HDL Function and Size in Patients with On-Target LDL Plasma Levels and a First-Onset ACS
Abstract
1. Introduction
2. Results
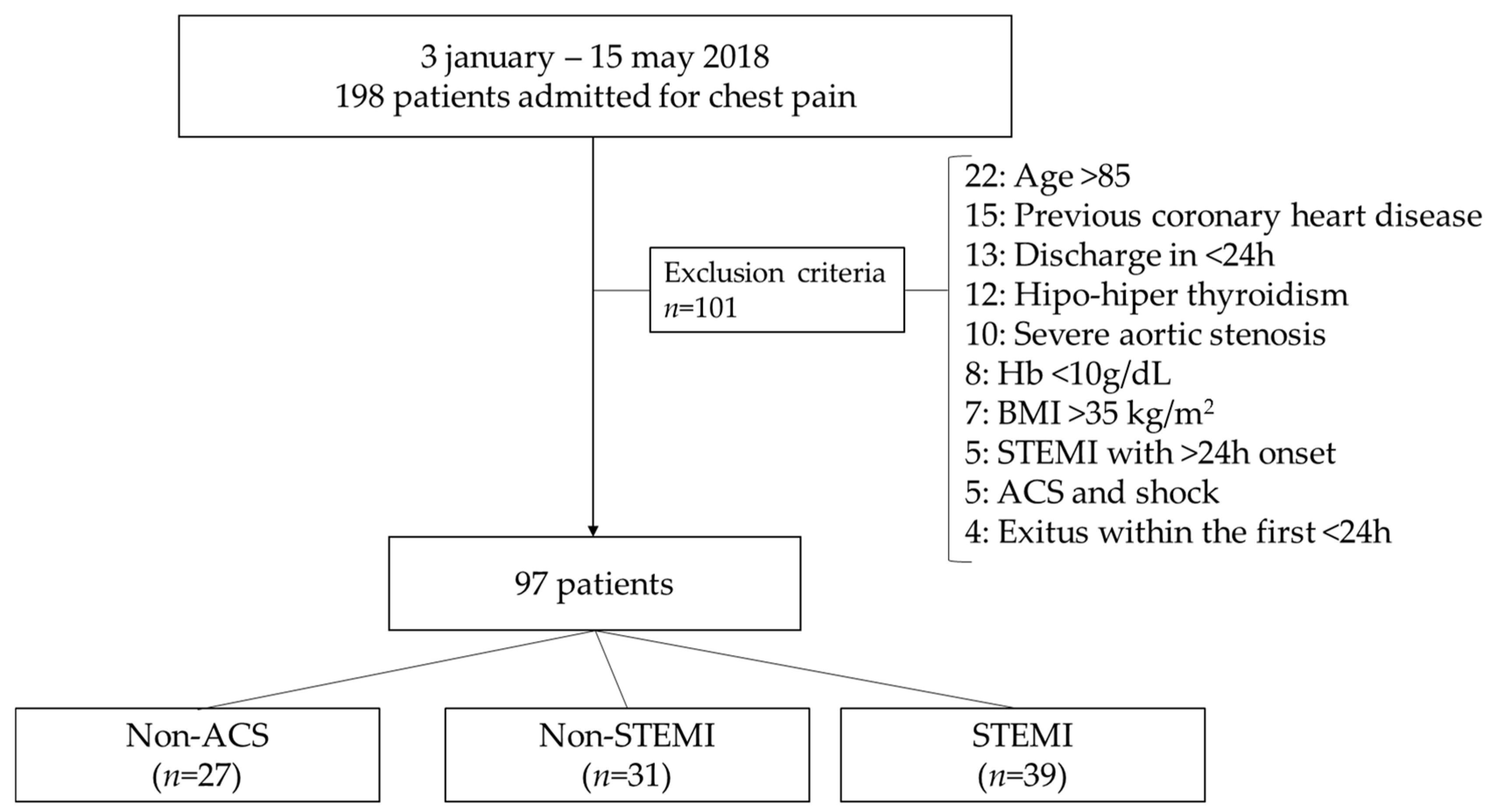
2.1. Patient Characteristics
2.2. Lipoprotein Particle Number and Size
2.3. Assays for Lipoprotein Functionality
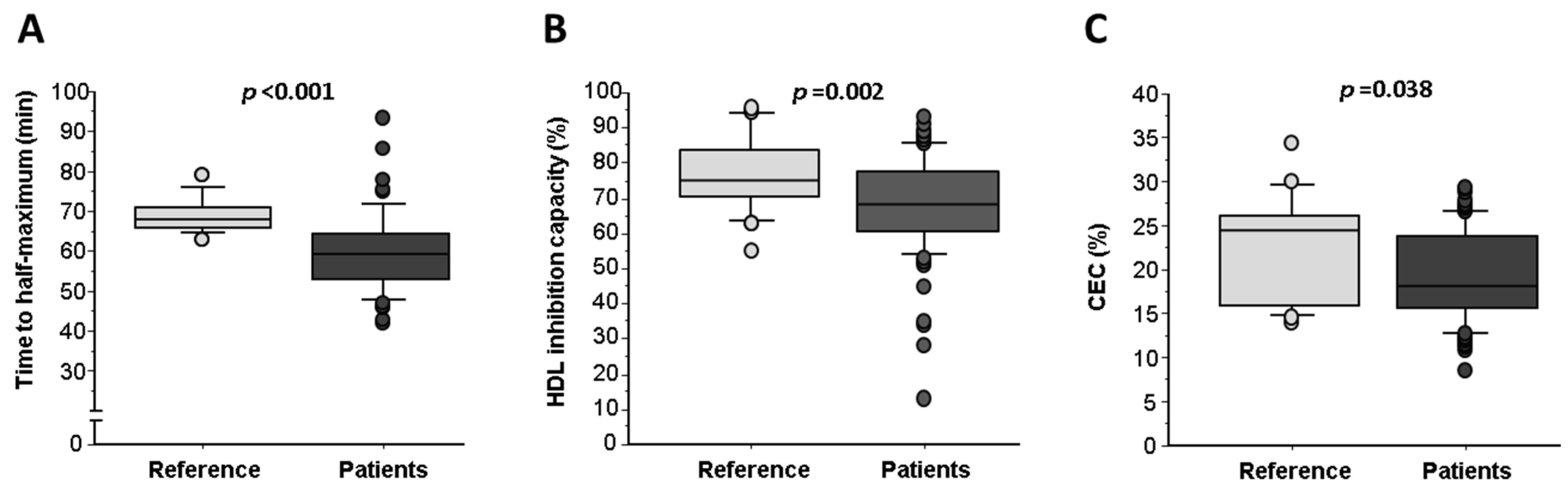
2.4. HDL Oxidation Inversely Correlates with Cholesterol Efflux Capacity (CEC)
3. Discussion
4. Materials and Methods
4.1. Clinical Diagnosis of Chest Pain Categories
4.2. Biochemical and Laboratory Parameters
4.3. LDL and HDL Sample Preparation and Purity Control
4.4. Conjugated Diene Assay
4.5. HDL Antioxidant Potential
4.6. HDL Cholesterol Efflux Capacity Assay
4.7. Lipoprotein Particle Number and Size Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef]
- Claessen, B.E.; Guedeney, P.; Gibson, C.M.; Angiolillo, D.J.; Cao, D.; Lepor, N.; Mehran, R. Lipid Management in Patients Presenting With Acute Coronary Syndromes: A Review. J. Am. Heart Assoc. 2020, 9, e018897. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Bittner, V. LDL Lowering After Acute Coronary Syndrome: Is Lower Better? Curr. Treat. Options Cardiovasc. Med. 2013, 15, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Moreno-Arribas, J.; Bartomeu-Gonzalez, V.; Agudo, P.; Miralles, B.; Masiá, M.D.; López-Palop, R.; Bertomeu-Martínez, V. Low Levels of High-Density Lipoproteins Cholesterol Are Independently Associated With Acute Coronary Heart Disease in Patients Hospitalized for Chest Pain. Rev. Esp. Cardiol. 2012, 65, 319–325. [Google Scholar] [CrossRef]
- Weverling-Rijnsburger, A.W.; Jonkers, I.J.; van Exel, E.; Gussekloo, J.; Westendorp, R.G. High-density vs. low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch. Intern. Med. 2003, 163, 1549–1554. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- de Goma, E.M.; Leeper, N.J.; Heidenreich, P.A. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J. Am. Coll. Cardiol. 2008, 51, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.; Bittner, V.; Fruchart, J.-C.; Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-melander, M.; Frikke-schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Joy, T.; Hegele, R.A. Is raising HDL a futile strategy for atheroprotection? Nat. Rev. Drug Discov. 2008, 7, 143–155. [Google Scholar] [CrossRef]
