Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine
Abstract
1. Introduction
2. Extracting Dibenzo[b,f]oxepines from Plants and Its Significance in Medicine
2.1. Pacharin
- a.
- the stem bark, stem wood, and roots of Bauhinia ungulate L. [7];
- b.
- c.
- the heartwood of Bauhinia racemosa Lamk. [10];
- d.
- the stem bark of Bauhinia aculeata L. [11];
- e.
- f.
- vine stems from Millettia dorwardi Collet Hemsl [14];
- g.
- the bark of Rhamnus caroliniana [15];
- h.
- the flowers of Cercis chinensis Bunge [16].

2.2. Bauhiniastatins 1–4
2.3. Bauhinoxepins A and B
2.4. Yagonine, Aristoyagonine
2.5. Secocularidine, Secocularine, Norsecocularidine
2.6. Secosarcocapnine, Norsecosarcocapnine, 4-Hydroxysecosarcocapnine
2.7. Artocarpol A, Artocarpols D-G (Figure 3)

2.8. Tournefolic Acid B, Tournefolic Acid B Methyl Ester, and Tournefolic Acid B Ethyl Ester (Figure 3)
2.9. The Physicochemical Approaches to Estimate Medical Activity
3. Biosynthesis of Dibenzo[b,f]oxepines
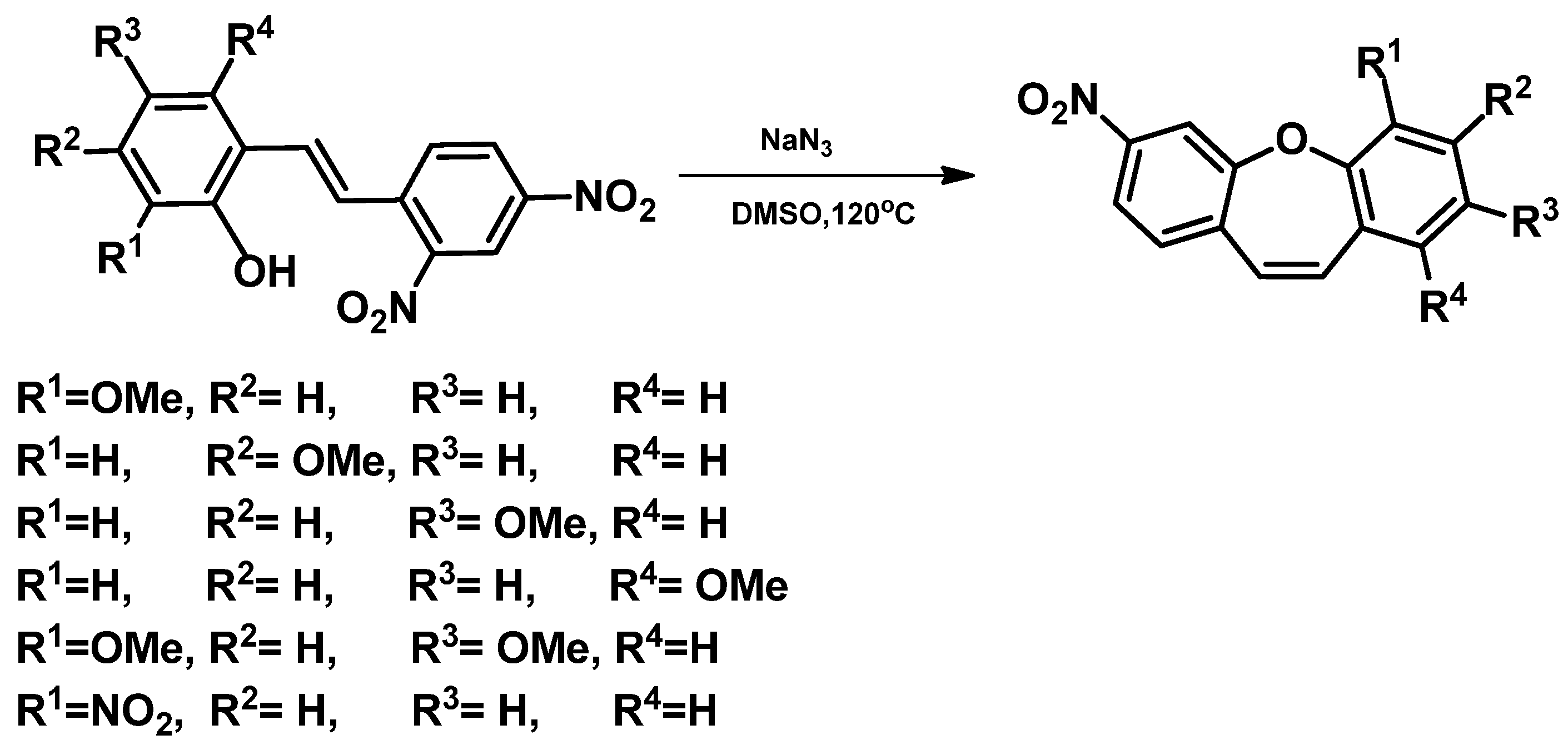
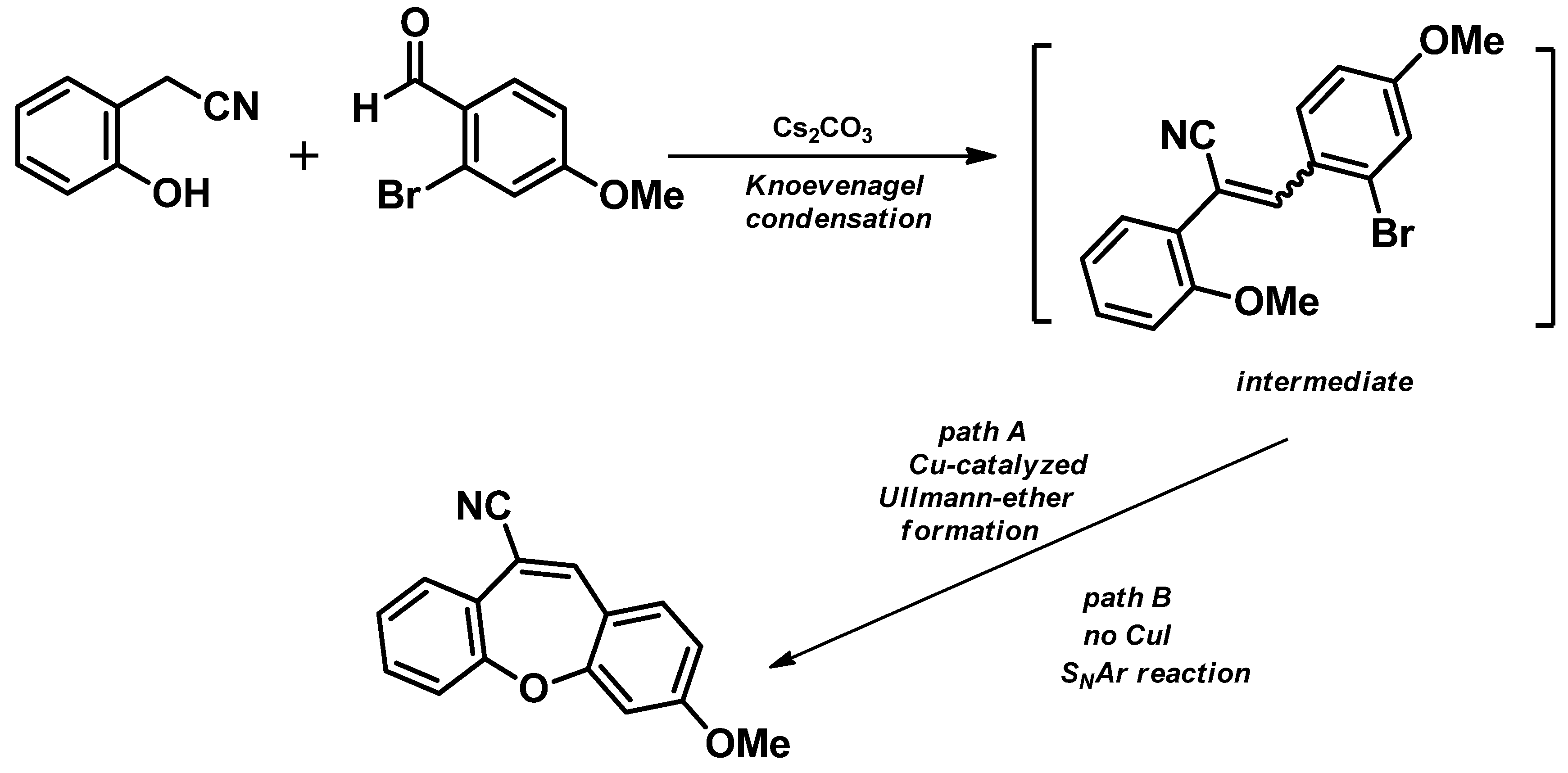
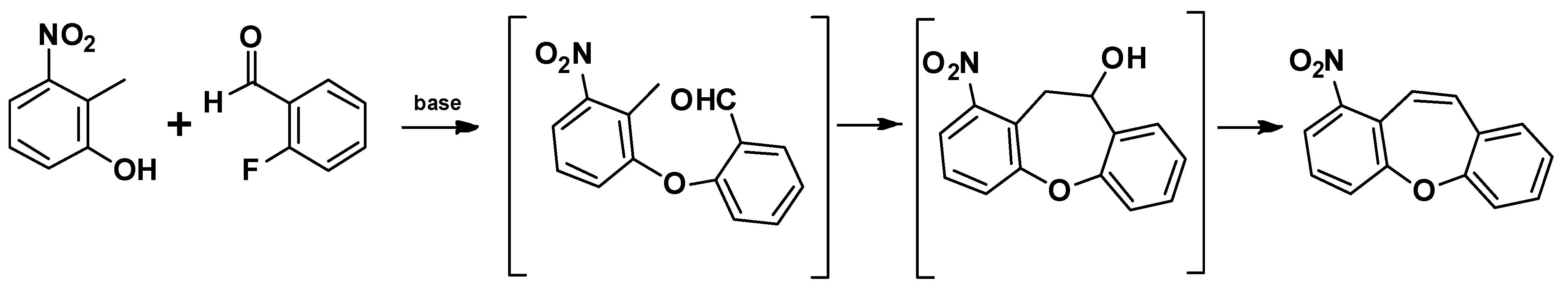
4. Structure of Dibenzo[b,f]oxepine and Synthesis of Dibenzo[b,f]oxepine Derivatives
- Wagner–Meerwein rearrangement [66]
- 3.
