Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology
Abstract
1. Introduction
2. SCD1 as a Regulator of Cardiac Function and Metabolism
2.1. Expression and Regulation of SCD Isoforms in the Heart
2.2. SCD1 Involvement in the Development of Cardiac Pathologies
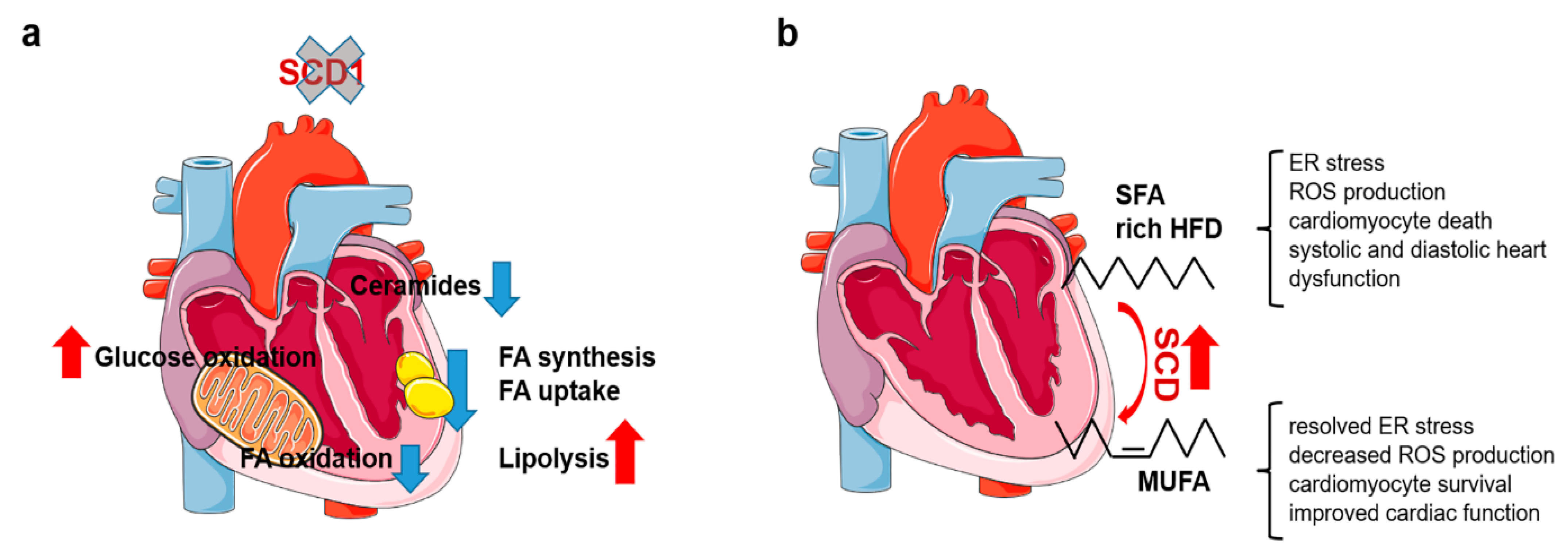
2.3. Reprogramming of Cardiac Metabolism in SCD1-Deficient Mice
2.4. Elevated SCD Activity Protects against Cardiotoxic Effects of SFA
3. SCD in Vessel (patho)Physiology
3.1. Regulation of SCD1 Expression in Vessels
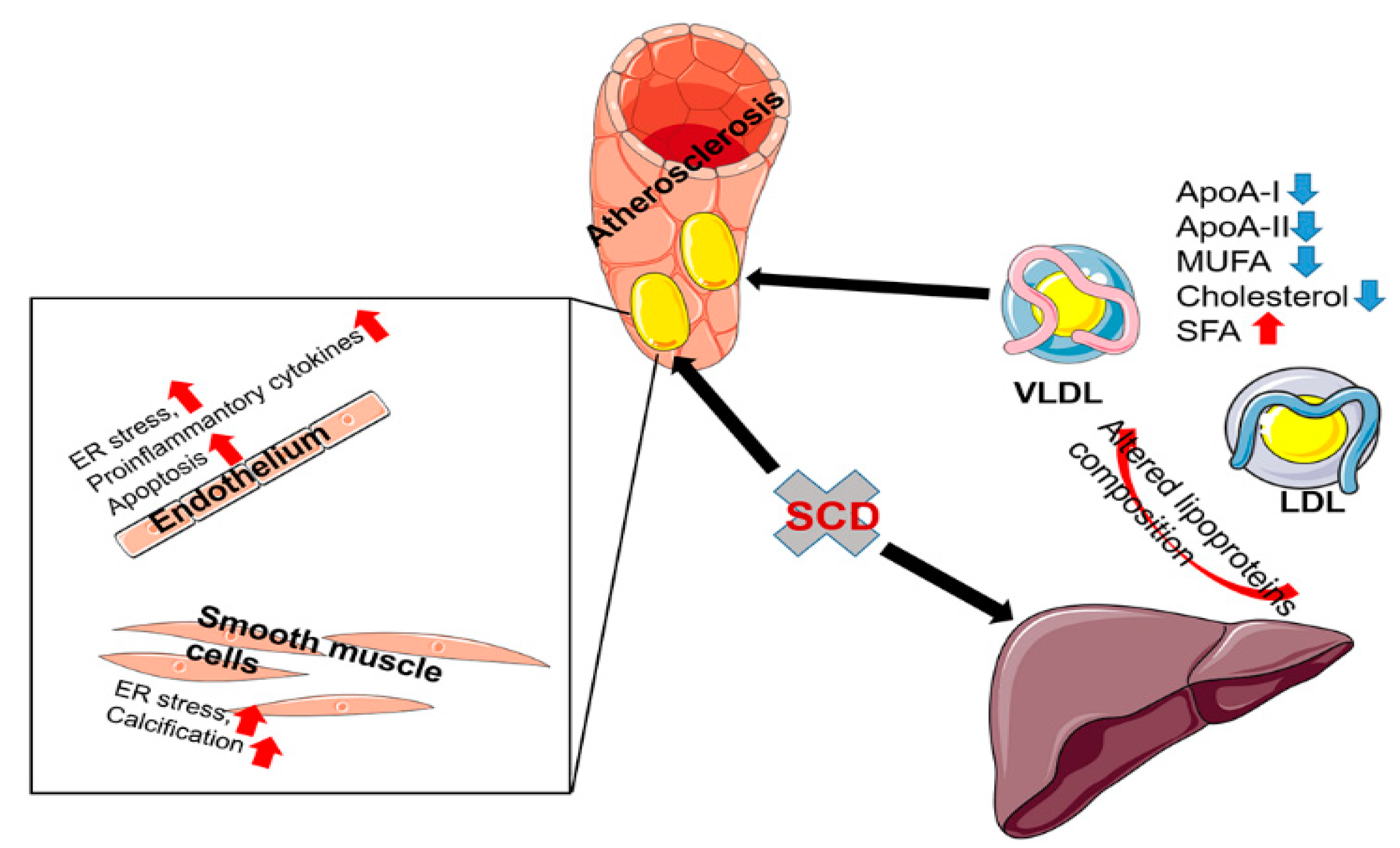
3.2. The Role of SCD1 in the Pathogenesis of Atherosclerosis
3.3. The Role of SCD1 in Vascular Smooth Muscle Cells Transdifferentiation
3.4. The Role of SCD1 in Endothelial Cells
3.5. The Role of SCD1 in Macrophages
4. Δ-9 Desaturation of Blood Fatty Acid as a Diagnostic Marker for Cardiovascular Disorders
4.1. Indirect Indicators of SCD Activity
4.2. SCD Systemic Activity and Cardiovascular Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Foryst-Ludwig, A.; Haemmerle, G.; Zechner, R. The role of adipose triglyceride lipase and cytosolic lipolysis in cardiac function and heart failure. Cell Rep. Med. 2020, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Popeijus, H.E.; van Otterdijk, S.D.; van der Krieken, S.E.; Konings, M.; Serbonij, K.; Plat, J.; Mensink, R.P. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2342–2349. [Google Scholar] [CrossRef]
- Razani, B.; Zhang, H.; Schulze, P.C.; Schilling, J.D.; Verbsky, J.; Lodhi, I.J.; Topkara, V.K.; Feng, C.; Coleman, T.; Kovacs, A.; et al. Fatty acid synthase modulates homeostatic responses to myocardial stress. J. Biol. Chem. 2011, 286, 30949–30961. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Wang, H.; Klein, M.G.; Zou, H.; Lane, W.; Snell, G.; Levin, I.; Li, K.; Sang, B.C. Crystal structure of human stearoyl–Coenzyme A desaturase in complex with substrate. Nat. Struct. Mol. Biol. 2015, 22, 581–585. [Google Scholar] [CrossRef]
- Bai, Y.; McCoy, J.G.; Levin, E.J.; Sobrado, P.; Rajashankar, K.R.; Fox, B.G.; Zhou, M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature 2015, 524, 252–256. [Google Scholar] [CrossRef]
- Miyazaki, M.; Jacobson, M.J.; Man, W.C.; Cohen, P.; Asilmaz, E.; Friedman, J.M.; Ntambi, J.M. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 2003, 278, 33904–33911. [Google Scholar] [CrossRef]
- Miyazaki, M.; Bruggink, S.M.; Ntambi, J.M. Identification of mouse palmitoyl-Coenzyme A Δ9-desaturase. J. Lipid Res. 2006, 47, 700–704. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Schmidt, R.E.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef]
- O’neill, L.M.; Guo, C.A.; Ding, F.; Phang, Y.X.; Liu, Z.; Shamsuzzaman, S.; Ntambi, J.M. Stearoyl-CoA desaturase-2 in murine development, metabolism, and disease. Int. J. Mol. Sci. 2020, 21, 8619. [Google Scholar] [CrossRef] [PubMed]