- Barter, P. HDL-C: Role as a risk modifier. Atheroscler. Suppl. 2011, 12, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: Inflammatory resolution versus thrombotic chaos. Ann. N. Y. Acad. Sci. 2012, 1254, 18–32. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Casaní, L.; Muñoz-García, N.; Joan-Babot, O.; Peña, E.; Aržanauskaitė, M.; Gutierrez, M.; Mendieta, G.; Padró, T.; Badimon, L.; et al. HDL (High-Density Lipoprotein) Remodeling and Magnetic Resonance Imaging-Assessed Atherosclerotic Plaque Burden: Study in a Preclinical Experimental Model. Arter. Thromb. Vasc. Biol. 2020, 40, 2481–2493. [Google Scholar] [CrossRef]
- Urbina, E.M.; McCoy, C.E.; Gao, Z.; Khoury, P.R.; Shah, A.S.; Dolan, L.M.; Kimball, T.R. Lipoprotein particle number and size predict vascular structure and function better than traditional lipids in adolescents and young adults. J. Clin. Lipidol. 2017, 11, 1023–1031. [Google Scholar] [CrossRef]
- Mora, S.; Otvos, J.D.; Rosenson, R.S.; Pradhan, A.; Buring, J.E.; Ridker, P.M. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 2010, 59, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibanez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Reiner, Z. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients: A clinical update. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 799–807. [Google Scholar] [CrossRef]
- Ozaki, Y.; Tanaka, A.; Nishiguchi, T.; Komukai, K.; Taruya, A.; Satogami, K.; Kashiwagi, M.; Kuroi, A.; Matsuo, Y.; Ino, Y.; et al. High-density lipoprotein cholesterol as a therapeutic target for residual risk in patients with acute coronary syndrome. PLoS ONE 2018, 13, e0200383. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, X.; Zhou, Q.; Zhang, Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 212. [Google Scholar] [CrossRef]
- Badimon, J.J.; Badimon, L.; Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 1990, 85, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.; Latorre, G.; Sentí, M.; Marrugat, J. Función antioxidante de las lipoproteínas de alta densidad: Un nuevo paradigma en la arteriosclerosis. The antioxidant function of high density lipoproteins: A new paradigm in atherosclerosis. Rev. Esp. Cardiol. 2004, 57, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef]
