CRISPR-Cas9-Mediated Mutation of Methyltransferase METTL4 Results in Embryonic Defects in Silkworm Bombyx mori
Abstract
1. Introduction
2. Results
2.1. CRISPR-Cas9 System Induced the Mutation of BmMETTL4
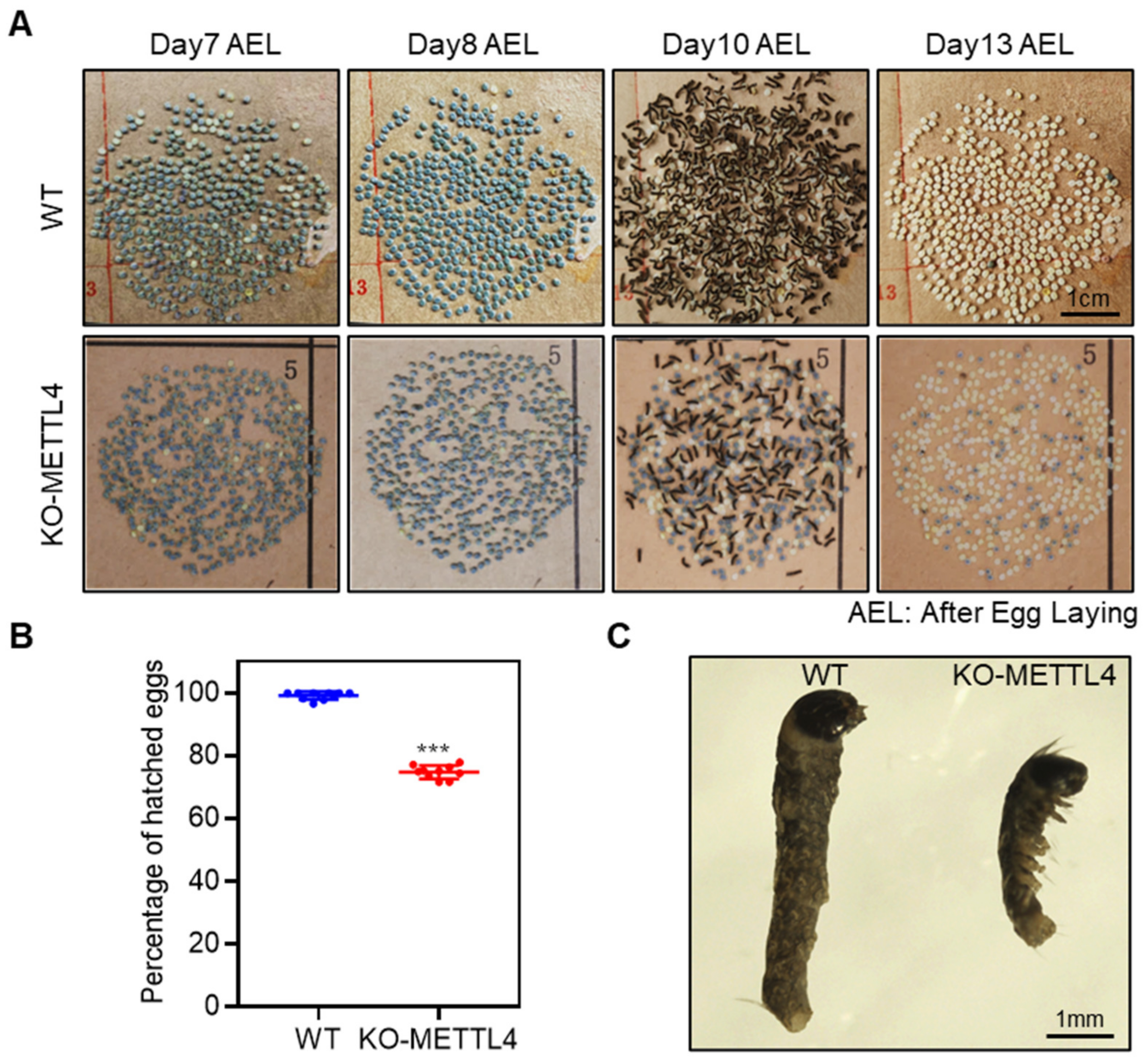
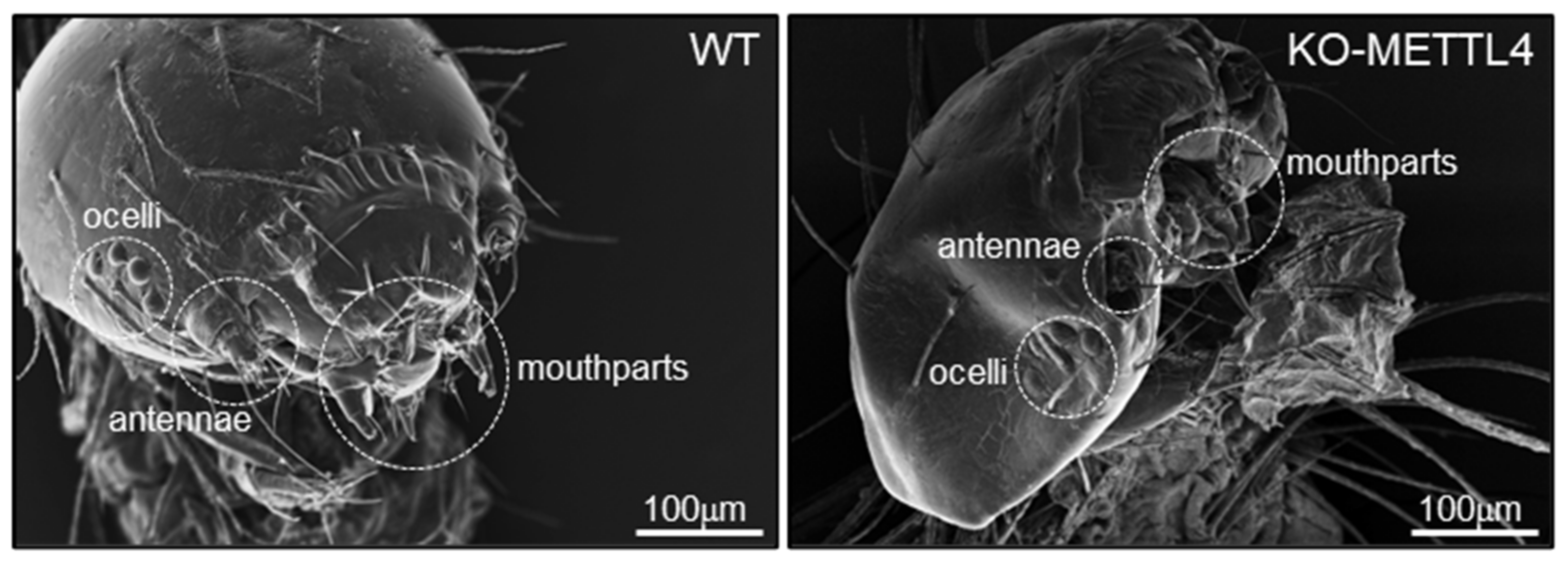
2.2. Depletion of BmMETTL4 Caused Developmental Defect of Silkworm Embryo
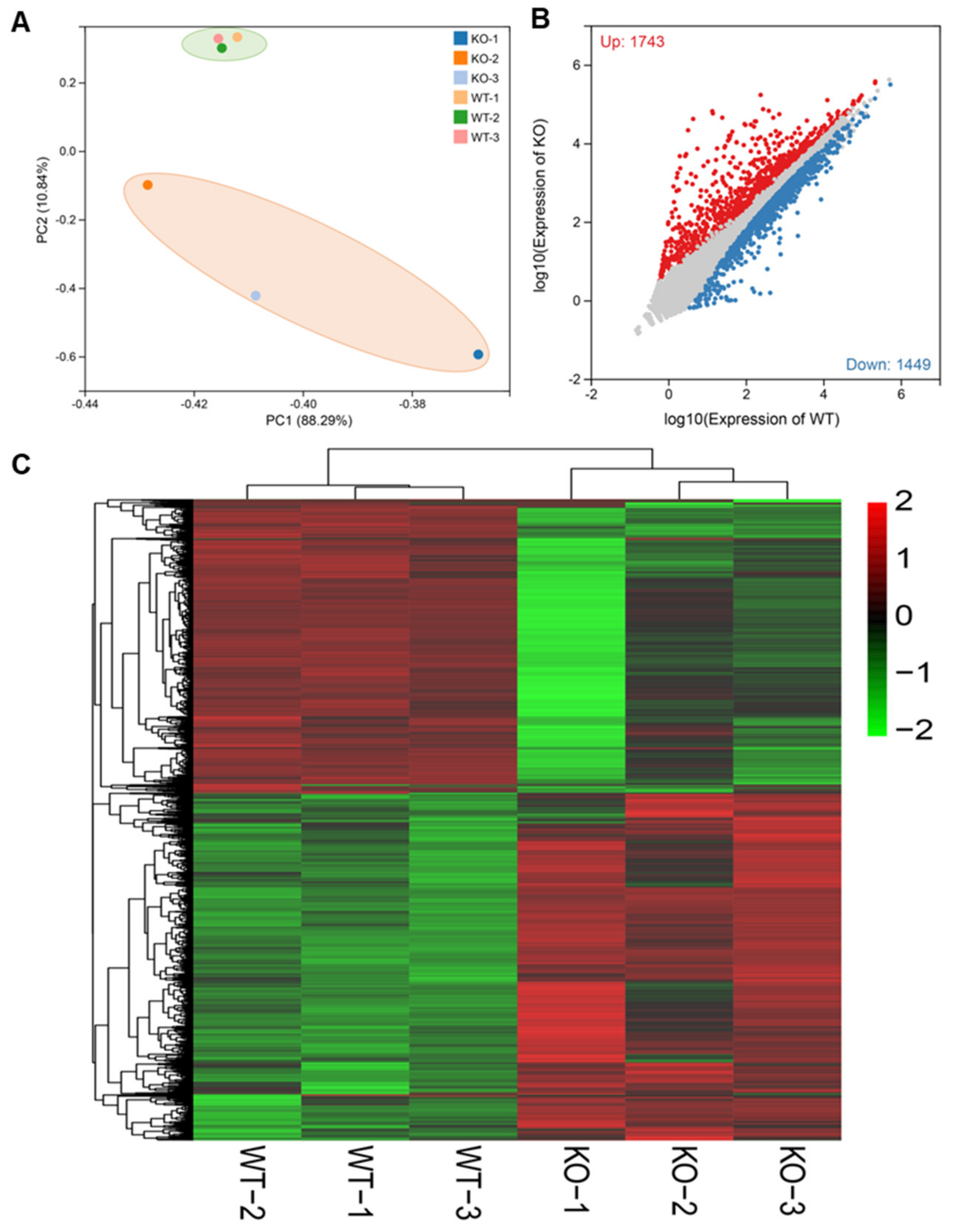
2.3. Depletion of BmMETTL4 Led to Great Changes in Transcriptome
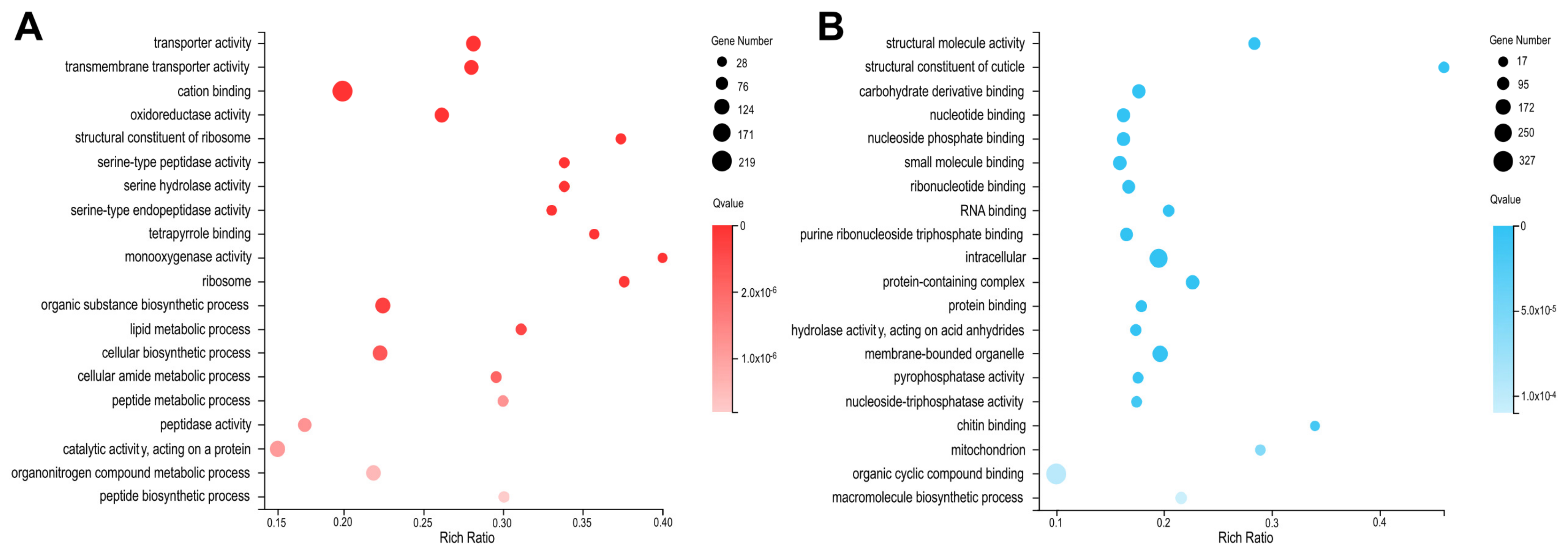
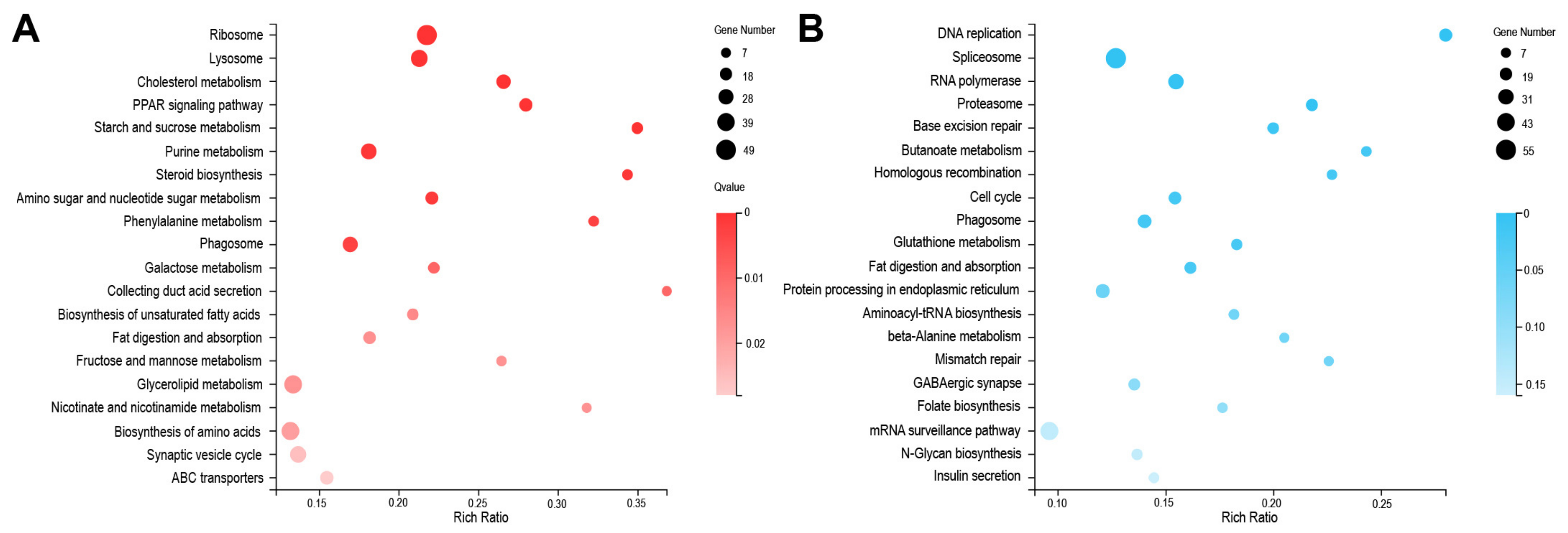
2.4. Depletion of BmMETTL4 Induced Differential GO and KEGG between Up-DEGs and Down-DEGs
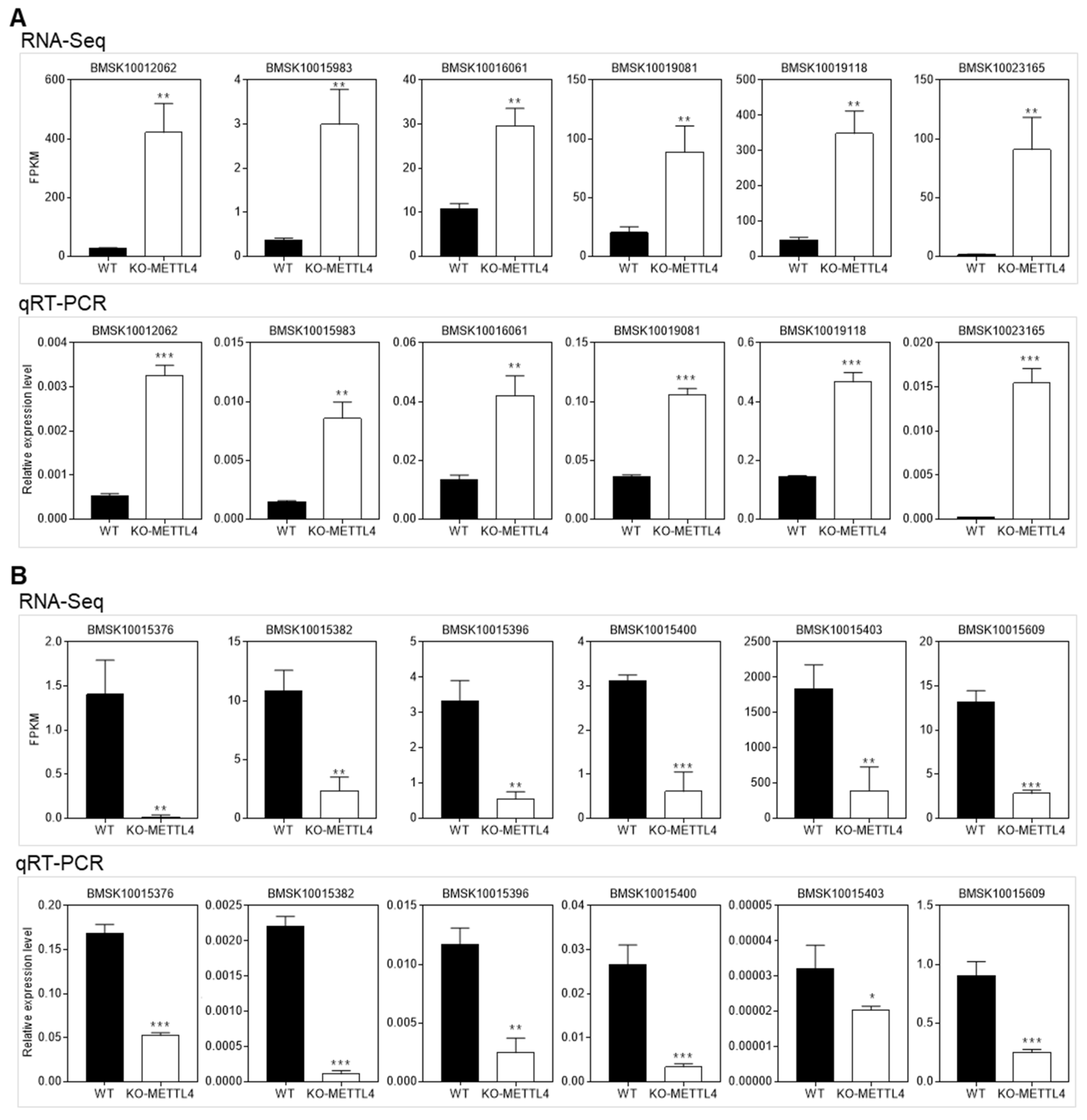
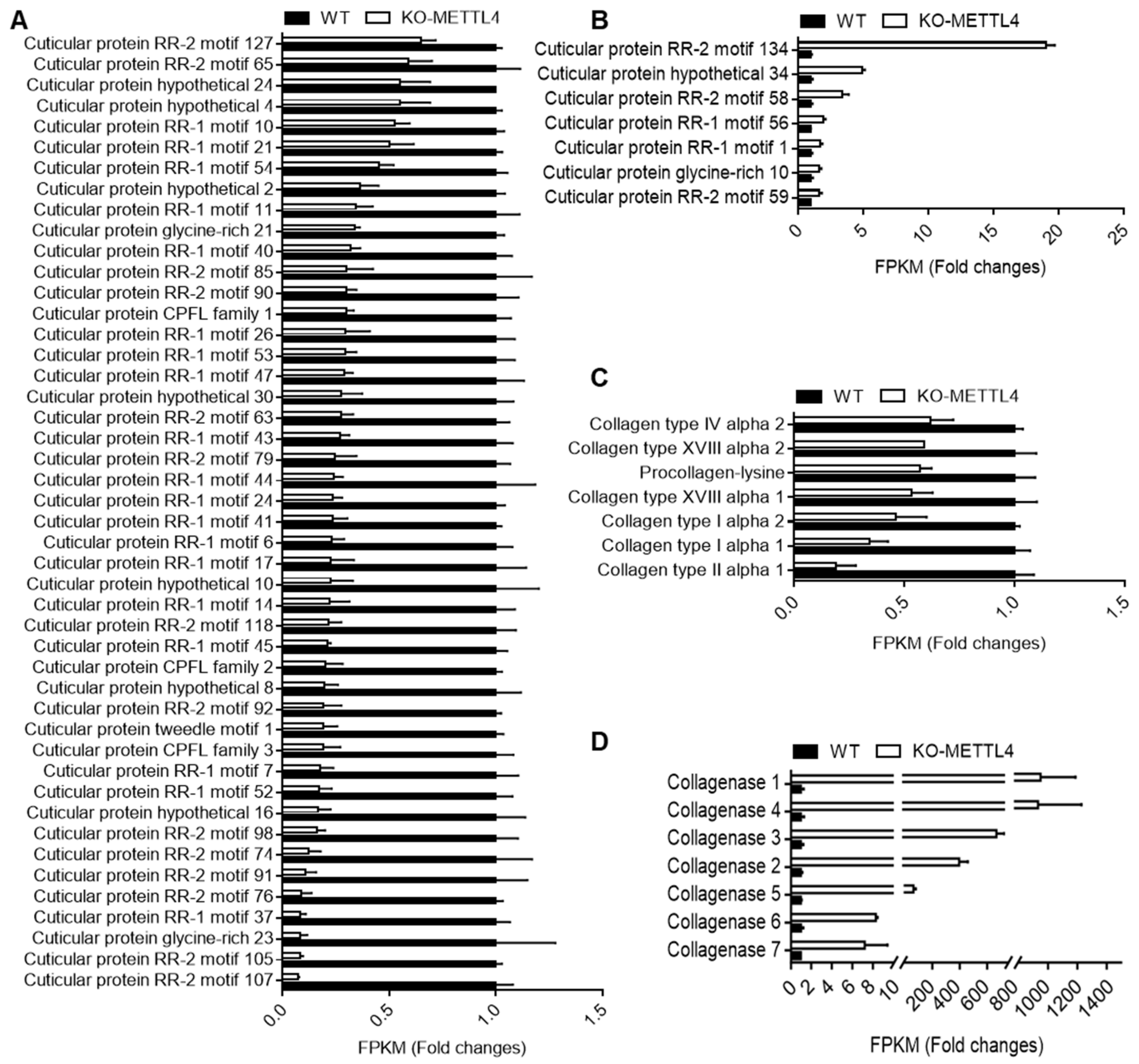
2.5. Down-Regulated Structural Constituents in BmMETTL4 Mutant May Result in Abnormal Silkworm Embryo
3. Discussion
4. Materials and Methods
4.1. Silkworm Strain
4.2. Plasmid Construction
4.3. Germline Transformation
4.4. Genomic DNA Extraction and Mutagenesis Analysis
4.5. Quantitative Real-Time PCR Analysis
4.6. Dot Blot Analysis
4.7. RNA-Seq Analysis
4.8. Photography and Scanning Electron Microscopy
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisenauer, A.; Kahng, L.S.; McCollum, S.; Shapiro, L. Bacterial DNA methylation: A cell cycle regulator? J. Bacteriol. 1999, 181, 5135–5139. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, D.M.