- Igal, R.A.; Sinner, D.I. Stearoyl-CoA desaturase 5 (SCD5), a Δ-9 fatty acyl desaturase in search of a function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158840. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.T.; Ntambi, J.M. Role of stearoyl-Coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008, 19, 248–256. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Z.; Liu, F.; Ellsworth, K.; Dallas-Yang, Q.; Wu, M.; Ronan, J.; Esau, C.; Murphy, C.; Szalkowski, D.; et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase–1. J. Clin. Investig. 2005, 115, 1030–1038. [Google Scholar] [CrossRef]
- Lee, S.H.; Dobrzyn, A.; Dobrzyn, P.; Rahman, S.M.; Miyazaki, M.; Ntambi, J.M. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J. Lipid Res. 2004, 45, 1674–1682. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Miyazaki, M.; Socci, N.D.; Hagge-Greenberg, A.; Liedtke, W.; Soukas, A.A.; Sharma, R.; Hudgins, L.C.; Ntambi, J.M.; Friedman, J.M. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002, 297, 240–243. [Google Scholar] [CrossRef]
- Liu, K.; Lin, L.; Li, Q.; Xue, Y.; Zheng, F.; Wang, G.; Zheng, C.; Du, L.; Hu, M.; Huang, Y.; et al. Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl. Acad. Sci. USA 2020, 117, 2462–2472. [Google Scholar] [CrossRef]
- Binczek, E.; Jenke, B.; Holz, B.; Günter, R.H.; Thevis, M.; Stoffel, W. Obesity resistance of the stearoyl-CoA desaturase-deficient (Scd1-/-) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol. Chem. 2007, 388, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef] [PubMed]
- Olichwier, A.; Balatskyi, V.V.; Wolosiewicz, M.; Ntambi, J.M.; Dobrzyn, P. Interplay between thyroid hormones and stearoyl-CoA desaturase 1 in the regulation of lipid metabolism in the heart. Int. J. Mol. Sci 2021, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007, 6, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Kim, Y.C.; Gray-Keller, M.P.; Attie, A.D.; Ntambi, J.M. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 2000, 275, 30132–30138. [Google Scholar] [CrossRef]
- Rogowski, M.P.; Flowers, M.T.; Stamatikos, A.D.; Ntambi, J.M.; Paton, C.M. SCD1 activity in muscle increases triglyceride PUFA content, exercise capacity, and PPARδ expression in mice. J. Lipid Res. 2013, 54, 2636–2646. [Google Scholar] [CrossRef]
- Volkmar, N.; Gawden-Bone, C.M.; Williamson, J.C.; Nixon-Abell, J.; West, J.A.; George-Hyslop, P.H.S.; Kaser, A.; Lehner, P.J. Regulation of membrane fluidity by RNF145-triggered degradation of the lipid hydrolase ADIPOR2. EMBO J. 2022, 41, e110777. [Google Scholar] [CrossRef] [PubMed]
- Janikiewicz, J.; Hanzelka, K.; Dziewulska, A.; Kozinski, K.; Dobrzyn, P.; Bernas, T.; Dobrzyn, A. Inhibition of SCD1 impairs palmitate-derived autophagy at the step of autophagosome-lysosome fusion in pancreatic β-cells. J. Lipid Res. 2015, 56, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Rios-Esteves, J.; Resh, M.D. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013, 4, 1072–1081. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Lee, S.H.; Dobrzyn, P.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 381–387. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Dobrzyn, P.; Lee, S.H.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Nat. Acad. Sci. USA 2003, 100, 11110–11115. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, P.; Sampath, H.; Dobrzyn, A.; Miyazaki, M.; Ntambi, J.M. Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 357–364. [Google Scholar] [CrossRef]
