Cardiac Functional and Structural Abnormalities in a Mouse Model of CDKL5 Deficiency Disorder
Abstract
1. Introduction
2. Results
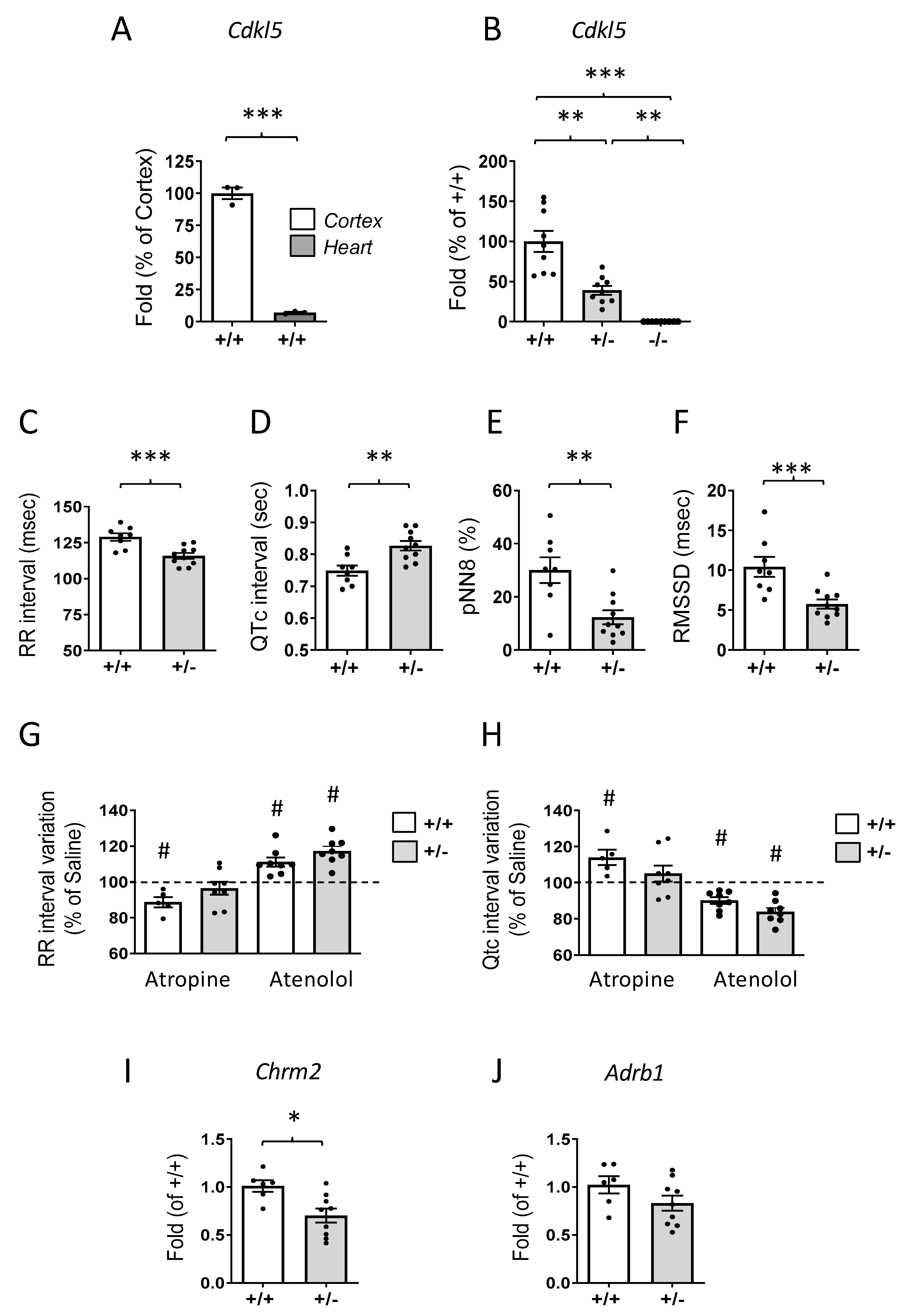
2.1. Prolonged QTc Interval and Elevated Heart Rate in Cdkl5 +/− Mice
2.2. Alterations of Voltage-Gated Channel Expression in the Hearts of Cdkl5 +/− Mice
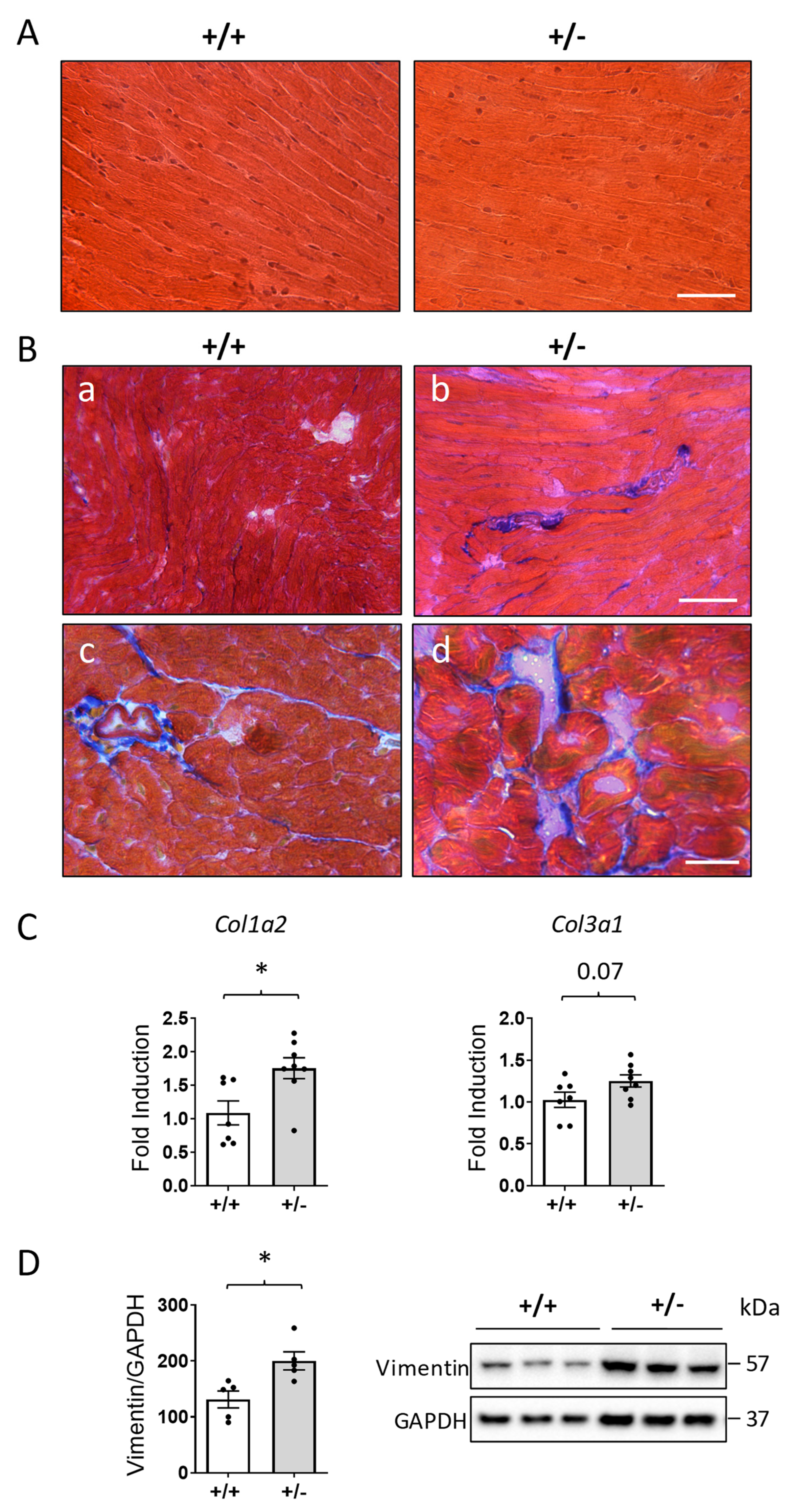
2.3. Increased Cardiac Fibrosis in Cdkl5 +/− Mice
2.4. Altered Gap Junction Organization and Connexin Expression in the Hearts of Cdkl5 +/− Mice
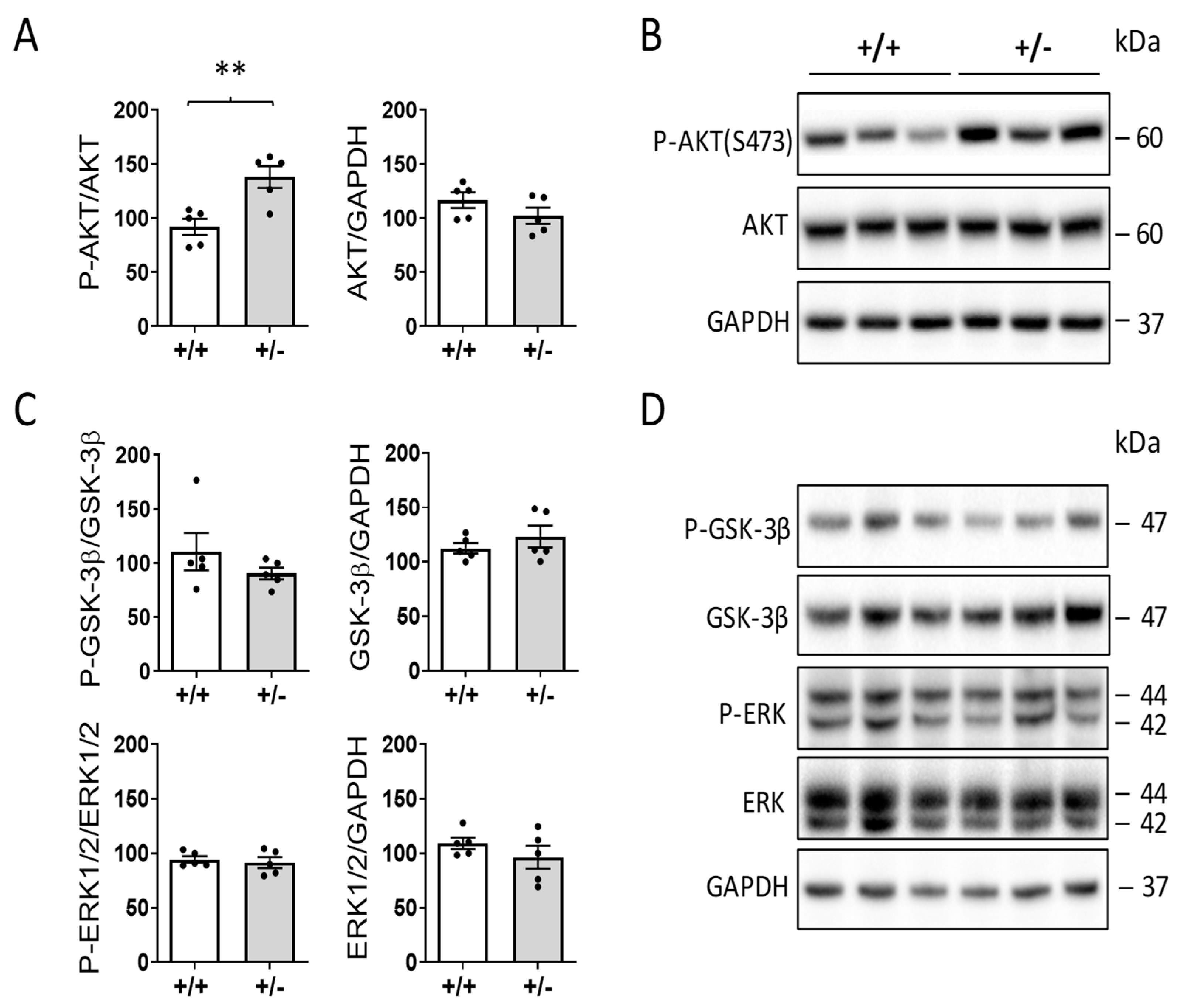
2.5. Increased AKT Activation in the Hearts of Cdkl5 +/− Mice
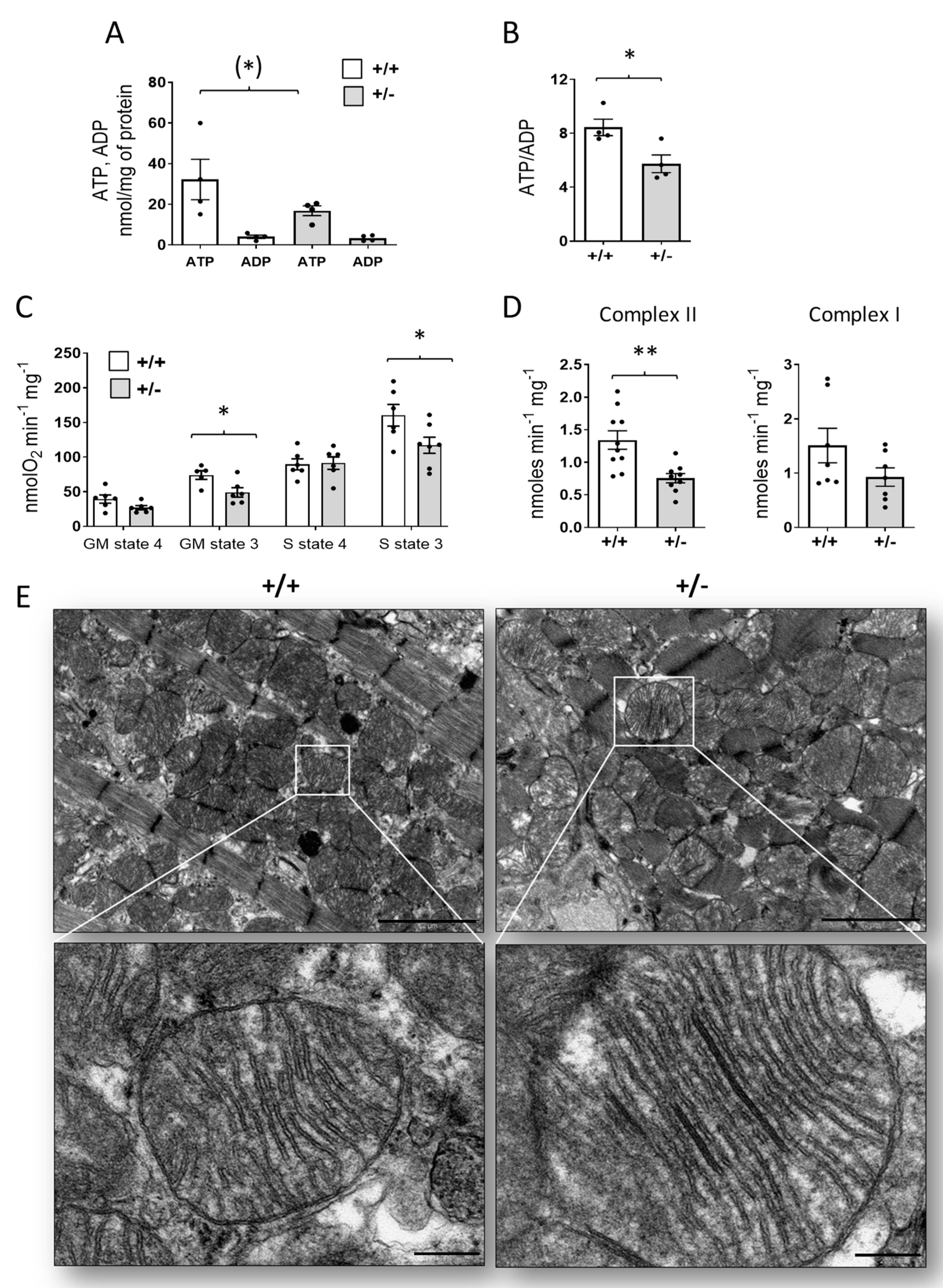
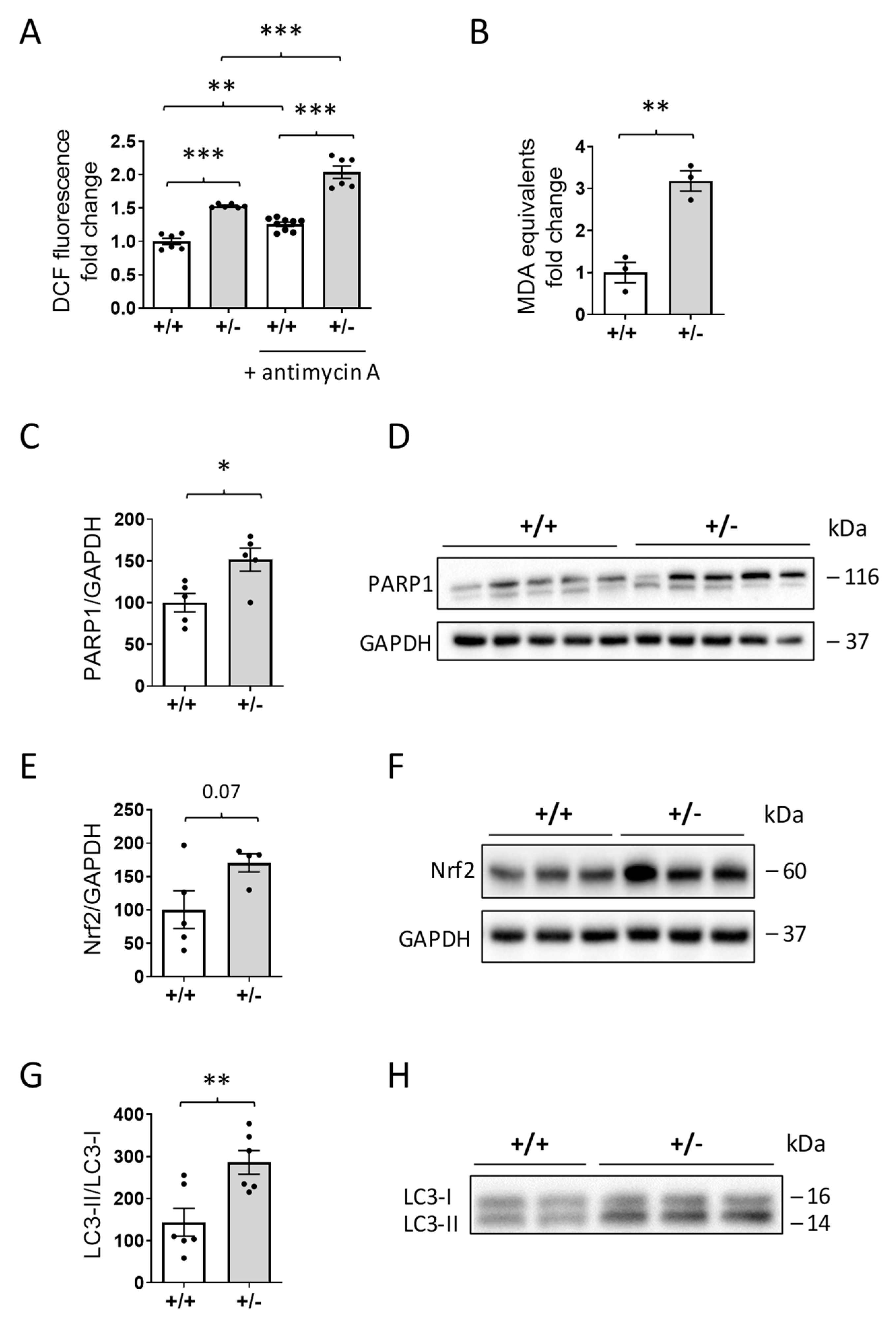
2.6. Mitochondrial Dysfunction in the Hearts of Cdkl5 +/− Mice
2.7. Increased ROS Production in the Hearts of Cdkl5 +/− Mice
3. Discussion
4. Materials and Methods
4.1. Animal Husbandry
4.2. Surgical Procedure, In Vivo Recording, and Data Analysis
4.3. Heart Dissection, Measurement, and Collection
4.4. RNA Isolation and RT-qPCR
4.5. Histological and Immunohistochemistry Procedures
4.5.1. Hematoxylin and Eosin (H&E)
4.5.2. Masson’s Trichrome Staining
4.5.3. Connexin 43, Actinin, and β-Catenin Staining
4.6. Image Acquisition and Measurements
Quantification of β-Catenin Staining Intensity and Areas
4.7. Western Blotting
4.8. Adenine Nucleotide Measurement
4.9. Mitochondrial Oxygen Consumption
4.10. Enzymatic Activities of the Mitochondrial Respiratory Chain
4.11. Measurement of ROS Production
4.12. Measurement of Lipid Peroxidation
4.13. Transmission Electron Microscopy
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fehr, S.; Wilson, M.; Downs, J.; Williams, S.; Murgia, A.; Sartori, S.; Vecchi, M.; Ho, G.; Polli, R.; Psoni, S.; et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur. J. Hum. Genet. 2013, 21, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Demarest, S.T.; Olson, H.E.; Moss, A.; Pestana-Knight, E.; Zhang, X.; Parikh, S.; Swanson, L.C.; Riley, K.D.; Bazin, G.A.; Angione, K.; et al. CDKL5 deficiency disorder: Relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia 2019, 60, 1733–1742. [Google Scholar] [CrossRef]
- Demarest, S.; Pestana-Knight, E.M.; Olson, H.E.; Downs, J.; Marsh, E.D.; Kaufmann, W.E.; Partridge, C.A.; Leonard, H.; Gwadry-Sridhar, F.; Frame, K.E.; et al. Severity Assessment in CDKL5 Deficiency Disorder. Pediatr. Neurol. 2019, 97, 38–42. [Google Scholar] [CrossRef]
- Lindy, A.S.; Stosser, M.B.; Butler, E.; Downtain-Pickersgill, C.; Shanmugham, A.; Retterer, K.; Brandt, T.; Richard, G.; McKnight, D.A. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018, 59, 1062–1071. [Google Scholar] [CrossRef]
- Olson, H.E.; Demarest, S.T.; Pestana-Knight, E.M.; Swanson, L.C.; Iqbal, S.; Lal, D.; Leonard, H.; Cross, J.H.; Devinsky, O.; Benke, T.A. Cyclin-Dependent Kinase-Like 5 Deficiency Disorder: Clinical Review. Pediatr. Neurol. 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Scala, E.; Ariani, F.; Mari, F.; Caselli, R.; Pescucci, C.; Longo, I.; Meloni, I.; Giachino, D.; Bruttini, M.; Hayek, G.; et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J. Med. Genet. 2005, 42, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.; Junaid, M.; Wong, K.; Demarest, S.; Downs, J. Exploring quality of life in individuals with a severe developmental and epileptic encephalopathy, CDKL5 Deficiency Disorder. Epilepsy Res. 2021, 169, 106521. [Google Scholar] [CrossRef]