- Preparation from 2-halogenobenzaldehydes [71]
- 4.
- Intramolecular aromatic nucleophilic substitution SNAr [58]
- 5.
- Knoevenagel condensation [72]
- 6.
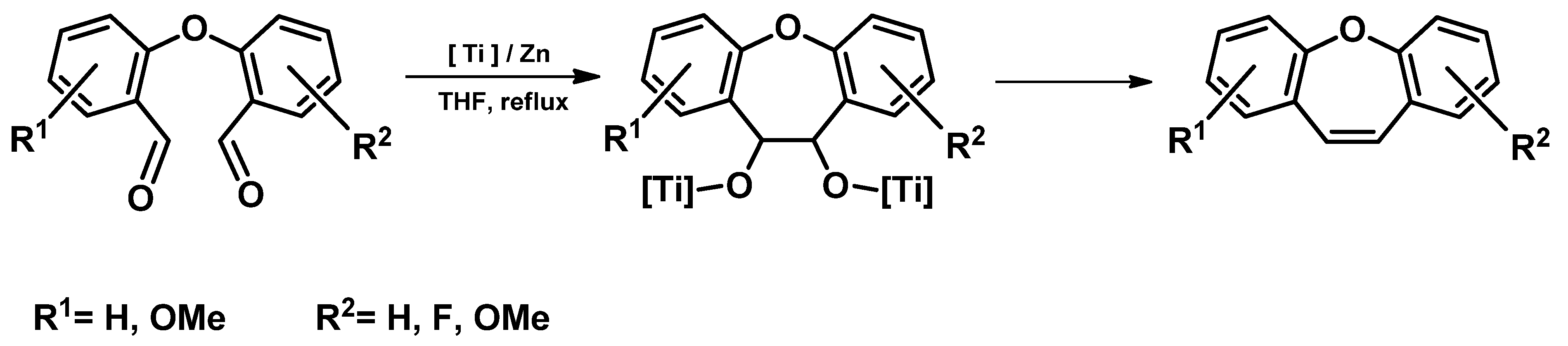
- Intramolecular McMurry reaction [74]
- 7.
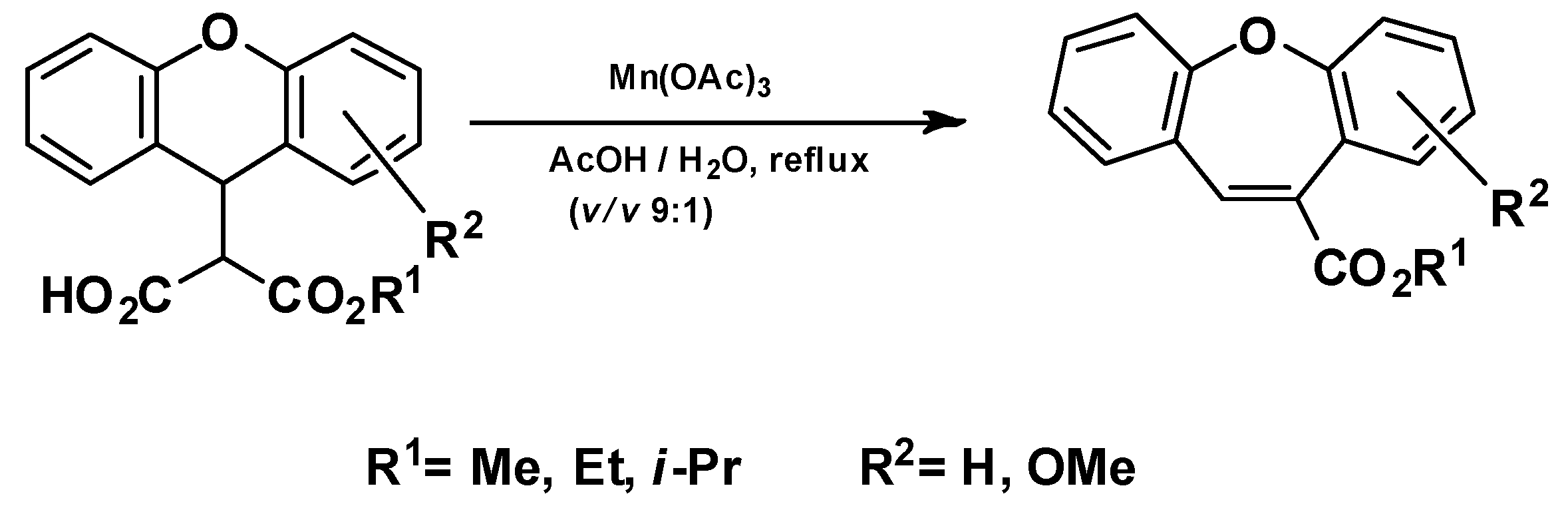
- Mn(III)-based oxidative radical rearrangement [75]
- 8.
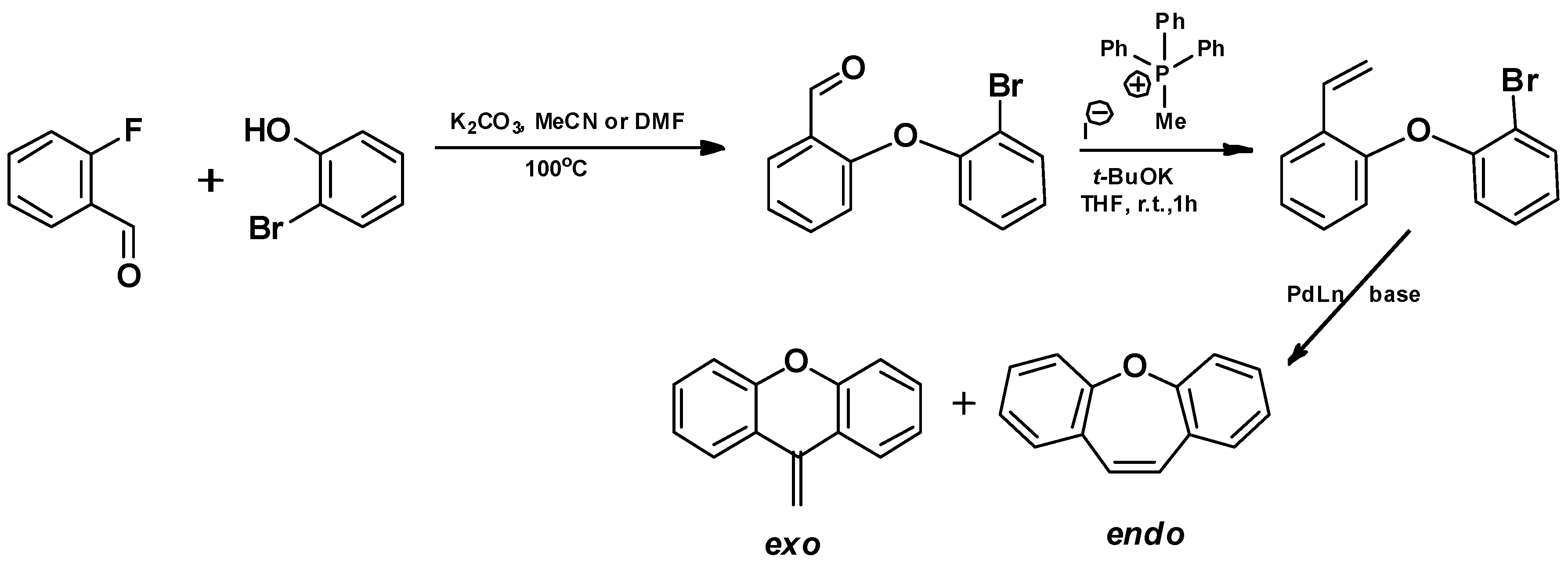
- Sequential Mizoroki–Heck reaction and Pd-catalyzed etherification [76]
- 9.
