Eptifibatide, an Older Therapeutic Peptide with New Indications: From Clinical Pharmacology to Everyday Clinical Practice
Abstract
1. Introduction
2. Hemostasis and the Role of the Platelets
- Vasoconstriction of the damaged vessel immediately after the injury to reduce blood loss;
- Adhesion, activation, and aggregation of platelets that form a platelet plug (usually referred to as primary hemostasis);
- Coagulation of protein factors and formation of a dense fibrin mesh (usually referred to as secondary hemostasis).
3. Antiplatelet Medications
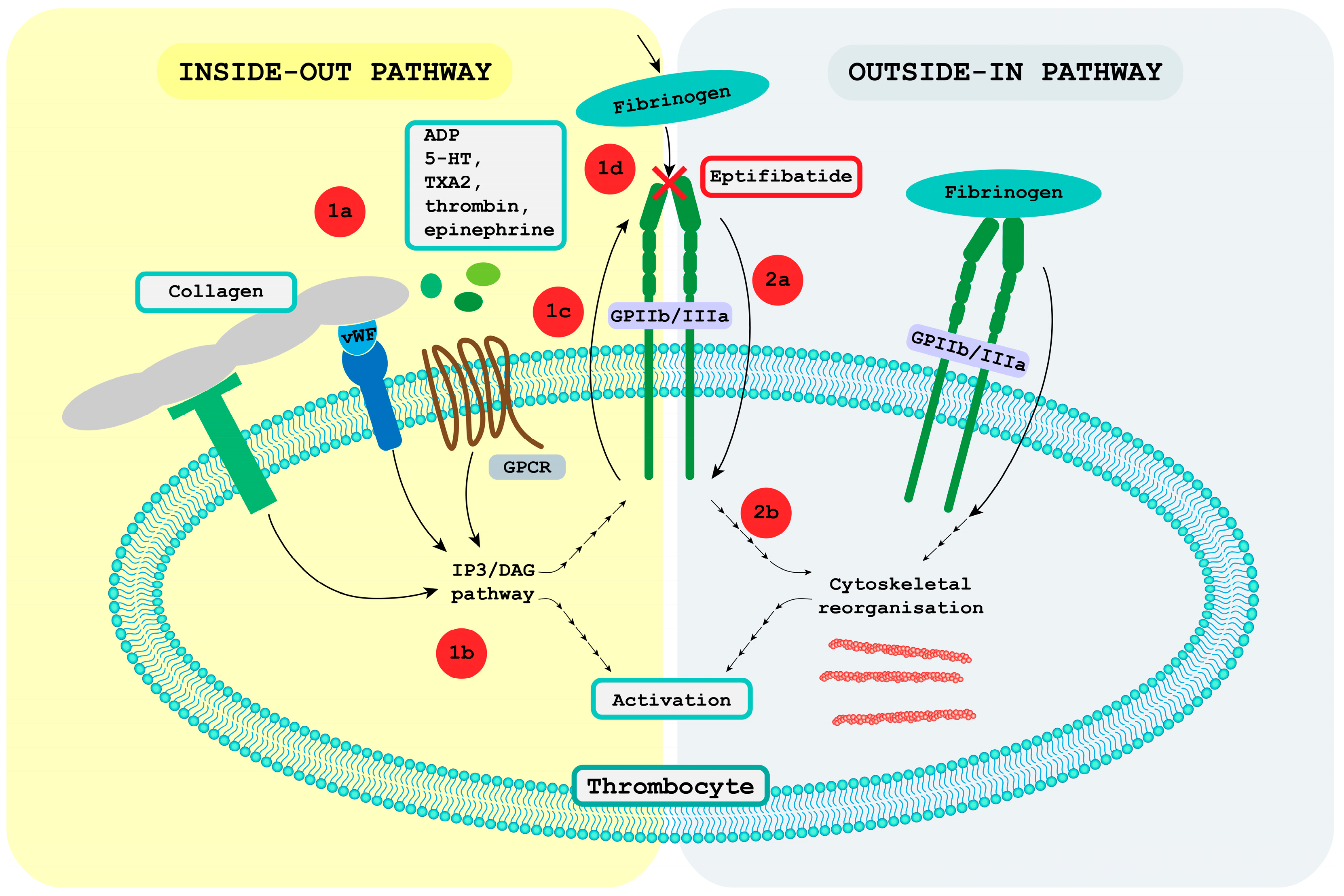
4. Receptor GPIIb/IIIa
4.1. Receptor GPIIb/IIIa Pathway
4.1.1. Inside-Out Pathway
4.1.2. Outside-in Pathway
5. Eptifibatide
5.1. Associated Drug Classes
5.1.1. GPIIb/IIIa Inhibitors
5.1.2. Snake Venom and Disintegrin Peptide Family
- Approximately 41–51 amino acid long peptides with four cysteine bridges (echistatin and obtustatin);
- Approximately 70 amino acid long peptides with six cysteine bridges (barbourin, flavoviridin, and atrolysin);
- Approximately 84 amino acid long peptides with seven cysteine bridges (bitistatin);
- Macromolecular complexes of usually noncovalently bound homodimers or heterodimers, which are 67 amino acids long and have 10 cysteines incorporated into the structure.