- Amendola, E.; Zhan, Y.; Mattucci, C.; Castroflorio, E.; Calcagno, E.; Fuchs, C.; Lonetti, G.; Silingardi, D.; Vyssotski, A.L.; Farley, D.; et al. Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS ONE 2014, 9, e91613. [Google Scholar] [CrossRef] [PubMed]
- Jhang, C.L.; Lee, H.Y.; Chen, J.C.; Liao, W. Dopaminergic loss of cyclin-dependent kinase-like 5 recapitulates methylphenidate-remediable hyperlocomotion in mouse model of CDKL5 deficiency disorder. Hum. Mol. Genet. 2020, 29, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kobayashi, S.; Fukaya, M.; Watanabe, A.; Murakami, T.; Hagiwara, M.; Sato, T.; Ueno, H.; Ogonuki, N.; Komano-Inoue, S.; et al. CDKL5 controls postsynaptic localization of GluN2B-containing NMDA receptors in the hippocampus and regulates seizure susceptibility. Neurobiol. Dis. 2017, 106, 158–170. [Google Scholar] [CrossRef]
- Schroeder, E.; Yuan, L.; Seong, E.; Ligon, C.; DeKorver, N.; Gurumurthy, C.B.; Arikkath, J. Neuron-Type Specific Loss of CDKL5 Leads to Alterations in mTOR Signaling and Synaptic Markers. Mol. Neurobiol. 2018, 56, 4151–4162. [Google Scholar] [CrossRef]
- Tang, S.; Wang, I.J.; Yue, C.; Takano, H.; Terzic, B.; Pance, K.; Lee, J.Y.; Cui, Y.; Coulter, D.A.; Zhou, Z. Loss of CDKL5 in Glutamatergic Neurons Disrupts Hippocampal Microcircuitry and Leads to Memory Impairment in Mice. J. Neurosci. 2017, 37, 7420–7437. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.T.; Allen, M.; Goffin, D.; Zhu, X.; Fairless, A.H.; Brodkin, E.S.; Siegel, S.J.; Marsh, E.D.; Blendy, J.A.; Zhou, Z. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 21516–21521. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, R.; Lupori, L.; Sagona, G.; Gennaro, M.; Della Sala, G.; Putignano, E.; Pizzorusso, T. Searching for biomarkers of CDKL5 disorder: Early-onset visual impairment in CDKL5 mutant mice. Hum. Mol. Genet. 2017, 26, 2290–2298. [Google Scholar] [CrossRef]
- Fuchs, C.; Trazzi, S.; Torricella, R.; Viggiano, R.; De Franceschi, M.; Amendola, E.; Gross, C.; Calza, L.; Bartesaghi, R.; Ciani, E. Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3beta signaling. Neurobiol. Dis. 2014, 70, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Putignano, E.; Chelini, G.; Melani, R.; Calcagno, E.; Michele Ratto, G.; Amendola, E.; Gross, C.T.; Giustetto, M.; Pizzorusso, T. Dendritic Spine Instability in a Mouse Model of CDKL5 Disorder Is Rescued by Insulin-like Growth Factor 1. Biol. Psychiatry 2016, 80, 302–311. [Google Scholar] [CrossRef]
- Ren, E.; Roncace, V.; Trazzi, S.; Fuchs, C.; Medici, G.; Gennaccaro, L.; Loi, M.; Galvani, G.; Ye, K.; Rimondini, R.; et al. Functional and Structural Impairments in the Perirhinal Cortex of a Mouse Model of CDKL5 Deficiency Disorder Are Rescued by a TrkB Agonist. Front. Cell. Neurosci. 2019, 13, 169. [Google Scholar] [CrossRef]
