Chromatin Remodeling Enzyme Cluster Predicts Prognosis and Clinical Benefit of Therapeutic Strategy in Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
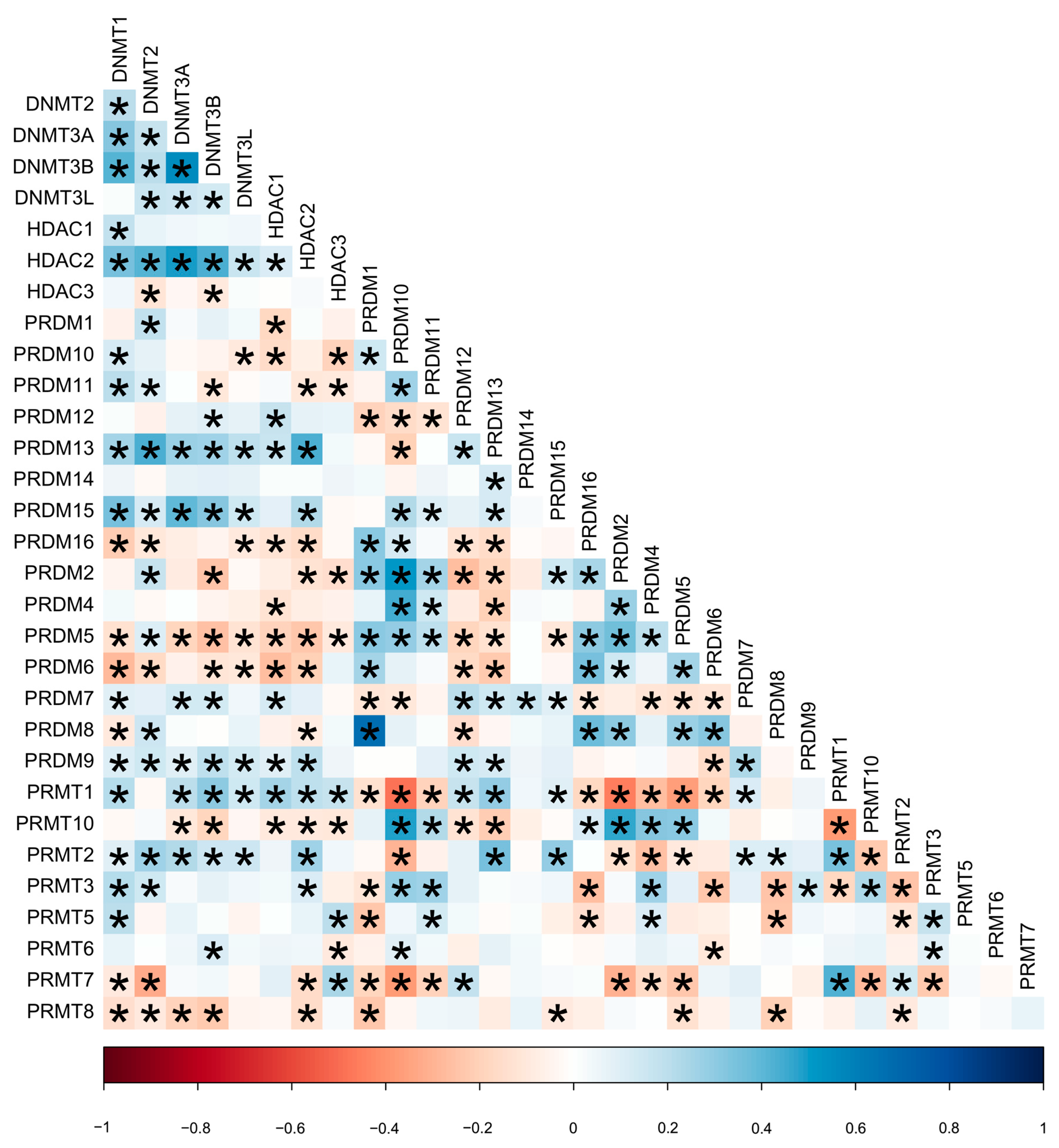
2.2. Hierarchical Clustering Analysis
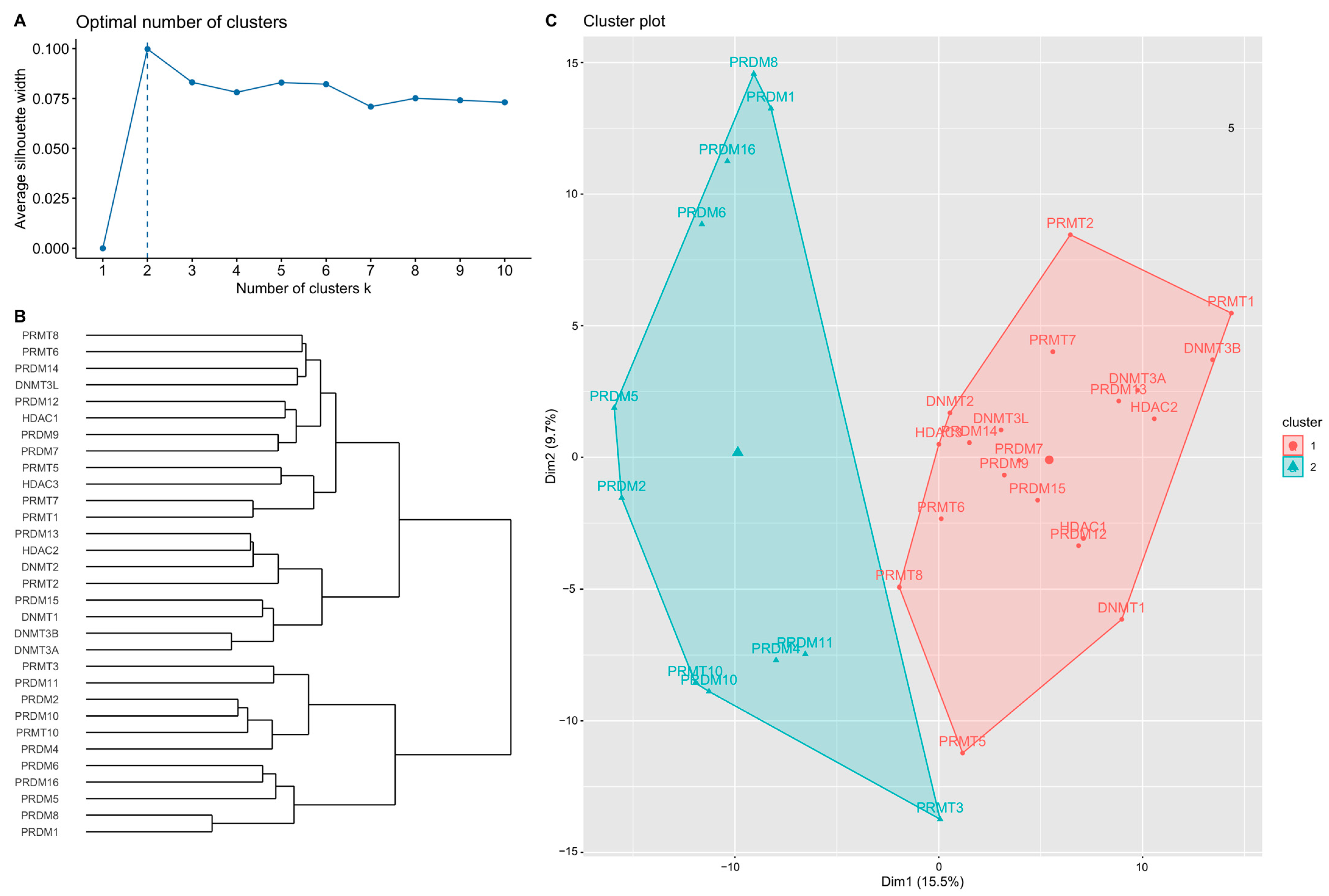
2.3. Two Clusters According to the Optimal Number in Analysis
2.4. Higher mRNA Expression of Target Genes in High-Risk Groups
2.5. High- and Low-Risk Groups Clinical Manifestation
2.6. Progression-Free Survival Is Lower in the High-Risk Group of GC1
2.7. mRNA Expression and Progression-Free Survival Analysis of GC1 High-Risk Group Target Genes According to TNBC and Non-TNBC Subgroups
3. Discussion
4. Material and Methods
4.1. Data Source
4.2. Messenger RNA (mRNA) Expression
4.3. Hierarchical Clustering
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G., Jr.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Trail, P.A.; Dubowchik, G.M.; Lowinger, T.B. Antibody drug conjugates for treatment of breast cancer: Novel targets and diverse approaches in ADC design. Pharmacol. Ther. 2018, 181, 126–142. [Google Scholar] [CrossRef]
- Blum, J.L.; Flynn, P.J.; Yothers, G.; Asmar, L.; Geyer, C.E., Jr.; Jacobs, S.A.; Robert, N.J.; Hopkins, J.O.; O'Shaughnessy, J.A.; Dang, C.T.; et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 2017, 35, 2647–2655. [Google Scholar] [CrossRef]
- Masood, S. Neoadjuvant chemotherapy in breast cancers. Womens Health 2016, 12, 480–491. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Mittendorf, E.A.; Clarke, C.A.; Lichtensztajn, D.Y.; Hunt, K.K.; Giordano, S.H. Incorporating Tumor Characteristics to the American Joint Committee on Cancer Breast Cancer Staging System. Oncologist 2017, 22, 1292–1300. [Google Scholar] [CrossRef]
- Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; Wang, Y.C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; van't Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Lænkholm, A.V.; Jensen, M.B.; Eriksen, J.O.; Rasmussen, B.B.; Knoop, A.S.; Buckingham, W.; Ferree, S.; Schaper, C.; Nielsen, T.O.; Haffner, T.; et al. PAM50 Risk of Recurrence Score Predicts 10-Year Distant Recurrence in a Comprehensive Danish Cohort of Postmenopausal Women Allocated to 5 Years of Endocrine Therapy for Hormone Receptor-Positive Early Breast Cancer. J. Clin. Oncol. 2018, 36, 735–740. [Google Scholar] [CrossRef]
