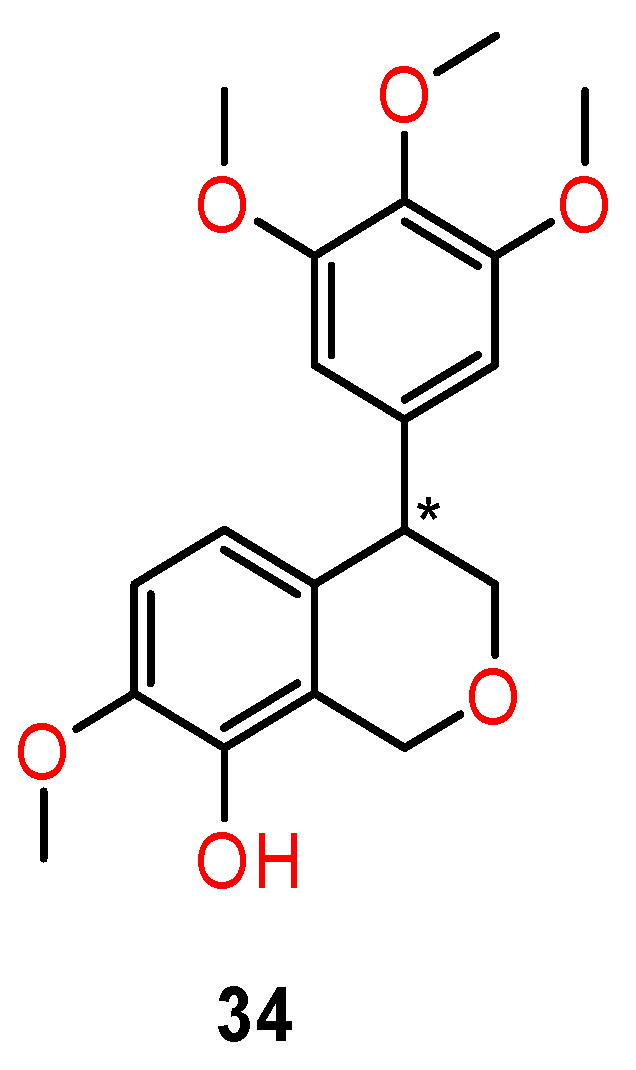
4.4. Chiral Anthramycin Derivatives
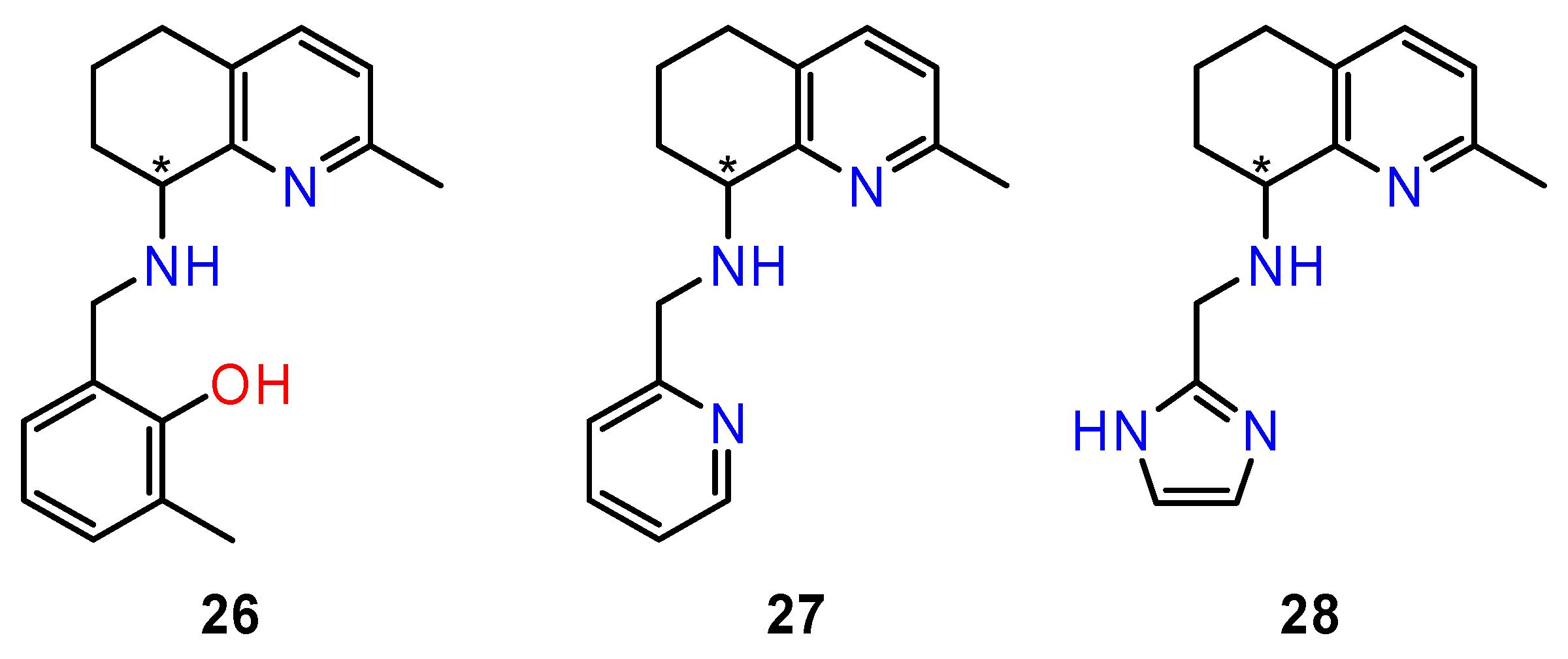
4.5. Derivatives of Tetrahydroquinolin-8-Amines