- Gennaccaro, L.; Fuchs, C.; Loi, M.; Pizzo, R.; Alvente, S.; Berteotti, C.; Lupori, L.; Sagona, G.; Galvani, G.; Gurgone, A.; et al. Age-Related Cognitive and Motor Decline in a Mouse Model of CDKL5 Deficiency Disorder is Associated with Increased Neuronal Senescence and Death. Aging Dis. 2021, 12, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Galvani, G.; Mottolese, N.; Gennaccaro, L.; Loi, M.; Medici, G.; Tassinari, M.; Fuchs, C.; Ciani, E.; Trazzi, S. Inhibition of Microglia Over-activation Restores Neuronal Survival in a Mouse Model of CDKL5 Deficent Disorder. J. Neuroinflamm. 2021, 18, 155. [Google Scholar] [CrossRef]
- Tassinari, M.; Mottolese, N.; Galvani, G.; Ferrara, D.; Gennaccaro, L.; Loi, M.; Medici, G.; Candini, G.; Rimondini, R.; Ciani, E.; et al. Luteolin Treatment Ameliorates Brain Development and Behavioral Performance in a Mouse Model of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2022, 23, 8719. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Xiong, Z.Q. Molecular and Synaptic Bases of CDKL5 Disorder. Dev. Neurobiol. 2019, 79, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.D.; Dando, O.; Landsberger, N.; Kilstrup-Nielsen, C.; Kind, P.C.; Bailey, M.E.; Cobb, S.R. Characterisation of CDKL5 Transcript Isoforms in Human and Mouse. PLoS ONE 2016, 11, e0157758. [Google Scholar] [CrossRef] [PubMed]
- Montini, E.; Andolfi, G.; Caruso, A.; Buchner, G.; Walpole, S.M.; Mariani, M.; Consalez, G.; Trump, D.; Ballabio, A.; Franco, B. Identification and characterization of a novel serine-threonine kinase gene from the Xp22 region. Genomics 1998, 51, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Striano, P.; Ferrer-Costa, C.; Campuzano, O.; Mates, J.; Del Olmo, B.; Iglesias, A.; Perez-Serra, A.; Mademont, I.; Pico, F.; et al. Targeted next-generation sequencing provides novel clues for associated epilepsy and cardiac conduction disorder/SUDEP. PLoS ONE 2017, 12, e0189618. [Google Scholar] [CrossRef]
- Amin, S.; Majumdar, A.; Mallick, A.A.; Patel, J.; Scatchard, R.; Partridge, C.A.; Lux, A. Caregiver’s perception of epilepsy treatment, quality of life and comorbidities in an international cohort of CDKL5 patients. Hippokratia 2017, 21, 130–135. [Google Scholar]
- Stansauk, J.; Fidell, A.; Benke, T.; Schaffer, M.; Demarest, S.T. Analysis of electrocardiograms in individuals with CDKL5 deficiency disorder. Am. J. Med. Genet. A 2022, 191, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Guideri, F.; Acampa, M.; DiPerri, T.; Zappella, M.; Hayek, Y. Progressive cardiac dysautonomia observed in patients affected by classic Rett syndrome and not in the preserved speech variant. J. Child. Neurol. 2001, 16, 370–373. [Google Scholar] [CrossRef]
- Julu, P.O.; Kerr, A.M.; Hansen, S.; Apartopoulos, F.; Jamal, G.A. Immaturity of medullary cardiorespiratory neurones leading to inappropriate autonomic reactions as a likely cause of sudden death in Rett’s syndrome. Arch. Dis. Child. 1997, 77, 464–465. [Google Scholar] [CrossRef]
- Van Niekerk, C.; Van Deventer, B.S.; du Toit-Prinsloo, L. Long QT syndrome and sudden unexpected infant death. J. Clin. Pathol. 2017, 70, 808–813. [Google Scholar] [CrossRef]
- Lajiness, J.D.; Conway, S.J. Origin, development, and differentiation of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2014, 70, 2–8. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, Y.; Ceylan-Isik, A.F.; Wold, L.E.; Nunn, J.M.; Ren, J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: Role of autophagy. Basic Res. Cardiol. 2011, 106, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Jakimiec, M.; Paprocka, J.; Smigiel, R. CDKL5 Deficiency Disorder-A Complex Epileptic Encephalopathy. Brain Sci. 2020, 10, 107. [Google Scholar] [CrossRef]