- Du, X.M.; Kim, M.J.; Hou, L.; Le Goff, W.; Chapman, M.J.; Van Eck, M.; Curtiss, L.K.; Burnett, J.R.; Cartland, S.P.; Quinn, C.M.; et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015, 116, 1133–1142. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arter. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, Y.; Li, S.; Cui, C.J.; Zhu, C.G.; Guo, Y.L.; Wu, N.Q.; Xu, R.X.; Liu, G.; Dong, Q.; et al. Large HDL Subfraction But Not HDL-C Is Closely Linked With Risk Factors, Coronary Severity and Outcomes in a Cohort of Nontreated Patients With Stable Coronary Artery Disease: A Prospective Observational Study. Medicine 2016, 95, e2600. [Google Scholar] [CrossRef]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. HDL Particle Subspecies and Their Association With Incident Type 2 Diabetes: The PREVEND Study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Ruidavets, J.B.; Genoux, A.; Ingueneau, C.; Najib, S.; Ferrières, J.; Perret, B.; Martinez, L.O. Serum level of HDL particles are independently associated with long-term prognosis in patients with coronary artery disease: The GENES study. Sci. Rep. 2020, 10, 8138. [Google Scholar] [CrossRef]
- Tanaka, S.; Diallo, D.; Delbosc, S.; Genève, C.; Zappella, N.; Yong-Sang, J.; Patche, J.; Harrois, A.; Hamada, S.; Denamur, E.; et al. High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann. Intensive. Care. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mao, L.; Chen, J.; Zhang, T.; Weng, S.; Guo, X.; Kuang, J.; Yu, B.; Peng, D. High-sensitivity CRP may be a marker of HDL dysfunction and remodeling in patients with acute coronary syndrome. Sci. Rep. 2021, 11, 11444. [Google Scholar] [CrossRef]
- Weissglas-Volkov, D.; Pajukanta, P. Genetic causes of high and low serum HDL-cholesterol. J. Lipid Res. 2010, 51, 2032–2057. [Google Scholar] [CrossRef]
- Hafiane, A.; Genest, J. High density lipoproteins: Measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015, 3, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 2014, 371, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Martínez Rey-Rañal, E.; Moreno, M.J.; Escribano, D.; Moreno-Arribas, J.; Quintanilla, M.A.; Zuazola, P.; Núñez, J.; Bertomeu-González, V. Predictive Value of Pro-BNP for Heart Failure Readmission after an Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 and SCORE2-op (No Date) European Society of Cardiology. 2021. Available online: https://www.escardio.org/Education/Practice-Tools/CVD-prevention-toolbox/SCORE-Risk-Charts (accessed on 28 November 2022).