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. An essential role for DNA adenine methylation in bacterial virulence. Science 1999, 284, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Wion, D.; Casadesus, J. N6-methyl-adenine: An epigenetic signal for DNA-protein interactions. Nat. Rev. Microbiol. 2006, 4, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Weyand, N.J.; Mahan, M.J. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 2001, 69, 7197–7204. [Google Scholar] [CrossRef]
- Fang, G.; Munera, D.; Friedman, D.I.; Mandlik, A.; Chao, M.C.; Banerjee, O.; Feng, Z.; Losic, B.; Mahajan, M.C.; Jabado, O.J.; et al. Genome-wide mapping of methylated adenine residues in pathogenic Escherichia coli using single-molecule real-time sequencing. Nat. Biotechnol. 2012, 30, 1232–1239. [Google Scholar] [CrossRef]
- Greer, E.L.; Blanco, M.A.; Gu, L.; Sendinc, E.; Liu, J.; Aristizabal-Corrales, D.; Hsu, C.H.; Aravind, L.; He, C.; Shi, Y. DNA Methylation on N6-Adenine in C. elegans. Cell 2015, 161, 868–878. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, H.; Liu, D.; Cheng, Y.; Liu, X.; Zhang, W.; Yin, R.; Zhang, D.; Zhang, P.; Liu, J.; et al. N6-methyladenine DNA modification in Drosophila. Cell 2015, 161, 893–906. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, L.; Cui, X.; Bao, S.; Geng, Y.; Yu, G.; Liang, F.; Xie, S.; Lu, T.; Gu, X.; et al. DNA N(6)-Adenine Methylation in Arabidopsis thaliana. Dev. Cell 2018, 45, 406–416.e3. [Google Scholar] [CrossRef]