- Fuchs, C.; Gennaccaro, L.; Trazzi, S.; Bastianini, S.; Bettini, S.; Lo Martire, V.; Ren, E.; Medici, G.; Zoccoli, G.; Rimondini, R.; et al. Heterozygous CDKL5 Knockout Female Mice Are a Valuable Animal Model for CDKL5 Disorder. Neural. Plast. 2018, 2018, 9726950. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, J.; Aromolaran, K.A.; Aromolaran, A.S. Neurological Disorders and Risk of Arrhythmia. Int. J. Mol. Sci. 2020, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Koschke, M.; Boettger, M.K.; Schulz, S.; Berger, S.; Terhaar, J.; Voss, A.; Yeragani, V.K.; Bar, K.J. Autonomy of autonomic dysfunction in major depression. Psychosom. Med. 2009, 71, 852–860. [Google Scholar] [CrossRef]
- Lopez-Santiago, L.F.; Meadows, L.S.; Ernst, S.J.; Chen, C.; Malhotra, J.D.; McEwen, D.P.; Speelman, A.; Noebels, J.L.; Maier, S.K.; Lopatin, A.N.; et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J. Mol. Cell. Cardiol. 2007, 43, 636–647. [Google Scholar] [CrossRef]
- Miura, T.; Tsuchihashi, K.; Yoshida, E.; Kobayashi, K.; Shimamoto, K.; Iimura, O. Electrocardiographic abnormalities in cerebrovascular accidents. Jpn. J. Med. 1984, 23, 22–26. [Google Scholar] [CrossRef]
- Guideri, F.; Acampa, M. Sudden death and cardiac arrhythmias in Rett syndrome. Pediatr. Cardiol. 2005, 26, 111. [Google Scholar] [CrossRef]
- Julu, P.O.; Kerr, A.M.; Apartopoulos, F.; Al-Rawas, S.; Engerstrom, I.W.; Engerstrom, L.; Jamal, G.A.; Hansen, S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch. Dis. Child. 2001, 85, 29–37. [Google Scholar] [CrossRef]
- Ming, X.; Julu, P.O.; Brimacombe, M.; Connor, S.; Daniels, M.L. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005, 27, 509–516. [Google Scholar] [CrossRef]
- Xiong, L.; Leung, T.W.H. Autonomic dysfunction in neurological disorders. Aging 2019, 11, 1903–1904. [Google Scholar] [CrossRef]
- Toledo, M.A.; Junqueira, L.F., Jr. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin. Auton. Res. 2010, 20, 11–17. [Google Scholar] [CrossRef]
- Aharon-Peretz, J.; Harel, T.; Revach, M.; Ben-Haim, S.A. Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer’s disease. Arch. Neurol. 1992, 49, 919–922. [Google Scholar] [CrossRef]
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac innervation and sudden cardiac death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef]
- Vanoli, E.; Schwartz, P.J. Sympathetic--parasympathetic interaction and sudden death. Basic Res. Cardiol. 1990, 85, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Q.; Tang, B.; Wu, Z.; Yang, T.; Tang, J. CDKL5 Deficiency Augments Inhibitory Input into the Dentate Gyrus That Can Be Reversed by Deep Brain Stimulation. J. Neurosci. 2021, 41, 9031–9046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, L.; Shen, C.; Hu, K.; Ge, J.; Sun, A. SCN5A Variants: Association With Cardiac Disorders. Front. Physiol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Hara, M.; Takahashi, T.; Mitsumasu, C.; Igata, S.; Takano, M.; Minami, T.; Yasukawa, H.; Okayama, S.; Nakamura, K.; Okabe, Y.; et al. Disturbance of cardiac gene expression and cardiomyocyte structure predisposes Mecp2-null mice to arrhythmias. Sci. Rep. 2015, 5, 11204. [Google Scholar] [CrossRef]