- Dziewulska, A.; Dobosz, A.M.; Dobrzyn, A.; Smolinska, A.; Kolczynska, K.; Ntambi, J.M.; Dobrzyn, P. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J. Cell. Physiol. 2020, 235, 1129–1140. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, J.; Kataoka, M.; Matsuhashi, T.; Katsumata, Y.; Shirakawa, K.; Yoshida, N.; Isobe, S.; Moriyama, H.; Goto, S.; et al. Sirt1 counteracts decrease in membrane phospholipid unsaturation and diastolic dysfunction during saturated fatty acid overload. J. Mol. Cell. Cardiol. 2019, 133, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Yokoyama, T.; Sekiguchi, K.; Iijima, D.; Sunaga, H.; Maniwa, M.; Ueno, M.; Iso, T.; Arai, M.; Kurabayashi, M. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS ONE 2012, 7, e33283. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.V.; Muller, C.J.F.; Huisamen, B.; Essop, M.F.; Louw, J. The transcription profile unveils the cardioprotective effect of aspalathin against lipid toxicity in an in vitro H9c2 model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Pintado, C.; Rubio, B.; Mazuecos, L.; López, V.; Fernández, A.; Salamanca, A.; Bárcena, B.; Fernández-Agulló, T.; Arribas, C.; et al. Central leptin regulates heart lipid content by selectively increasing PPAR β/δ expression. J. Endocrinol. 2018, 236, 43–56. [Google Scholar] [CrossRef]
- AbdAlla, S.; Fu, X.; Elzahwy, S.S.; Klaetschke, K.; Streichert, T.; Quitterer, U. Up-regulation of the cardiac lipid metabolism at the onset of heart failure. Cardiovasc. Hematol. Agents Med. Chem. 2011, 9, 190–206. [Google Scholar] [CrossRef]
- Abd Alla, J.; Jamous, Y.F.; Quitterer, U. Stearoyl-CoA desaturase (SCD) induces cardiac dysfunction with cardiac lipid overload and angiotensin II AT1 receptor protein up-regulation. Int. J. Mol. Sci. 2021, 22, 9883. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Pyrkowska, A.; Duda, M.K.; Bednarski, T.; Maczewski, M.; Langfort, J.; Dobrzyn, A. Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 1348–1358. [Google Scholar] [CrossRef]
- Bednarski, T.K.; Duda, M.K.; Dobrzyn, P. Alterations of lipid metabolism in the heart in spontaneously hypertensive rats precedes left ventricular hypertrophy and cardiac dysfunction. Cells 2022, 11, 3032. [Google Scholar] [CrossRef]
- Cui, H.; Kohsaka, A.; Waki, H.; Bhuiyan, M.E.R.; Gouraud, S.S.; Maeda, M. Metabolic cycles are linked to the cardiovascular diurnal rhythm in rats with essential hypertension. PLoS ONE 2011, 6, e17339. [Google Scholar] [CrossRef] [PubMed]
- Miklankova, D.; Markova, I.; Hüttl, M.; Zapletalova, I.; Poruba, M.; Malinska, H. Metformin affects cardiac arachidonic acid metabolism and cardiac lipid metabolite storage in a prediabetic rat model. Int. J. Mol. Sci. 2021, 22, 7680. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Jiang, H.; Trent, C.M.; Yuen, J.J.; Narayanasamy, S.; Curley, R.W.; Harrison, E.H.; Goldberg, I.J.; Maurer, M.S.; Blaner, W.S. Cardiac dysfunction in β-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1675–H1684. [Google Scholar] [CrossRef]