- Sestak, I.; Martín, M.; Dubsky, P.; Kronenwett, R.; Rojo, F.; Cuzick, J.; Filipits, M.; Ruiz, A.; Gradishar, W.; Soliman, H.; et al. Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res. Treat. 2019, 176, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Noordhoek, I.; Treuner, K.; Putter, H.; Zhang, Y.; Wong, J.; Meershoek-Klein Kranenbarg, E.; Duijm-de Carpentier, M.; van de Velde, C.J.H.; Schnabel, C.A.; Liefers, G.J. Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR(+) Early-stage Breast Cancer for 10 Years of Endocrine Therapy. Clin. Cancer Res. 2021, 27, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Schmitt, F. An Update on Breast Cancer Multigene Prognostic Tests-Emergent Clinical Biomarkers. Front. Med. 2018, 5, 248. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Bayani, J.; Marshall, A.; Dunn, J.A.; Campbell, A.; Cunningham, C.; Sobol, M.S.; Hall, P.S.; Poole, C.J.; Cameron, D.A.; et al. Comparing Breast Cancer Multiparameter Tests in the OPTIMA Prelim Trial: No Test Is More Equal Than the Others. J. Natl. Cancer Inst. 2016, 108, djw050. [Google Scholar] [CrossRef]
- Bianchini, G.; Qi, Y.; Alvarez, R.H.; Iwamoto, T.; Coutant, C.; Ibrahim, N.K.; Valero, V.; Cristofanilli, M.; Green, M.C.; Radvanyi, L.; et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers. J. Clin. Oncol. 2010, 28, 4316–4323. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Dworkin, A.M.; Huang, T.H.; Toland, A.E. Epigenetic alterations in the breast: Implications for breast cancer detection, prognosis and treatment. Semin. Cancer Biol. 2009, 19, 165–171. [Google Scholar] [CrossRef]
- Stefansson, O.A.; Esteller, M. Epigenetic modifications in breast cancer and their role in personalized medicine. Am. J. Pathol. 2013, 183, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics 2012, 4, 5. [Google Scholar] [CrossRef]
- Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Sorrentino, A.; Fiore, D.; Proto, M.C.; Moncharmont, B.; Gazzerro, P.; Bifulco, M.; Abbondanza, C. Multifaceted Role of PRDM Proteins in Human Cancer. Int. J. Mol. Sci. 2020, 21, 2648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Cyprian, F.S.; Akhtar, S.; Gatalica, Z.; Vranic, S. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: A new clinical paradigm in the treatment of triple-negative breast cancer. Bosn. J. Basic Med. Sci. 2019, 19, 227–233. [Google Scholar] [CrossRef]