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.S.H.; Frezza, C.; Gurdon, J.B. Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat. Struct. Mol. Biol. 2016, 23, 24–30. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Luo, G.Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y.; et al. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 2016, 7, 13052. [Google Scholar] [CrossRef]
- Xiao, C.L.; Zhu, S.; He, M.; Chen, D.; Zhang, Q.; Chen, Y.; Yu, G.; Liu, J.; Xie, S.Q.; Luo, F.; et al. N(6)-Methyladenine DNA Modification in the Human Genome. Mol. Cell 2018, 71, 306–318.e7. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Z.; Cui, X.; Ji, C.; Li, Y.; Zhang, P.; Liu, J.; Riaz, A.; Yao, P.; Liu, M.; et al. N(6)-Methyladenine DNA Methylation in Japonica and Indica Rice Genomes and Its Association with Gene Expression, Plant Development, and Stress Responses. Mol. Plant 2018, 11, 1492–1508. [Google Scholar] [CrossRef]
- Hao, Z.; Wu, T.; Cui, X.; Zhu, P.; Tan, C.; Dou, X.; Hsu, K.W.; Lin, Y.T.; Peng, P.H.; Zhang, L.S.; et al. N(6)-Deoxyadenosine Methylation in Mammalian Mitochondrial DNA. Mol. Cell 2020, 78, 382–395.e8. [Google Scholar] [CrossRef]
