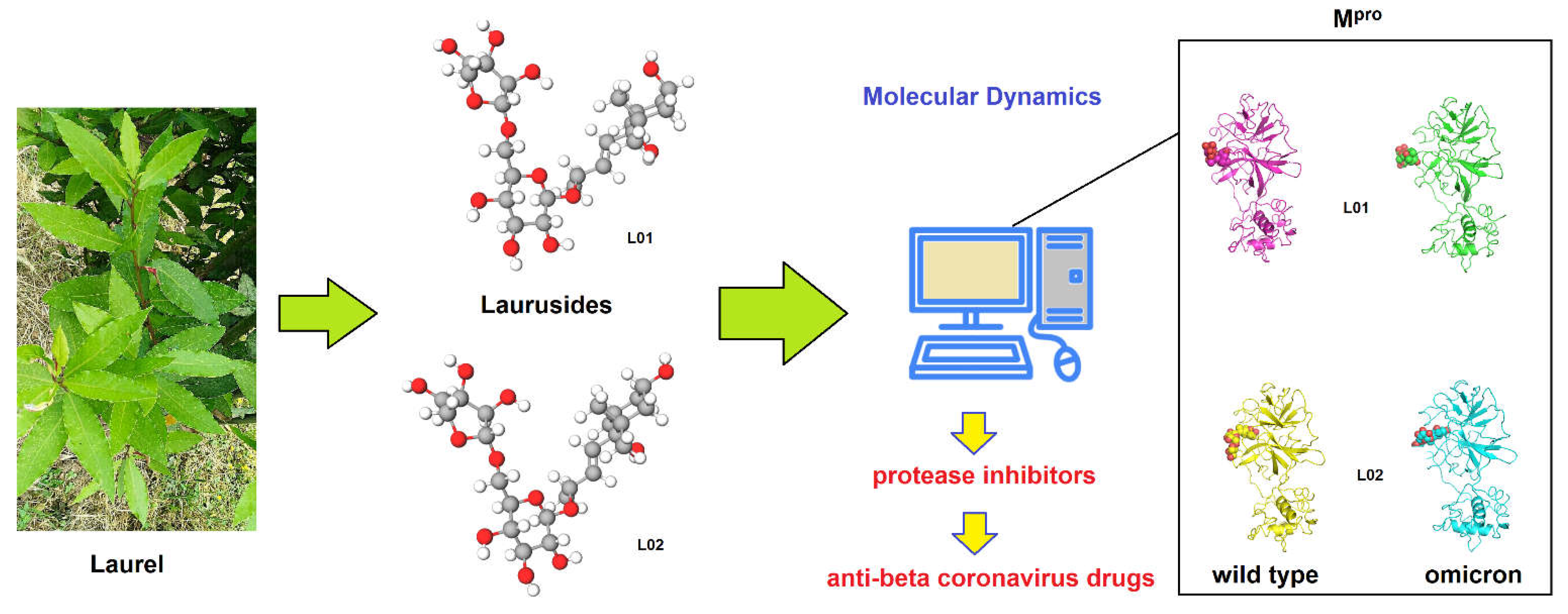
Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study
Abstract
1. Introduction
2. Results and Discussion
3. Methods
- L01 [IUPAC name: (2R,3E)-4-[(1S,4R,6R)-1,4-Dihydroxy-2,2,6-trimethylcyclohexyl]-3-buten-2-yl 6-O-[(2R,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)tetrahydro-2-furanyl]-β-D-glucopyranoside ] SMILES:
- C[C@H](C=C[C@@]1(O)[C@H](C)C[C@@H](O)CC1(C)C)O[C@@H]3O[C@H](CO[C@@H]2OC[C@](O)(CO)[C@H]2O)[C@@H](O)[C@H](O)[C@H]3O
- L02 [IUPAC name: (2R,3E)-4-[(1S,4S,6R)-1,4-Dihydroxy-2,2,6-trimethylcyclohexyl]-3-buten-2-yl 6-O-[(2R,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)tetrahydro-2-furanyl]-β-D-glucopyranoside ] SMILES:
- C[C@@H]1C[C@@H](CC([C@]1(/C=C/[C@@H](C)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CO[C@H]3[C@@H]([C@](CO3)(CO)O)O)O)O)O)O)(C)C)O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolles, M.; Donaldson, E.; Baric, R. SARS-CoV and emergent coronaviruses: Viral determinants of interspecies transmission. Curr. Opin. Virol. 2011, 1, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Haidar, G.; Mellors, J.W. COVID-19: Challenges of viral variants. Annu. Rev. Med. 2023, 74, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Krukiewicz, K.; Yahya, G.; Haq, M.U.; Maryam, S.; Mosbah, R.A.; Saber, S.; Alrouji, M. The breadth of bacteriophages contributing to the development of the phage-based vaccines for COVID-19: An ideal platform to design the multiplex vaccine. Int. J. Mol. Sci. 2023, 24, 1536. [Google Scholar] [CrossRef]
- Aaby, P.; Netea, M.G.; Benn, C.S. Beneficial non-specific effects of live vaccines against COVID-19 and other unrelated infections. Lancet Infect. Dis. 2023, 23, e34–e42. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Less COVID-19 deaths in southern and insular Italy explained by forest bathing, Mediterranean environment, and antiviral plant volatile organic compounds. Environ. Chem. Lett. 2021, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.; Roviello, G.N. Anti-coronavirus vaccines: Past investigations on SARS-CoV-1 and MERS-CoV, the approved vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARS-CoV-2 Infection. Curr. Med. Chem. 2022, 29, 4–18. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Borbone, N.; Piccialli, I.; Falanga, A.P.; Piccialli, V.; Roviello, G.N.; Oliviero, G. Nucleic Acids as Biotools at the Interface between Chemistry and Nanomedicine in the COVID-19 Era. Int. J. Mol. Sci. 2022, 23, 4359. [Google Scholar] [CrossRef]
- Malik, N.; Dhuldhaj, U.P. Available drug therapies on COVID-19 and its side effects: An overview. Cell. Mol. Biomed. Rep. 2023, 3, 41–61. [Google Scholar]
- Gantla, M.R.; Tsigelny, I.F.; Kouznetsova, V.L. Repurposing of drugs for combined treatment of COVID-19 cytokine storm using machine learning. Med. Drug Discov. 2023, 17, 100148. [Google Scholar] [CrossRef]
- Ouni, L.; Ramazani, A. In Silico Screening of Some Anti-Cancer Drugs Against the Main Protease of COVID-19 Using Molecular Docking. Lett. Org. Chem. 2023, 20, 77–90. [Google Scholar]
