Adaptive Stimulations in a Biophysical Network Model of Parkinson’s Disease
Abstract
1. Introduction
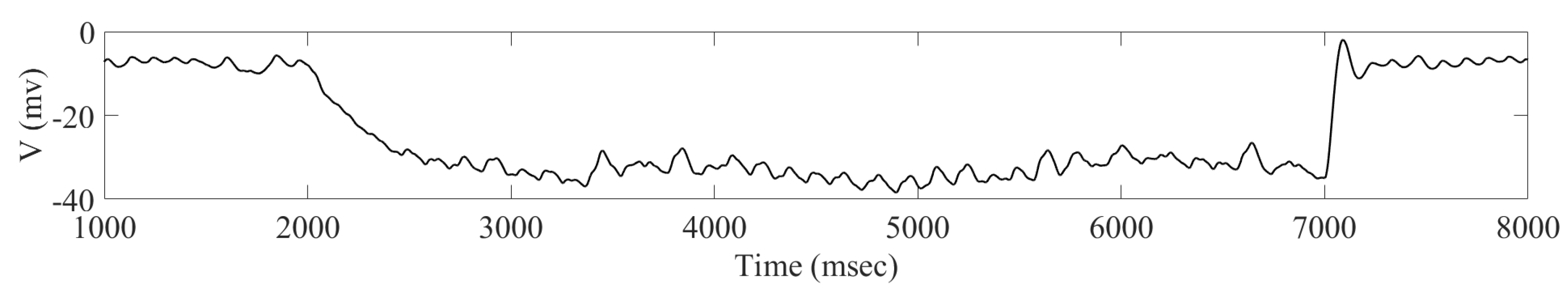
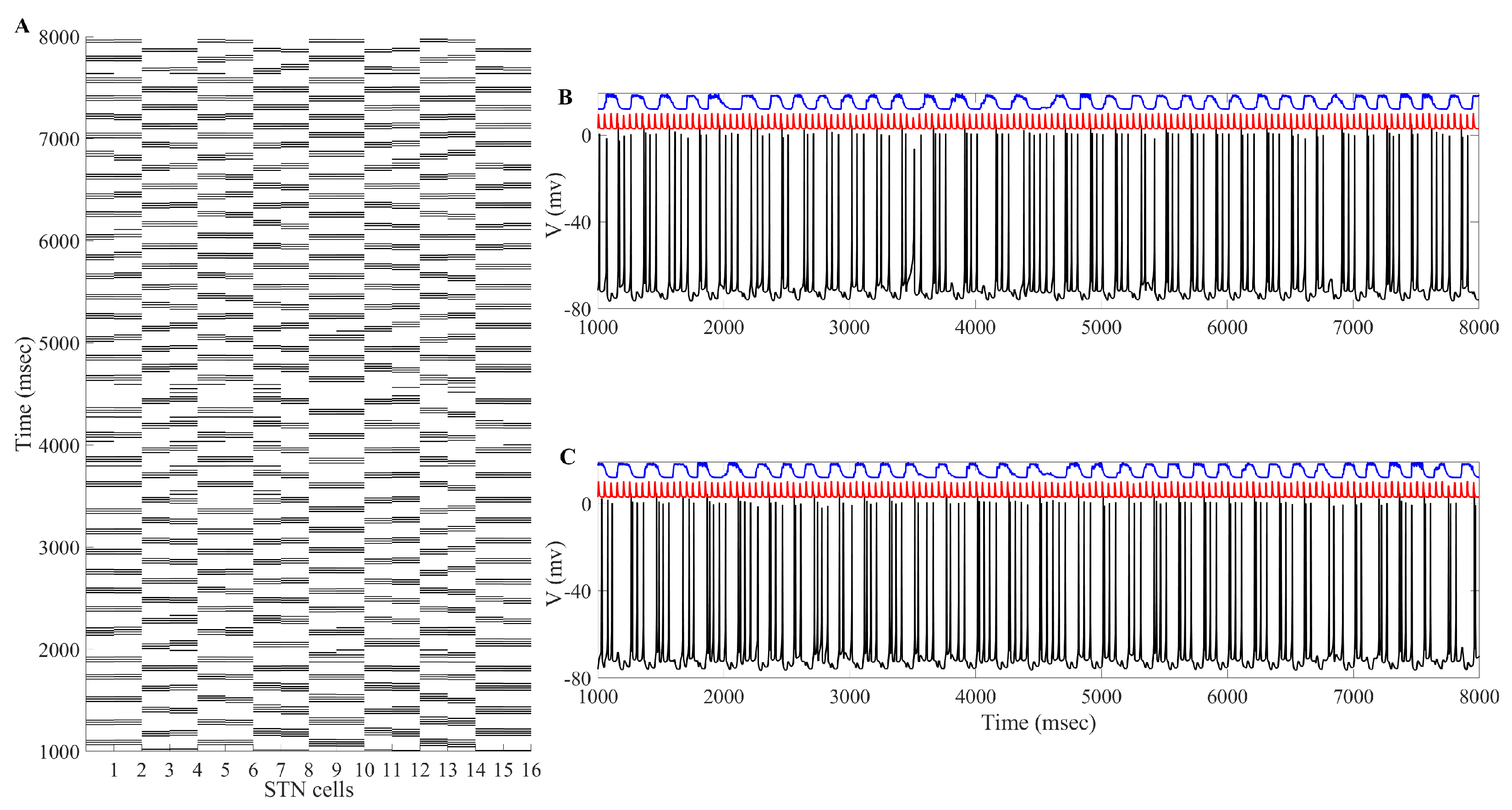
2. Results
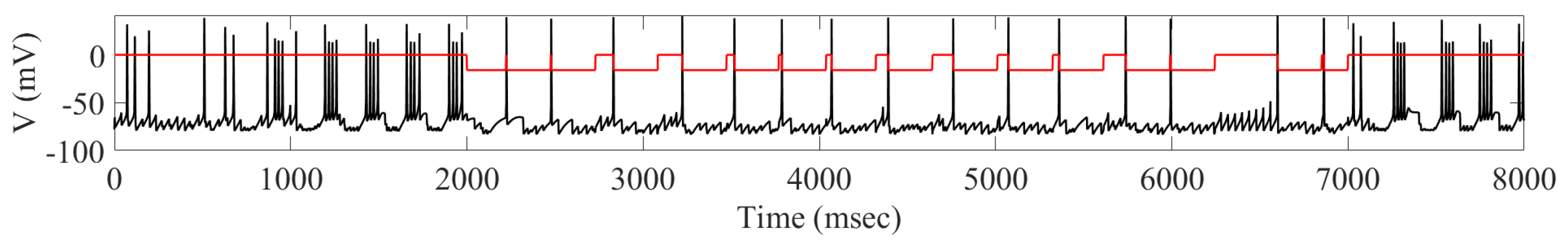
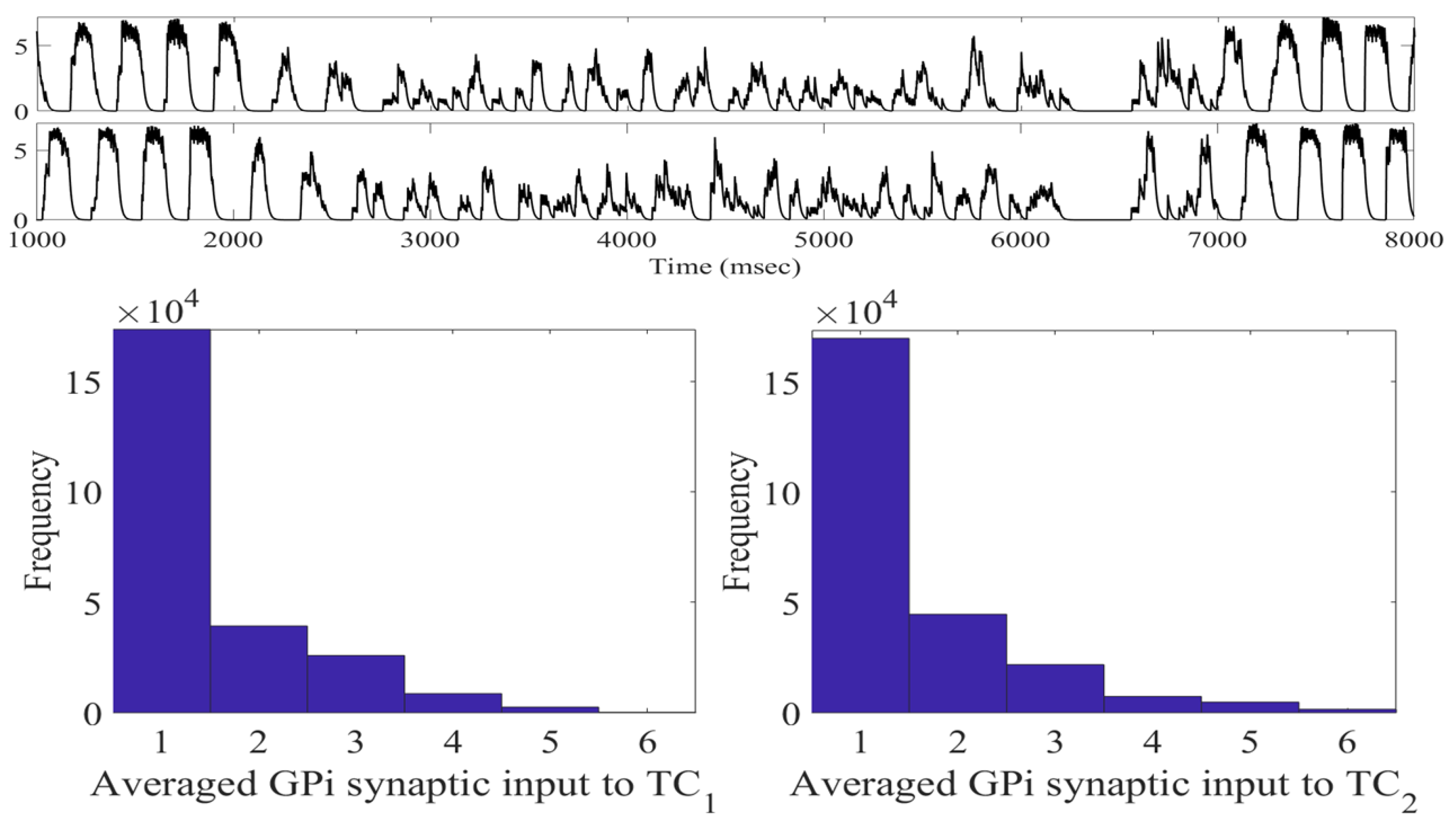
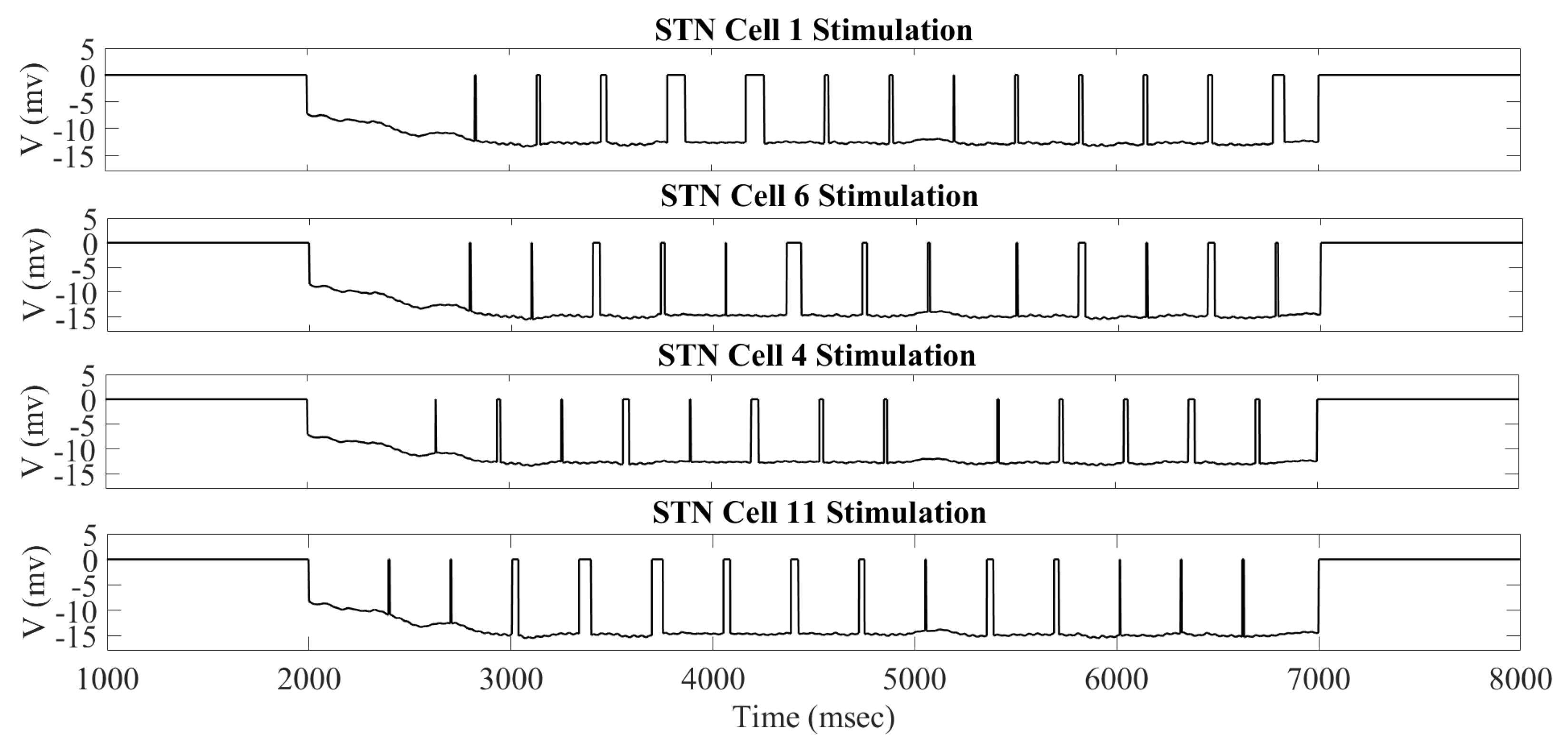
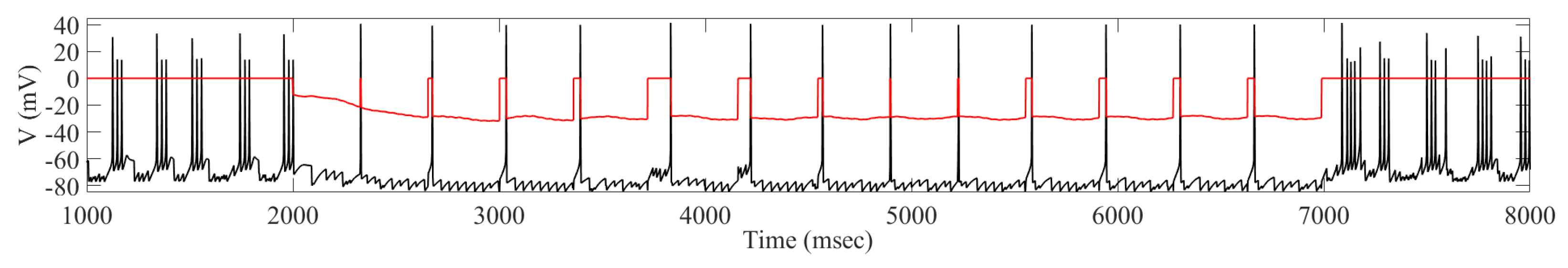
2.1. Adaptive Constant Pulse DBS
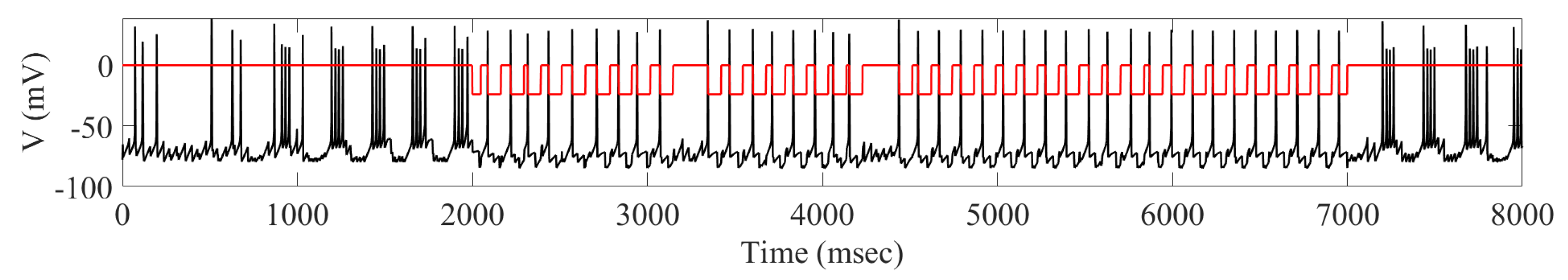
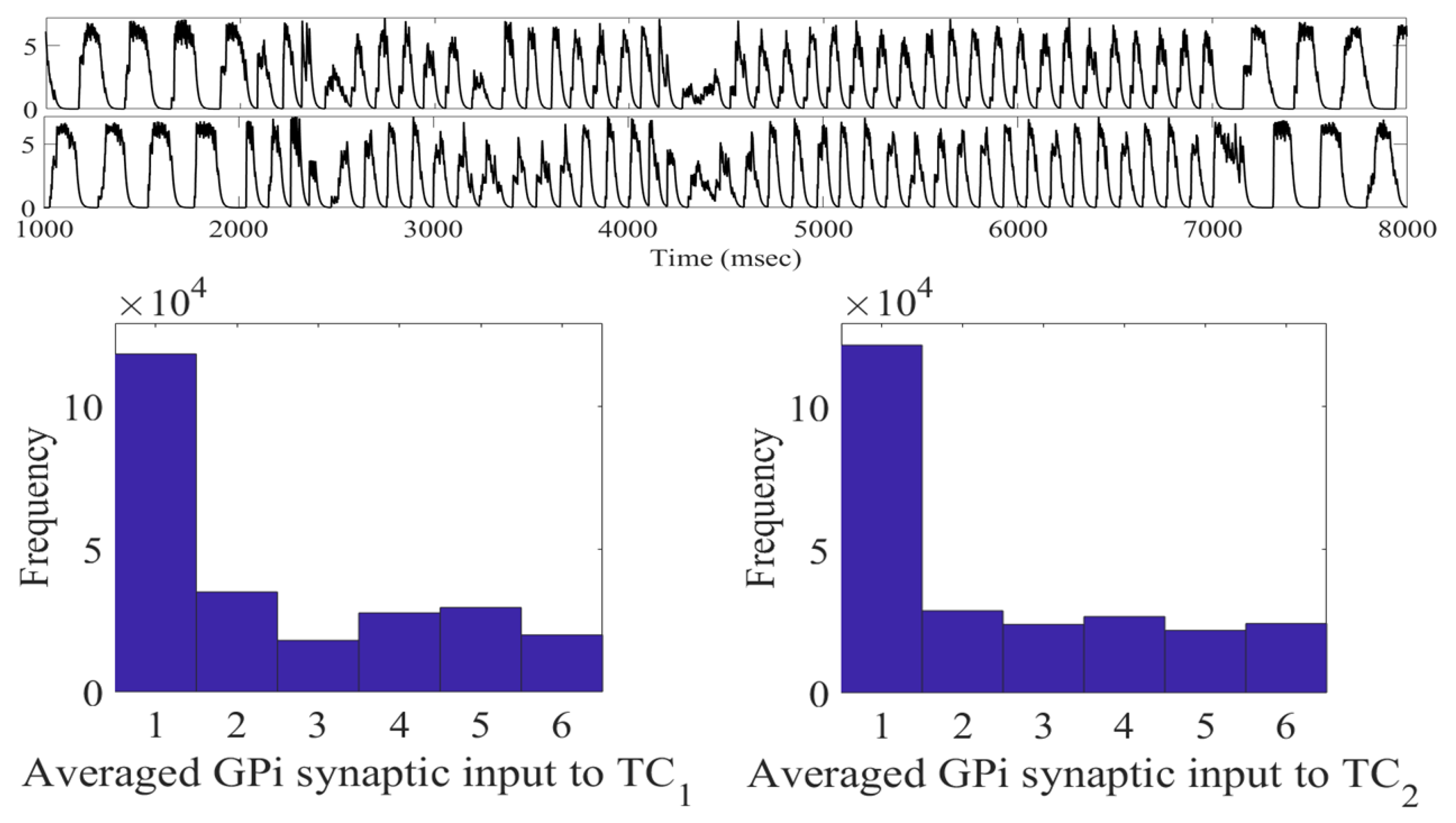
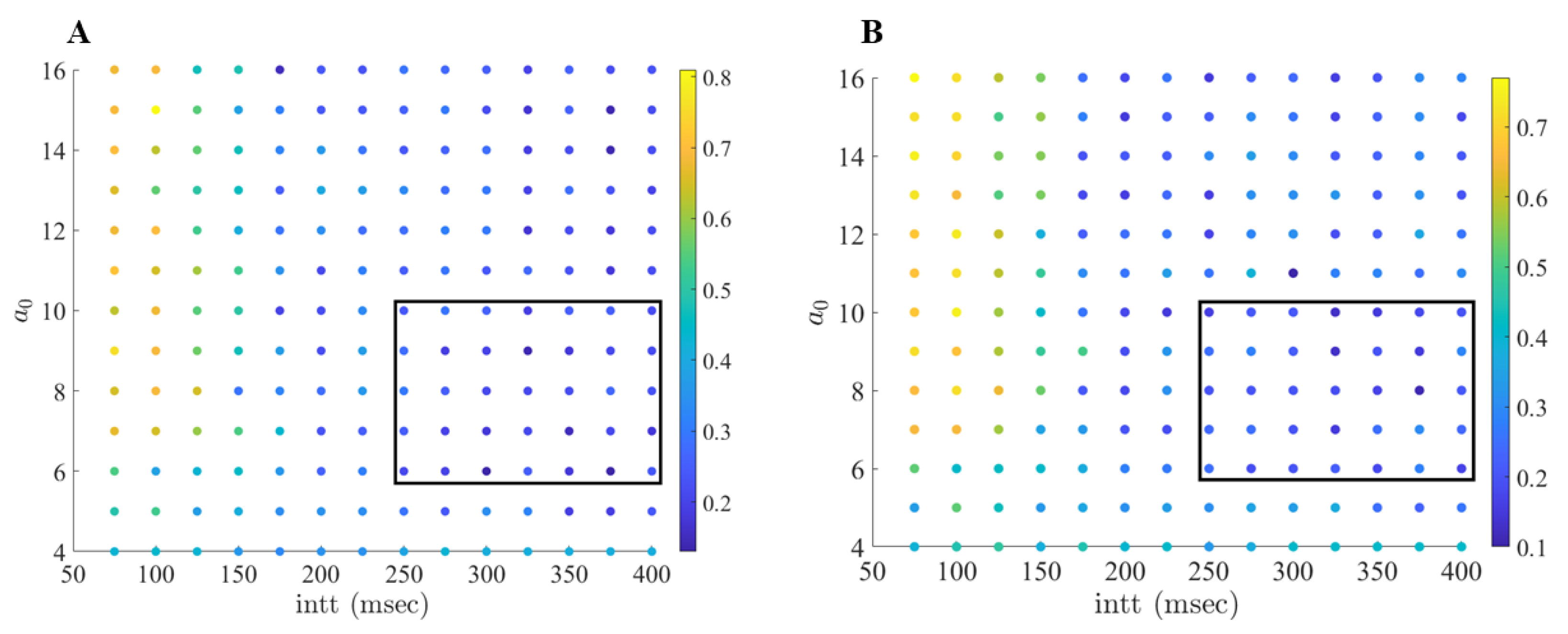
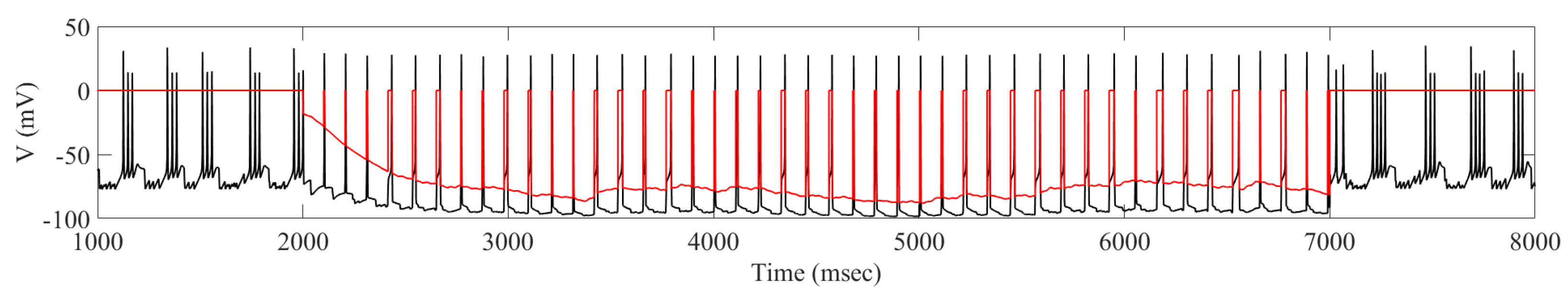
2.2. Adaptive Multi-Site LFP Stimulation
2.3. DBS for Heterogeneous TC Cells
3. Discussion
4. Methods and Materials
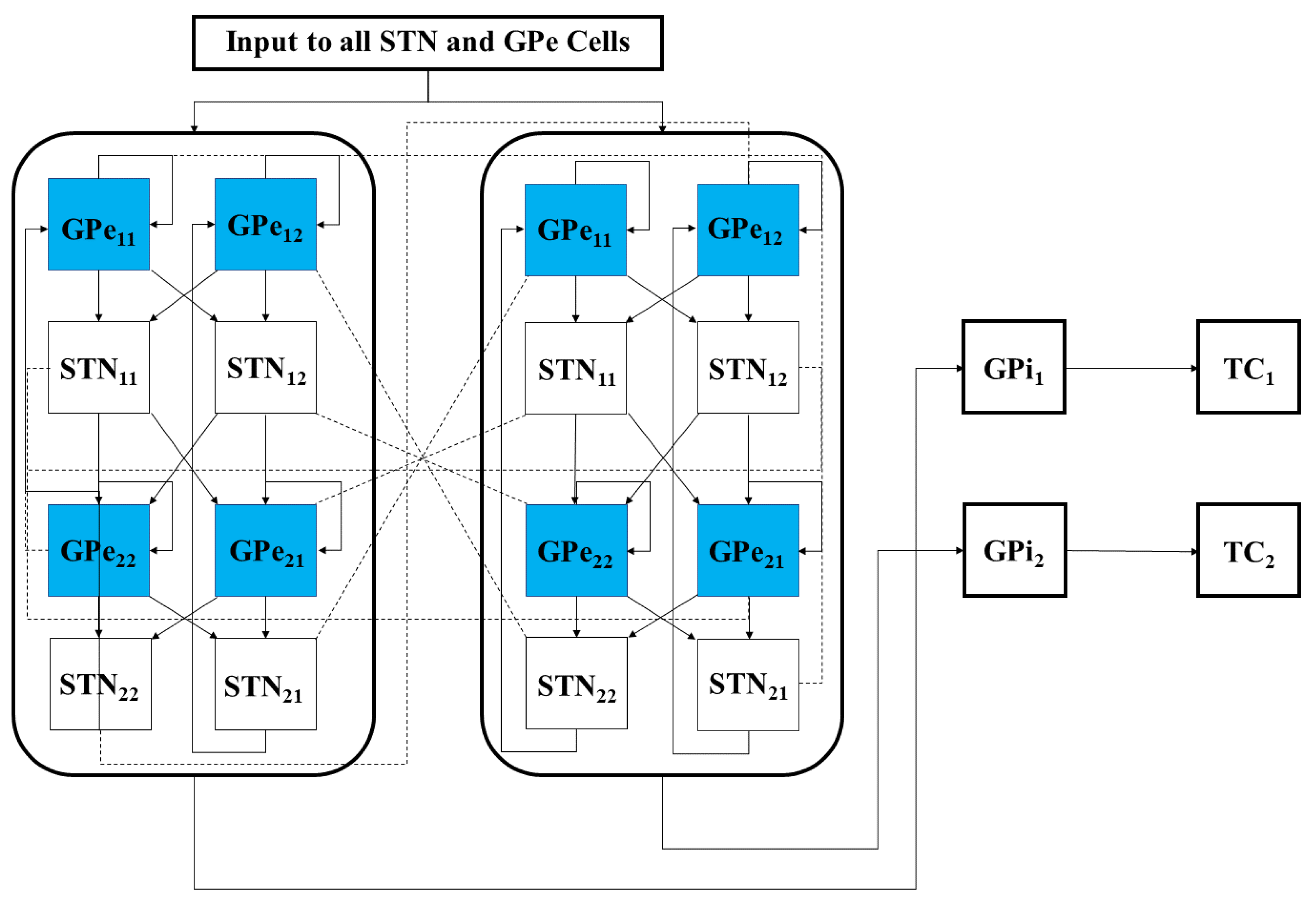
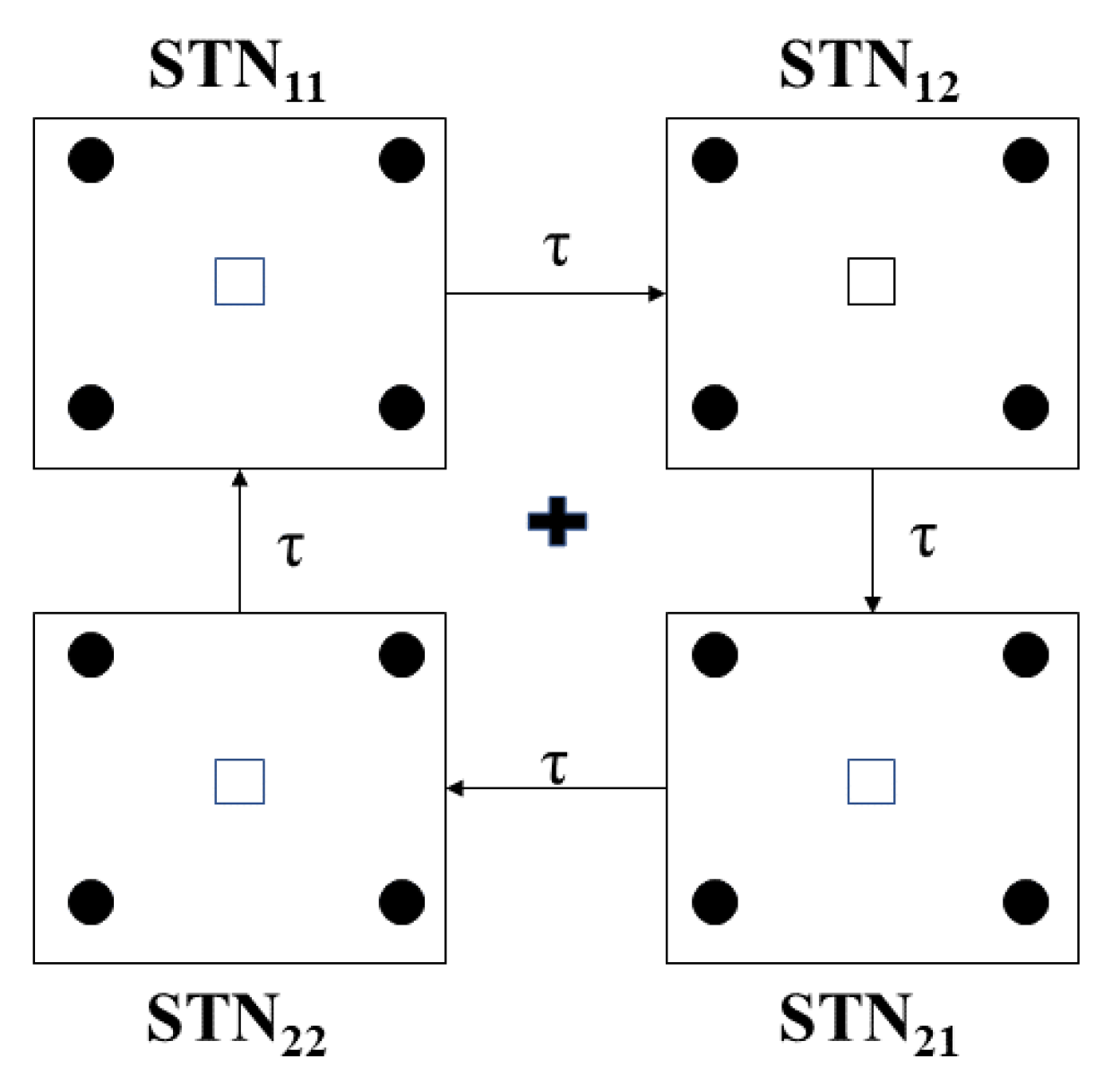
4.1. The Network Model
4.1.1. Architecture of Coupling between Individual Neurons
4.1.2. Averaged GPi Synaptic Input to TC
4.1.3. TC Relay Responses and Error Index
4.2. Adaptive Constant Pulse DBS
4.3. Adaptive Multi-Site LFP Stimulation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aDBS | adaptive deep brain stimulation |
| acDBS | adaptive constant pulse deep brain stimulation |
| aLFPDBS | adaptive local field potential deep brain stimulation |
| DBS | deep brain stimulation |
| GP | globus pallidus |
| GPe | external segment of the globus pallidus |
| GPi | internal segment of the globus pallidus |
| HF | conventional high-frequency stimulation |
| PD | Parkinson’s disease |
| LFP | local field potential |
| STN | subthalamic nucleus |
| TC | thalamacortical neurons |
Appendix A
- Functions for TC neurons in system (6):
- Parameters for TC neurons:
- GPi currents:
- GPi equations and functions:
- GPi parameters:
- STN currents:
- STN equations and functions:
- STN Parameters:
- GPe currents:
- GPe equations and functions:
- GPe parameters:
References