- Severs, N.J.; Bruce, A.F.; Dupont, E.; Rothery, S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 2008, 80, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lerner, D.L.; Yamada, K.A.; Schuessler, R.B.; Saffitz, J.E. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation 2000, 101, 547–552. [Google Scholar] [CrossRef]
- Bonda, T.A.; Szynaka, B.; Sokolowska, M.; Dziemidowicz, M.; Winnicka, M.M.; Chyczewski, L.; Kaminski, K.A. Remodeling of the intercalated disc related to aging in the mouse heart. J. Cardiol. 2016, 68, 261–268. [Google Scholar] [CrossRef]
- Hayflick, L. Biological aging is no longer an unsolved problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Scapagini, G.; Curro, D.; Giuffrida Stella, A.M.; De Marco, C.; Butterfield, D.A.; Calabrese, V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front. Biosci. 2007, 12, 1107–1123. [Google Scholar] [CrossRef]
- Pecorelli, A.; Belmonte, G.; Meloni, I.; Cervellati, F.; Gardi, C.; Sticozzi, C.; De Felice, C.; Signorini, C.; Cortelazzo, A.; Leoncini, S.; et al. Alteration of serum lipid profile, SRB1 loss, and impaired Nrf2 activation in CDKL5 disorder. Free Radic. Biol. Med. 2015, 86, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Hayek, J.; De Felice, C.; Valacchi, G. Increased levels of 4HNE-protein plasma adducts in Rett syndrome. Clin. Biochem. 2011, 44, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, S.; De Felice, C.; Signorini, C.; Zollo, G.; Cortelazzo, A.; Durand, T.; Galano, J.M.; Guerranti, R.; Rossi, M.; Ciccoli, L.; et al. Cytokine Dysregulation in MECP2- and CDKL5-Related Rett Syndrome: Relationships with Aberrant Redox Homeostasis, Inflammation, and omega-3 PUFAs. Oxid. Med. Cell. Longev. 2015, 2015, 421624. [Google Scholar] [CrossRef]
- Cortelazzo, A.; de Felice, C.; Leoncini, S.; Signorini, C.; Guerranti, R.; Leoncini, R.; Armini, A.; Bini, L.; Ciccoli, L.; Hayek, J. Inflammatory protein response in CDKL5-Rett syndrome: Evidence of a subclinical smouldering inflammation. Inflamm. Res. 2017, 66, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Vigli, D.; Rusconi, L.; Valenti, D.; La Montanara, P.; Cosentino, L.; Lacivita, E.; Leopoldo, M.; Amendola, E.; Gross, C.; Landsberger, N.; et al. Rescue of prepulse inhibition deficit and brain mitochondrial dysfunction by pharmacological stimulation of the central serotonin receptor 7 in a mouse model of CDKL5 Deficiency Disorder. Neuropharmacology 2019, 144, 104–114. [Google Scholar] [CrossRef]
- Carli, S.; Chaabane, L.; Butti, C.; De Palma, C.; Aimar, P.; Salio, C.; Vignoli, A.; Giustetto, M.; Landsberger, N.; Frasca, A. In vivo magnetic resonance spectroscopy in the brain of Cdkl5 null mice reveals a metabolic profile indicative of mitochondrial dysfunctions. J. Neurochem. 2021, 157, 1253–1269. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Massey, S.; Stait, T.; Ellery, M.; Reljic, B.; Formosa, L.E.; Quigley, A.; Dottori, M.; Thorburn, D.; Stroud, D.A.; et al. Abnormalities of mitochondrial dynamics and bioenergetics in neuronal cells from CDKL5 deficiency disorder. Neurobiol. Dis. 2021, 155, 105370. [Google Scholar] [CrossRef]