- Costanzo, V.; Gilhen-Baker, M.; Beresford-Kroeger, D.; Roviello, G.N. Tree-inhabiting polypore fungi as sources of a cornucopia of bioactive compounds. Future Microbiol. 2022, 17, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’Approach. Int. J. Environ. Res. Public Health 2021, 19, 273. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In silico investigation on the interaction of chiral phytochemicals from opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Baker, S.; Gilhen-Baker, M.; Roviello, G.N. The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques. Int. J. Environ. Res. Public Health 2023, 20, 793. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. Forest-bathing and physical activity as weapons against COVID-19: A review. Environ. Chem. Lett. 2022, 20, 131–140. [Google Scholar] [CrossRef]
- Gilhen-Baker, M.; Roviello, V.; Beresford-Kroeger, D.; Roviello, G.N. Old growth forests and large old trees as critical organisms connecting ecosystems and human health. A review. Environ. Chem. Lett. 2022, 20, 1529–1538. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G.N.; Lichtfouse, E. River therapy. Environ. Chem. Lett. 2022, 20, 2729–2734. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. The healing power of clean rivers: In silico evaluation of the antipsoriatic potential of apiin and hyperoside plant metabolites contained in river waters. Int. J. Environ. Res. Public Health 2022, 19, 2502. [Google Scholar] [CrossRef]
- Shao, T.; Verma, H.K.; Pande, B.; Costanzo, V.; Ye, W.; Cai, Y.; Bhaskar, L.V.K.S. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front. Physiol. 2021, 12, 751374. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Sillanpaa, M.; Rezakhani, Y.; Wang, C. Review of concerned SARS-CoV-2 variants like Alpha (B. 1.1. 7), Beta (B. 1.351), Gamma (P. 1), Delta (B. 1.617. 2), and Omicron (B. 1.1. 529), as well as novel methods for reducing and inactivating SARS-CoV-2 mutants in wastewater treatment facilities. J. Hazard. Mater. Adv. 2022, 7, 100140. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Dargham, S.R.; Loka, S.; Shaik, R.M.; Chemaitelly, H.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Yassine, H.M.; Al-Khatib, H.A. Coronavirus disease 2019 disease severity in children infected with the omicron variant. Clin. Infect. Dis. 2022, 75, e361–e367. [Google Scholar] [CrossRef]
- Pacchiarini, N.; Sawyer, C.; Williams, C.; Sutton, D.; Roberts, C.; Simkin, F.; King, G.; McClure, V.; Cottrell, S.; Clayton, H. Epidemiological analysis of the first 1000 cases of SARS-CoV-2 lineage BA. 1 (B. 1.1. 529, Omicron) compared with co-circulating Delta in Wales, UK. Influenza Other Respir. Viruses 2022, 16, 986–993. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef]
- Hu, Q.; Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Zhang, Y.W.; Huang, P.; Ge, G.B. The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19. MedComm 2022, 3, e151. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Jedrzejczak, R.; Stols, L.; Langan, P.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Structural plasticity of SARS-CoV-2 3CL Mpro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020, 11, 3202. [Google Scholar] [CrossRef]