5. Inhibitors of Microtubule Polymerisation
5.1. Combretastatin A-4 Analogues
5.2. Analogues of 4-Arylisochromenes
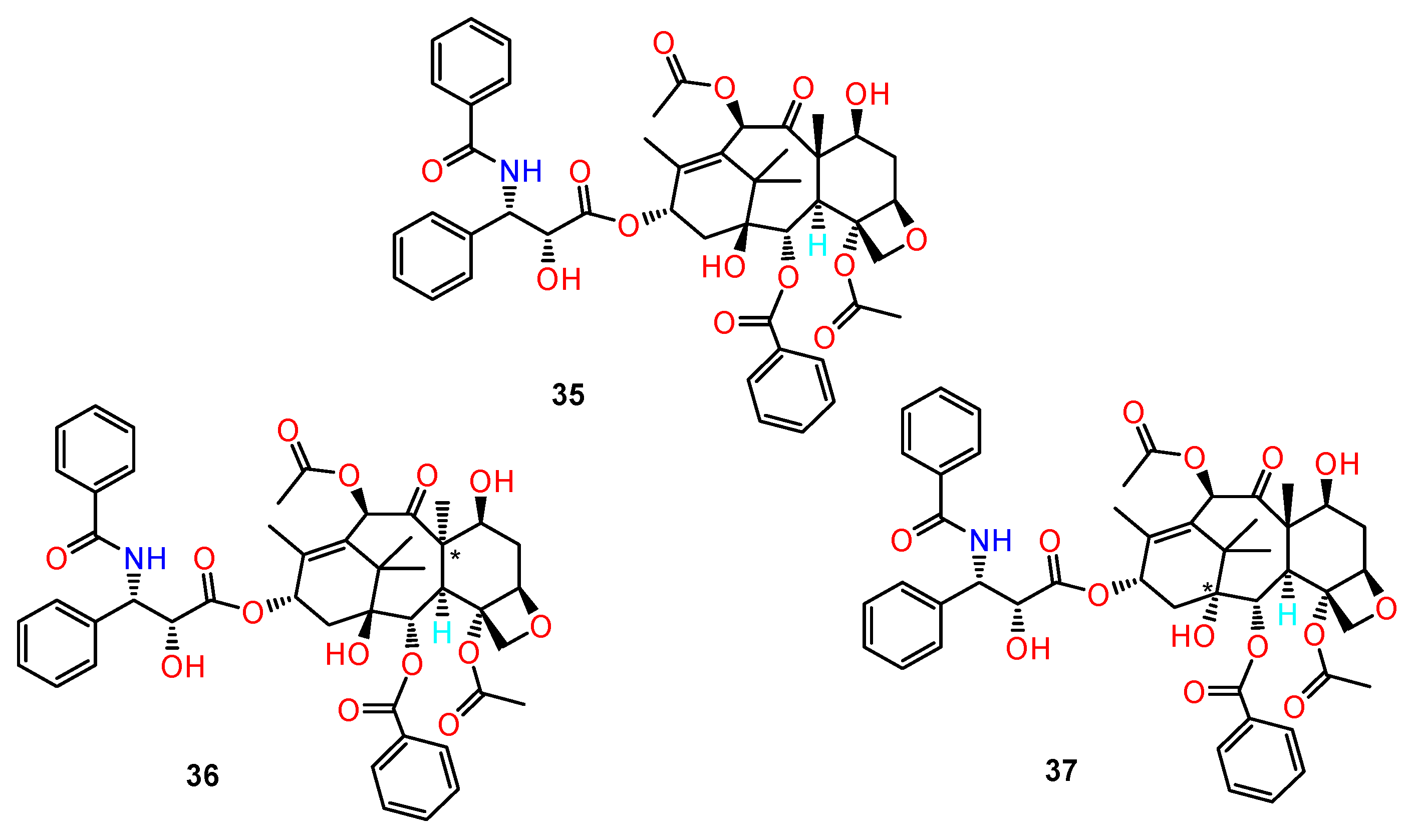
5.3. Taxol Isomers
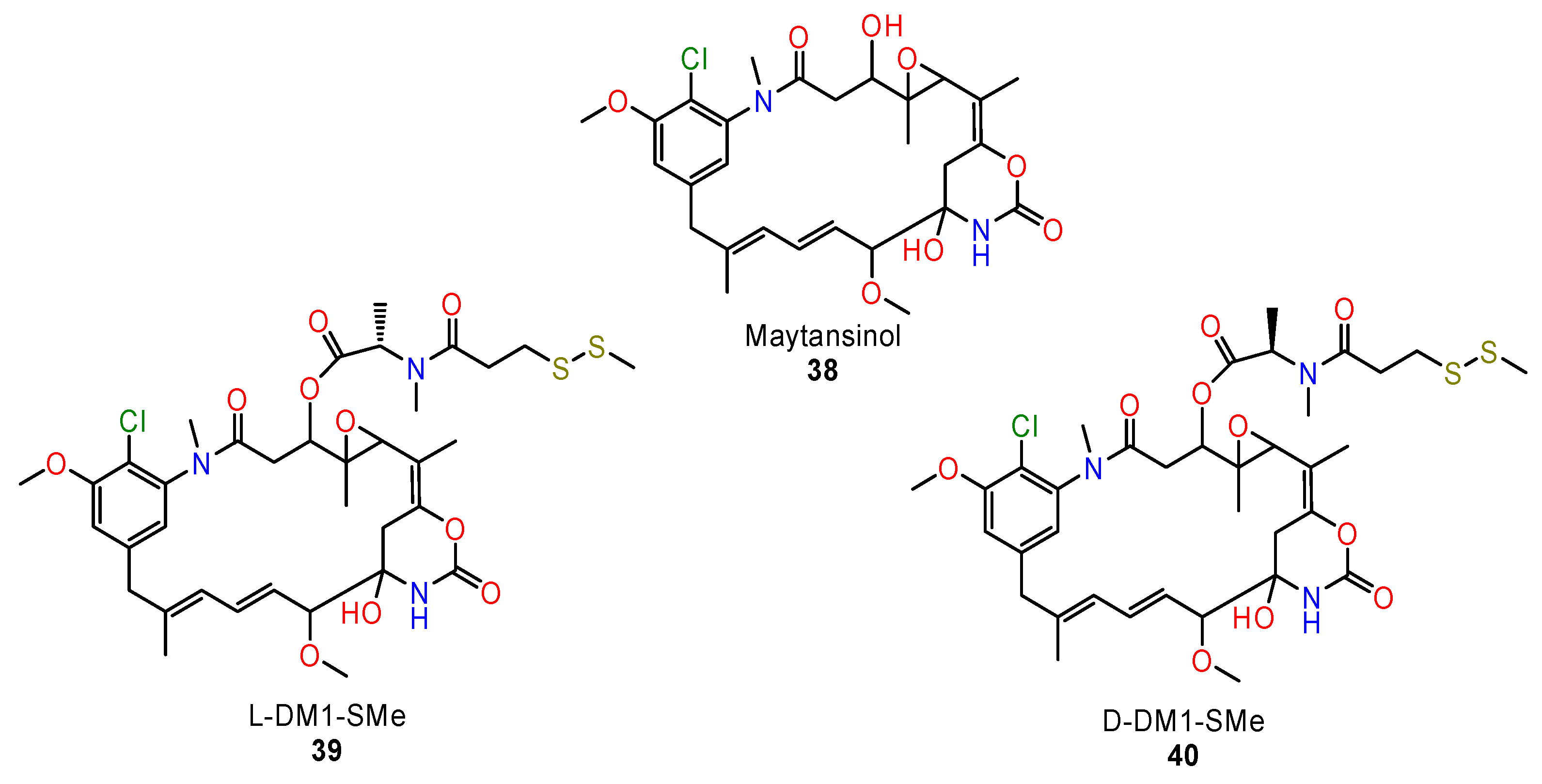