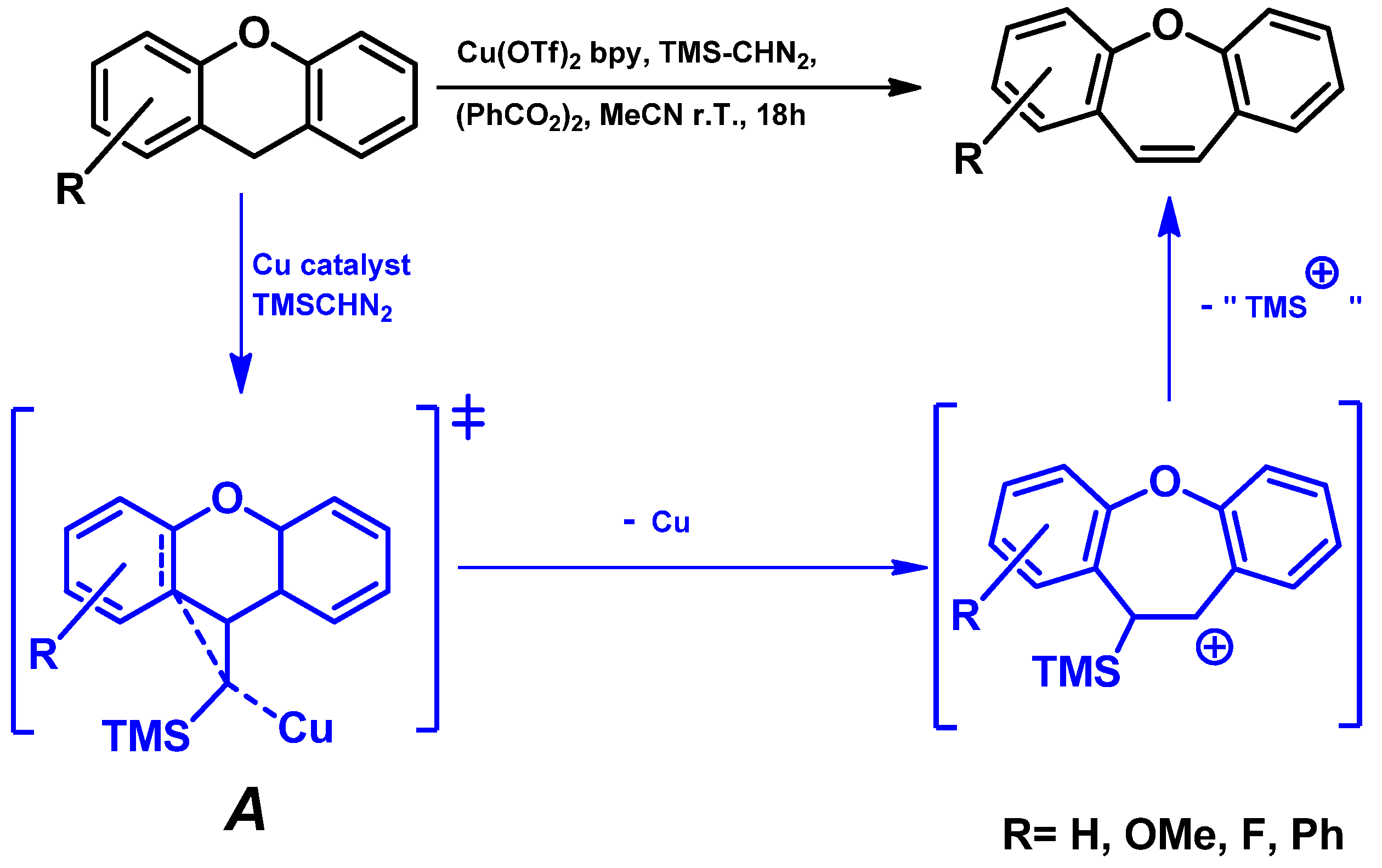
- Oxidative CH bond functionalization and ring expansion with TMSCHN2 [77]
5. Active Synthetic Dibenzo[b,f]oxepin Derivatives
6. Potential of Dibenzo[b,f]oxepines as Microtubule Inhibitors
7. The Perspective for Applications of Dibenzo[b,f]oxepine Derivatives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ram, V.J.; Sethi, A.; Nath, M.; Pratap, R. Chapter 3—Seven-Membered Heterocycles. In The Chemistry of Heterocycles Chemistry of Six-to-Eight-Membered N, O, S, P and Se Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 393–425. [Google Scholar]
- Rosowsky, A. (Ed.) The Chemistry of Heterocyclic Compounds; Seven-Membered Heterocyclic Compounds Containing Oxygen and Sulfur; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1972; Volume XXVI. [Google Scholar]
- Beleńki, L.I. Comprehensive Heterocyclic Chemistry III. 13.02—Oxepanes and Oxepines; Elsevier: Amsterdam, The Netherlands, 2008; Volume 13, pp. 45–95. [Google Scholar]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS initiative for open knowledge management in natural products research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.M.; de Carvalho, J.L.; da Silva, H.C.; Lemos, T.L.G.; Arriaga, A.M.C.; Braz-Filho, R.; Militao, G.C.G.; Silva, T.D.S.; Ribeiro, P.R.V.; Santiago, G.M.P. New Cytotoxic Bibenzyl and Other Constituents from Bauhinia ungulata L. (Fabaceae). Chem. Biodivers. 2016, 13, 1630–1635. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Iwamoto, C.; Usami, Y.; Yamada, T.; Ohishi, H.; Cragg, G.M. Antineoplastic Agents. 551. Isolation and Structures of Bauhiniastatins 1–4 from Bauhinia purpurea. J. Nat. Prod. 2006, 69, 323–327. [Google Scholar] [CrossRef]
- Boonphong, S.; Puangsombat, P.; Baramee, A.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Bioactive Compounds from Bauhinia purpurea Possessing Antimalarial, Antimycobacterial, Antifungal, Anti-inflammatory, and Cytotoxic Activities. J. Nat. Prod. 2007, 70, 795–801. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Reddy, A.V.R.; Reddy, D.S.K.; Ward, R.S.; Adhikesavalu, D.; Cameron, T.S. Pacharin: A new dibenzo(2,3-6,7)oxepin derivative from Bauhinia racemosa Lamk. Tetrahedron 1984, 40, 4245–4252. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Sri Tjahjandarie, T. Antioxidant activity of two isomeric benzoxepin derivatives from the stembark of Bauhinia aculeata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
- Góis, R.W.S.; de Sousa, L.M.; da Silva, H.C.; da Silva, F.E.F.; Pimenta, A.T.A.; Lima, M.A.S.; Arriaga, A.M.C.; Lemos, T.L.G.; Braz-Filho, R.; Militãod, G.C.G.; et al. Chemical constituents from Bauhinia acuruana and their cytotoxicity. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2017, 27, 711–715. [Google Scholar] [CrossRef]
- De Maria Souza, S.; Santos de Souza, L.; Silva, V.R.; Pereira Soares, M.B.; Bezerra, D.P.; Wagner da Silva Gois, R.; da Silva, H.C.; Pinheiro Santiago, G.M.; Militao, G.C.G. Natural Dibenzo[b,f]oxepines, Pacharin and Bauhiniastatin-1, Isolated from Bauhinia acuruana Induce Apoptosis on Breast Cancer Cells via MCL-1 Protein Reduction. Planta Med. 2023, 89, 286–294. [Google Scholar] [CrossRef]
- Chen, K.; Tang, H.; Zheng, L.; Wang, L.; Xue, L.; Peng, A.; Tang, M.; Long, C.; Chen, X.; Yeb, H.; et al. Identification of compounds with cytotoxic activity from Millettia dorwardi Coll. Et. Hemsl. Phytochem. Lett. 2018, 25, 60–64. [Google Scholar] [CrossRef]
- Bindu Mekala, A.; Satyal, P.; Setzer, W.N. Phytochemicals from the Bark of Rhamnus caroliniana. Nat. Prod. Commun. 2017, 12, 403–406. [Google Scholar]
- Mu, L.; Li, J.; Yang, J.; Zhang, D. New dibenz[b,f]oxepins from Cercis chinensis Bunge. J. Asian Nat. Prod. Res. 2007, 9, 649–653. [Google Scholar] [CrossRef]
- Gois, R.W.S.; De Sousa, L.M.; Lemos, T.L.G.; Arriaga, A.M.C.; Andrade-Neto, M.; Santi-ago, G.M.P.; Ferreira, Y.S.; Alves, P.B.; De Jesus, H.C.R. Chemical composition and larvicidal effects of essential oil from Bauhinia acuruana (Moric) against Aedes aegypti. J. Essent. Oil Res. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Góis, R.W.S.; De Sousa, L.M.; Santiago, G.M.P.; Romero, N.R.; Lemos, T.L.G.; Arriaga, A.M.C.; Braz-Filho, R. Larvicidal activity against Aedes aegypti of pacharin from Bauhinia acuruana. Parasitol. Res. 2013, 112, 2753–2757. [Google Scholar] [CrossRef]
- Narita, K.; Nakamura, K.; Abe, Y.; Katoh, T. Total Synthesis of Bauhinoxepin J: A Biologically Active Dibenzo[b,f]oxepin Isolated from Bauhinia purpurea. Eur. J. Org. Chem. 2011, 26, 4985–4988. [Google Scholar] [CrossRef]
- Prudhomme, V.; Cucca, M.; Nauton, L.; Andrieu, E.; Fereyrolles, M.; Lamoine, S.; Michelin, C.; Bennis, K.; Collin, A.; De Ceuninck, F.; et al. Design, synthesis and biological evaluation of conformationnally-restricted analogues of E7010 as inhibitors of tubulin assembly (ITA) and vascular disrupting agents (VDA). Eur. J. Med. Chem. 2022, 244, 114809. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhou, P.; Liang, L.; Cao, Y.; Qiao, J.; Li, X.; Teng, Z.; Wang, L. Nanogel-Incorporated Injectable Hydrogel for Synergistic Therapy Based on Sequential Local Delivery of Combretastatin-A4 Phosphate (CA4P) and Doxorubicin (DOX). ACS Appl. Mater. Interfaces 2018, 10, 18560–18573. [Google Scholar] [CrossRef]
- Kittakoop, P.; Nopichai, S.; Thongon, N.; Charoenchai, P.; Thebtaranonth, Y. Bauhinoxepins A and B: New Antimycobacterial Dibenzo[b,f]oxepins from Bauhinia saccocalyx. Helv. Chim. Acta 2004, 87, 175–179. [Google Scholar] [CrossRef]
- De Lera, A.R.; Suau, R.; Castedo, L. Synthetic approaches to cularines. II. Ullmann condensation. J. Heterocycl. Chem. 1987, 24, 313–319. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, M.; Yoo, M.; Kim, J.E.; Lee, H.K.; Heo, J.-N.; Lee, C.O.; Yoo, M.; Junga, K.-Y.; Yun, C.-S.; et al. A natural compound, aristoyagonine, is identified as a potent bromodomain inhibitor by mid-throughput screening. Biochem. Biophys. Res. Commun. 2018, 503, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Boente, J.M.; Castedo, L.; Domínguez, D.; Fariña, A.; Rodriguez de Lera, A.; Villaverde, C. Secocularines: A new group of isoquinoline related alkaloids present in the fumariaceae. Tetrahedron Lett. 1984, 25, 889–892. [Google Scholar] [CrossRef]
- Castedo, L.; Dominguez, D.; Lopez, S.; Tojo, E.; Villaverde, C. Five new secocularine alkaloids have been isolated from Sarcocapnos species and their structures elucidated by spectroscopic studies and chemical correlations. Heterocycles 1987, 26, 591–594. [Google Scholar] [CrossRef]
- Castedo, L.; López, S.; Rodríguez De Lera, A.; Villaverde, C. Alkaloids of Sarcocapnos crassifolia subsp. speciosa. Phytochemistry 1989, 28, 251–257. [Google Scholar] [CrossRef]