- McIntyre, C.C.; Hahn, P. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol. Dis. 2010, 38, 329–337. [Google Scholar] [CrossRef]
- Wichmann, T.; DeLong, M.R. Deep brain stimulation for neurologic disorders. Neuron 2006, 52, 197–204. [Google Scholar] [CrossRef]
- Benabid, A.L.; Chabardes, S.; Mitrofanis, J.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009, 8, 67–81. [Google Scholar] [CrossRef]
- Volkmann, J. Deep brain stimulation for the treatment of Parkinson’s disease. J. Clin. Neurophys. 2004, 21, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, C.; Omel’chenko, O.; Popovych, O.V.; Maistrenko, Y.; Tass, P.A. Control of spatially patterned synchrony with multisite delayed feedback. Phys. Rev. E 2007, 76, 066209. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Obeso, J.A.; Lang, A.E.; Houeto, J.-L.; Pollak, P.; Rehncrona, S.; Kulisevsky, J.; Albanese, A.; Volkmann, J.; Hariz, M.I.; et al. Bilateral deep brain stimulation in Parkinson’s disease: A multicentre study with 4 years follow-up. Brain 2005, 128, 2240–2249. [Google Scholar] [CrossRef]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shea-Brown, E.; Rabitz, H.; Greenwald, B.; Kosut, R. Optimal deep brain stimulation of the subthalamic nucleus—A computational study. J. Comput. Neurosci. 2007, 23, 265–282. [Google Scholar] [CrossRef]
- Feng, X.; Shea-Brown, E.; Rabitz, H.; Greenwald, B.; Kosut, R. Toward closed-loop optimization of deep brain stimulation for Parkinson’s disease: Concepts and lessons from a computational model. J. Neuroeng. 2007, 4, L14–L21. [Google Scholar] [CrossRef]
- Parastarfeizabadi, M.; Kouzani, A.Z. Advances in closed-loop deep brain stimulation devices. J. Neuroeng. Rehabil. 2017, 14, 79. [Google Scholar] [CrossRef]