- Yao, B.; Li, Y.; Wang, Z.; Chen, L.; Poidevin, M.; Zhang, C.; Lin, L.; Wang, F.; Bao, H.; Jiao, B.; et al. Active N(6)-Methyladenine Demethylation by DMAD Regulates Gene Expression by Coordinating with Polycomb Protein in Neurons. Mol. Cell 2018, 71, 848–857.e6. [Google Scholar] [CrossRef]
- Xie, Q.; Wu, T.P.; Gimple, R.C.; Li, Z.; Prager, B.C.; Wu, Q.; Yu, Y.; Wang, P.; Wang, Y.; Gorkin, D.U.; et al. N(6)-methyladenine DNA Modification in Glioblastoma. Cell 2018, 175, 1228–1243.e20. [Google Scholar] [CrossRef]
- Boulias, K.; Greer, E.L. Means, mechanisms and consequences of adenine methylation in DNA. Nat. Rev. Genet. 2022, 23, 411–428. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, S.; Wang, X.; Zhu, Y.; Chen, Z.; Chen, D. N6-methyladenine functions as a potential epigenetic mark in eukaryotes. Bioessays 2015, 37, 1155–1162. [Google Scholar] [CrossRef]
- Iyer, L.M.; Zhang, D.; Aravind, L. Adenine methylation in eukaryotes: Apprehending the complex evolutionary history and functional potential of an epigenetic modification. Bioessays 2016, 38, 27–40. [Google Scholar] [CrossRef]
- Fedeles, B.I.; Singh, V.; Delaney, J.C.; Li, D.; Essigmann, J.M. The AlkB Family of Fe(II)/alpha-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond. J. Biol. Chem. 2015, 290, 20734–20742. [Google Scholar] [CrossRef]
- Wu, T.P.; Wang, T.; Seetin, M.G.; Lai, Y.; Zhu, S.; Lin, K.; Liu, Y.; Byrum, S.D.; Mackintosh, S.G.; Zhong, M.; et al. DNA methylation on N(6)-adenine in mammalian embryonic stem cells. Nature 2016, 532, 329–333. [Google Scholar] [CrossRef]