- Basu, P.; Alibhai, F.J.; Tsimakouridze, E.V.; Singh, R.K.; Paglialunga, S.; Holloway, G.P.; Martino, T.A.; Bakovic, M. Male-specific cardiac dysfunction in CTP:phosphoethanolamine cytidylyltransferase (Pcyt2)-deficient mice. Mol. Cell. Biol. 2015, 35, 2641–2657. [Google Scholar] [CrossRef] [PubMed]
- Helle, E.; Priest, J.R. Maternal obesity and diabetes mellitus as risk factors for congenital heart disease in the offspring. J. Am. Heart Assoc. 2020, 9, e011541. [Google Scholar] [CrossRef]
- Turdi, S.; Ge, W.; Hu, N.; Bradley, K.M.; Wang, X.; Ren, J. Interaction between maternal and postnatal high fat diet leads to a greater risk of myocardial dysfunction in offspring via enhanced lipotoxicity, IRS-1 serine phosphorylation and mitochondrial defects. J. Mol. Cell. Cardiol. 2013, 55, 117–129. [Google Scholar] [CrossRef]
- Kolwicz, S.C.; Liu, L.; Goldberg, I.J.; Tian, R. Enhancing cardiac triacylglycerol metabolism improves recovery from ischemic stress. Diabetes 2015, 64, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Kappel, B.A.; Carnevale, D.; Cavalera, M.; Mavilio, M.; Arisi, I.; Fardella, V.; Cifelli, G.; Casagrande, V.; Rizza, S.; et al. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol. Metab. 2015, 4, 741–752. [Google Scholar] [CrossRef]
- Campbell, F.M.; Kozak, R.; Wagner, A.; Altarejos, J.Y.; Dyck, J.R.B.; Belke, D.D.; Severson, D.L.; Kelly, D.P.; Lopaschuk, G.D. A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: Reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J. Biol. Chem. 2002, 277, 4098–4103. [Google Scholar]
- Cohen, E.D.; Yee, M.; Porter, G.A.; Ritzer, E.; McDavid, A.N.; Brookes, P.S.; Pryhuber, G.S.; O’Reilly, M.A. Neonatal hyperoxia inhibits proliferation and survival of atrial cardiomyocytes by suppressing fatty acid synthesis. JCI Insight 2021, 6, 140785. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Dobrzyn, A.; Miyazaki, M.; Ntambi, J.M. Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. J. Lipid Res. 2010, 51, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Gan, A.-M.; Tracz-Gaszewska, Z.; Ellert-Miklaszewska, A.; Navrulin, V.O.; Ntambi, J.M.; Dobrzyn, P. Stearoyl-CoA desaturase regulates angiogenesis and energy metabolism in ischemic cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 10459. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, J.; Kataoka, M.; Matsuhashi, T.; Katsumata, Y.; Shirakawa, K.; Yoshida, N.; Isobe, S.; Moriyama, H.; Goto, S.; et al. Decrease in membrane phospholipids unsaturation correlates with myocardial diastolic dysfunction. PLoS ONE 2018, 13, e0208396. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Endo, J.; Kataoka, M.; Matsuhashi, T.; Katsumata, Y.; Shirakawa, K.; Isobe, S.; Moriyama, H.; Goto, S.; Shimanaka, Y.; et al. Palmitate induces cardiomyocyte death via inositol requiring enzyme-1 (IRE1)-mediated signaling independent of X-box binding protein 1 (XBP1). Biochem. Biophys. Res. Commun. 2020, 526, 122–127. [Google Scholar] [CrossRef]