- Bouthour, W.; Megevand, P.; Donoghue, J.; Luscher, C.; Birbaumer, N.; Krack, P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Popovych, O.V.; Tass, P.A. Adaptive delivery of continuous and delayed feedback deep brain stimulation—A computational study. Sci. Rep. 2019, 9, 10585. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Ludvic, Z.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive Deep Brain Stimulation in Advanced Parkinson Disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [PubMed]
- de Castro, D.L.; Aroso, M.; Aguiar, A.P.; Grayden, D.B.; Aguiar, P. A novel closed-loop control algorithm to disrupt pathological neuronal oscillations—Implementation and validation in vitro. bioRxiv 2022. [Google Scholar] [CrossRef]
- Piña-Fuentes, D.; van Dijk, J.M.C.; van Zijl, J.C.; Moes, H.R.; van Laar, T.; Oterdoom, D.M.; Little, S.; Brown, P.; Beudel, M. Acute effects of adaptive deep brain stimulation in Parkinson’s disease. Brain Stimul. 2020, 13, 1507–1516. [Google Scholar] [CrossRef]
- Guidetti, M.; Marceglia, S.; Loh, A.; Harmsen, I.; Meoni, S.; Foffani, G.; Lozano, A.; Moro, E.; Volkmann, J.; Priori, A. Clinical perspectives of deep brain stimulation. Brain Stimul. 2021, 14, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Vissani, M.; Isaias, I.U.; Mazzoni, A. Deep brain stimulation: A review of the open neural engineering challenges. J. Neural Eng. 2020, 17, 051002. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Nguyen, T.; Peterman, K.; Khawaldeh, S.; Debove, I.; Shah, S.; Torrecillos, F.; Tan, H.; Pogosyan, A.; Lachenmayer, M.; et al. Combining Multimodal Biomarkers to Guide Deep Brain Stimulation Programming in Parkinson Disease. Neuromodulation 2022, 26, 1–13. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Guo, Y.; Rubin, J.E. Multi-site Stimulation of Subthalamic Nucleus Diminishes Thalamocortical Relay Errors in a Biophysical Network. Neural Netw. 2011, 24, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, C.; Popovych, O.; Tass, P.A. Effectively desynchronizing brain stimulation based on a coordinated delayed feedback stimulation via several sites: A computational study. Biol. Cybern. 