- Zimmer, A.S.; Gillard, M.; Lipkowitz, S.; Lee, J.M. Update on PARP Inhibitors in Breast Cancer. Curr. Treat. Options Oncol. 2018, 19, 21. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lu, Z.; Luo, R.Z.; Zhang, X.; Seto, E.; Liao, W.S.; Yu, Y. Multiple histone deacetylases repress tumor suppressor gene ARHI in breast cancer. Int. J. Cancer 2007, 120, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Chen, Y.Y.; Scott, G.K.; Devries, S.; Chin, K.; Benz, C.C.; Waldman, F.M.; Hwang, E.S. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin. Cancer Res. 2009, 15, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cao, R.X.; Zu, X.Y.; Hong, T.; Yang, J.; Liu, L.; Xiao, X.H.; Ding, W.J.; Zhao, Q.; Liu, J.H.; et al. Identification and characterization of novel spliced variants of PRMT2 in breast carcinoma. FEBS J. 2012, 279, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cao, R.X.; Liu, J.H.; Liu, Y.B.; Wang, J.; Liu, L.P.; Chen, Y.J.; Yang, J.; Zhang, Q.H.; Wu, Y.; et al. Nuclear loss of protein arginine N-methyltransferase 2 in breast carcinoma is associated with tumor grade and overexpression of cyclin D1 protein. Oncogene 2014, 33, 5546–5558. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, Y.J.; Chen, L.; Shen, Y.Y.; Zhang, Q.H.; Yang, J.; Cao, R.X.; Zu, X.Y.; Wen, G.B. PRMT2β, a C-terminal splice variant of PRMT2, inhibits the growth of breast cancer cells. Oncol. Rep. 2017, 38, 1303–1311. [Google Scholar] [CrossRef]
- Oh, T.G.; Wang, S.M.; Muscat, G.E. Therapeutic Implications of Epigenetic Signaling in Breast Cancer. Endocrinology 2017, 158, 431–447. [Google Scholar] [CrossRef]
- Hernandez, S.J.; Dolivo, D.M.; Dominko, T. PRMT8 demonstrates variant-specific expression in cancer cells and correlates with patient survival in breast, ovarian and gastric cancer. Oncol. Lett. 2017, 13, 1983–1989. [Google Scholar] [CrossRef]
- Yao, R.; Jiang, H.; Ma, Y.; Wang, L.; Wang, L.; Du, J.; Hou, P.; Gao, Y.; Zhao, L.; Wang, G.; et al. PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 2014, 74, 5656–5667. [Google Scholar] [CrossRef]