- Minville-Walz, M.; Gresti, J.; Pichon, L.; Bellenger, S.; Bellenger, J.; Narce, M.; Rialland, M. Distinct regulation of stearoyl-CoA desaturase 1 gene expression by cis and trans C18:1 fatty acids in human oortic smooth muscle cells. Genes Nutr. 2012, 7, 209–216. [Google Scholar] [CrossRef]
- da Silva, M.S.; Julien, P.; Bilodeau, J.F.; Barbier, O.; Rudkowska, I. Trans fatty acids suppress TNF-α-induced inflammatory gene expression in endothelial (HUVEC) and hepatocellular carcinoma (HepG2) cells. Lipids 2017, 52, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kris-Etherton, P.M.; Thompson, J.T.; Hannon, D.B.; Gillies, P.J.; vanden Heuvel, J.P. Alpha-linolenic acid increases cholesterol efflux in macrophage-derived foam cells by decreasing stearoyl CoA desaturase 1 expression: Evidence for a farnesoid-X-receptor mechanism of action. J. Nutr. Biochem. 2012, 23, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Weigert, C.; Staiger, H.; Rittig, K.; Cegan, A.; Lutz, P.; Machicao, F.; Häring, H.U.; Schleicher, E. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 339–349. [Google Scholar] [CrossRef]
- Qin, X.; Tian, J.; Zhang, P.; Fan, Y.; Chen, L.; Guan, Y.; Fu, Y.; Zhu, Y.; Chien, S.; Wang, N. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc. Res. 2007, 74, 506–514. [Google Scholar] [CrossRef]
- Huang, K.C.; Chuang, P.Y.; Hsieh, R.Z.; Chen, C.N.; Chang, S.F.; Su, Y.P. Stearoyl-CoA desaturase-1 attenuates the high shear force damage effect on human MG63 osteosarcoma cells. Int. J. Mol. Sci. 2020, 21, 4720. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 2018, 62, 1800322. [Google Scholar] [CrossRef]
- Khorram, O.; Han, G.; Bagherpour, R.; Magee, T.R.; Desai, M.; Ross, M.G.; Chaudhri, A.A.; Toloubeydokhti, T.; Pearce, W.J. Effect of maternal undernutrition on vascular expression of micro and messenger RNA in newborn and aging offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.L.E.; van Eck, M.; Hildebrand, R.B.; Wong, B.W.C.; Bissada, N.; Ruddle, P.; Kontush, A.; Hussein, H.; Pouladi, M.A.; Chapman, M.J.; et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 341–347. [Google Scholar] [CrossRef]
- Brown, J.M.; Chung, S.; Sawyer, J.K.; Degirolamo, C.; Alger, H.M.; Nguyen, T.; Zhu, X.; Duong, M.N.; Wibley, A.L.; Shah, R.; et al. Inhibition of stearoyl-Coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation 2008, 118, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, X.; Mai, H.; Li, G.; Lin, Z.; Jie, W.; Li, K.; Zhou, H.; Wei, S.; Hu, L.; et al. SCD Rs41290540 single-nucleotide polymorphism modifies MiR-498 binding and is associated with a decreased risk of coronary artery disease. Mol. Genet. Genomic Med. 2020, 8, e1136. [Google Scholar] [CrossRef]
- Savransky, V.; Jun, J.; Li, J.; Nanayakkara, A.; Fonti, S.; Moser, A.B.; Steele, K.E.; Schweitzer, M.A.; Patil, S.P.; Bhanot, S.; et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl Coenzyme A desaturase. Circ. Res. 2008, 103, 1173–1180. [Google Scholar] [CrossRef]