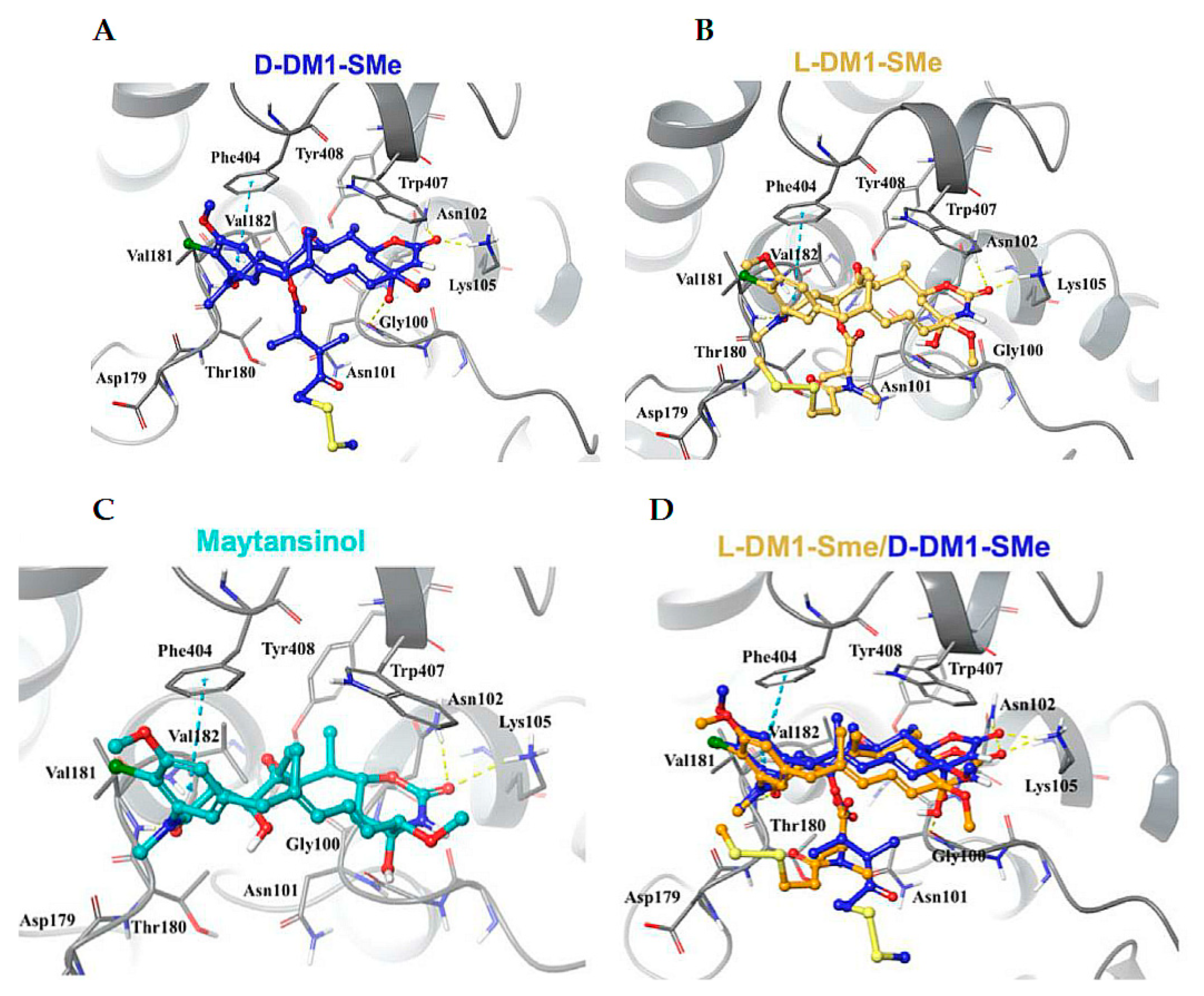
5.4. Maytansinoids
6. Proteasome Inhibitors
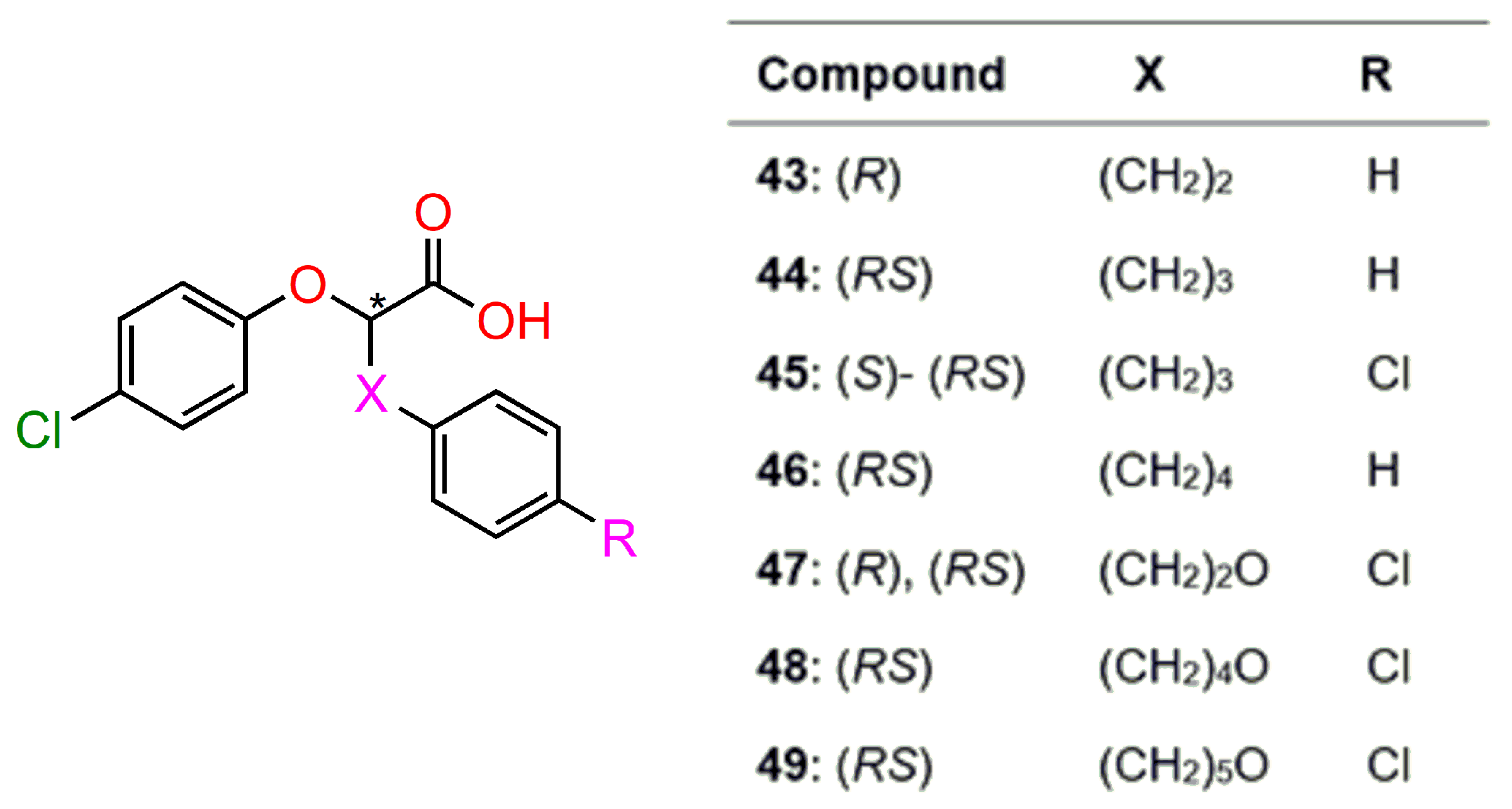
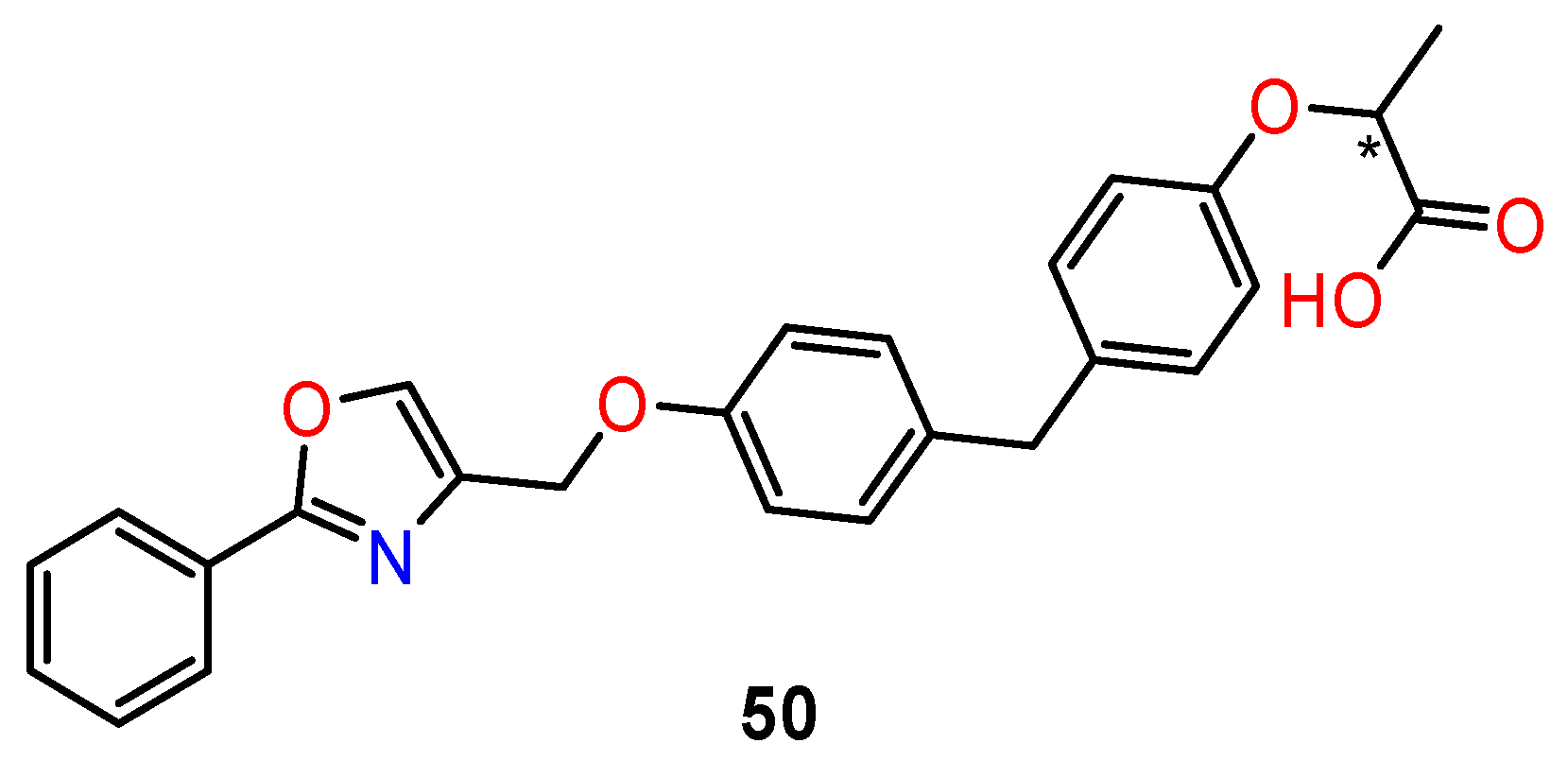
7. PPARs Proliferator
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Health Statistics and Information Systems. 2021. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 17 February 2023).