- Eram, M.S.; Bustos, S.P.; Lima-Fernandes, E.; Siarheyeva, A.; Senisterra, G.; Hajian, T.; Chau, I.; Duan, S.; Wu, H.; Dombrovski, L.; et al. Trimethylation of histone H3 lysine 36 by human methyltransferase PRDM9 protein. J. Biol. Chem. 2014, 289, 12177–12188. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.; Houle, A.A.; Hussin, J.G.; Awadalla, P. The impact of recombination on human mutation load and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Paigen, K.; Petkov, P.M. PRDM9 and Its Role in Genetic Recombination. Trends Genet. 2018, 34, 291–300. [Google Scholar] [CrossRef]
- Ding, B.; Yan, L.; Zhang, Y.; Wang, Z.; Zhang, Y.; Xia, D.; Ye, Z.; Xu, H. Analysis of the role of mutations in the KMT2D histone lysine methyltransferase in bladder cancer. FEBS Open Bio. 2019, 9, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.E.; Zheng, H.; Saad, M.A.; Rahimy, M.; Ku, J.; Kuo, S.Z.; Honda, T.K.; Wang-Rodriguez, J.; Xuan, Y.; Korrapati, A.; et al. The non-coding landscape of head and neck squamous cell carcinoma. Oncotarget 2016, 7, 51211–51222. [Google Scholar] [CrossRef]
- Reid, A.G.; Nacheva, E.P. A potential role for PRDM12 in the pathogenesis of chronic myeloid leukaemia with derivative chromosome 9 deletion. Leukemia 2004, 18, 178–180. [Google Scholar] [CrossRef]
- Sorrentino, A.; Federico, A.; Rienzo, M.; Gazzerro, P.; Bifulco, M.; Ciccodicola, A.; Casamassimi, A.; Abbondanza, C. PR/SET Domain Family and Cancer: Novel Insights from the Cancer Genome Atlas. Int. J. Mol. Sci. 2018, 19, 3250. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, H.; He, T.; Yang, J.; Tao, H.; Wang, Y.; Hu, Q. Overexpression of PRDM13 inhibits glioma cells via Rho and GTP enzyme activation protein. Int. J. Mol. Med. 2018, 42, 966–974. [Google Scholar] [CrossRef]
- Mzoughi, S.; Fong, J.Y.; Papadopoli, D.; Koh, C.M.; Hulea, L.; Pigini, P.; Di Tullio, F.; Andreacchio, G.; Hoppe, M.M.; Wollmann, H.; et al. PRDM15 is a key regulator of metabolism critical to sustain B-cell lymphomagenesis. Nat. Commun. 2020, 11, 3520. [Google Scholar] [CrossRef]