- Jagtap, S.; Thanos, J.M.; Fu, T.; Wang, J.; Lalonde, J.; Dial, T.O.; Feiglin, A.; Chen, J.; Kohane, I.; Lee, J.T.; et al. Aberrant mitochondrial function in patient-derived neural cells from CDKL5 deficiency disorder and Rett syndrome. Hum. Mol. Genet. 2019, 28, 3625–3636. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.E.; Ng, Y.S.; White, K.; Davey, T.; Mannella, C.; Falkous, G.; Feeney, C.; Schaefer, A.M.; McFarland, R.; Gorman, G.S.; et al. The Spectrum of Mitochondrial Ultrastructural Defects in Mitochondrial Myopathy. Sci. Rep. 2016, 6, 30610. [Google Scholar] [CrossRef] [PubMed]
- Stadhouders, A.M.; Jap, P.H.; Winkler, H.P.; Eppenberger, H.M.; Wallimann, T. Mitochondrial creatine kinase: A major constituent of pathological inclusions seen in mitochondrial myopathies. Proc. Natl. Acad. Sci. USA 1994, 91, 5089–5093. [Google Scholar] [CrossRef] [PubMed]
- Sugden, P.H.; Clerk, A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid. Redox Signal. 2006, 8, 2111–2124. [Google Scholar] [CrossRef]
- Barth, S.; Glick, D.; Macleod, K.F. Autophagy: Assays and artifacts. J. Pathol. 2010, 221, 117–124. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2019, 38, e101812. [Google Scholar] [CrossRef]
- Bueno, O.F.; De Windt, L.J.; Tymitz, K.M.; Witt, S.A.; Kimball, T.R.; Klevitsky, R.; Hewett, T.E.; Jones, S.P.; Lefer, D.J.; Peng, C.F.; et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000, 19, 6341–6350. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Walker, A.; Singh, A.; Tully, E.; Woo, J.; Le, A.; Nguyen, T.; Biswal, S.; Sharma, D.; Gabrielson, E. Nrf2 signaling and autophagy are complementary in protecting breast cancer cells during glucose deprivation. Free Radic. Biol. Med. 2018, 120, 407–413. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair. 2019, 81, 102651. [Google Scholar] [CrossRef]
- Bai, P.; Nagy, L.; Fodor, T.; Liaudet, L.; Pacher, P. Poly(ADP-ribose) polymerases as modulators of mitochondrial activity. Trends Endocrinol. Metab. 2015, 26, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, W.; Zhang, Y.; Zhang, F.; Huang, K. PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription. Cell Death Dis. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Szyller, J.; Jagielski, D.; Bil-Lula, I. Antioxidants in Arrhythmia Treatment-Still a Controversy? A Review of Selected Clinical and Laboratory Research. Antioxidants 2022, 11, 1109. [Google Scholar] [CrossRef]
- Jeong, E.M.; Liu, M.; Sturdy, M.; Gao, G.; Varghese, S.T.; Sovari, A.A.; Dudley, S.C., Jr. Metabolic stress, reactive oxygen species, and arrhythmia. J. Mol. Cell. Cardiol. 2012, 52, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Shah, A.K.; Dhalla, N.S. Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias. Int. J. Mol. Sci. 2020, 21, 4200. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Alvente, S.; Berteotti, C.; Bastianini, S.; Lo Martire, V.; Matteoli, G.; Silvani, A.; Zoccoli, G. Autonomic mechanisms of blood pressure alterations during sleep in orexin/hypocretin-deficient narcoleptic mice. Sleep 2021, 44, zsab022. [Google Scholar] [CrossRef] [PubMed]
- Lo Martire, V.; Silvani, A.; Alvente, S.; Bastianini, S.; Berteotti, C.; Valli, A.; Zoccoli, G. Modulation of sympathetic vasoconstriction is critical for the effects of sleep on arterial pressure in mice. J. Physiol. 2018, 596, 591–608. [Google Scholar] [CrossRef]