- Frotáis, P.; Arbaoui, J.; Bakkali, E.-H. Effects of various isoquinoline alkaloids on in vitro 3H-dopamine uptake by rat striatal synaptosomes. J. Nat. Prod. 1995, 58, 1475–1484. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the lsoquinoline Alkaloids. Nat. Prod. Rep. 1989, 6, 405–432. [Google Scholar] [CrossRef]
- Tojo, E.; Dominguez, D.; Castedo, L. Alkaloids from Sarcocapnos enneaphylla. Phytochemistry 1991, 30, 1005–1010. [Google Scholar] [CrossRef]
- Phillipson, D.; Roberts, M.F.; Zenk, M.H. (Eds.) The Chemistry and Biology of Iso Quinoline Alkaloids; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1985; ISBN 978-3-642-70130-6. [Google Scholar]
- Kumari, S.; Sharma, A.; Sharma, U. A cularine-type isoquinoline alkaloid from the root part of Cissampelos pareira. Nat. Prod. Res. 2022; in press. [Google Scholar] [CrossRef]
- Chung, M.-I.; Ko, H.-H.; Yen, M.-H.; Lin, C.-N.; Yang, S.-Z.; Tsao, L.-T.; Wang, J.-P. Artocarpol A, A Novel Constituent with Potent Anti-Inflammatory Effect, Isolated from Artocarpus rigida. Helv. Chim. Acta 2000, 83, 1200–1204. [Google Scholar] [CrossRef]
- Ko, H.H.; Lin, C.N.; Yang, S.Z. New Constituents of Artocarpus rigida. Helv. Chim. Acta 2000, 83, 3000–3005. [Google Scholar] [CrossRef]
- Ko, H.-H.; Yang, S.-Z.; Lin, C.-N. Artocarpol F, A Phenolic Compound with A Novel Skeleton, Isolated from Artocarpus rigida. Tetrahedron Lett. 2001, 42, 5269–5270. [Google Scholar] [CrossRef]
- Lu, Y.H.; Lin, C.-N.; Ko, H.-H.; Yang, S.-Z.; Tsao, L.T.; Wang, J.-P. Two Novel and Anti-Inflammatory Constituents of Artocarpus rigida. Helv. Chim. Acta 2002, 85, 1626–1632. [Google Scholar] [CrossRef]
- Lu, Y.H.; Lin, C.-N.; Ko, H.-H.; Yang, S.-Z.; Tsao, L.T.; Wang, J.-P. Novel Anti-Inflammatory Constituents of Artocarpus rigida. Helv. Chim. Acta 2003, 86, 2566–2572. [Google Scholar] [CrossRef]
- Kuan, Y.-H.; Lin, R.-H.; Tsao, L.-T.; Lin, C.-N.; Wang, J.-P. Artocarpol A stimulation of superoxide anion generation in neutrophils involved the activation of PLC, PKC and p38 mitogen-activated PK signaling pathways. Br. J. Pharm. 2005, 145, 460–468. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Chang, Y.-Y.; Kuo, Y.-H.; Shiao, M.-S. Anti-Lipid-Peroxidative Principles from Tournefortia sarmentosa. J. Nat. Prod. 2002, 65, 745–747. [Google Scholar] [CrossRef]
- Chen, P.-C.; Tsai, W.-J.; Ueng, Y.-F.; Tzeng, T.-T.; Chen, H.-L.; Zhu, P.-R.; Huang, C.-H.; Shiao, Y.-J.; Li, W.-T. Neuroprotective and Antineuroinflammatory Effects of Hydroxyl-Functionalized Stilbenes and 2-Arylbenzo[b]furans. J. Med. Chem. 2017, 60, 4062–4073. [Google Scholar] [CrossRef]
- Wang, C.N.; Pan, H.C.; Lin, Y.L.; Chi, C.W.; Shiao, Y.J. Ester derivatives of tournefolic acid B attenuate N methyl-D-aspartatemediated excitotoxicity in rat cortical neurons. Mol. Pharmacol. 2006, 69, 950–959. [Google Scholar] [CrossRef]
- Chi, C.W.; Wang, C.N.; Lin, Y.L.; Chen, C.F.; Shiao, Y.J. Tournefolic acid B methyl ester attenuates glutamate-induced toxicity by blockade of ROS accumulation and abrogating the activation of caspases and JNK in rat cortical neurons. J. Neurochem. 2005, 92, 692–700. [Google Scholar] [CrossRef]
- Chi, C.W.; Lin, Y.L.; Wang, Y.H.; Chen, C.F.; Wang, C.N.; Shiao, Y.J. Tournefolic acid B attenuates amyloid beta proteinmediated toxicity by abrogating the calcium overload in mitochondria and retarding the caspase 8-truncated Bid-cytochrome c pathway in rat cortical neurons. Eur. J. Pharmacol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tsay, H.J.; Lai, T.H.; Tzeng, T.T.; Shiao, Y.J. Lithospermic acid attenuates 1-methyl-4-phenylpyridine-induced neurotoxicity by blocking neuronal apoptotic and neuroinflammatory pathways. J. Biomed. Sci. 2015, 22, 37. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today. Technol. 2004, 1, 337341. [Google Scholar] [CrossRef] [PubMed]
- Daniel, F.V.; Stephen, R.J.; Cheng, H.; Smith, B.R.; Keith, W.W.; Kenneth, D.K.; Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; et al. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar]
- Kenny, P.W. Hydrogen-Bond Donors in Drug Design. J. Med. Chem. 2022, 65, 14261–14275. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N.C.; Fersht, A.R. Hydrogen bonding in enzymatic catalysis analysed by protein engineering. Nature 1985, 316, 656–657. [Google Scholar] [CrossRef]
- Tromans, R.A.; Carter, T.S.; Chabanne, L.; Crump, M.P.; Li, H.; Matlock, J.V.; Orchard, M.G.; Davis, A.P. A biomimetic receptorfor glucose. Nat. Chem. 2019, 11, 52–56. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bonnet, J.; Colotte, M.; Coudy, D.; Couallier, V.; Portier, J.; Morin, B.; Tuffet, S. Chain and conformation stability of solid-state DNA: Implications for room temperature storage. Nucleic Acids Res. 2010, 38, 1531–1546. [Google Scholar] [CrossRef]
- Campello, M.J.; Castedo, L.; Dominguez, D.; Rodriguez de Lera, A.; Saá, J.M.; Suau, R.; Tojo, E.; Vidal, M.C. New oxidized isocularine alkaloids from sarcocapnos plants. Tetrahedron Lett. 1984, 25, 5933–5936. [Google Scholar] [CrossRef]
- Castedo, L.; Suau, R.; Mouriño, A. A revised structure for pontevedrine. Tetrahedron Lett. 1976, 17, 501–502. [Google Scholar] [CrossRef]
- Drake, J.A.G.; Jones, D.W. The structure of dibenz[b,f]oxepin. Acta Crystallogr. B Struct. Cryst. Cryst. Chem. 1982, 38, 200–203. [Google Scholar] [CrossRef]
- Shukla, D.; Wan, P. Evidence for a planar cyclically conjugated 8π system in the excited state: Large Stokes shift observed for dibenz[b,f]oxepin fluorescence. J. Am. Chem. Soc. 1993, 115, 2990–2991. [Google Scholar] [CrossRef]
- Wrzesiński, M.; Mielecki, D.; Szczeciński, P.; Grzesiuk, E.; Krawczyk, H. Synthesis of derivatives of methoxydibenzo[b,f]oxepine in the presence of sodium azide. Tetrahedron 2016, 72, 3877–3884. [Google Scholar]
- Toldo, J.; El Bakouri, O.; Sola, M.; Norrby, P.-O.; Ottosson, H. Is Excited-State Aromaticity a Driving Force for Planarization of Dibenzannelated 8π-Electron Heterocycles? ChemPlusChem 2019, 84, 712–721. [Google Scholar] [CrossRef]
- Ottosson, H. Organic photochemistry: Exciting excited-state aromaticity. Nat. Chem. 2012, 4, 969–971. [Google Scholar] [CrossRef]
- Kotani, R.; Liu, L.; Kumar, P.; Kuramochi, H.; Tahara, T.; Liu, P.; Osuka, A.; Karadakov, P.B.; Saito, S. Controlling the S1 Energy Profile by Tuning Excited-State Aromaticity. J. Am. Chem. Soc. 2020, 142, 14985–14992. [Google Scholar] [CrossRef]
- Pschorr, R.; Knöffler, C. Űber die Konstitution des Morphothebains II. Synthese des durch Abbau von Morphothebain erhaltenen Tetramethoxyphenanthrens. Liebigs Ann. Chem. 1911, 382, 50–61. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Moreno, D.A.; García-Viguera, C. Maqui berry vs. Sloe berry–liquor-based beverage for new development. Nat. Prod. Commun. 2015, 10, 81–82. [Google Scholar] [CrossRef]