- Mzoughi, S.; Zhang, J.; Hequet, D.; Teo, S.X.; Fang, H.; Xing, Q.R.; Bezzi, M.; Seah, M.K.Y.; Ong, S.L.M.; Shin, E.M.; et al. PRDM15 safeguards naive pluripotency by transcriptionally regulating WNT and MAPK-ERK signaling. Nat. Genet. 2017, 49, 1354–1363. [Google Scholar] [CrossRef]
- Moelans, C.B.; de Weger, R.A.; Monsuur, H.N.; Vijzelaar, R.; van Diest, P.J. Molecular profiling of invasive breast cancer by multiplex ligation-dependent probe amplification-based copy number analysis of tumor suppressor and oncogenes. Mod. Pathol. 2010, 23, 1029–1039. [Google Scholar] [CrossRef]
- Moelans, C.B.; van der Groep, P.; Hoefnagel, L.D.C.; van de Vijver, M.J.; Wesseling, P.; Wesseling, J.; van der Wall, E.; van Diest, P.J. Genomic evolution from primary breast carcinoma to distant metastasis: Few copy number changes of breast cancer related genes. Cancer Lett. 2014, 344, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, N.; Toyota, M.; Suzuki, H.; Honma, T.; Fujikane, T.; Ohmura, T.; Nishidate, T.; Ohe-Toyota, M.; Maruyama, R.; Sonoda, T.; et al. Gene amplification and overexpression of PRDM14 in breast cancers. Cancer Res. 2007, 67, 9649–9657. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Shakoori, A.; Zarnogi, S.; Sun, X.; Varma, S.; Lee, E.; Shokrani, B.; Laiyemo, A.O.; Washington, K.; Brim, H. Reduced Representation Bisulfite Sequencing Determination of Distinctive DNA Hypermethylated Genes in the Progression to Colon Cancer in African Americans. Gastroenterol. Res. Pract. 2016, 2016, 2102674. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, M.O.; Bryan, R.T.; Emes, R.D.; Glossop, J.R.; Luscombe, C.; Cheng, K.K.; Zeegers, M.P.; James, N.D.; Devall, A.J.; Mein, C.A.; et al. Quantitative genome-wide methylation analysis of high-grade non-muscle invasive bladder cancer. Epigenetics 2016, 11, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Brinkman, P.; Boksem, R.J.; Rhodius, R.J.; Witte, B.I.; Zwinderman, A.H.; Heideman, D.A.; Duin, S.; Koning, R.; Steenbergen, R.D.; et al. Combined sputum hypermethylation and eNose analysis for lung cancer diagnosis. J. Clin. Pathol. 2014, 67, 707–711. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef]




| Characteristics | Overall |
|---|---|
| Diagnosis age (years), mean ± SD | 55.9 ± 12.4 |
| Age group ≥ 50 years | 329 (68%) |
| Age group < 50 years | 157 (32%) |
| Subtypes | |