5.2. Biochemical Structure
5.3. Pharmacodynamics
5.4. Pharmacokinetics
5.5. Clinical Applications
5.5.1. Acute Coronary Syndromes: Angina Pectoris, STEMI, and NSTEMI
Angina Pectoris and non-ST Elevation Myocardial Infarction
ST-Elevated Myocardial Infarction
5.5.2. Percutaneous Coronary Intervention
5.5.3. Bridging Strategy for Patients Undergoing Surgery after Coronary Stent Insertion
5.5.4. Ischemic Stroke and Carotid and Intracranial Aneurysm Stenting
5.5.5. Septic Shock
5.6. Contraindications
5.7. Administration and Dosages
5.8. Adverse Effects and Interactions
5.8.1. Adverse Effects
5.8.2. Interactions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharm. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Angell, Y.; Holford, M.; Moos, W.H. Building on success: A bright future for peptide therapeutics. Protein Pept. Lett. 2018, 25, 1044–1050. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Fisher, E.; Pavlenko, K.; Vlasov, A.; Ramenskaya, G. Peptide-based therapeutics for oncology. Pharm. Med. 2019, 33, 9–20. [Google Scholar] [CrossRef]
- Sloan, L.A. Review of Glucagon-Like Peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus in patients with chronic kidney disease and their renal effects. J. Diabetes 2019, 11, 938–948. [Google Scholar] [CrossRef]
- Peterson, S.C.; Barry, A.R. Effect of Glucagon-like Peptide-1 receptor agonists on all-cause mortality and cardiovascular outcomes: A meta-analysis. Curr. Diabetes Rev. 2018, 14, 273–279. [Google Scholar] [CrossRef]
- Li, X.-F.; Liu, C.-F.; Rao, G.-W. Monoclonal antibodies, small molecule inhibitors and antibody-drug conjugates as HER2 inhibitors. Curr. Med. Chem. 2021, 28, 3339–3360. [Google Scholar] [CrossRef]
- Vuong, H.G.; Ho, A.T.N.; Tran, T.T.K.; Capdevila, J.; Benekli, M.; Nakazawa, T.; Katoh, R.; Kondo, T. Efficacy and toxicity of sorafenib in the treatment of advanced medullary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2019, 41, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Cabot, P.J.; Moyle, P.M. Glucagon-Like Peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019, 16, 2278–2295. [Google Scholar] [CrossRef] [PubMed]
- Hackenberger, C.P.R.; Dawson, P.E.; Chen, Y.-X.; Hojo, H. Modern peptide and protein chemistry: Reaching new heights. J. Org. Chem. 2020, 85, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Grieco, P.; Gomez-Monterrey, I. Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials. Arch. Biochem. Biophys. 2019, 662, 15–32. [Google Scholar] [CrossRef]
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharmacol. 2017, 7, 526. [Google Scholar] [CrossRef]
- Lenasi, H. Hemostaza. Med. Razgl. 2017, 56, 197–214. [Google Scholar]
- Scridon, A. Platelets and their role in hemostasis and thrombosis—From physiology to pathophysiology and therapeutic implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- LaPelusa, A.; Dave, H.D. Physiology, Hemostasis. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Jamasbi, J.; Ayabe, K.; Goto, S.; Nieswandt, B.; Peter, K.; Siess, W. Platelet receptors as therapeutic targets: Past, present and future. Thromb. Haemost. 2017, 117, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jandrot-Perrus, M. New Advances in treating thrombotic diseases: GPVI as a platelet drug target. Drug Discov. Today 2014, 19, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Metharom, P.; Berndt, M.C.; Baker, R.I.; Andrews, R.K. Current State and Novel Approaches of Antiplatelet Therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Sattar, Y.; Jamil, R.T. Eptifibatide. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sampat, P.J.; Wadhwa, R. Prasugrel. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wiviott, S.D.; Antman, E.M.; Braunwald, E. Prasugrel. Circulation 2010, 122, 394–403. [Google Scholar] [CrossRef]
- Danielak, D.; Karaźniewicz-Łada, M.; Główka, F. Ticagrelor in Modern Cardiology—An up-to-Date Review of Most Important Aspects of Ticagrelor Pharmacotherapy. Expert Opin. Pharmacother. 2018, 19, 103–112. [Google Scholar] [CrossRef]
- Juneja, S.; Gupta, K.; Kaushal, S. Ticagrelor: An Emerging Oral Antiplatelet Agent. J. Pharm. Pharm. 2013, 4, 78–80. [Google Scholar] [CrossRef]
- De Luca, L.; Steg, P.G.; Bhatt, D.L.; Capodanno, D.; Angiolillo, D.J. Cangrelor: Clinical Data, Contemporary Use, and Future Perspectives. J. Am. Heart Assoc. 2021, 10, e022125. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Lopez, R.A.; Hai, O. Antiplatelet Medications. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stoffer, K.; Bistas, K.G.; Reddy, V.; Shah, S. Abciximab. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tummala, R.; Rai, M.P. Glycoprotein IIb/IIIa Inhibitors. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Layne, K.; Ferro, A. Antiplatelet Therapy in Acute Coronary Syndrome. Eur. Cardiol. 2017, 2017 12, 33–37. [Google Scholar] [CrossRef]