- Ugwu, D.I.; Eze, F.U.; Ezugwu, J.A.; Attah, S.I.; Ugwu, M.C.; Ekoh, O.C.; Nyoyoko, I.F. Chemotherapeutic Importance of Oxepines. Int. J. Chem. Sci. 2021, 19, 401. [Google Scholar]
- Yadav, S.; Ramarao, J.; Suresh, S. Recent Advances on the Development of Synthetic Strategies to Access Dibenzoxepine Derivatives. Synthesis 2023, 55, 369–399. [Google Scholar] [CrossRef]
- Storz, T.; Vangrevelinghe, E.; Dittmar, P. Synthesis and Wagner-Meerwein Rearrangement of 9-(α-Hydroxyalkyl)xanthenes to 10-Substituted Dibenz[b,f]oxepins: Scope, Limitations and ab initio Calculations. Synthesis 2005, 15, 2562–2570. [Google Scholar] [CrossRef]
- Olivera, R.; San Martin, R.; Churruca, F.; Dominguez, E. Revisiting the Ullmann—Ether Reaction: A Concise and Amenable Synthesis of Novel Dibenzoxepino [4,5-d]pyrazoles by Intramolecular Etheration of 4,5-(o,o′-Halohydroxy)arylpyrazoles. J. Org. Chem. 2002, 67, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Irie, A.; Nakamura, H.; Hino, K.; Uno, H.; Nishimura, H. Nonsteroidal antiinflammatory agents. 1. 10,11-Dihydro-11-oxodibenz[b,f] oxepinacetic acids and related compounds. J. Med. Chem. 1982, 25, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Bharath, Y.; Thirupathi, B.; Ranjit, G.; Mohapatra, D.K. An Efficient Synthesis of Dibenzo[b,f]oxepins by Ring-Closing Metathesis. Asian J. Org. Chem. 2013, 2, 848–851. [Google Scholar] [CrossRef]
- Matsuda, T.; Sato, S. Synthesis of Dibenzoheteropines of Group 13–16 Elements via Ring-Closing Metathesis. J. Org. Chem. 2013, 78, 3329–3335. [Google Scholar] [CrossRef]
- Choi, Y.L.; Lim, H.S.; Lim, H.J.; Heo, J.-N. One-Pot Transition-Metal-Free Synthesis of Dibenzo[b,f]oxepins from 2-Halobenzaldehydes. Org. Lett. 2012, 14, 5102–5105. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; He, Q.; Xie, Y.; Yang, C. Copper-Assisted/Copper-Free Synthesis of Functionalized Dibenzo[b,f]oxepins and Their Analogs via a One-Pot Tandem Reaction. Helv. Chim. Acta 2013, 96, 296–308. [Google Scholar] [CrossRef]
- Taweesak, P.; Thongaram, P.; Kraikruan, P.; Thanetchaiyakup, A.; Chuanopparat, N.; Hsieh, H.-P.; Uang, B.-J.; Ngernmeesri, P. One-Pot Synthesis of Dibenzo[b,f]oxepines and Total Synthesis of Bauhinoxepin C. J. Org. Chem. 2021, 86, 1955–1963. [Google Scholar] [CrossRef]
- Moreno, D.R.R.; Giorgi, G.; Salas, C.O.; Tapia, R.A. New Short Strategy for the Synthesis of the Dibenz[b,f]oxepin Scaffold. Molecules 2013, 18, 14797–14806. [Google Scholar] [CrossRef]
- Cong, Z.; Miki, T.; Urakawa, O.; Nishino, H. Synthesis of Dibenz[b,f]oxepins via Manganese(III)-Based Oxidative 1,2-Radical Rearrangement. J. Org. Chem. 2009, 74, 3978–3981. [Google Scholar] [CrossRef]
- Jepsen, T.H.; Larsen, M.; Jørgensen, M.; Nielsen, M.B. Three-Step Synthesis of (Thio)xanthene and Dibenzothiepine/Dibenzoxepine by an Intramolecular Mizoroki-Heck Reaction of Diaryl (Thio)Ethers. Synlett 2012, 23, 418–422. [Google Scholar]
- Stopka, T.; Marzo, L.; Zurro, M.; Janich, S.; Wrthwein, E.-U.; Daniliuc, C.G.; Aleman, J.; Mancheno, O.G. Oxidative CH Bond Functionalization and Ring Expansion with TMSCHN2: A Copper(I)-Catalyzed Approach to Dibenzoxepines and Dibenzoazepines. Angew. Chem. Int. Ed. 2015, 54, 5049–5053. [Google Scholar] [CrossRef]
- Phillips, S.T.; de Paulis, T.; Neergaard, J.R.; Baron, B.M.; Siegel, B.W.; Seeman, P.; Van Tol, H.H.M.; Guan, H.-C.; Smith, H.E. Binding of 5H-Dibenzo[a,d]cycloheptene and Dibenz[b,f]oxepin Analogs of Clozapine to Dopamine and Serotonin Receptors. J. Med. Chem. 1995, 38, 708–714. [Google Scholar] [CrossRef]
- Harris, T.W.; Smith, H.E.; Mobley, P.L.; Manier, D.H.; Sulser, F. Affinity of 10-(4-Methylpiperazino)dibenz[b,f]oxepins for Clozapine and Spiroperidol Binding Sites in Rat Brain. J. Med. Chem. 1982, 25, 855–858. [Google Scholar] [CrossRef]
- Schindler, W.; Blattner, H. 10-(Aminomethyl)- and (Aminoethyl)-dibenz(b,f)oxepins. U.S. Patent 3,641,056, 8 February 1972. [Google Scholar]
- Ong, H.H.; Profitt, J.A.; Anderson, V.B.; Spaulding, T.C.; Wilker, J.C.; Geyer, H.M.; Kruse, H. Tricyclics with Analgesic and Antidepressant Activity. 1. [(Alkylamino) ethyl]thio] dibenz[b,f]-oxepins and 10,11-Dihydro Derivatives. J. Med. Chem. 1980, 23, 494–501. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Alonso, J.M.; Andrés, J.I.; Cid, J.M.; Fernández, J.; Iturrino, L.; Megens, A. Synthesis of 2-N,N-Dimethylaminomethyl-2,3,3a,12b-tetrahydrodibenzo-[b,f] furo [2,3-d]-oxepin Derivatives as Potential Anxiolytic Agents. Chem. Pharm. Bull. 2004, 52, 262–265. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Alonso, J.M.; Cid, J.M.; Font, L.M.; Megens, A. Synthesis of 2-N,N-dimethylaminomethyl-2,3,3a,12b-tetrahydro dibenzo[b,f]furo [2,3-d]oxepine derivatives as potential anxiolytic agents. Part 2: Substitutions by methyl groups on the tetrahydrofuran ring. Farmaco 2005, 60, 241–248. [Google Scholar] [CrossRef]
- Bartolomé, J.M.; Alcudia, A.; Andrés, J.I.; Cid, J.M.; García, M.; Megens, A.; Toledo, M.A.; Trabanco, A.A. Novel 2-N,N-dimethylaminomethyl-2,3,3a,12b-tetrahydrodibenzo [b,f]furo [2,3-d]oxepin derivatives displaying combined norepinephrine reuptake inhibition and 5-HT2A/2C receptor antagonism. Bioorg. Med. Lett. 2005, 15, 2898–2901. [Google Scholar] [CrossRef]
- Rupčić, R.; Modrić, M.; Hutinec, A.; Čikoš, A.; Stanić, B.; Mesić, M.; Pešić, D.; Merćep, M. Novel Tetracyclic Imidazole Derivatives: Synthesis, Dynamic NMR Study, and Anti-Inflammatory Evaluation. J. Heterocycl. Chem. 2010, 47, 640–656. [Google Scholar] [CrossRef]
- Kiyama, R.; Honma, T.; Hayashi, K.; Ogawa, M.; Hara, M.; Fujimoto, M.; Fujishita, T. Novel Angiotensin II Receptor Antagonists. Design, Synthesis, and in Vitro Evaluation of Dibenzo-[a,d]cycloheptene and Dibenzo[b,f]oxepin Derivatives. Searching for Bioisosteres of Biphenyltetrazole Using a Three-Dimensional Search Technique. J. Med. Chem. 1995, 38, 2728–2741. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A. Combination Treatments for Alzheimer’s Disease and Similar Diseases. U.S. Patent S 2010/0292157 A1, 18 November 2010. [Google Scholar]
- Acton, D.; Hill, G.; Tait, B.S. Tricyclic Triarylethylene Antiestrogens: Dibenz[b,f] oxepins, Dibenzo[b,f ]thiepins, Dibenzo-[a,e]cyclooctenes, and Dibenzo[b,f]thiocins. J. Med. Chem. 1983, 26, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Identifier: NCT01805024 “Congenital Muscular Dystrophy Ascending Multiple Dose Cohort Study Analyzing Pharmacokinetics at Three Dose Levels in Children and Adolescents with Assessment of Safety and Tolerability of Omigapil (CALLISTO) (CALLISTO)”. Available online: https://ClinicalTrials.gov (accessed on 22 June 2023).