- Hard Coronary Heart Disease (10-Year Risk). 2001. Available online: https://www.framinghamheartstudy.org/fhs-risk-functions/hard-coronary-heart-disease-10-year-risk/ (accessed on 28 November 2022).
- Havel, R.J.; Eder, H.A.; Bradgon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Franco, E.; Pérez, A.; Ribas, V.; Sánchez-Hernández, J.A.; Blanco-Vaca, F.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Standardization of a method to evaluate the antioxidant capacity of high-density lipoproteins. Int. J. Biomed. Sci. 2009, 5, 402–410. [Google Scholar] [PubMed]
- Esterbauer, H.; Striegl, G. Continuous Monitoring of in Vitro Oxidation of Human Low Density Lipoprotein. Free. Radic. Biol. Med. 1989, 6, 67–75. [Google Scholar]
- Valkonen, M.; Kuusi, T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J. Lipid Res. 1997, 38, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Yeum, K.J.; Russell, R.M.; Krinsky, N.I. A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free. Radic. Biol. Med. 2001, 31, 1043–1050. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef]
- Mallol, R.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Cañellas, N.; Brezmes, J.; Plana, N.; Masana, L. Surface fitting of 2D diffusion-edited 1H NMR spectroscopy data for the characterization of human plasma lipoproteins. Metabolomics 2011, 7, 572–582. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef] [PubMed]

| ACS (n = 70) | p-Value | |||
|---|---|---|---|---|
| Non-ACS (n = 27) | NSTEMI (n = 31) | STEMI (n = 39) | ||
| Age (years, mean ± SEM) | 65.8 ± 2.6 | 70.3 ± 1.9 | 60.0 ± 1.7 *‡ | 0.001 |
| Male/Female, n | 14/13 | 26/5 | 32/7 | 0.007 |
| Weight (Kg) | 84.1 ± 5.1 | 79.5 ± 2.7 | 85.2 ± 2.5 | 0.375 |
| BMI (Kg/m2, mean ± SEM) | 29.6 ± 1.4 | 27.7 ± 0.6 | 28.7 ± 0.7 | 0.354 |
| Risk Factor, n (%) | ||||
| Diabetes Mellitus | 8 (30%) | 10 (32%) | 6 (15%) | 0.210 |
| Hypertension | 15 (55%) | 25 (81%) | 15 (38%) | 0.002 |