2005, 93, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tass, P. A model of desynchronizing deep brain stimulation with a demand-controlled reset of neural subpopulations. Biol. Cybern. 2003, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.M.; Valizadeh, A.; Tass, P.A. Decoupling of interacting neuronal populations by time-shifted stimulation through spike-timing-dependent plasticity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Fleming, J.E.; Dunn, E.; Lowery, M. Simulation of closed-loop deep brain stimulation schemes for suppression of pathological beta oscillations in Parkinson’s disease. Front. Neurosci. 2020, 14, 166. [Google Scholar] [CrossRef]
- Fleming, J.E.; Orłowski, J.; Lowery, M.; Chaillet, A. Self-tuning deep brain stimulation controller for suppression of beta oscillations: Analytical derivation and numerical validation. Front. Neurosci. 2020, 14, 639. [Google Scholar] [CrossRef]
- Khaledi-Nasab, A.; Kromer, J.A.; Tass, P.A. Long-lasting desynchronization of plastic neural networks by random reset stimulation. Front. Physiol. 2021, 11, 622620. [Google Scholar] [CrossRef]
- Kromer, J.; Khaledi-Nasab, A.; Tass, P.A. Impact of number of stimulation sites on long-lasting desynchronization effects of coordinated reset stimulation. Chaos 2020, 30, 083134. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, G.; Zhou, C.; Zhu, X.; Zhang, W.; Wang, J.; Li, H.; Wu, H.; Fietkiewicz, C.; Loparo, K.A. Closing the loop of DBS using the beta oscillations in cortex. Cogn. Neurodyn. 2021, 15, 1157–1167. [Google Scholar] [CrossRef]
- Popovych, O.V.; Lysyansky, B.; Rosenblum, M.; Pikovsky, A.; Tass, P.A. Pulsatile Desynchronizing Delayed Feedback for Closed-Loop Deep Brain Stimulation. PLoS ONE 2017, 12, e0173363. [Google Scholar] [CrossRef]