- Bastianini, S.; Lo Martire, V.; Alvente, S.; Berteotti, C.; Matteoli, G.; Rullo, L.; Stamatakos, S.; Silvani, A.; Candeletti, S.; Romualdi, P.; et al. Early-life nicotine or cotinine exposure produces long-lasting sleep alterations and downregulation of hippocampal corticosteroid receptors in adult mice. Sci. Rep. 2021, 11, 23897. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Joviano-Santos, J.V.; Santos-Miranda, A.; Menezes-Filho, J.E.R.; Soto-Blanco, B.; Cruz, J.S.; Guatimosim, C.; Melo, M.M. Non-invasive ECG recording and QT interval correction assessment in anesthetized rats and mice. Pesquisa. Veterinaria. Brasileira. 2019, 39, 409–415. [Google Scholar] [CrossRef]
- Silvani, A.; Bastianini, S.; Berteotti, C.; Franzini, C.; Lenzi, P.; Lo Martire, V.; Zoccoli, G. Dysregulation of heart rhythm during sleep in leptin-deficient obese mice. Sleep 2010, 33, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Laude, D.; Baudrie, V.; Elghozi, J.L. Effects of atropine on the time and frequency domain estimates of blood pressure and heart rate variability in mice. Clin. Exp. Pharmacol. Physiol. 2008, 35, 454–457. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jones, D.P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J. Chromatogr. 1981, 225, 446–449. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fato, R.; Bergamini, C.; Bortolus, M.; Maniero, A.L.; Leoni, S.; Ohnishi, T.; Lenaz, G. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim. Biophys. Acta 2009, 1787, 384–392. [Google Scholar] [CrossRef]
- Reilly, C.A.; Aust, S.D. Measurement of lipid peroxidation. Curr. Protoc. Toxicol. 2001, 1, 2–4. [Google Scholar] [CrossRef]
- Mucerino, S.; Di Salle, A.; Alessio, N.; Margarucci, S.; Nicolai, R.; Melone, M.A.; Galderisi, U.; Peluso, G. Alterations in the carnitine cycle in a mouse model of Rett syndrome. Sci. Rep. 2017, 7, 41824. [Google Scholar] [CrossRef]
- Cheng, H.; Charles, I.; James, A.F.; Abdala, A.P.; Hancox, J.C. QT(c) interval and ventricular action potential prolongation in the Mecp2(Null/+) murine model of Rett syndrome. Physiol. Rep. 2022, 10, e15437. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.D.; Wang, T.; Mike, E.; Herrera, J.; Beavers, D.L.; Huang, T.W.; Ward, C.S.; Skinner, S.; Percy, A.K.; Glaze, D.G.; et al. Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: Implication for therapy in Rett syndrome. Sci. Transl. Med. 2011, 3, 113ra125. [Google Scholar] [CrossRef]
- Kumar, A.; Jaryal, A.; Gulati, S.; Chakrabarty, B.; Singh, A.; Deepak, K.K.; Pandey, R.M.; Gupta, N.; Sapra, S.; Kabra, M.; et al. Cardiovascular Autonomic Dysfunction in Children and Adolescents With Rett Syndrome. Pediatr. Neurol. 2017, 70, 61–66. [Google Scholar] [CrossRef]
- Clark, B.C.; Kopp, A.; Morey, W.; Djukic, A. Serial follow-up of corrected QT interval in Rett syndrome. Dev. Med. Child. Neurol. 2020, 62, 833–836. [Google Scholar] [CrossRef]
- Mari, F.; Azimonti, S.; Bertani, I.; Bolognese, F.; Colombo, E.; Caselli, R.; Scala, E.; Longo, I.; Grosso, S.; Pescucci, C.; et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum. Mol. Genet. 2005, 14, 1935–1946. [Google Scholar] [CrossRef]