- Zimmermann, K.; Waldmeier, P.C.; Tatton, W.G. Dibenzoxepines as treatments for neurodegenerative diseases. Pure Appl. Chem. 1999, 71, 2039–2046. [Google Scholar] [CrossRef]
- Sagot, Y.; Toni, N.; Perrelet, D.; Lurot, S.; King, B.; Rixner, H.; Mattenberger, L.; Waldmeier, P.C.; Kato, A.C. An orally active anti-apoptotic molecule (CGP 3466B) preservesmitochondria and enhances survival in an animal model ofmotoneuron disease. Br. J. Pharmacol. 2000, 131, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-K.; Vázquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732. [Google Scholar] [CrossRef]
- Ansari, M.; Hussain, M.; Arun, A.; Chakravarti, B.; Konwar, R.; Hajela, K. Synthesis of targeted dibenzo[b,f]thiepines and dibenzo[b,f]oxepines as potential lead molecules with promising anti-breast cancer activity. Eur. J. Med. Chem. 2015, 99, 113–124. [Google Scholar] [CrossRef]
- Agnew, M.N.; Rizwaniuk, A.; Ong, H.H.; Wichmann, J.K. Nuclear hydroxylated metabolite of fluradoline (2-fluoro-11-[(β-methylamino)ethylthio]dibenz[b,f]oxepin hydrochloride). Identification and synthesis. J. Heterocycl. Chem. 1986, 23, 265–269. [Google Scholar] [CrossRef]
- Jinno, S.; Okita, T. Synthesis of an Antioxidant Having a Dibenz[b,f]oxepine Skeleton. Heterocycles 1999, 51, 303–314. [Google Scholar]
- Cloos, P.A.C.; Jensen, F.R.; Boissy, P.; Stahlhut, M. Compounds Modulating the Activity of Gapdh and/or Iamt. Patent WO2004039773A8, 13 May 2004. [Google Scholar]
- Roeder, T.; Nathanson, J.A. Characterization of Insect Neuronal Octopamine Receptors (OA3 Receptors). Neurochem. Res. 1993, 18, 921–925. [Google Scholar] [CrossRef]
- Hammond, J.; Cai, D.; Verhey, K. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 2008, 20, 71–76. [Google Scholar] [CrossRef]
- Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Cancer. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef]
- Steinmetz, M.; Prota, A. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Lowe, S.; Lin, A. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Garbicz, D.; Tobiasz, P.; Borys, F.; Poterała, M.; Grzesiuk, E.; Krawczyk, H. The stilbene and dibenzo[b,f]oxepine derivatives as anticancer compounds. Biomed. Pharmacother. 2020, 123, 109781. [Google Scholar] [CrossRef]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Kobauri, P.; Galenkamp, N.S.; Schulte, A.M.; de Vries, J.; Simeth, N.A.; Maglia, G.; Thallmair, S.; Kolarski, D.A.; Szymanski, W.; Feringa, B.L. Hypothesis-Driven, Structure-Based Design in Photopharmacology: The Case of eDHFR Inhibitors. J. Med. Chem. 2022, 65, 4798–4817. [Google Scholar] [CrossRef]