- Masuda, M.; Ting, T.C.; Levi, M.; Saunders, S.J.; Miyazaki-Anzai, S.; Miyazaki, M. Activating transcription factor 4 regulates stearate-induced vascular calcification. J. Lipid Res. 2012, 53, 1543–1552. [Google Scholar] [CrossRef]
- Masuda, M.; Miyazaki-Anzai, S.; Keenan, A.L.; Okamura, K.; Kendrick, J.; Chonchol, M.; Offermanns, S.; Ntambi, J.M.; Kuro-O, M.; Miyazaki, M. Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity. J. Clin. Investig. 2015, 125, 4544–4558. [Google Scholar] [CrossRef]
- Kageyama, A.; Matsui, H.; Ohta, M.; Sambuichi, K.; Kawano, H.; Notsu, T.; Imada, K.; Yokoyama, T.; Kurabayashi, M. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-ΚB, novel targets of eicosapentaenoic acid. PLoS ONE 2013, 8, e68197. [Google Scholar] [CrossRef]
- Sunaga, H.; Matsui, H.; Anjo, S.; Syamsunarno, M.R.A.A.; Koitabashi, N.; Iso, T.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; Kurabayashi, M. Elongation of long-chain fatty acid family member 6 (Elovl6)-driven fatty acid metabolism regulates vascular smooth muscle cell phenotype through AMP-activated protein kinase/Krüppel-like factor 4 (AMPK/KLF4) signaling. J. Am. Heart Assoc. 2016, 5, e004014. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.A.; Walker, C.L.; Xu, Z.; Whitley, P.; Pavlina, T.M.; Hise, M.; Zaloga, G.P.; Siddiqui, R.A. Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J. Lipid Res. 2010, 51, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Z.; Tao, J.; Dou, B.; Gao, M.; Liu, Y. Overexpression of stearoyl-CoA desaturase 1 in bone marrow mesenchymal stem cells enhance the expression of induced endothelial cells. Lipids Health Dis. 2014, 13, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Kurdi-Haidar, B.; Oram, J.F. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 2004, 45, 972–980. [Google Scholar] [CrossRef]
- Sun, Y.; Hao, M.; Luo, Y.; Liang, C.P.; Silver, D.L.; Cheng, C.; Maxfield, F.R.; Tall, A.R. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J. Biol. Chem. 2003, 278, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fuentes, P.; García-Otín, A.L.; Calvo, L.; Gómez-Coronado, D.; Civeira, F.; Cenarro, A. Atorvastatin decreases stearoyl-CoA desaturase gene expression in THP-1 macrophages incubated with oxidized LDL. Lipids 2009, 44, 115–123. [Google Scholar] [CrossRef]
- Kim, S.R.; Jeon, S.Y.; Lee, S.M. The association of cardiovascular risk factors with saturated fatty acids and fatty acid desaturase indices in erythrocyte in middle-aged Korean adults. Lipids Health Dis. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Alsharari, Z.D.; Riserus, U.; Leander, K.; Sjogren, P.; Carlsson, A.C.; Vikstrom, M.; Laguzzi, F.; Gigante, B.; Cederholm, T.; Dr Faire, U.; et al. Serum fatty acids, desaturase activities and abdominal obesity—a population-based study of 60-year old men and women. PLoS ONE 2017, 12, e0170684. [Google Scholar] [CrossRef]
- Warensjö, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; de Faire, U.; Risérus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef]