- Caravez, J.C.; Iyer, K.S.; Kavthe, R.D.; Kincaid, J.R.; Lipshutz, B.H. A 1-pot synthesis of the SARS-CoV-2 Mpro Inhibitor Nirmatrelvir, the key ingredient in Paxlovid. Org. Lett. 2022, 24, 9049–9053. [Google Scholar] [CrossRef]
- Catlin, N.; Bowman, C.; Campion, S.; Cheung, J.; Nowland, W.; Sathish, J.; Stethem, C.; Updyke, L.; Cappon, G. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models. Reprod. Toxicol. 2022, 108, 56–61. [Google Scholar] [CrossRef]
- Falanga, A.P.; Terracciano, M.; Oliviero, G.; Roviello, G.N.; Borbone, N. Exploring the Relationship between G-Quadruplex Nucleic Acids and Plants: From Plant G-Quadruplex Function to Phytochemical G4 Ligands with Pharmaceutic Potential. Pharmaceutics 2022, 14, 2377. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo [c] phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem.-Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Gopalakrishnakone, P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid. -Based Complement. Altern. Med. 2010, 7, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Degner, S.C.; Papoutsis, A.J.; Romagnolo, D.F. Health benefits of traditional culinary and medicinal Mediterranean plants. In Complementary and Alternative Therapies and the Aging Population; Elsevier: Amsterdam, The Netherlands, 2009; pp. 541–562. [Google Scholar]
- Mauriello, L.M.; Artz, K. Culinary medicine: Bringing healthcare into the kitchen. Am. J. Health Promot. 2019, 33, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Martínez, J.P.Q.; Campos, M.R.S. Bioactive Compounds and Functional foods as coadjuvant therapy for thrombosis. Food Funct. 2023, 14, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef]
- García-Gurrola, A.; Wall-Medrano, A.; Olivas-Aguirre, M.A.; Olivas-Aguirre, F.J.; Escobar-Puentes, A.A. Immunomodulatory Properties of Nutraceuticals and Functional Foods. In Nutraceuticals and Functional Foods in Immunomodulators; Springer: Singapore, 2022; pp. 21–72. [Google Scholar]
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Abass, K.; Hakkola, J.; Atawia, A.R.; Farag, M.A. The wild Egyptian artichoke as a promising functional food for the treatment of hepatitis C virus as revealed via UPLC-MS and clinical trials. Food Funct. 2016, 7, 3006–3016. [Google Scholar] [CrossRef]
- Calder, P.C.; Kew, S. The immune system: A target for functional foods? Br. J. Nutr. 2002, 88, S165–S176. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Turning the tide: Natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Ricci, A.; Roviello, G.N. Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV. Life 2023, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Duc, N.V.; Luyen, B.T.T.; Van Oanh, H.; Jang, H.J.; Huong, T.T.; Kim, Y.H.; Thao, N.P. Megastigmane and abscisic acid glycosides from the leaves of Laurus nobilis L. Phytochem. Lett. 2019, 33, 1–5. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Lower COVID-19 mortality in Italian forested areas suggests immunoprotection by Mediterranean plants. Environ. Chem. Lett. 2021, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.E.; Noell, S.; Plotnikova, O.; Ferre, R.; Liu, W.; Bolanos, B.; Fennell, K.; Nicki, J.; Craig, T.; Zhu, Y. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J. Biol. Chem. 2022, 298, 101972. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Nchiozem-Ngnitedem, V.-A.; Melchert, D.; Fobofou, S.A. Demystifying racemic natural products in the homochiral world. Nat. Rev. Chem. 2022, 6, 806–822. [Google Scholar] [CrossRef]