- Pianowski, Z.L. (Ed.) Molecular Photoswitches: Chemistry, Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Kirchner, S.; Pianowski, Z. Photopharmacology of Antimitotic Agents. Int. J. Mol. Sci. 2022, 23, 5657. [Google Scholar] [CrossRef]
- Tobiasz, P.; Borys, F.; Borecka, M.; Krawczyk, H. Synthesis of building blocks with dibenzo[b,f] oxepin for use in photopharmacology. Int. J. Mol. Sci. 2021, 22, 11033. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Sobel, J.; Krawczyk, H. Synthesis and study of dibenzo[b,f] oxepine combined with fluoroazobenzenes—New photoswitches for application in biological systems. Molecules 2022, 27, 5836. [Google Scholar] [CrossRef] [PubMed]
- Gale, T.V.; Horton, T.M.; Hoffmann, A.R.; Branco, L.M.; Garry, R.F. Host proteins identified in extracellular viral particles as targets for broad-spectrum antiviral inhibitors. J. Proteome Res. 2019, 18, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Childs-Disney, J.L.; Yang, X.; Gibaut, M.R.Q.; Tong, Y.; Batey, R.T.; Disney, M.D. Targeting RNA structures with small molecules. Nat. Rev. Drug Discov. 2022, 21, 736–762. [Google Scholar] [CrossRef] [PubMed]
- Connelly, C.M.; Numata, T.; Boer, R.E.; Moon, M.H.; Sinniah, R.S.; Barchi, J.J.; Ferré-D’Amaré, A.R.; Schneekloth, J.S., Jr. Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure. Nat. Commun. 2019, 10, 1501. [Google Scholar] [CrossRef]
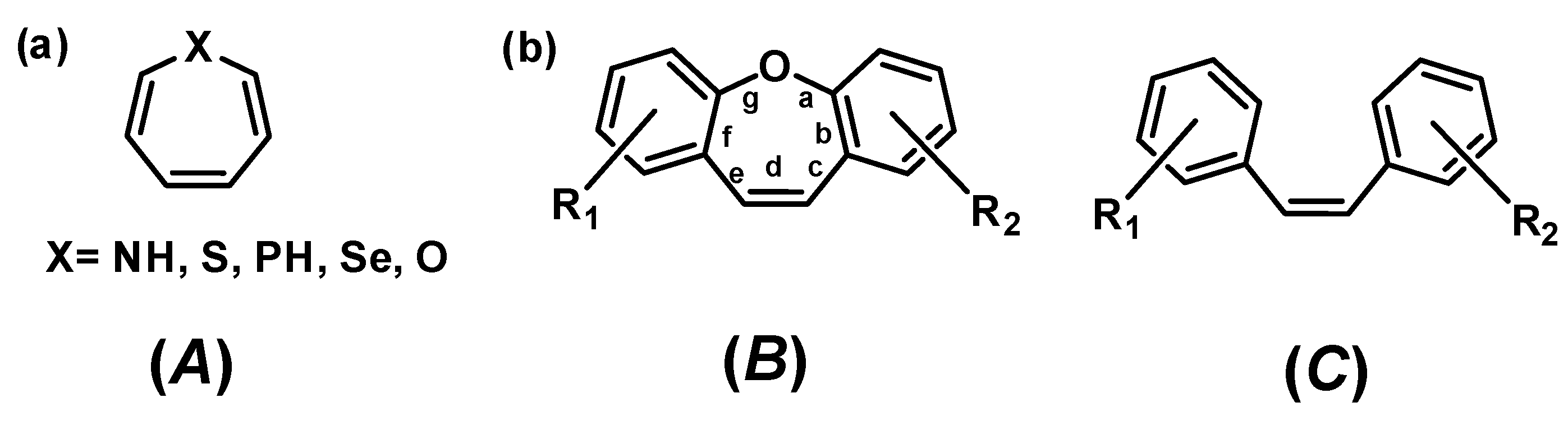

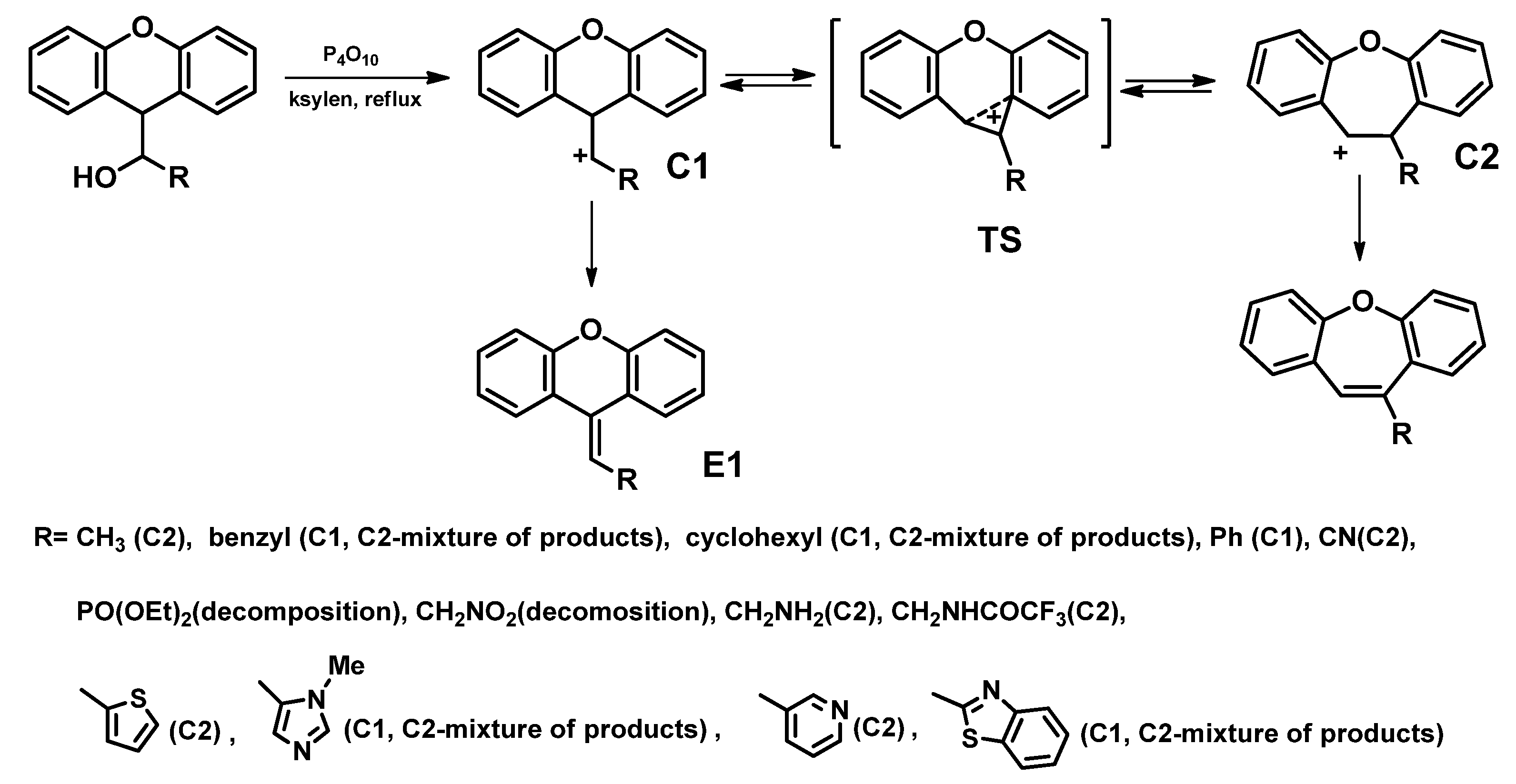
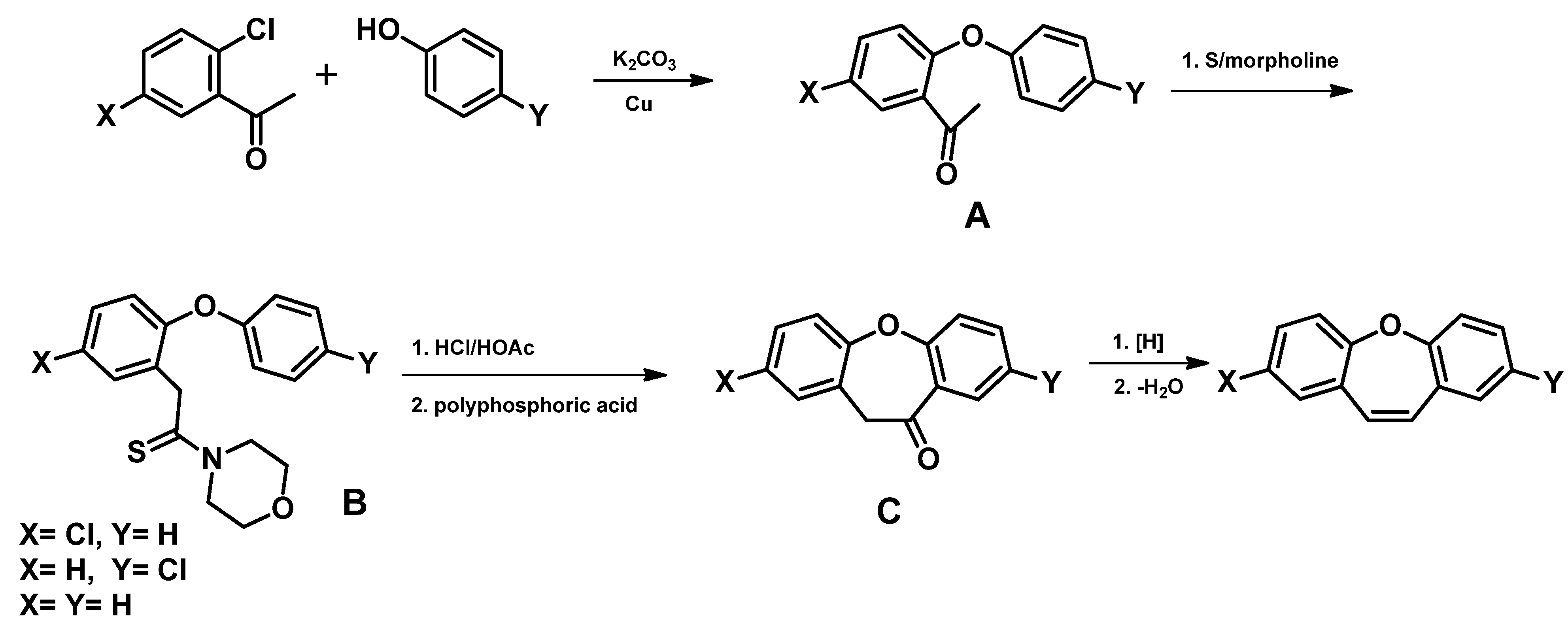
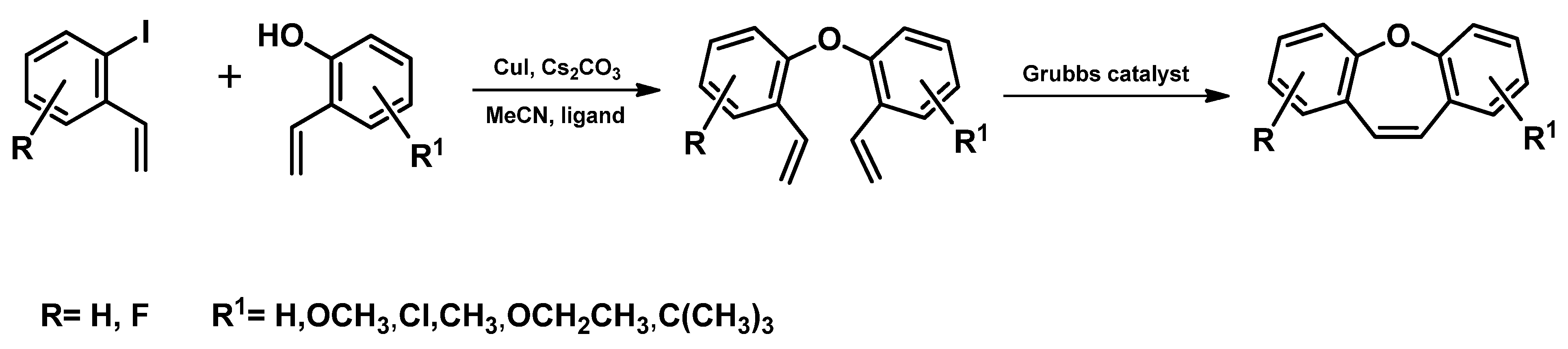
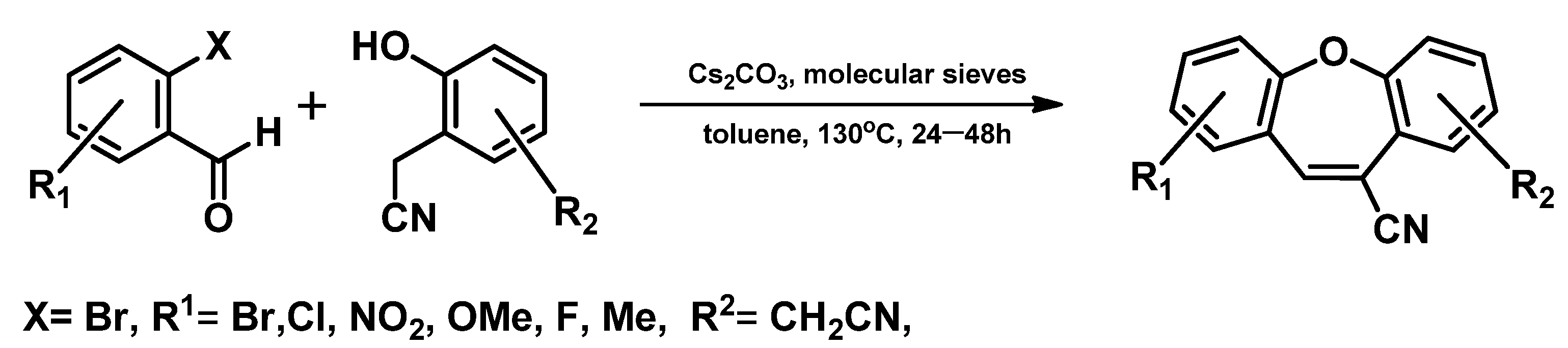



| Entry | Name of Compound | XLogP3-AA | TPSA [Ų] | Hydrogen Bond Donor Count | Hydrogen Bond Acceptor Count |
|---|---|---|---|---|---|
| 1 | Pacharin | 3.6 | 58.9 | 2 | 4 |
| 2 | Bauhiniastatin 1 | 2.5 | 72.8 | 1 | 5 |
| 3 | Bauhiniastatin 2 | 3.5 | 68.2 | 2 | 5 |
| 4 | Bauhiniastatin 3 | 3.5 | 68.2 | 2 | 5 |
| 5 | Bauhiniastatin 4 | 3.6 | 58.9 | 2 | 4 |
| 6 | Bauhinoxepin A | 4.5 | 58.9 | 2 | 4 |
| 7 | Bauhinoxepin B | 5.5 | 58.9 | 2 | 4 |
| 8 | Yagonine | 2.6 | 74.3 | 0 | 6 |
| 9 | Aristoyagonine | 2.7 | 57.2 | 0 | 5 |
| 10 | Secocularidine | 3.8 | 51.2 | 1 | 5 |
| 11 | Secocularine | 4.2 | 40.2 | 0 | 5 |