- Popovych, O.V.; Tass, P.A. Multisite Delayed Feedback for Electrical Brain Stimulation. Front. Physiol. 2018, 9, 00046. [Google Scholar] [CrossRef]
- Rouhani, E.; Fathi, Y. Robust multi-input multi-output adaptive fuzzy terminal sliding mode control of deep brain stimulation in Parkinson’s disease: A simulation study. Sci. Rep. 2021, 11, 21169. [Google Scholar] [CrossRef]
- Spiliotis, K.; Starke, J.; Franz, D.; Richter, A.; Köhling, R. Deep brain stimulation for movement disorder treatment: Exploring frequency-dependent efficacy in a computational network model. Biol. Cybern. 2022, 116, 93–116. [Google Scholar] [CrossRef]
- Spiliotis, K.; Butenko, K.; van Rienen, U.; Starke, J.; Köhling, R. Complex network measures reveal optimal targets for deep brain stimulation and identify clusters of collective brain dynamics. Front. Phys. 2022, 10, 951724. [Google Scholar] [CrossRef]
- Toth, K.; Wilson, D. Control of coupled neural oscillations using near-periodic inputs. Chaos 2022, 32, 033130. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z. Multiple-site deep brain stimulation with delayed rectangular waveforms for Parkinson’s disease. Electron. Res. Arch. 2021, 29, 3471–3487. [Google Scholar]
- Guo, Y.; Rubin, J.E.; McIntyre, C.C.; Vitek, J.L.; Terman, D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J. Neurophysiol. 2008, 99, 1477–1492. [Google Scholar] [CrossRef]
- Terman, D.; Rubin, J.E.; Yew, A.C.; Wilson, C.J. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J. Nerurosci. 2002, 22, 2963–2976. [Google Scholar] [CrossRef]
- Rubin, J.E.; Terman, D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rythmicity in a computational model. J. Comput. Neurosci. 2004, 16, 211–235. [Google Scholar] [CrossRef]
- Bergman, H.; Wichmann, T.; Karmon, B.; DeLong, M. The primate subthalamic nucleus. II. Neuronal activity in the mptp model of parkinsonism. J. Neurophysiol. 1994, 72, 507–520. [Google Scholar] [CrossRef]