- Owen, C.; Lukacik, P.; Strain-Damerell, C.; Douangamath, A.; Powell, A.; Fearon, D.; Brandao-Neto, J.; Crawshaw, A.; Aragao, D.; Williams, M. SARS-CoV-2 main protease with unliganded active site (2019-nCoV, coronavirus disease 2019, COVID-19). PDB Entry 2020. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Falanga, A.P.; Terracciano, M.; D’Ambrosio, C.; Piccialli, G.; Oliviero, G.; Roviello, G.N.; Borbone, N. CD, UV, and In Silico Insights on the Effect of 1, 3-Bis (1′-uracilyl)-2-propanone on Serum Albumin Structure. Biomolecules 2022, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, P.L.; Riccardi, C.; Palumbo, R.; Gale, T.F.; Musumeci, D.; Roviello, G.N. Self-assembly of thyminyl l-tryptophanamide (TrpT) building blocks for the potential development of drug delivery nanosystems. J. Nanostruct. Chem. 2023. [Google Scholar] [CrossRef]
- Ivani, I.; Dans, P.D.; Noy, A.; Pérez, A.; Faustino, I.; Hospital, A.; Walther, J.; Andrio, P.; Goñi, R.; Balaceanu, A. Parmbsc1: A refined force field for DNA simulations. Nat. Methods 2016, 13, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Berendsen, H.; Grigera, J.; Straatsma, T. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Esposito, C.L.; Autiero, I.; Sandomenico, A.; Li, H.; Bassal, M.A.; Ibba, M.L.; Wang, D.; Rinaldi, L.; Ummarino, S.; Gaggi, G. Targeted systematic evolution of an RNA platform neutralizing DNMT1 function and controlling DNA methylation. Nat. Commun. 2023, 14, 99. [Google Scholar] [CrossRef]
- Ronda, L.; Tonelli, A.; Sogne, E.; Autiero, I.; Spyrakis, F.; Pellegrino, S.; Abbiati, G.; Maffioli, E.; Schulte, C.; Piano, R.; et al. Rational Design of a User-Friendly Aptamer/Peptide-Based Device for the Detection of Staphylococcus aureus. Sensors 2020, 20, 4977. [Google Scholar] [CrossRef]
- Piacenti, V.; Langella, E.; Autiero, I.; Nolan, J.C.; Piskareva, O.; Adamo, M.F.; Saviano, M.; Moccia, M. A combined experimental and computational study on peptide nucleic acid (PNA) analogues of tumor suppressive miRNA-34a. Bioorg. Chem. 2019, 91, 103165. [Google Scholar] [CrossRef]
- Autiero, I.; Ruvo, M.; Improta, R.; Vitagliano, L. The intrinsic flexibility of the aptamer targeting the ribosomal protein S8 is a key factor for the molecular recognition. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1006–1016. [Google Scholar] [CrossRef]
- Abraham, M.; Spoel, D.V.; Lindahl, E.; Hess, B. GROMACS User Manual Version 2019. Available online: http://www.gromacs.org (accessed on 11 March 2023).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Turner, P.; Stambulchik, E.; Unix-like, A. Grace (Plotting Tool); Grace 5.1.23; Grace Development Team: Weizmann Institute of Science, Israel, 2012. [Google Scholar]
- DeLano, W.L. The PyMOL Molecular Graphics System. 2002. Available online: http://www.pymol.org/ (accessed on 11 March 2023).