- Schmit, K.; Dolor, R.J.; Jones, W.S.; Vemulapalli, S.; Hasselblad, V.; Subherwal, S.; Heidenfelder, B.; Patel, M.R. Comparative Effectiveness Review of Antiplatelet Agents in Peripheral Artery Disease. J. Am. Heart Assoc. 2014, 3, e001330. [Google Scholar] [CrossRef]
- Devabhakthuni, S.; Seybert, A.L. Oral Antiplatelet Therapy for the Management of Acute Coronary Syndromes: Defining the Role of Prasugrel. Crit. Care Nurse 2011, 31, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin AIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Solh, T.; Botsford, A.; Solh, M. Glanzmann’s thrombasthenia: Pathogenesis, diagnosis, and current and emerging treatment options. J. Blood Med. 2015, 6, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wu, X.; Luo, Q.; Wei, G.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Zi, J.; Ju, W.; et al. NLRP3 regulates platelet integrin AIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 2018, 103, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, M.; Shen, H.; You, T.; Ding, Z.; Cui, Q.; Ma, Z.; Yang, F.; Xie, Z.; Shi, H.; et al. Clinical and molecular insights into glanzmann’s thrombasthenia in China. Clin. Genet. 2018, 94, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Amirkhosravi, A.; Amaya, M.; Siddiqui, F.; Biggerstaff, J.P.; Meyer, T.V.; Francis, J.L. Blockade of GpIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets 1999, 10, 285–292. [Google Scholar] [CrossRef]
- Nieswandt, B.; Varga-Szabo, D.; Elvers, M. Integrins in platelet activation. J. Thromb. Haemost. 2009, 7, 206–209. [Google Scholar] [CrossRef]
- Ahn, J.-M.; Kassees, K.; Lee, T.-K.; Manandhar, B.; Yousif, A.M. Strategy and Tactics for Designing Analogs: Biochemical Characterization of the Large Molecules. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 66–115. ISBN 978-0-12-803201-5. [Google Scholar]
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake venoms in drug discovery: Valuable therapeutic tools for life saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Eptifibatide. DB00063. Available online: https://go.drugbank.com/drugs/DB00063 (accessed on 1 March 2023).
- Cannon, C.P. Oral glycoprotein IIb/IIIa inhibition—Great idea, but it didn’t work. Am. J. Med. 2002, 112, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Newby, L.K.; Califf, R.M.; White, H.D.; Harrington, R.A.; Van de Werf, F.; Granger, C.B.; Simes, R.J.; Hasselblad, V.; Armstrong, P.W. The failure of orally administered glycoprotein IIb/IIIa inhibitors to prevent recurrent cardiac events. Am. J. Med. 2002, 112, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; McCabe, C.H.; Wilcox, R.G.; Langer, A.; Caspi, A.; Berink, P.; Lopez-Sendon, J.; Toman, J.; Charlesworth, A.; Anders, R.J.; et al. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation 2000, 102, 149–156. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Serruys, P.; Knudtson, M.; van Es, G.A.; Timmis, G.C.; van der Zwaan, C.; Kleiman, J.; Gong, J.; Roecker, E.B.; Dreiling, R.; et al. Long-Term Treatment with a platelet glycoprotein-receptor antagonist after percutaneous coronary revascularization. Excite trial investigators. Evaluation of oral xemilofiban in controlling thrombotic events. N. Engl. J. Med. 2000, 342, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Second SYMPHONY Investigators. Randomized trial of aspirin, sibrafiban, or both for secondary prevention after acute coronary syndromes. Circulation 2001, 103, 1727–1733. [Google Scholar] [CrossRef]
- Topol, E.J.; Easton, J.D.; Amarenco, P.; Califf, R.; Harrington, R.; Graffagnino, C.; Davis, S.; Diener, H.C.; Ferguson, J.; Fitzgerald, D.; et al. Design of the blockade of the glycoprotein IIb/IIIa receptor to avoid vascular occlusion (BRAVO) trial. Am. Heart J. 2000, 139, 927–933. [Google Scholar] [CrossRef]
- Mousa, S.A.; Khurana, S.; Forsythe, M.S. Comparative in vitro efficacy of different platelet glycoprotein IIb/IIIa antagonists on platelet-mediated clot strength induced by tissue factor with use of thromboelastography: Differentiation among glycoprotein IIb/IIIa antagonists. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1162–1167. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. From snake venom’s disintegrins and C-Type lectins to anti-platelet drugs. Toxins 2019, 11, 303. [Google Scholar] [CrossRef]
- Hawgood, B.J. Abbé Felice Fontana (1730–1805): Founder of modern toxinology. Toxicon 1995, 33, 591–601. [Google Scholar] [CrossRef]
- Phillips, D.R.; Scarborough, R.M. Clinical pharmacology of eptifibatide. Am. J. Cardiol. 1997, 80, 11B–20B. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.P.D. RCSB PDB—7THO: Integrin AlaphIIBbeta3 Complex with Eptifibatide. Available online: https://www.rcsb.org/structure/7THO (accessed on 1 March 2023).