| Obesity (>30%) | 8 (30%) | 7 (23%) | 13 (33%) | 0.517 |
| Dyslipidaemia | 11 (41%) | 17 (55%) | 11 (28%) | 0.078 |
| Smoking habits | 4 (15%) | 5 (16%) | 22 (56%) | <0.001 |
| COPD | 0 (0%) | 3 (10%) | 2 (5%) | 0.251 |
| Lipid profile | ||||
| Total cholesterol (mg/dL) | 182.2 ± 8.6 | 167.5 ± 6.6 | 169.1 ± 5.6 | 0.292 |
| Triglycerides (mg/dL) | 140.3 ± 11.1 | 154.2 ± 21.4 | 143.6 ± 11.7 | 0.811 |
| LDL-C (mg/dL) | 102.4 ± 8.4 | 95.8 ± 5.4 | 101.8 ± 5.2 | 0.715 |
| HDL-C (mg/dL) | 52.0 ± 2.8 | 44.3 ± 2.4 * | 39.4 ± 1.7 * | <0.001 |
| ApoA-I (mg/ml) | 1.8 ± 0.1 | 1.5 ± 0.1 * | 1.4 ± 0.1 * | <0.001 |
| HDL-C/LDL-C | 0.61 ± 0.07 | 0.50 ± 0.04 | 0.43 ± 0.03 * | 0.021 |
| Triglycerides/HDL-C | 3.1 ± 0.3 | 4.2 ± 0.8 | 4.1 ± 0.5 | 0.395 |
| Non-HDL-C (mg/dL) | 130.2 ± 8.8 | 123.1 ± 6.7 | 129.6 ± 5.7 | 0.734 |
| Lp(a) (mg/dL) | 27.3 ± 5.9 | 35.1 ± 6.1 | 49.7 ± 7.3 * | 0.058 |
| Cardiac parameters | ||||
| BNP (pg/mL) | 401 ± 220 | 1600 ± 556 | 2082 ± 594 * | 0.081 |
| Troponin-I (ng/L) | 720 ± 484 | 9355 ± 3208 | 83,307 ± 17,824 *‡ | <0.001 |
| LVEF (%) | 59.4 ± 1.5 | 57.2 ± 1.5 | 51.7 ± 1.9 *‡ | 0.007 |
| Hepatic and renal parameters | ||||
| GOT (UI/l) | 24.2 ± 2.8 | 40.2 ± 8.1 | 66.2 ± 11.4 *‡ | 0.006 |
| GPT (UI/l) | 24.7 ± 3.9 | 31.6 ± 5.1 | 40.1 ± 4.0 * | 0.050 |
| Creatinine (mg/dL) | 0.8 ± 0.06 | 1.0 ± 0.05 * | 0.9 ± 0.04 | 0.060 |
| Medication at admission, n (%) | ||||
| Acetylsalicylic acid | 7 (26%) | 7 (23%) | 2 (5%) | 0.044 |
| Clopidogrel | 2 (7%) | 0 (0%) | 1 (3%) | 0.259 |
| Angiotensin II receptor blocker | 12 (44%) | 14 (45%) | 8 (20%) | 0.048 |
| ACE inhibitor | 2 (7%) | 4 (13%) | 2 (5%) | 0.493 |
| Diuretics | 7 (26%) | 6 (19%) | 4 (10%) | 0.245 |
| Calcium antagonists | 2 (7%) | 8 (26%) | 2 (5%) | 0.022 |
| Beta-Blockers | 8 (30%) | 9 (29%) | 1 (3%) | 0.004 |
| Omeprazole | 6 (22%) | 11 (35%) | 3 (8%) | 0.016 |
| Statins | 8 (30%) | 11 (35%) | 9 (23%) | 0.521 |
| Fibrates | 1 (4%) | 1 (3%) | 2 (5%) | 0.916 |
| OADs | 4 (15%) | 7 (23%) | 3 (8%) | 0.212 |
| Metformin | 5 (16%) | 4 (13%) | 2 (5%) | 0.228 |
| DPP-4 inhibitors | 2 (7%) | 2 (6%) | 1 (3%) | 0.631 |
| Others, n (%) | ||||
| Coronary stent | 0 (0%) | 7 (23%) | 12 (31%) | 0.007 |
| Cathetherization/Revascularization | 8 (30%) | 15 (48%) | 32 (82%) | <0.001 |