- Kweon, S.M.; Chen, Y.; Moon, E.; Kvederaviciute, K.; Klimasauskas, S.; Feldman, D.E. An Adversarial DNA N(6)-Methyladenine-Sensor Network Preserves Polycomb Silencing. Mol. Cell 2019, 74, 1138–1147.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Zhang, Q.; Li, B.; Lu, C.; Li, W.; Cheng, T.; Xia, Q.; Zhao, P. DNA methylation on N6-adenine in lepidopteran Bombyx mori. Biochim. Biophys Acta Gene Regul. Mech. 2018, 1861, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, S.; Wang, X.; Chang, J.; Gao, J.; Shi, R.; Zhang, J.; Lu, W.; Liu, Y.; Zhao, P.; et al. Highly efficient multiplex targeted mutagenesis and genomic structure variation in Bombyx mori cells using CRISPR/Cas9. Insect Biochem. Mol. Biol. 2014, 49, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, Y.; Liu, Y.; Chang, J.; Zhang, T.; Wang, X.; Shi, R.; Lu, W.; Xia, X.; Zhao, P.; et al. An integrated CRISPR Bombyx mori genome editing system with improved efficiency and expanded target sites. Insect Biochem. Mol. Biol. 2017, 83, 13–20. [Google Scholar] [CrossRef]
- Futahashi, R.; Okamoto, S.; Kawasaki, H.; Zhong, Y.S.; Iwanaga, M.; Mita, K.; Fujiwara, H. Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1138–1146. [Google Scholar] [CrossRef]