- Bank, R.P.D. RCSB PDB—2VDN: Re-Refinement of Integrin AlphaIIbBeta3 Headpiece Bound to Antagonist Eptifibatide. Available online: https://www.rcsb.org/structure/2VDN (accessed on 1 March 2023).
- Bank, R.P.D. RCSB PDB—7U60: Integrin AlaphIIBbeta3 Complex with CRGDfV. Available online: https://www.rcsb.org/structure/7U60 (accessed on 1 March 2023).
- PubChem Eptifibatide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/123610 (accessed on 9 January 2023).
- Disintegrin Barbourin—Sistrurus Miliarius Barbouri (Dusky Pigmy Rattlesnake) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P22827/entry (accessed on 1 March 2023).
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Rose, J.W.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Design of potent and specific integrin antagonists. peptide antagonists with high specificity for glycoprotein IIb-IIIa. J. Biol. Chem. 1993, 268, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, Y.; Jiao, Z.; Wu, M.; Li, C.; Liu, J.; Liu, C.; Hu, Y.; Li, Q.; Zhang, H. Clinical evaluation of the tolerability, pharmacokinetics, and inhibition of platelet aggregation of eptifibatide in healthy chinese subjects. Clin. Pharmacol. Drug Dev. 2020, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Pancioli, A.M.; Broderick, J.; Brott, T.; Tomsick, T.; Khoury, J.; Bean, J.; del Zoppo, G.; Kleindorfer, D.; Woo, D.; Khatri, P.; et al. The combined approach to lysis utilizing eptifibatide and Rt-PA in acute ischemic stroke: The clear stroke trial. Stroke 2008, 39, 3268–3276. [Google Scholar] [CrossRef]
- Adeoye, O.; Sucharew, H.; Khoury, J.; Vagal, A.; Schmit, P.A.; Ewing, I.; Levine, S.R.; Demel, S.; Eckerle, B.; Katz, B.; et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke 2015, 46, 2529–2533. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, G. Safety of glycoprotein IIb-IIIa inhibitors used in stroke-related treatment: A systematic review and meta-analysis. Clin. Appl. Thromb./Hemost. 2020, 26, 1076029620942594. [Google Scholar] [CrossRef]
- Luo, L.; Lin, J.; Deng, Y.; Li, Z.; Yuan, Y.; Zhang, W. Treatment of progressive ischemic stroke with low-dose eptifibatide: A retrospective case-control study. Exp. Ther. Med. 2022, 25, 22. [Google Scholar] [CrossRef]
- Berthelsen, R.E.; Ostrowski, S.R.; Bestle, M.H.; Johansson, P.I. Co-administration of iloprost and eptifibatide in septic shock (CO-ILEPSS)-a randomised, controlled, double-blind investigator-initiated trial investigating safety and efficacy. Crit. Care 2019, 23, 301. [Google Scholar] [CrossRef]
- Cheddad El Aouni, M.; Magro, E.; Abdelrady, M.; Nonent, M.; Gentric, J.C.; Ognard, J. Safety and Efficacy of Cangrelor Among Three antiplatelet regimens during stent-assisted endovascular treatment of unruptured intracranial aneurysm: A single-center retrospective study. Front. Neurol. 2022, 13, 727026. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Liu, H. Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: A systematic review and meta-analysis of prospective studies. J. Thromb. Thrombolysis 2022, 53, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ma, K.; Xiang, R.; Han, B.; Chang, J.; Zuo, Z.; Luo, Y.; Mao, M. Efficacy and safety of a bridging strategy that uses intravenous platelet glycoprotein receptor inhibitors for patients undergoing surgery after coronary stent implantation: A meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, A.; Lin, Y.; Dannenberg, L.; Parco, C.; Schulze, V.; Brockmeyer, M.; Jung, C.; Heinen, Y.; Perings, S.; Zeymer, U.; et al. Routine glycoprotein IIb/IIIa inhibitor therapy in st-segment elevation myocardial infarction: A meta-analysis. Can. J. Cardiol. 2019, 35, 1576–1588. [Google Scholar] [CrossRef]