- Nini, A.; Feingold, A.; Slovin, H.; Bergman, H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J. Neurophysiol. 1995, 74, 1800–1805. [Google Scholar] [CrossRef]
- Boraud, T.; Bezard, E.; Guehl, D.; Bioulac, B.; Gross, C. Effects of L-dopa on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998, 787, 157–160. [Google Scholar] [CrossRef]
- Wichmann, T.; Bergman, H.; Starr, P.; Subramanian, T.; Watts, R.; DeLong, M. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp. Brain Res. 1999, 125, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Magnin, M.; Morel, A.; Jeanmonod, D. Single-unit analysis of the pallidum, thalamus, and subthalamic nucleus in parkinsonian patients. Neuroscience 2000, 96, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Vaadia, E.; Bergman, H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and tremulous 1-methyl-4- phenyl-1,2,3,6 tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 2000, 20, 8559–8571. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Oliviero, A.; Mazzone, P.; Insola, A.; Tonali, P.; Lazzaro, V.D. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 2001, 21, 1033–1038. [Google Scholar] [CrossRef]
- Levy, R.; Hutchison, W.; Lozano, A.; Dostrovsky, J. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J. Neurosci. 2003, 20, 7766–7775. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, J.; Rubchinsky, L.; Sigvardt, K.; Wheelock, V.; Pappas, C. Temporal evolution of oscillations and synchrony in GPi/muscle pairs in Parkinson’s disease. J. Neurophysiol. 2005, 93, 1569–1584. [Google Scholar] [CrossRef]
- Wichmann, T.; Soares, J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J. Neurophysiol. 2006, 95, 2120–2133. [Google Scholar] [CrossRef]