- Blagosklonny, M.V. Matching targets for selective cancer therapy. Drug Discov. Today 2003, 8, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Alama, A.; Orengo, A.M.; Ferrini, S.; Gangemi, R. Targeting cancer-initiating cell drug-resistance: A roadmap to a new-generation of cancer therapies? Drug Discov. Today 2012, 17, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell. Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Dembic, Z. Antitumor drugs and their targets. Molecules 2020, 25, 5776. [Google Scholar] [CrossRef]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 16, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.E. Design and synthesis of analogues of natural products. Org. Biomol. Chem. 2015, 13, 5302–5343. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Boldi, A.M. (Ed.) Combinatorial Synthesis of Natural Product-Based Libraries; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sun, H.; Liu, Z.; Zhao, H.; Ang, E.L. Recent advances in combinatorial biosynthesis for drug discovery. Drug Des. Dev. Ther. 2015, 9, 823–833. [Google Scholar]
- Kumar, S.V.; Saravanan, D.; Kumar, B.; Jayakumar, A. An update on prodrugs from natural products. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. 1), S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Du, J.; Tang, X.L. Natural products against cancer: A comprehensive bibliometric study of the research projects, publications, patents and drugs. J. Cancer Res. Ther. 2014, 10, C27–C37. [Google Scholar]
- Wang, Y.F.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Natural taxanes: Developments since 1828. Chem. Rev. 2011, 111, 7652–7709. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current perspectives on taxanes: Focus on their bioactivity, delivery and combination therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Lei, L.; Wang, X.J.; Tang, S.C. Novel taxanes in development: Hopes or hypes? Crit. Rev. Oncol. Hematol. 2022, 176, 103727. [Google Scholar] [CrossRef]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, recent advances and future prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.; Xu, H. Recent advances in the chemistry and biology of podophyllotoxins. Chem. Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, P.; Keglevich, A.; Hazai, L. New anticancer vinca alkaloids in the last decade—A mini-review. Curr. Org. Chem. 2021, 25, 1224–1234. [Google Scholar] [CrossRef]
- Levêque, D.; Jehl, F. Molecular pharmacokinetics of Catharanthus (vinca) alkaloids. J. Clin. Pharmacol. 2007, 47, 579–588. [Google Scholar] [CrossRef]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of the in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Li, W.-Q.; Morris-Natschke, S.L.; Qian, K.; Yang, L.; Zhu, G.-X.; Wu, X.-B.; Chen, A.-L.; Zhang, S.-Y.; Nan, X.; et al. Perspectives on biologically active camptothecin derivatives. Med. Res. Rev. 2015, 35, 753–789. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.S.; Adhikari, N.; Jha, T.; Gayen, S. A review on camptothecin analogs with promising cytotoxic profile, anti-cancer agents in medicinal chemistry. Anticancer Agents Med. Chem. 2018, 18, 1796–1814. [Google Scholar] [CrossRef] [PubMed]
- Giddings, L.A.; Newman, D.J. Microbial natural products: Molecular blueprints for antitumor drugs. J. Ind. Microbiol. Biotechnol. 2013, 40, 1181–1210. [Google Scholar] [CrossRef]
- Olsufyeva, E.N.; Yankovskaya, V.S. Main trends in the design of semi-synthetic antibiotics of a new generation. Russ. Chem. Rev. 2020, 89, 339–378. [Google Scholar] [CrossRef]
- Gross, H. Genomic mining—A concept for the discovery of new bioactive natural products. Curr. Opin. Drug Discov. Devel. 2009, 12, 207–219. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sorolla, M.A.; Gopal Krishnan, P.D.; Sorolla, A. From seabed to bedside: A review on promising marine anticancer compounds. Biomolecules 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Matulja, D.; Wittine, K.; Malatesti, N.; Laclef, S.; Turks, M.; Markovic, K.M.; Ambrožić, G.; Marković, D. Marine natural products with high anticancer activities. Curr. Med. Chem. 2020, 27, 1243–1307. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine anticancer agents: An overview with a particular focus on their chemical classes. Marine Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Blackmond, D.G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, a002147. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-Q.; Zhang, J.-G.; Cheng, J.-F. Overview of Chirality and Chiral Drugs. In Chiral Drugs: Chemistry and Biological Action; Lin, G.-Q., You, Q.-D., Cheng, J.-F., Eds.; John Wiley & Sons: New York, NY, USA, 2011; pp. 5–21. [Google Scholar]
- Burton, A.S.; Berger, E.L. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef]
- Pahwa, R.; Chhabra, J.; Kumar, R.; Narang, R. Melphalan: Recent insights on synthetic, analytical and medicinal aspects. Eur. J. Med. Chem. 2022, 238, 114494. [Google Scholar] [CrossRef]
- Shelley, M.D.; Hartley, L.; Groundwater, P.W.; Fish, R.G. Structure-activity studies on gossypol in tumor cell lines. Anticancer Drugs 2000, 11, 209–216. [Google Scholar] [CrossRef]