- Yang, J.; Song, W.; Li, C.; Fang, C.; Zhang, Y.; Wang, Q.; Zhang, M.; Qian, G. Comparative study of collagen distribution in the dermis of the embryonic carapace of soft- and hard-shelled cryptodiran turtles. J. Morphol. 2021, 282, 543–552. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, X.; Feng, Y.; Zheng, L.; Zhao, M.; Huang, M. Secretion of collagenases by Saccharomyces cerevisiae for collagen degradation. Biotechnol. Biofuels Bioprod. 2022, 15, 89. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Y.; Wang, Y.; Gao, T.; Ma, Z.; Yang, Y.; Zhang, P.; Yi, F.; Zhan, J.; Zhang, H.; et al. Regulation of Adipocyte Differentiation by METTL4, a 6mA Methylase. Sci. Rep. 2020, 10, 8285. [Google Scholar] [CrossRef]
- Chen, H.; Gu, L.; Orellana, E.A.; Wang, Y.; Guo, J.; Liu, Q.; Wang, L.; Shen, Z.; Wu, H.; Gregory, R.I.; et al. METTL4 is an snRNA m(6)Am methyltransferase that regulates RNA splicing. Cell Res. 2020, 30, 544–547. [Google Scholar] [CrossRef]
- Gu, L.; Wang, L.; Chen, H.; Hong, J.; Shen, Z.; Dhall, A.; Lao, T.; Liu, C.; Wang, Z.; Xu, Y.; et al. CG14906 (mettl4) mediates m(6)A methylation of U2 snRNA in Drosophila. Cell Discov. 2020, 6, 44. [Google Scholar] [CrossRef]
- Jacobs, C.G.; Rezende, G.L.; Lamers, G.E.; van der Zee, M. The extraembryonic serosa protects the insect egg against desiccation. Proc. Biol. Sci. 2013, 280, 20131082. [Google Scholar] [CrossRef]