| Triple negative breast cancer (TNBC) | 106 (22%) |
| Luminal A | 224 (46.1%) |
| Luminal B | 116 (23.9%) |
| HER2 type | 40 (8.2%) |
| Pathological stage | |
| Stage I | 87 (18%) |
| Stage II | 300 (62%) |
| Stage III | 99 (20%) |
| IIIA | 70 (14.4%) |
| IIIB | 6 (1.2%) |
| IIIC | 23 (4.7%) |
| Stage IV | 0 (0%) |
| Tumor size | |
| T1 (<2 cm) | 144 (29.6%) |
| T2 (2 cm–5 cm) | 303 (62.3%) |
| T3 (>5 cm) | 32 (6.6%) |
| T4 (direct to chest wall or skin) | 7 (1.4%) |
| Lymph node status | |
| Non-lymph node invasion (LN0) | 225 (46%) |
| Lymph node invasion (LN+) | 261 (54%) |
| N1 (1–3) | 178 (36.6%) |
| N2 (4–9) | 59 (12.1%) |
| N3 (≥10) | 24 (4.9%) |
| Treatment | |
| Radiotherapy (RT) | 305 (63%) |
| Chemotherapy (CT) | 386 (79%) |
| Treatment subgroup | |
| RT alone | 52 (10.7%) |
| CT alone | 133 (27.4%) |
| Chemotherapy + Radiotherapy | 253 (52%) |
| Survival outcome | |
| Died | 29 (6%) |
| Disease progressed | 45 (9.3%) |
| Genes | mRNA Expression | Genes | mRNA Expression |
|---|---|---|---|
| DNMT1 | 1.75 (−1.75, 6.11) | PRDM2 | −1.53 (−8.84, 2.85) |
| DNMT2 | −2.06 (−8.62, 5.47) | PRDM4 | 0.53 (−7.29, 6.63) |
| DNMT3A | 1.96 (−1.48, 6.86) | PRDM5 | −2.83 (−11.00, 3.04) |
| DNMT3B | 2.19 (−2.45, 7.70) | PRDM6 | −0.13 (−4.58, 6.60) |
| DNMT3L | −3.12 (−3.12, 31.38) | PRDM7 | -1.86 (-1.86, 6.63) |
| HDAC1 | 0.96 (−5.19, 4.74) | PRDM8 | −2.58 (−9.36, 3.47) |
| HDAC2 | 1.38 (−4.16, 10.61) | PRDM9 | −14.32 (−14.32, 56.65) |
| HDAC3 | 0.17 (−6.33, 5.73) | PRMT1 | 0.97 (−2.07, 4.77) |
| PRDM1 | 0.34 (−4.65, 4.93) | PRMT10 | −1.51 (−8.33, 2.63) |
| PRDM10 | −0.38 (−4.63, 3.37) | PRMT2 | −1.14 (−4.80, 7.08) |
| PRDM11 | −1.70 (−6.01, 2.43) | PRMT3 | 0.80 (−5.64, 7.34) |
| PRDM12 | 1.11 (−2.13, 7.80) | PRMT5 | 0.60 (−5.05, 8.60) |
| PRDM13 | −2.24 (−2.24, 21.56) | PRMT6 | 0.66 (−6.83, 4.13) |
| PRDM14 | −5.16 (−5.16, 10.82) | PRMT7 | −0.001 (−3.79, 6.10) |
| PRDM15 | 0.50 (−3.15, 4.44) | PRMT8 | −0.76 (−1.64, 14.55) |
| PRDM16 | −2.80 (−6.56, 2.62) |
| Genes | Gene Cluster 1 | p | |
|---|---|---|---|
| Low-Risk (n = 389) | High-Risk (n = 97) | ||
| Gene cluster 1 included | |||
| DNMT1 | 1.59 (−1.75, 6.11) | 2.39 (−0.68, 5.77) | <0.001 |
| DNMT2 | −2.37 (−8.62, 3.27) | 0.01 (−7.78, 5.47) | <0.001 |
| DNMT3A | 1.74 (−1.48, 5.55) | 3.15 (−0.68, 6.86) | <0.001 |