- Kim, O.Y.; Lim, H.H.; Lee, M.J.; Kim, J.Y.; Lee, J.H. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 366–374. [Google Scholar] [CrossRef]
- Warensjö, E.; Risérus, U.; Gustafsson, I.B.; Mohsen, R.; Cederholm, T.; Vessby, B. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Berglund, L. Indices of fatty acid desaturase activity in healthy human subjects: Effects of different types of dietary fat. Br. J. Nutr. 2013, 110, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Vinknes, K.J.; Elshorbagy, A.K.; Nurk, E.; Drevon, C.A.; Gjesdal, C.G.; Tell, G.S.; Nygård, O.; Vollset, S.E.; Refsum, H. Plasma stearoyl-CoA desaturase indices: Association with lifestyle, diet, and body composition. Obesity 2013, 21, E294–E302. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, G.M.; Cabrera-Mulero, R.; Rojo-Martínez, G.; Gómez-Zumaquero, J.M.; Chaves, F.J.; de Marco, G.; Soriguer, F.; Castaño, L.; Morcillo, S. Polymorphisms in the SCD1 gene are associated with indices of stearoyl CoA desaturase activity and obesity: A prospective study. Mol. Nutr. Food Res. 2013, 57, 2177–2184. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Veenstra, J.; Westra, J.; Disselkoen, C.; Koch, K.; McKenzie, K.A.; O’Bott, J.; vander Woude, J.; Fischer, K.; Shearer, G.C.; et al. A genome-wide association study of red-blood cell fatty acids and ratios incorporating dietary covariates: Framingham Heart Study Offspring Cohort. PLoS ONE 2018, 13, e0194882. [Google Scholar] [CrossRef]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Warensjö, E.; Sundström, J.; Vessby, B.; Cederholm, T.; Risérus, U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am. J. Clin. Nutr. 2008, 88, 203–209. [Google Scholar] [CrossRef]
- Temme, E.H.M.; Mensink, R.P.; Hornstra, G. Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am. J. Clin. Nutr. 1996, 63, 897–903. [Google Scholar] [CrossRef]
- Ebbesson, S.O.E.; Devereux, R.B.; Cole, S.; Ebbesson, L.O.E.; Fabsitz, R.R.; Haack, K.; Harris, W.S.; Howard, W.J.; Laston, S.; Lopez-Alvarenga, J.C.; et al. Heart rate is associated with red blood cell fatty acid concentration: The Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Am. Heart J. 2010, 159, 1020–1025. [Google Scholar] [CrossRef]
- Yang, B.; Ding, F.; Wang, F.L.; Yan, J.; Ye, X.W.; Yu, W.; Li, D. Association of serum fatty acid and estimated desaturase activity with hypertension in middle-aged and elderly Chinese population. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Sundström, J.; Lind, L.; Vessby, B.; Andrén, B.; Aro, A.; Lithell, H.O. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation 2001, 103, 836–841. [Google Scholar] [CrossRef]
- Wiberg, B.; Sundström, J.; Árnlöv, J.; Terént, A.; Vessby, B.; Zethelius, B.; Lind, L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: A community-based study with long-term follow-up. Stroke 2006, 37, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Nettleton, J.A.; Folsom, A.R. Plasma fatty acid composition and incident heart failure in middle-aged adults: The atherosclerosis risk in communities (ARIC) study. Am. Heart J. 2008, 156, 965–974. [Google Scholar] [CrossRef]