- Bertani, I.; Rusconi, L.; Bolognese, F.; Forlani, G.; Conca, B.; De Monte, L.; Badaracco, G.; Landsberger, N.; Kilstrup-Nielsen, C. Functional consequences of mutations in CDKL5, an X-linked gene involved in infantile spasms and mental retardation. J. Biol. Chem. 2006, 281, 32048–32056. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Franco, B.; Rosner, M.R. CDKL5/Stk9 kinase inactivation is associated with neuronal developmental disorders. Hum. Mol. Genet. 2005, 14, 3775–3786. [Google Scholar] [CrossRef] [PubMed]
- Kameshita, I.; Sekiguchi, M.; Hamasaki, D.; Sugiyama, Y.; Hatano, N.; Suetake, I.; Tajima, S.; Sueyoshi, N. Cyclin-dependent kinase-like 5 binds and phosphorylates DNA methyltransferase 1. Biochem. Biophys. Res. Commun. 2008, 377, 1162–1167. [Google Scholar] [CrossRef] [PubMed]

| Cdkl5 +/+ | Cdkl5 +/− | Cdkl5 −/− | |
|---|---|---|---|
| RR interval (msec) | 128.9 ± 2.6 | 115.7 ± 2.1 ** | 118.4 ± 3.1 * |
| QTc interval (sec) | 0.749 ± 0.016 | 0.827 ± 0.015 ** | 0.820 ± 0.022 * |
| pNN8 (%) | 30.3 ± 4.3 | 12.3 ± 2.7 *** | 8.2 ± 2.8 *** |
| RMSSD (msec) | 10.4 ± 1.3 | 5.8 ± 0.6 ** | 5.9 ± 0.9 ** |
| Genes | Cdkl5 +/+ | Cdkl5 +/− | p |
|---|---|---|---|
| Kcnq1 | 1.00 ± 0.119 (n = 7) | 1.08 ± 0.048 (n = 9) | n.s. |
| Kcnh2 | 1.00 ± 0.055 (n = 7) | 0.88 ± 0.032 (n = 9) | n.s. |
| Kcnj2 | 1.00 ± 0.069 (n = 7) | 0.99 ± 0.037 (n = 9) | n.s. |
| Scn5a | 1.00 ± 0.054 (n = 5) | 0.87 ± 0.030 (n = 5) | * |
| Hcn4 | 1.00 ± 0.067 (n = 7) | 0.76 ± 0.039 (n = 9) | * |
| Cdkl5 +/+ | Cdkl5 +/− | p | |
|---|---|---|---|
| Heart weight (mg) | 110.83 ± 3.24 | 105.89 ± 2.27 | n.s. |
| Body weight (g) | 22.19 ± 0.27 | 21.74 ± 0.31 | n.s. |
| Heart/body weight (mg/g) | 4.98 ± 0.12 | 4.87 ± 0.07 | n.s. |
| Atrioventricular distance (cm) | 0.51 ± 0.01 | 0.52 ± 0.03 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loi, M.; Bastianini, S.; Candini, G.; Rizzardi, N.; Medici, G.; Papa, V.; Gennaccaro, L.; Mottolese, N.; Tassinari, M.; Uguagliati, B.; et al. Cardiac Functional and Structural Abnormalities in a Mouse Model of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2023, 24, 5552. https://doi.org/10.3390/ijms24065552
Loi M, Bastianini S, Candini G, Rizzardi N, Medici G, Papa V, Gennaccaro L, Mottolese N, Tassinari M, Uguagliati B, et al. Cardiac Functional and Structural Abnormalities in a Mouse Model of CDKL5 Deficiency Disorder. International Journal of Molecular Sciences. 2023; 24(6):5552. https://doi.org/10.3390/ijms24065552
Chicago/Turabian StyleLoi, Manuela, Stefano Bastianini, Giulia Candini, Nicola Rizzardi, Giorgio Medici, Valentina Papa, Laura Gennaccaro, Nicola Mottolese, Marianna Tassinari, Beatrice Uguagliati, and et al. 2023. "Cardiac Functional and Structural Abnormalities in a Mouse Model of CDKL5 Deficiency Disorder" International Journal of Molecular Sciences 24, no. 6: 5552. https://doi.org/10.3390/ijms24065552
APA StyleLoi, M., Bastianini, S., Candini, G., Rizzardi, N., Medici, G., Papa, V., Gennaccaro, L., Mottolese, N., Tassinari, M., Uguagliati, B., Berteotti, C., Martire, V. L., Zoccoli, G., Cenacchi, G., Trazzi, S., Bergamini, C., & Ciani, E. (2023). Cardiac Functional and Structural Abnormalities in a Mouse Model of CDKL5 Deficiency Disorder. International Journal of Molecular Sciences, 24(6), 5552. https://doi.org/10.3390/ijms24065552