- Jung, K.; Florin, E.; Patil, K.R.; Caspers, J.; Rubbert, C.; Eickhoff, C.; Popovych, O. Whole-brain dynamic modelling for classification of Parkinson’s disease. Brain Commun. 2023, 5, fcac331. [Google Scholar] [CrossRef]
- van Wijk, B.; de Bie, R.; Beudel, M. A systematic review of local field potential physiomarkers in Parkinson’s disease: From clinical correlations to adaptive deep brain stimulation algorithms. J. Neurol. 2023, 270, 1162–1170. [Google Scholar] [CrossRef]
- An, Q.; Yin, Z.; Ma, R.; Fan, H.; Xu, Y.; Gan, Y.; Gao, Y.; Meng, F.; Yang, A.; Jiang, Y.; et al. Adaptive deep brain stimulation for Parkinson’s disease: Looking back at the past decade on motor outcomes. J. Neurol. 2022, 270, 1371–1387. [Google Scholar] [CrossRef]
- Rosin, B.; Slovik, M.; Mitelman, R.; Rivlin-Etzion, M.; Haber, S.; Israel, Z.; Vaadia, E.; Bergman, H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 2011, 72, 370–384. [Google Scholar] [CrossRef]
- Hamani, C.; Richter, E.; Schwalb, J.; Lozano, A. Bilateral subthalamic nucleus stimulation for Parkinson’s disease:a systematic review of the clinical literature. Neurosurgery 2005, 56, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.D.; Jeremy, A.F.; Jérôme, B. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr. Opin. Neurobiol. 2006, 16, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Urbain, N.; Gervasoni, D.; Souliere, F.; Lobo, L.; Rentero, N.; Windels, F.; Astier, B.; Savasta, M.; Fort, P.; Renaud, B. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. Eur. J. Neurosci. 2000, 12, 3361–3374. [Google Scholar] [CrossRef]
- Urbain, N.; Rentero, N.; Gervasoni, D.; Renaud, B.; Chouvet, G. The switch of subthalamic neurons from an irregular to a bursting pattern does not solely depend on their GABAergic inputs in the anesthetic-free rat. J. Neurosci. 2002, 22, 8665–8675. [Google Scholar] [CrossRef]