- Daura, X.; Gademann, K.; Jaun, B.; Seebach, D.; Van Gunsteren, W.F.; Mark, A.E. Peptide folding: When simulation meets experiment. Angew. Chem. Int. Ed. 1999, 38, 236–240. [Google Scholar] [CrossRef]
- Roviello, G.N.; Moccia, M.; Sapio, R.; Valente, M.; Bucci, E.M.; Castiglione, M.; Pedone, C.; Perretta, G.; Benedetti, E.; Musumeci, D. Synthesis, characterization and hybridization studies of new nucleo-gamma-peptides based on diaminobutyric acid. J. Pept. Sci. 2006, 12, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Omodei, D.; Vicidomini, C.; Roviello, G.N. Willardiine and its synthetic analogues: Biological aspects and implications in peptide chemistry of this nucleobase amino acid. Pharmaceuticals 2022, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, G.N. Potential Anti-SARS-CoV-2 Molecular Strategies. Molecules 2023, 28, 2118. [Google Scholar] [CrossRef] [PubMed]
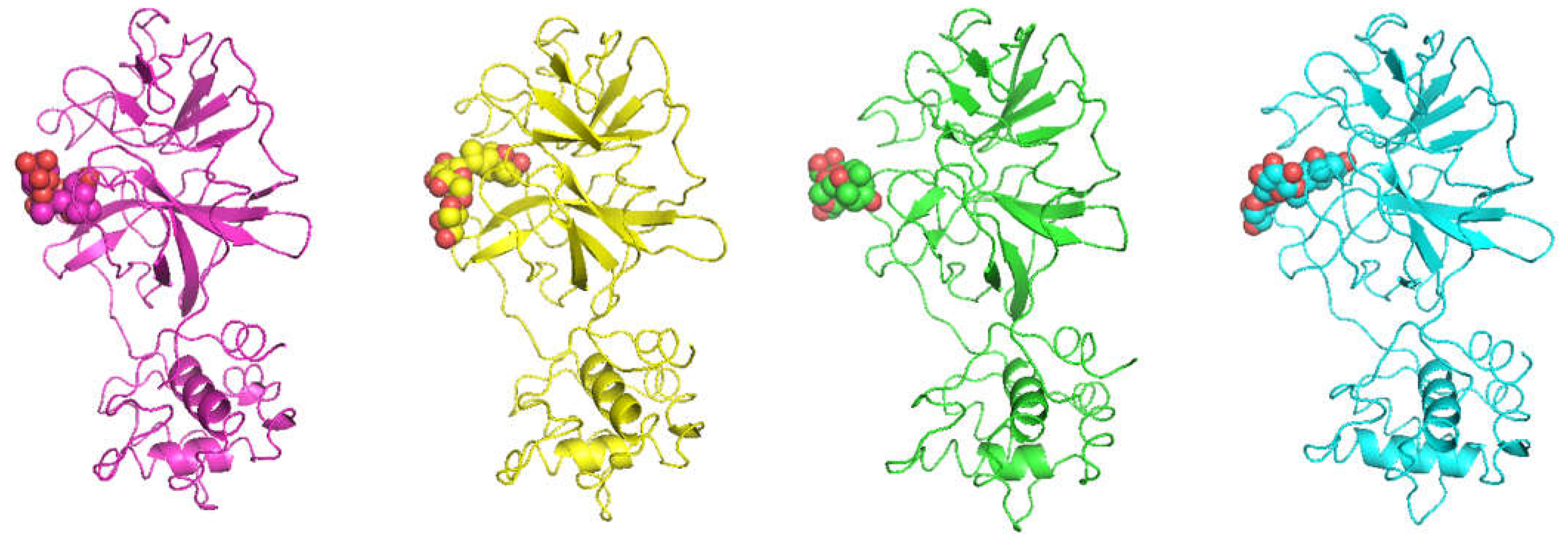


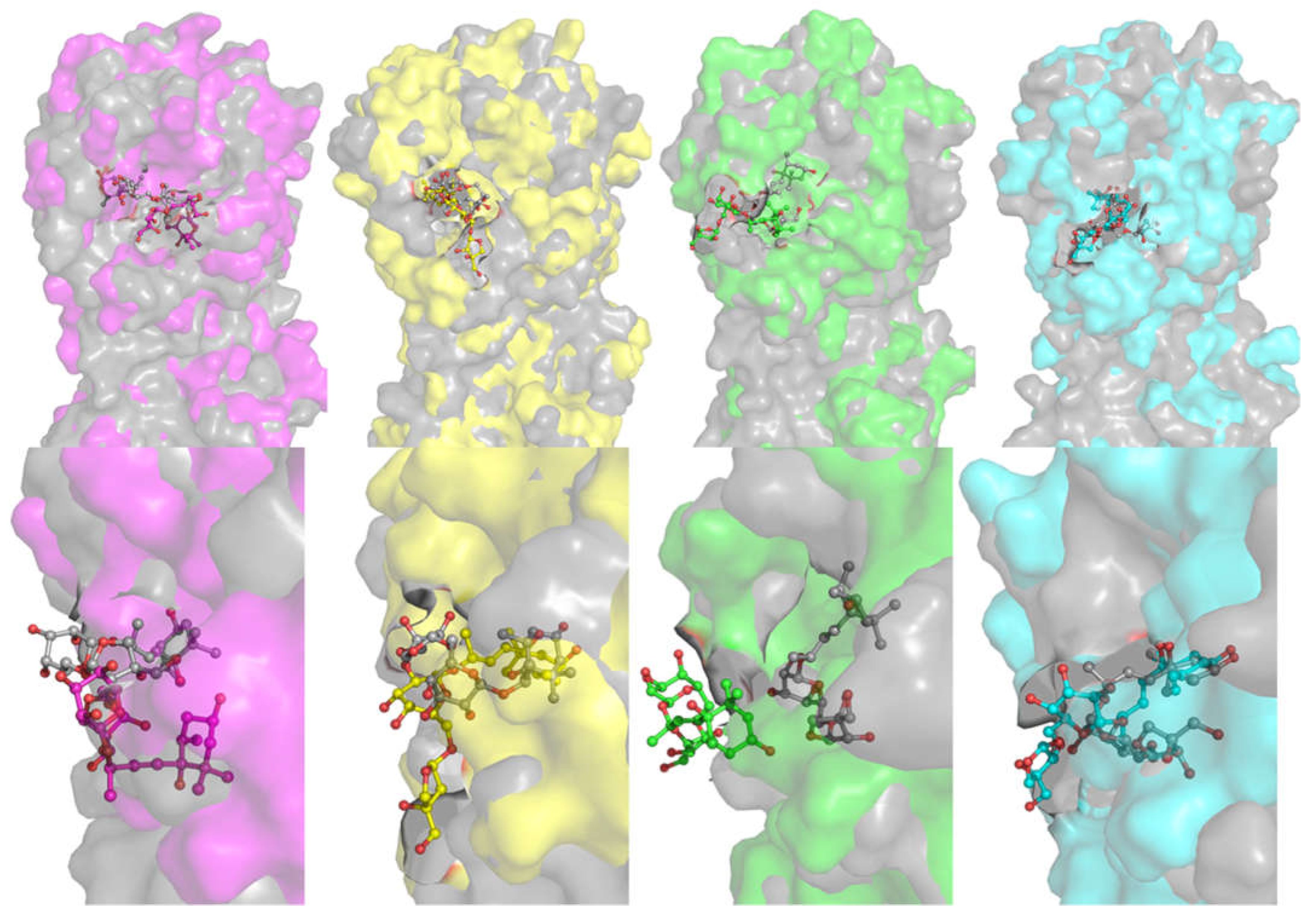
| Occurrence (%) | |||||