- Easson, L.H.; Stedman, E. Studies on the relationship between chemical constitution and physiological action: Molecular dissymmetry and physiological activity. Biochem. J. 1933, 27, 1257–1266. [Google Scholar] [CrossRef]
- Patel, B.K.; Hutt, A.J. Stereoselectivity in Drug Action and Disposition: An Overview. In Chirality in Drug Design and Development, 1st ed.; Reddy, I.K., Mehvar, R., Eds.; CRC Press: New York, NY, USA, 2004; pp. 139–190. [Google Scholar]
- Agrawal, Y.K.; Bhatt, H.G.; Raval, H.G.; Oza, P.M.; Gogoi, P.J. Chirality—A new era of therapeutics. Mini-Rev. Med. Chem. 2007, 7, 451–460. [Google Scholar] [CrossRef]
- Čižmáriková, R.; Valentová, J.; Horáková, R. Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis. Open Chem. 2020, 18, 628–647. [Google Scholar]
- Smith, S.W. Chiral toxicology: It’s the same thing… only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Čižmáriková, R.; Habala, L.; Valentová, J.; Markuliak, M. Survey of pharmacological activity and pharmacokinetics of selected β-adrenergic blockers in regard to their stereochemistry. Appl. Sci. 2019, 9, 625. [Google Scholar] [CrossRef]
- Čižmáriková, R.; Čižmárik, J.; Valentová, J.; Habala, L.; Markuliak, M. Chiral aspects of local anesthetics. Molecules 2020, 25, 2738. [Google Scholar] [CrossRef] [PubMed]
- Hutt, A.; Valentová, J. The chiral switch: The development of single enantiomer drugs from racemates. Acta Fac. Pharm. Univ. Comenianae 2003, 50, 1–16. [Google Scholar]
- Hutt, A.J. Chirality and pharmacokinetics: An area of neglected dimensionality? Drug Metabol. Drug Interact. 2007, 22, 79–112. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in drug pharmacokinetics and toxicity: Pharmacological relevance and analytical methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Gu, J.; Sui, Z.; Fang, C.; Tan, Q. Stereochemical considerations in pharmacokinetic processes of representative antineoplastic agents. Drug. Metab. Rev. 2017, 49, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Shah, U.; Patel, S. Drug stereochemistry: A prodigy for pharmacology and drug development. Curr. Drug. Discov. Technol. 2020, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A. Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.V.; Briand, L.E. Relevance and bio-catalytic strategies for the kinetic resolution of ketoprofen towards dexketoprofen. Crit. Rev. Biotechnol. 2018, 38, 778–800. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rao, C.V.; Seibert, K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996, 56, 4566–4569. [Google Scholar]
- Caban-Toktas, S.; Sahin, A.; Lule, S.; Esendagli, G.; Vural, I.; Karlı Oguz, K.; Soylemezoglu, F.; Mut, M.; Dalkara, T.; Khan, M.; et al. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int. J. Pharm. 2020, 578, 119076. [Google Scholar] [CrossRef]
- Wechter, W.J.; Kantoci, D.; Murray, E.D., Jr.; Quiggle, D.D.; Leipold, D.D.; Gibson, K.M.; McCracken, J.D. R-flurbiprofen chemoprevention and treatment of intestinal adenomas in the APC(Min)/+ mouse model: Implications for prophylaxis and treatment of colon cancer. Cancer Res. 1997, 57, 4316–4324. [Google Scholar]
- Wechter, W.J.; Leipold, D.D.; Murray, E.D., Jr.; Quiggle, D.; McCracken, J.D.; Barrios, R.S.; Greenberg, N.M. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000, 60, 2203–2208. [Google Scholar]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Wang, Y.; Huang, H.; Zhang, Q.; Zhang, P. Chirality in metal-based anticancer agents. Dalton Trans. 2018, 47, 4017–4026. [Google Scholar] [CrossRef]
- Romero, M.J.; Sadler, P.J. Chirality in Organometallic Anticancer Complexes. In Bioorganometallic Chemistry; Jaouen, G., Salmain, M., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 85–116. [Google Scholar]
- Valentová, J.; Lintnerová, L. Chirality in Anticancer Agents. In Current Topics in Chirality—From Chemistry to Biology; Akitsu, T., Ed.; IntechOpen: London, UK, 2021; pp. 139–153. [Google Scholar]
- Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M.E. Chiral flavonoids as antitumor agents. Pharmaceuticals 2021, 14, 1267. [Google Scholar] [CrossRef]
- Shagufta, I.A. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and cancer: From natural sources to mechanisms of action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive marine xanthones: A review. Mar. Drugs 2022, 20, 58. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From natural products to new synthetic small molecules: A journey through the world of xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone biosynthetic pathway in plants: A review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.S.; Niranjana, S.R.; Girish, K.S. An overview on genus garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.B.; Bussmann, R.W.; Hart, R.H.; Li, P.; Bai, Y.J.; Long, C.L. Garcinia in Southern China: Ethnobotany, management, and niche modeling. Econ. Bot. 2017, 70, 416–430. [Google Scholar] [CrossRef]
- Jia, C.; Xue, J.; Gong, C.; Li, X.; Li, D.; Li, Z.; Hua, H. Chiral resolution and anticancer effect of xanthones from Garcinia paucinervis. Fitoterapia 2018, 127, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Carraro, M.L.; Ribeiro, J.; Araújo, J.; Tiritan, M.E.; Pinto, M.M.M. Synthetic chiral derivatives of xanthones: Biological activities and enantioselectivity studies. Molecules 2019, 24, 791. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Atwell, G.J.; Baguley, B.C.; Boyd, M.; Thomsen, L.L.; Zhuang, L.; Denny, W.A. Potential antitumor agents. 63. Structure-activity relationships for side-chain analogues of the colon 38 active agent 9-oxo-9H-xanthene-4-acetic acid. J. Med. Chem. 1991, 34, 2864–2870. [Google Scholar] [CrossRef]