- Rinzel, J. Bursting oscillations in an excitable membrane model. In Ordinary and Partial Differential Equations; Sleeman, B., Jarvis, R., Eds.; Springer: New York, NY, USA, 1985; pp. 304–316. [Google Scholar]
- Castro-Alamancos, M.; Calcagnotto, M. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: In vitro and in vivo. J. Neurophysiol. 2001, 85, 1489–1497. [Google Scholar] [CrossRef]
- Plenz, D.; Kitai, S. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 1999, 400, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Park, C.; Terman, D.; Wilson, C. Transitions between irregular and rhythmic firing patterns in excitatory-inhibitory neuronal networks. J. Comput. Neurosci. 2007, 23, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Elder, C.; Okun, M.; Patrick, S.; Vitek, J. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J. Neurosci. 2003, 23, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Lindén, H.; Pettersen, K.H.; Einevoll, G.T. Intrinsic dendritic filtering gives low-pass power spectra of local field potentials. J. Comput. Neurosci. 2010, 29, 423–444. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojsavljevic, T.; Guo, Y.; Macaluso, D. Adaptive Stimulations in a Biophysical Network Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 5555. https://doi.org/10.3390/ijms24065555
Stojsavljevic T, Guo Y, Macaluso D. Adaptive Stimulations in a Biophysical Network Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(6):5555. https://doi.org/10.3390/ijms24065555
Chicago/Turabian StyleStojsavljevic, Thomas, Yixin Guo, and Dominick Macaluso. 2023. "Adaptive Stimulations in a Biophysical Network Model of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 6: 5555. https://doi.org/10.3390/ijms24065555
APA StyleStojsavljevic, T., Guo, Y., & Macaluso, D. (2023). Adaptive Stimulations in a Biophysical Network Model of Parkinson’s Disease. International Journal of Molecular Sciences, 24(6), 5555. https://doi.org/10.3390/ijms24065555