- Svendsen, K.; Olsen, T.; Nordstrand Rusvik, T.C.; Ulven, S.M.; Holven, K.B.; Retterstøl, K.; Telle-Hansen, V.H. Fatty acid profile and estimated desaturase activities in whole blood are associated with metabolic health. Lipids Health Dis. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Ebbesson, S.O.E.; Lopez-Alvarenga, J.C.; Okin, P.M.; Devereux, R.B.; Tejero, M.E.; Harris, W.S.; Ebbesson, L.O.E.; Maccluer, J.W.; Wenger, C.; Laston, S.; et al. Heart rate is associated with markers of fatty acid desaturation: The GOCADAN study. Int. J. Circumpolar. Health 2012, 71, 17343. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shearer, G. Plasma oleic-to-stearic acid ratio and future heart failure risk: From MESA. Curr. Dev. Nutr. 2020, 4, 637. [Google Scholar] [CrossRef]
- Jun, J.C.; Drager, L.F.; Najjar, S.S.; Gottlieb, S.S.; Brown, C.D.; Smith, P.L.; Schwartz, A.R.; Polotsky, V.Y. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep 2011, 34, 1207–1213. [Google Scholar] [CrossRef]
| Population | Parameter of Systemic SCD Activity | Association with Cardiovascular Phenotype | Reference |
|---|---|---|---|
| Middle-aged indigenous Alaskans of both sexes | Plasma 16:1/16:0 | Positive association with heart rate | [95] |
| Middle-aged indigenous Alaskans of both sexes | Erythrocytes 16:1/16:0 | Negative association with heart rate | [95] |
| Middle-aged Koreans of both sexes | Erythrocytes 18:1/18:0 | Positive correlation with systolic and diastolic blood pressure | [77] |
| Men born between 1920 and 1924 and examined at 50, 60, 70, 77, and 82 years | Serum cholesterol esters 16:1/16:0 | Increased hazard ratios of total and cardiovascular mortality | [87] |
| Seven-year follow-up study of middle-and elderly-aged subjects of both sexes | 18:1/18:0 | Increased hazard ratios of heart failure | [93] |
| Prospective study of middle-aged white subjects of both sexes from Minnesota, USA | Plasma cholesterol esters 16:1/16:0 | Increased hazard ratios of heart failure | [80] |
| Middle-and elderly-aged Korean men | Serum phospholipids 18:1/18:0 | Positive correlation with arterial stiffness | [90] |
| Middle-and elderly-aged Chinese of both sexes | Total serum lipids 16:1/16:0 | Increased odd ratio of hypertension | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatskyi, V.V.; Dobrzyn, P. Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology. Int. J. Mol. Sci. 2023, 24, 5531. https://doi.org/10.3390/ijms24065531
Balatskyi VV, Dobrzyn P. Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology. International Journal of Molecular Sciences. 2023; 24(6):5531. https://doi.org/10.3390/ijms24065531
Chicago/Turabian StyleBalatskyi, Volodymyr V., and Pawel Dobrzyn. 2023. "Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology" International Journal of Molecular Sciences 24, no. 6: 5531. https://doi.org/10.3390/ijms24065531
APA StyleBalatskyi, V. V., & Dobrzyn, P. (2023). Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology. International Journal of Molecular Sciences, 24(6), 5531. https://doi.org/10.3390/ijms24065531