- Zhou, S.; Kestell, P.; Baguley, B.C.; Paxton, J.W. 5,6-dimethylxanthenone-4-acetic acid (DMXAA): A new biological response modifier for cancer therapy. Investig. New Drugs 2002, 20, 281–295. [Google Scholar] [CrossRef]
- Daei Farshchi Adli, A.; Jahanban-Esfahlan, R.; Seidi, K.; Samandari-Rad, S.; Zarghami, N. An overview on Vadimezan (DMXAA): The vascular disrupting agent. Chem. Biol. Drug Des. 2018, 91, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Zhao, Z.; Yang, J.; Chen, K. Research on effect of minor bupleurum decoction of proliferation and apoptosis of esophageal cancer cell strain eca-109 cell. Pak. J. Pharm. Sci. 2014, 27, 1675–1679. [Google Scholar]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor activity in vivo and vitro of new chiral derivatives of baicalin and induced apoptosis via the PI3K/Akt signaling pathway. Mol. Ther. Oncolytics 2020, 19, 67–78. [Google Scholar] [CrossRef]
- Pabiś, S.; Kula, J. Synthesis and bioactivity of (R)-ricinoleic acidderivatives: A review. Curr. Med. Chem. 2016, 23, 4037–4056. [Google Scholar] [CrossRef]
- Montes D’Oca, C.D.R.M.; Coelho, T.; Marinho, T.G.; Lopes Hack, C.R.; da Costa Duarte, R.; da Silva, P.A.; Montes D’Orca, M.G. Synthesis and antituberculosis activity of new fatty acid amides. Bioorg. Med. Chem. Lett. 2010, 20, 5255–5257. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Piovesan, L.A.; Montes D’Oca, C.R.; Lopes Hack, C.R.; Treptow, T.G.M.; Rodrigues, M.O.; Vendramini-Costa, D.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Montes D’Orca, M.G. Antiproliferative activity of synthetic fatty acid amides from renewable resources. Bioorg. Med. Chem. 2015, 23, 340–347. [Google Scholar] [CrossRef]
- Matysiak, S.; Chmiel, A.; Skolimowski, J.; Kula, J.; Pasternak, B.; Blaszczyk, A. Synthesis and cytotoxicity of (R)- and (S)-ricinoleic acid amides and their acetates. Chirality 2017, 29, 616–622. [Google Scholar] [CrossRef]
- Blaszczy, A.; Matysiak, S.; Kula, J.; Szostakiewicz, K.; Karkusiewicz, Z. Cytotoxic and genotoxic effects of (R)- and (S)-ricinoleic acid derivatives. Chirality 2020, 32, 998–1007. [Google Scholar] [CrossRef]
- Suckling, C. From multiply active natural product to candidate drug? Antibacterial (and other) minor groove binders for DNA. Future Med. Chem. 2012, 4, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Rao, M.V.; Laxman, N.; Ramesh, G.; Reddy, G.S.K. Recent developments in the design, synthesis and structure-activity relationship studies of pyrrolo[2,1-c][1,4]benzodiazepines as DNA-interactive antitumour antibiotics. Curr. Med. Chem. Anticancer Agents 2002, 2, 215–254. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, A.; Psurski, M.; Bagiński, M.; Bieszczad, B.; Mroczkowska, M.; Wilczek, M.; Czajkowska, J.; Trzybiński, D.; Woźniak, K.; Wietrzyk, J. Novel (S)-1,3,4,12a-tetrahydropyrazino[2,1-c][1,4]benzodiazepine-6,12(2H,11H)-dione derivatives: Selective inhibition of MV-4-11 biphenotypic B myelomonocytic leukemia cells’ growth is accompanied by reactive oxygen species overproduction and apoptosis. Bioorg. Med. Chem. Lett. 2018, 28, 618–625. [Google Scholar] [CrossRef]
- Bentley, K.W. Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef]
- Jantová, S.; Repický, A.; Letašiová, S.; Cipák, L. 4-Amino-3-acetylquinoline-induced apoptosis of murine L1210 leukemia cells involves ROS-mitochondrial-mediated death signaling and activation of p38 MAPK. Cell Biochem. Funct. 2008, 26, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Yang, Q.-X.; Teng, Y.-N.; Chen, S.; Gao, W.-J.; Guo, J.; Hsu, P.-L.; Liu, Y.; Morris-Natschke, S.L.; Hung, C.-C.; et al. Development of novel amino-quinoline-5,8-dione derivatives as NAD(P)H:quinone oxidoreductase 1 (NQO1) inhibitors with potent antiproliferative activities. Eur. J. Med. Chem. 2018, 154, 199–209. [Google Scholar] [CrossRef]
- Facchetti, G.; Christodoulou, M.S.; Mendoza, L.B.; Cusinato, F.; Dalla Via, L.; Rimoldi, I. Biological properties of new chiral 2-methyl-5,6,7,8-tetrahydroquinolin-8-amine-based compounds. Molecules 2020, 25, 5561. [Google Scholar] [CrossRef]
- Jordan, A.; Hadfield, J.A.; Lawrence, N.J.; McGown, A.T. Tubulin as a target for anticancer drugs: Agents which interact with the mitotic spindle. Med. Res. Rev. 1998, 18, 259–296. [Google Scholar] [CrossRef]
- Sherbet, G.V. Combretastatin analogues in cancer biology: A prospective view. Cell. Biochem. 2020, 121, 2127–2138. [Google Scholar] [CrossRef]
- Zhou, P.; Liang, Y.; Zhang, H.; Jiang, H.; Feng, K.; Xu, P.; Wang, J.; Wang, X.; Ding, K.; Luo, C.; et al. Design, synthesis, biological evaluation and cocrystal structures with tubulin of chiral β-lactam bridged combretastatin A-4 analogues as potent antitumor agents. Eur. J. Med. Chem. 2018, 144, 817–842. [Google Scholar] [CrossRef]