- Saleiro, C.; Teixeira, R.; De Campos, D.; Lopes, J.; Oliveiros, B.; Costa, M.; Gonçalves, L. Glycoprotein IIb/IIIa Inhibitors for Cardiogenic Shock Complicating Acute Myocardial Infarction: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Intensive Care 2020, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and treatment of acute coronary syndromes: A review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Singh, A.; Museedi, A.S.; Grossman, S.A. Acute Coronary Syndrome. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators Inhibition of Platelet Glycoprotein IIb/IIIa with Eptifibatide in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 1998, 339, 436–443. [Google Scholar] [CrossRef]
- Gibson, C.M.; Kirtane, A.J.; Murphy, S.A.; Rohrbeck, S.; Menon, V.; Lins, J.; Kazziha, S.; Rokos, I.; Shammas, N.W.; Palabrica, T.M.; et al. Early initiation of eptifibatide in the emergency department before primary percutaneous coronary intervention for st-segment elevation myocardial infarction: Results of the time to integrilin therapy in acute myocardial infarction (TITAN)-TIMI 34 trial. Am. Heart J. 2006, 152, 668–675. [Google Scholar] [CrossRef]
- Ellis, S.G.; Tendera, M.; de Belder, M.A.; van Boven, A.J.; Widimsky, P.; Janssens, L.; Andersen, H.R.; Betriu, A.; Savonitto, S.; Adamus, J.; et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N. Engl. J. Med. 2008, 358, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- ten Berg, J.M.; van ’t Hof, A.W.J.; Dill, T.; Heestermans, T.; van Werkum, J.W.; Mosterd, A.; van Houwelingen, G.; Koopmans, P.C.; Stella, P.R.; Boersma, E.; et al. Effect of early, pre-hospital initiation of high bolus dose tirofiban in patients with ST-segment elevation myocardial infarction on short- and long-term clinical outcome. J. Am. Coll. Cardiol. 2010, 55, 2446–2455. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Kanic, V.; Kompara, G.; Suran, D. GP IIb/IIIa receptor inhibitors in mechanically ventilated patients with cardiogenic shock due to myocardial infarction in the era of potent P2Y12 receptor antagonists. J. Clin. Med. 2022, 11, 7426. [Google Scholar] [CrossRef]
- Myrda, K.; Gąsior, M.; Dudek, D.; Nawrotek, B.; Niedziela, J.; Wojakowski, W.; Gierlotka, M.; Grygier, M.; Stępińska, J.; Witkowski, A.; et al. One-year outcome of glycoprotein IIb/IIIa inhibitor therapy in patients with myocardial infarction-related cardiogenic shock. J. Clin. Med. 2021, 10, 5059. [Google Scholar] [CrossRef] [PubMed]
- Hoole, S.P.; Bambrough, P. Recent advances in percutaneous coronary intervention. Heart 2020, 106, 1380–1386. [Google Scholar] [CrossRef]
- Ahmad, M.; Mehta, P.; Reddivari, A.K.R.; Mungee, S. Percutaneous coronary intervention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: Executive summary. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Impact-II Investigators. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Lancet 1997, 349, 1422–1428. [Google Scholar] [CrossRef]
- Giugliano, R.P.; White, J.A.; Bode, C.; Armstrong, P.W.; Montalescot, G.; Lewis, B.S.; van ’t Hof, A.; Berdan, L.G.; Lee, K.L.; Strony, J.T.; et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N. Engl. J. Med. 2009, 360, 2176–2190. [Google Scholar] [CrossRef]
- Stone, G.W.; McLaurin, B.T.; Cox, D.A.; Bertrand, M.E.; Lincoff, A.M.; Moses, J.W.; White, H.D.; Pocock, S.J.; Ware, J.H.; Feit, F.; et al. Bivalirudin for patients with acute coronary syndromes. N. Engl. J. Med. 2006, 355, 2203–2216. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Rubboli, A.; Patti, G. What is the role for glycoprotein IIb/IIIa inhibitor use in the catheterization laboratory in the current era? Curr. Vasc. Pharmacol. 2018, 16, 451–458. [Google Scholar] [CrossRef]
- Friedland, S.; Eisenberg, M.J.; Shimony, A. Meta-analysis of randomized controlled trials of intracoronary versus intravenous administration of glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention for acute coronary syndrome. Am. J. Cardiol. 2011, 108, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, A.; Shemirani, H.; Amirpour, A.; Kermani-Alghoraishi, M. The effect of intracoronary versus intralesional injection of eptifibatide on myocardial perfusion outcomes during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction; a randomized clinical trial study. ARYA Atheroscler. 2019, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyl, J.S.; Newsome, A.S.; Hollis, I.B. Perioperative bridging with glycoprotein IIb/IIIa Inhibitors versus cangrelor: Balancing efficacy and safety. Ann. Pharmacother. 2019, 53, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Dargham, B.B.; Baskar, A.; Tejani, I.; Cui, Z.; Chauhan, S.; Sum-Ping, J.; Weideman, R.A.; Banerjee, S. Intravenous antiplatelet therapy bridging in patients undergoing cardiac or non-cardiac surgery following percutaneous coronary intervention. Cardiovasc. Revascularization Med. 2019, 20, 805–811. [Google Scholar] [CrossRef]