|---|---|---|---|---|---|
| W1 | W2 | O1 | O2 | ||
| Thr | 24 | 8 | |||
| Thr | 25 | 8 | |||
| Thr | 26 | 12 | |||
| His | 41* | 8 | 5 | 9 | |
| Thr | 45 | 3 | |||
| Ser | 46 | 11 | 3 | ||
| Tyr | 54 | 17 | |||
| Met | 49 | 16 | |||
| Asn | 142 | 13 | 58 | 13 | |
| Gly | 143 | 2 | 13 | ||
| Cys | 145 * | 4 | |||
| His | 164 * | 20 | 7 | 68 | |
| Glu | 166 * | 24 | 18 | 2 | 83 |
| Asp | 187 | 100 | |||
| Arg | 188 | 63 | 2 | 13 | |
| Gln | 189* | 23 | 5 | 41 | 2 |
| Thr | 190 | 12 | 7 | ||
| Gln | 192 | 73 | 11 | 9 | |
| MD | Protein Atom | Ligand Atom | Occurrence (%) | |||
|---|---|---|---|---|---|---|
| w1 | His | 164 * | O | L01 | O2/O3 | 20 |
| w2 | Asn | 142 | ND2 | L02 | O1 | 26 |
| Asn | 142 | OD1 | L02 | O4/O57/O8 | 32 | |
| Asp | 187 | OD1/2 | L02 | O2 | 100 | |
| Arg | 188 | O | L02 | O3 | 63 | |
| Gln | 192 | O | L02 | O9 | 32 | |
| Gln | 192 | N | L02 | O8 | 34 | |
| o1 | - | - | - | - | - | - |
| o2 | His | 164 * | ND1 | L02 | O2 | 66 |
| Glu | 166 * | OE1/2 | L02 | O8/O9 | 21 | |
| Glu | 166 * | N | L02 | O10 | 33 | |
| Glu | 166 * | N | L02 | O7 | 21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autiero, I.; Roviello, G.N. Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study. Int. J. Mol. Sci. 2023, 24, 5511. https://doi.org/10.3390/ijms24065511
Autiero I, Roviello GN. Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study. International Journal of Molecular Sciences. 2023; 24(6):5511. https://doi.org/10.3390/ijms24065511
Chicago/Turabian StyleAutiero, Ida, and Giovanni N. Roviello. 2023. "Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study" International Journal of Molecular Sciences 24, no. 6: 5511. https://doi.org/10.3390/ijms24065511
APA StyleAutiero, I., & Roviello, G. N. (2023). Interaction of Laurusides 1 and 2 with the 3C-like Protease (Mpro) from Wild-Type and Omicron Variant of SARS-CoV-2: A Molecular Dynamics Study. International Journal of Molecular Sciences, 24(6), 5511. https://doi.org/10.3390/ijms24065511