- Vargas, H.C.; Farnesi, L.C.; Martins, A.J.; Valle, D.; Rezende, G.L. Serosal cuticle formation and distinct degrees of desiccation resistance in embryos of the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus. J. Insect Physiol. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Jacobs, C.G.; Braak, N.; Lamers, G.E.; van der Zee, M. Elucidation of the serosal cuticle machinery in the beetle Tribolium by RNA sequencing and functional analysis of Knickkopf1, Retroactive and Laccase2. Insect Biochem. Mol. Biol. 2015, 60, 7–12. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Liang, W.; Dang, Z.; Wang, S.; Zhang, Y.; Zhao, P.; Lu, Z. Cytokine receptor DOME controls wing disc development in Bombyx mori. Insect Biochem. Mol. Biol. 2022, 148, 103828. [Google Scholar] [CrossRef]
- Stemmer, M.; Thumberger, T.; Del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS ONE 2015, 10, e0124633. [Google Scholar] [CrossRef]
- Ma, S.; Wang, X.; Fei, J.; Liu, Y.; Duan, J.; Wang, F.; Xu, H.; Zhao, P.; Xia, Q. Genetic marking of sex using a W chromosome-linked transgene. Insect Biochem. Mol. Biol. 2013, 43, 1079–1086. [Google Scholar] [CrossRef]
- Lu, C.; Li, Z.; Zhang, W.; Guo, H.; Lan, W.; Shen, G.; Xia, Q.; Zhao, P. SUMOylation of Translationally Regulated Tumor Protein Modulates Its Immune Function. Front. Immunol. 2022, 13, 807097. [Google Scholar] [CrossRef]