- Yun, A.N.; Toyoda, A.Y.; Solomon, E.J.; Roberts, R.J.; Ji, C.S. Safety and Efficacy of Periprocedural Bridging With Cangrelor Versus Eptifibatide. J. Cardiovasc. Pharm. 2022, 79, 383–389. [Google Scholar] [CrossRef]
- Rana, A.; Yu, S.; Reid-Herrera, S.; Kamen, S.; Hunter, K.; Shaikh, H.; Jovin, T.; Thon, O.R.; Patel, P.; Siegler, J.E.; et al. Eptifibatide use in ischemic stroke patients undergoing endovascular thrombectomy: A matched cohort analysis. Front. Neurol 2022, 13, 939215. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Sun, X.; Cheng, H.; Burgin, W.S.; Luo, W.; Jia, W.; Liu, Y.; He, W.; Geng, X.; Zhu, L.; et al. Combined approach to eptifibatide and thrombectomy in acute ischemic stroke because of large vessel occlusion: A matched-control analysis. Stroke 2022, 53, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Osteraas, N.D.; Crowley, R.W.; Panos, N.; Dafer, R.M. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 105021. [Google Scholar] [CrossRef]
- Horev, A.; Zlotnik, Y.; Borodetsky, V.; Biederko, R.; Star, M.; Zvenigorodsky, V.; Shelef, I.; Ifergane, G. Adjunctive treatment with low dose intra-arterial eptifibatide and intravenous aspirin during carotid stenting: A case series. J. Clin. Neurosci. 2021, 84, 29–32. [Google Scholar] [CrossRef]
- Jacobi, J. The pathophysiology of sepsis-2021 update: Part 1, immunology and coagulopathy leading to endothelial injury. Am. J. Health-Syst. Pharm. 2022, 79, 329–337. [Google Scholar] [CrossRef]
- Agrawal, H.; Aggarwal, K.; Littrell, R.; Velagapudi, P.; Turagam, M.K.; Mittal, M.; Alpert, M.A. Pharmacological and non pharmacological strategies in the management of coronary artery disease and chronic kidney disease. Curr. Cardiol. Rev. 2015, 11, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, M.; Mendoza, D.; Pettei, T.; Grayver, E. Eptifibatide and cirrhosis: Rethinking GPIIb-IIIa inhibitors for acute coronary syndrome in the setting of liver dysfunction. Cardiol. Res. 2014, 5, 191–194. [Google Scholar] [CrossRef]
- Boersma, E.; Harrington, R.A.; Moliterno, D.J.; White, H.; Théroux, P.; Van de Werf, F.; de Torbal, A.; Armstrong, P.W.; Wallentin, L.C.; Wilcox, R.G.; et al. Platelet glycoprotein IIb/IIIa Inhibitors in acute coronary syndromes: A meta-analysis of all major randomised clinical trials. Lancet 2002, 359, 189–198. [Google Scholar] [CrossRef]
- Alamin, M.A.; Al-Mashdali, A.; Al Kindi, D.I.; Elshaikh, E.A.; Othman, F. Eptifibatide-induced acute profound thrombocytopenia: A case report. Medicine 2022, 101, e28243. [Google Scholar] [CrossRef]
- Kamar, K.; MacDougall, K.; Alsheikh, M.; Parylo, S.; Skaradinskiy, Y. A rare case of eptifibatide-induced thrombocytopenia. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Gheith, Z.; Kilani, A.; Nguyen, T. Eptifibatide-induced severe thrombocytopenia after ST-elevation myocardial infarction (STEMI): A case report. Cureus 2022, 14, e29549. [Google Scholar] [CrossRef]
- Byrd, G.; Custovic, S.; Byrd, D.; Ingrassia Miano, D.; Bathla, J.; Attallah, A. Acute profound thrombocytopenia induced by eptifibatide causing diffuse alveolar hemorrhage. Case Rep. Crit. Care 2021, 2021, e8817067. [Google Scholar] [CrossRef]
- Golden, T.; Ghazala, S.; Wadeea, R.; Junna, S. Abciximab-induced acute profound thrombocytopenia postpercutaneous coronary intervention. Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Sharma, A.; Ferguson, C.; Bainey, K.R. Thrombocytopenia in acute coronary syndromes: Etiologies and proposed management. Can. J. Cardiol. 2015, 31, 809–811. [Google Scholar] [CrossRef]