| DNMT3B | 1.71 (−2.45, 7.31) | 3.64 (0.26, 7.70) | <0.001 |
| DNMT3L | −3.12 (−3.12, 10.90) | −3.12 (−3.12, 31.38) | <0.001 |
| HDAC1 | 0.89 (−5.19, 4.74) | 1.22 (−0.81, 3.89) | <0.001 |
| HDAC2 | 0.77 (−4.16, 8.34) | 4.61 (−1.65, 10.61) | 0.076 |
| HDAC3 | 0.11 (−6.33, 5.73) | 0.55 (−3.36, 4.82) | <0.001 |
| PRDM7 | −1.86 (−1.86, 6.63) | −1.86 (−1.86, 5.33) | <0.001 |
| PRDM9 | −14.32 (−14.32, 7.42) | −14.32 (−14.32, 56.65) | <0.001 |
| PRDM12 | 0.94 (−2.13, 7.80) | 1.90 (−2.13, 6.70) | <0.001 |
| PRDM13 | −2.24 (−2.24, 13.99) | 3.12 (−2.24, 21.56) | 0.373 |
| PRDM14 | −5.16 (−5.16, 10.82) | −5.16 (−5.16, 2.22) | <0.001 |
| PRDM15 | 0.35 (−3.15, 3.16) | 1.35 (−1.35, 4.44) | <0.001 |
| PRMT1 | 0.72 (−2.07, 4.47) | 1.81 (−1.95, 4.77) | <0.001 |
| PRMT2 | −1.30 (−4.80, 3.34) | 0.25 (−3.32, 7.08) | 0.077 |
| PRMT5 | 0.67 (−5.05, 5.48) | 0.33 (−3.67, 8.60) | 0.229 |
| PRMT6 | 0.66 (−6.83, 4.13) | 0.63 (−3.88, 3.37) | 0.308 |
| PRMT7 | 0.002 (−3.75, 6.10) | −0.14 (−3.79, 3.80) | 0.011 |
| PRMT8 | −0.68 (−1.64, 14.55) | −0.92 (−1.64, 10.46) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-Y.; Moi, S.-H.; Hou, M.-F.; Luo, C.-W.; Pan, M.-R. Chromatin Remodeling Enzyme Cluster Predicts Prognosis and Clinical Benefit of Therapeutic Strategy in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 5583. https://doi.org/10.3390/ijms24065583
Kuo C-Y, Moi S-H, Hou M-F, Luo C-W, Pan M-R. Chromatin Remodeling Enzyme Cluster Predicts Prognosis and Clinical Benefit of Therapeutic Strategy in Breast Cancer. International Journal of Molecular Sciences. 2023; 24(6):5583. https://doi.org/10.3390/ijms24065583
Chicago/Turabian StyleKuo, Chia-Yu, Sin-Hua Moi, Ming-Feng Hou, Chi-Wen Luo, and Mei-Ren Pan. 2023. "Chromatin Remodeling Enzyme Cluster Predicts Prognosis and Clinical Benefit of Therapeutic Strategy in Breast Cancer" International Journal of Molecular Sciences 24, no. 6: 5583. https://doi.org/10.3390/ijms24065583
APA StyleKuo, C.-Y., Moi, S.-H., Hou, M.-F., Luo, C.-W., & Pan, M.-R. (2023). Chromatin Remodeling Enzyme Cluster Predicts Prognosis and Clinical Benefit of Therapeutic Strategy in Breast Cancer. International Journal of Molecular Sciences, 24(6), 5583. https://doi.org/10.3390/ijms24065583