| 12 | Secosarcocapnine | 4.2 | 40.2 | 0 | 5 |
| 13 | Norsecosarcocapnine | 3.7 | 49 | 1 | 5 |
| 14 | Artocarpol D | 5.4 | 58.9 | 2 | 4 |
| 15 | Artocarpol E | 7.9 | 69.9 | 3 | 4 |
| 16 | Artocarpol F | 5.1 | 77.4 | 2 | 6 |
| 17 | Tournefolic acid B | 2.8 | 107 | 4 | 6 |
| 18 | Tournefolic acid B methyl ester | 3.2 | 96.2 | 3 | 6 |
| 19 | Tournefolic acid B ethyl ester | 3.5 | 96.2 | 3 | 6 |
| 20 | Combretastatin A-4 | 3.7 | 57.2 | 1 | 5 |
| Compound | Pharmacological Activities | Figure on Which the Structure Was Placed |
|---|---|---|
| 1 | antipsychotic properties | Figure 4 |
| 2a–c | antipsychotic properties | Figure 4 |
| 3a–d | sedative, anticonvulsant, and anesthetic-enhancing properties | Figure 4 |
| 4 | anxiolytic properties | Figure 4 |
| 5a,b | anti-inflammatory activity | Figure 4 |
| 6 | AII (angiotensin II) receptor antagonist | Figure 4 |
| 7 | antiestrogenic agent (moderately active) | Figure 4 |
| 8 | phase 1 clinical trials with pediatric and adolescent congenital muscular dystrophy; reduce the level of high-level Tau protein | Figure 4 |
| 9 | strong inhibitory effect on the proliferation of breast cancer cells of the MDA-MB-231 line | Figure 4 |
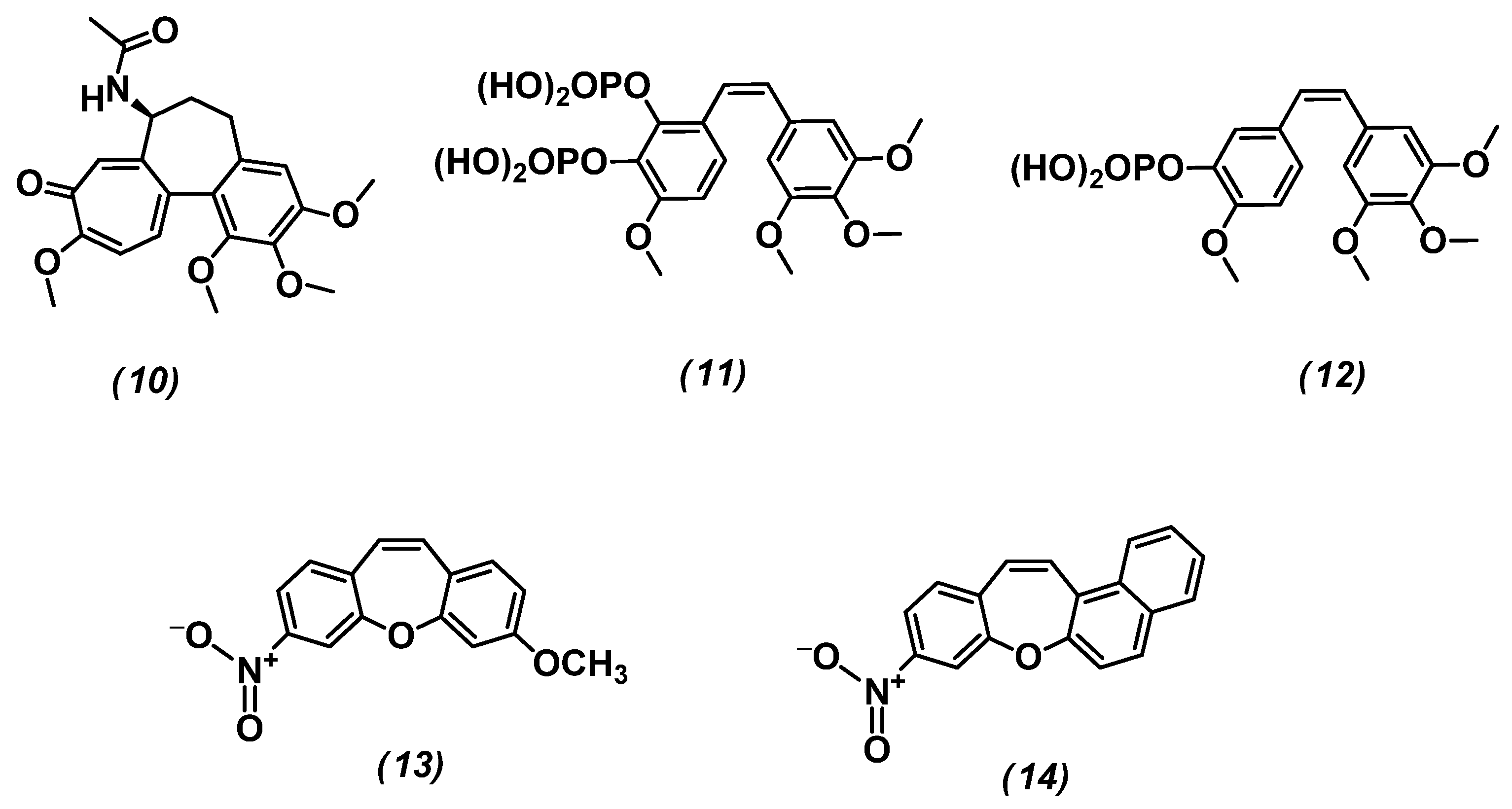
| 13 | the strongest cytotoxic effect against HeLa and U87 cancerous cells lines | Figure 5 |
| 14 | the strongest cytotoxic effect against HeLa and U87 cancerous cells lines | Figure 5 |
| 15a–h | In silico studies showed that compounds 15a–h and 16a–h interact with the colchicine binding site in tubulin with a part of the dibenzo[b,f]oxepine, in a part of the azo switch, or both at the same time. | Figure 7 |
| 16a–h | Figure 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, H. Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine. Int. J. Mol. Sci. 2023, 24, 12066. https://doi.org/10.3390/ijms241512066
Krawczyk H. Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine. International Journal of Molecular Sciences. 2023; 24(15):12066. https://doi.org/10.3390/ijms241512066
Chicago/Turabian StyleKrawczyk, Hanna. 2023. "Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine" International Journal of Molecular Sciences 24, no. 15: 12066. https://doi.org/10.3390/ijms241512066
APA StyleKrawczyk, H. (2023). Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine. International Journal of Molecular Sciences, 24(15), 12066. https://doi.org/10.3390/ijms241512066