- Dong, M.; Liu, F.; Zhou, H.; Zhai, S.; Yan, B. Novel natural product and privileged scaffold-based tubulin inhibitors targeting the colchicine binding site. Molecules 2016, 21, 1375. [Google Scholar] [CrossRef]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Raza, K.; Kaushik, L.; Malik, R.; Arora, S.; Katare, O.P. Role of colloidal drug delivery carriers in Taxane-mediated chemotherapy: A review. Curr. Pharm. Des. 2016, 22, 5127–5143. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Arnst, J. When Taxol met tubulin. J. Biol. Chem. 2020, 295, 13994–13995. [Google Scholar] [CrossRef] [PubMed]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Stiles, L.; Nelson, D.J. Molecular dynamics simulations of complexes between wild-type and mutant anthrax protective antigen variants and a model anthrax toxin receptor. J. Biomol. Struct. Dyn. 2005, 22, 503–519. [Google Scholar] [CrossRef]
- Ghadari, R.; Alavi, F.S.; Zahedi, M. Evaluation of the effect of the chiral centers of Taxol on binding to β-tubulin: A docking and molecular dynamics simulation study. Comput. Biol. Chem. 2015, 56, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Dong, Y.H.; Wang, D.F.; Liu, Z.P. Tubulin maytansine site binding ligands and their applications as MTAs and ADCs for cancer therapy. Curr. Med. Chem. 2020, 27, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Widdison, W.C.; Wilhelm, S.D.; Cavanagh, E.E.; Whiteman, K.R.; Leece, B.A.; Kovtun, Y.; Goldmacher, V.S.; Xie, H.; Steeves, R.M.; Lutz, R.J.; et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J. Med. Chem. 2006, 49, 4392–4408. [Google Scholar] [CrossRef]
- Li, W.; Huang, M.; Li, Y.; Xia, A.; Tan, L.; Zhang, Z.; Wang, Y.; Yang, J. C3 ester side chain plays a pivotal role in the antitumor activity of Maytansinoids. Biochem. Biophys. Res. Commun. 2021, 566, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Nunes, A.T.; Annunziata, C.M. Proteasome inhibitors: Structure and function. Semin. Oncol. 2017, 44, 377–380. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome inhibitor drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef]
- Kaplan, G.S.; Torcun, C.C.; Grune, T.; Ozer, N.Z.; Karademir, B. Proteasome inhibitors in cancer therapy: Treatment regimen and peripheral neuropathy as a side effect. Free Rad. Biol. Med. 2017, 103, 1–13. [Google Scholar] [CrossRef]
- Anchoori, R.K.; George, L.; Tseng, S.-H.; Lam, B.; Polkampally, S.; Amiano, A.D.; Foran, P.; Tsingine, H.; Samanapally, H.; Velasquez, F.C.; et al. Chirality and asymmetry increase the potency of candidate ADRM1/RPN13 inhibitors. PLoS ONE 2021, 16, e0256937. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Botta, M.; Audano, M.; Sahebkar, A.; Sirtori, C.R.; Mitro, N.; Ruscica, M. PPAR agonists and metabolic syndrome: An established role? Int. J. Mol. Sci. 2018, 19, 1197. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. The role of PPARs in disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.-D. PPAR beta/delta and the hallmarks of cancer. Cells 2020, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-γ modulators as current and potential cancer treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Yang, K.; Chi, T.; Liao, Z.; Wei, P. PPAR-α modulators as current and potential cancer treatments. Front. Oncol. 2021, 11, 599995. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Tamura, T.; Henmi, K.; Kuboyama, T.; Yanagisawa, A.; Matsubara, M.; Takahashi, Y.; Suzuki, M.; Saito, J.I.; Ueno, K.; et al. Development of dihydrodibenzooxepine peroxisome proliferator-activated receptor (PPAR) gamma ligands of a novel binding mode as anticancer agents: Effective mimicry of chiral structures by olefinic E/Z-isomers. J. Med. Chem. 2018, 61, 10067–10083. [Google Scholar] [CrossRef]
- Sabatino, L.; Ziccardi, P.; Cerchia, C.; Muccillo, L.; Piemontese, L.; Loiodice, F.; Colantuoni, V.; Lupo, A.; Lavecchia, A. Chiral phenoxyacetic acid analogues inhibit colon cancer cell proliferation acting as PPARγ partial agonists. Sci. Rep. 2019, 9, 5434. [Google Scholar] [CrossRef]
- Piemontese, L.; Cerchia, C.; Laghezza, A.; Ziccardi, P.; Sblano, S.; Tortorella, P.; Iacobazzi, V.; Infantino, V.; Convertini, P.; Dal Piaz, F.; et al. New diphenylmethane derivatives as peroxisome proliferator-activated receptor alpha/gamma dual agonists endowed with anti-proliferative effects and mitochondrial activity. Eur. J. Med. Chem. 2017, 127, 379–397. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentová, J.; Lintnerová, L.; Miklášová, N.; Oboňová, B.; Habala, L. Analogues of Anticancer Natural Products: Chiral Aspects. Int. J. Mol. Sci. 2023, 24, 5679. https://doi.org/10.3390/ijms24065679
Valentová J, Lintnerová L, Miklášová N, Oboňová B, Habala L. Analogues of Anticancer Natural Products: Chiral Aspects. International Journal of Molecular Sciences. 2023; 24(6):5679. https://doi.org/10.3390/ijms24065679
Chicago/Turabian StyleValentová, Jindra, Lucia Lintnerová, Natalia Miklášová, Bianka Oboňová, and Ladislav Habala. 2023. "Analogues of Anticancer Natural Products: Chiral Aspects" International Journal of Molecular Sciences 24, no. 6: 5679. https://doi.org/10.3390/ijms24065679
APA StyleValentová, J., Lintnerová, L., Miklášová, N., Oboňová, B., & Habala, L. (2023). Analogues of Anticancer Natural Products: Chiral Aspects. International Journal of Molecular Sciences, 24(6), 5679. https://doi.org/10.3390/ijms24065679