- Lu, F.; Wei, Z.; Luo, Y.; Guo, H.; Zhang, G.; Xia, Q.; Wang, Y. SilkDB 3.0: Visualizing and exploring multiple levels of data for silkworm. Nucleic Acids Res. 2020, 48, D749–D755. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Chen, F.; Zhou, M.; Lan, W.; Zhang, W.; Shen, G.; Lin, P.; Xia, Q.; Zhao, P.; Li, Z. CRISPR-Cas9-Mediated Mutation of Methyltransferase METTL4 Results in Embryonic Defects in Silkworm Bombyx mori. Int. J. Mol. Sci. 2023, 24, 3468. https://doi.org/10.3390/ijms24043468
Guo H, Chen F, Zhou M, Lan W, Zhang W, Shen G, Lin P, Xia Q, Zhao P, Li Z. CRISPR-Cas9-Mediated Mutation of Methyltransferase METTL4 Results in Embryonic Defects in Silkworm Bombyx mori. International Journal of Molecular Sciences. 2023; 24(4):3468. https://doi.org/10.3390/ijms24043468
Chicago/Turabian StyleGuo, Hao, Feng Chen, Mingyi Zhou, Weiqun Lan, Wenchang Zhang, Guanwang Shen, Ping Lin, Qingyou Xia, Ping Zhao, and Zhiqing Li. 2023. "CRISPR-Cas9-Mediated Mutation of Methyltransferase METTL4 Results in Embryonic Defects in Silkworm Bombyx mori" International Journal of Molecular Sciences 24, no. 4: 3468. https://doi.org/10.3390/ijms24043468
APA StyleGuo, H., Chen, F., Zhou, M., Lan, W., Zhang, W., Shen, G., Lin, P., Xia, Q., Zhao, P., & Li, Z. (2023). CRISPR-Cas9-Mediated Mutation of Methyltransferase METTL4 Results in Embryonic Defects in Silkworm Bombyx mori. International Journal of Molecular Sciences, 24(4), 3468. https://doi.org/10.3390/ijms24043468