- Gao, C.; Boylan, B.; Bougie, D.; Gill, J.C.; Birenbaum, J.; Newman, D.K.; Aster, R.H.; Newman, P.J. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcgammaRIIa and the integrin Beta3 cytoplasmic domain. J. Clin. Investig. 2009, 119, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R. Drug-induced immune thrombocytopenia: Incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology 2014, 30, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Hashmi, S.; Chaus, A.; Hertsberg, A.; Ehrenpreis, E.D. Complications and management of eptifibatide-induced thrombocytopenia. Ann. Pharmacother. 2021, 55, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, H.; Vahabi, Z.; Shojaeifard, M.; Mirzadeh, F.S. Diffuse alveolar hemorrhage; an under-diagnosed and rare complication of glycoprotein IIb/IIIa inhibitors. J. Cardiovasc. Thorac. Res. 2022, 14, 201–204. [Google Scholar] [CrossRef] [PubMed]

| Drug | Eptifibatide | Prasugrel | Ticagrelor | Cangrelor | Abciximab |
|---|---|---|---|---|---|
| Indication | acute coronary syndrome (ACS) | ACS | ACS | ACS | ACS |
| Main Contraindications | active bleeding, thrombocytopenia, severe, uncontrolled hypertension, major surgery or trauma within the prior six weeks, renal failure | active bleeding, cerebrovascular insult (CVI), transient ischemic attack (TIA), severe liver failure | active bleeding, concomitant use of ticagrelor with strong CYP3A4 inhibitors, severe liver failure | active bleeding, CVI, TIA | active bleeding, thrombocytopenia, vasculitis, severe uncontrolled hypertension, intracranial tumour, major surgery or trauma in the last six weeks |
| Drug Type and Mechanisms | glycoprotein IIb/IIIa receptor inhibitor | thienopyridine prodrug, an irreversible antagonist of the ADP P2Y12-receptor | cyclopentyl-triazolo-pyrimidine drug, direct-acting P2Y12-receptor antagonist | adenosine triphosphateanalogue, reversibly binds to P2Y12-receptor | glycoprotein IIb/IIIa receptor inhibitor |
| Time to Peak Action | 15 min | 30 min | 1.5 h | 2 min | 30 min |
| Half-Life | 2 h | 7 h | 7 h | 3–6 min | 30 min |
| Dose Administration | a double bolus of 180 μg/kg i.v. in 10 min, followed by an infusion of 2.0 μg/kg/min for up to 18 h | loading dose 60 mg, maintenance dose 10 mg/day | loading dose 180 mg, maintenance dose 90 mg/12 h | bolus 30 μg/kg injection then 4 μg/kg/min infusion | bolus 0.25 mg/kg injection then 0.125 μg/kg/min infusion for 12 h |
| Route/Dosing Interval | intravenous | oral, once daily | oral, twice daily | intravenous | intravenous |
| Major Side Effects | bleeding, thrombocytopenia | bleeding, thrombotic thrombocytopenic purpura, headache, dizziness | bleeding, dyspnea, hyperuricemia | bleeding, dyspnea | bleeding, chest pain, hypotension, injection site pain, abdominal pain, nausea, vomiting |
| Excretion | excreted in urine | ~70% of the dose is excreted in urine and ~30% in the faeces, as inactive metabolites | metabolized in the liver | ~60% of the dose is excreted in urine and ~35% in faeces | excreted in urine |
| Authors | Research Design | Number of Studies and Patients | Results | Main Findings |
|---|---|---|---|---|
| Liu et al., 2022 [74] | Analysis of studies using tirofiban and eptifibatide in patients with acute ischemic stroke (AIS). Use of the modified Rankin scale (mRS) to evaluate favorable and functional outcomes. | Twelve studies (two randomized control trials and ten prospective cohort studies) with 2926 patients. | Treatment with tirofiban or eptifibatide might increase mortality (relative risk (RR) = 0.84, 95% confidence interval (CI) 0.71–0.99, P = 0.121) but had no effects on the favorable outcome (RR = 1.09, 95% CI 0.89–1.35, p = 0.411), functional outcome (RR = 1.12, 95% CI 0.98–1.28, p = 0.010), and last available NIHSS (WMD = −2.32, 95% CI −5.14 to 0.50, p = 0.106). | Adding tirofiban or eptifibatide to thrombolysis/thrombectomy was not significantly associated with a favorable outcome (mRS = 0–1) nor a functional outcome (mRS = 0–2) in patients with AIS at 3 months but might be associated with higher mortality, possibly due to fatal intracranial hemorrhage. |
| Wu et al., 2022 [75] | The use of glycoprotein IIb/IIIa inhibitors (GPI) in patients requiring surgery after coronary stent placement. The primary outcome was the success rate of no major adverse cardiovascular events, and the secondary outcome the success rate of no reoperations for bleeding management. | Ten studies with 382 patients (four studies with eptifibatide with 167 patients). | For the primary endpoint, the success rate was 97.7% (95% CI 94.4–98.0%) for GPI and 95.8% (95% CI 90.4–99.4%) for eptifibatide. For secondary endpoints, the success rate was 98.0% (95% CI 94.8–99.9%) for GPI and 95.3% (95% CI 88.5–99.4%) for eptifibatide. | GPI are safe and effective as a bridging strategy for patients that require surgery following recent stent implantation. |
| Zhu et al., 2020 [70] | Analysis of safety of GPI in AIS. The two main outcomes analyzed were RR of death and 90-day intracerebral hemorrhage (ICH). | Twenty studies with 3700 patients | The RR values of symptomatic ICH for abciximab and eptifibatide were 4.26 (1.89, 9.59) and 0.17 (0.04, 0.69). | Eptifibatide can be considered safe in low doses and if used in suitable patients. Its use showed no statistically significant effect on any ICH and death. The incidence of symptomatic ICH was reduced after the administration of eptifibatide. |
| Karathanos et al., 2019 [76] | The use of GPI in selected patients with ST-elevation myocardial infarction (STEMI). | Twenty-one studies with 8585 patients. | A significant reduction in all-cause mortality at 30 days (2.4% [GPI] vs. 3.2%; risk ratio RR = 0.72; p = 0.01) and 6 months (3.7% vs. 4.8%; RR = 0.76; p = 0.02), and a reduction in recurrent myocardial infarction (1.1% vs. 2.1%; RR = 0.55; p = 0.0006). | GPI administration in STEMI decreased mortality, although that was mostly shown in pre-prasugrel/ticagrelor studies. Trials utilizing modern STEMI treatment are required to validate these results. |
| Saleiro et al., 2020 [77] | Use of GPIs as an adjunctive therapy in myocardiac infarct, complicated by cardiogenic shock. Analyzed outcomes were mortality, angiographic success, and bleeding events. | Seven studies with 1216 patients. | A 45% relative reduction in the odds of death at 30 days (pooled OR 0.55; 95% CI 0.35–0.85; I2 = 57%; p = 0.007) and a 49% reduction in the odds of death at 1 year (pooled OR 0.51; 95% CI 0.32–0.82; I2 = 58%; p = 0.005). Increased probability of achieving better flow (pooled OR, 2.05; 95% CI 1.37–3.05; I2 = 37%, p = 0.0004). Major bleeding events were not increased with GPI therapy (pooled OR, 1.0; 95% CI 0.55–1.83; I2 = 1%, p = 0.99). | GPIs are efficient as adjunct therapy and are associated with better short-term and long-term survival. They also didn’t increase the risk of bleeding. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonin, G.; Klen, J. Eptifibatide, an Older Therapeutic Peptide with New Indications: From Clinical Pharmacology to Everyday Clinical Practice. Int. J. Mol. Sci. 2023, 24, 5446. https://doi.org/10.3390/ijms24065446
Tonin G, Klen J. Eptifibatide, an Older Therapeutic Peptide with New Indications: From Clinical Pharmacology to Everyday Clinical Practice. International Journal of Molecular Sciences. 2023; 24(6):5446. https://doi.org/10.3390/ijms24065446
Chicago/Turabian StyleTonin, Gašper, and Jasna Klen. 2023. "Eptifibatide, an Older Therapeutic Peptide with New Indications: From Clinical Pharmacology to Everyday Clinical Practice" International Journal of Molecular Sciences 24, no. 6: 5446. https://doi.org/10.3390/ijms24065446
APA StyleTonin, G., & Klen, J. (2023). Eptifibatide, an Older Therapeutic Peptide with New Indications: From Clinical Pharmacology to Everyday Clinical Practice. International Journal of Molecular Sciences, 24(6), 5446. https://doi.org/10.3390/ijms24065446