| Risk Scores (%) | ||||
| Framingham risk score | 10.4 ± 1.4 | 17.9 ± 1.2 | 12.1 ± 1.1 | <0.001 |
| European Heart Score | 2.7 ± 0.3 | 4.6 ± 0.3 | 3.1 ± 0.3 | <0.001 |
| ACS (n = 69) | p-Value | |||
|---|---|---|---|---|
| Non-ACS (n = 25) | NSTEMI (n = 30) | STEMI (n = 39) | ||
| Triglyceride content in lipoprotein particles (mg/dL) | ||||
| VLDL-TG | 85.6 ± 6.9 | 77.2 ± 5.6 | 87.1 ± 6.5 | 0.504 |
| IDL-TG | 11.4 ± 0.3 | 11.2 ± 0.5 | 12.1 ± 0.6 | 0.428 |
| LDL-TG | 14.1 ± 0.9 | 13.4 ± 0.7 | 15.0 ± 0.7 | 0.312 |
| HDL-TG | 13.9 ± 0.5 | 12.2 ± 0.5 | 12.3 ± 0.7 | 0.148 |
| VLDL particle number (nmol/L) | ||||
| Small VLDL-P | 54.7 ± 4.7 | 48.9 ± 3.8 | 54.8 ± 4.3 | 0.549 |
| Medium VLDL-P | 5.7 ± 0.4 | 5.6 ± 0.3 | 6.8 ± 0.4 ‡ | 0.066 |
| Large VLDL-P | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 0.488 |
| Total VLDL-P | 61.9 ± 5.1 | 55.9 ± 4.2 | 63.1 ± 4.7 | 0.513 |
| LDL particle number (nmol/L) | ||||
| Small LDL-P | 691.9 ± 28.6 | 642.8 ± 19.6 | 677.5 ± 24.7 | 0.387 |
| Medium LDL-P | 367.3 ± 32.4 | 312.8 ± 17.1 | 336.7 ± 14.9 | 0.225 |
| Large LDL-P | 194.3 ± 9.9 | 184.9 ± 4.8 | 191.3 ± 4.9 | 0.594 |
| Total LDL-P | 1253.5 ± 62.7 | 1140.5 ± 35.2 | 1205.5 ± 40.6 | 0.257 |
| HDL particle number (nmol/L) | ||||
| Small HDL-P | 18.3 ± 0.8 | 14.8 ± 0.7 * | 13.8 ± 0.6 * | <0.001 |
| Medium HDL-P | 10.6 ± 0.4 | 10.7 ± 0.3 | 10.0 ± 0.2 | 0.171 |
| Large HDL-P | 0.31 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.501 |
| Total HDL-P | 29.2 ± 1.0 | 25.8 ± 0.8 * | 24.1 ± 0.6 * | <0.001 |
| Average particle diameter (nm) | ||||
| VLDL | 42.1 ± 0.05 | 42.1 ± 0.04 | 42.2 ± 0.04 * | 0.119 |
| LDL | 21.0 ± 0.06 | 21.0 ± 0.04 | 21.0 ± 0.04 | 0.913 |
| HDL | 8.3 ± 0.02 | 8.4 ± 0.02 * | 8.4 ± 0.02 * | 0.004 |
| Patients (n = 97) | p-Value | |||
|---|---|---|---|---|
| Reference Group (n = 31) | Non-ACS (n = 27) | ACS (n = 70) | ||
| HDL functionality | ||||
| TRAP (% HDL inhibition capacity) | 76.9 ± 1.9 | 66.3 ± 3.2 * | 68.9 ± 1.5 * | 0.005 |
| Cholesterol Efflux (% CE) | 22.3 ± 1.4 | 19.7 ± 1.0 | 19.5 ± 0.6 * | 0.116 |
| HDL baseline oxidation (RFU) | 4.8 ± 0.3 | 5.0 ± 0.4 | 5.2 ± 0.2 | 0.671 |
| LDL susceptibility to oxidation | ||||
| CD max (nmol CD/mg LDL) | 363 ± 4.1 | 369 ± 13.0 | 345 ± 6.7 ‡ | 0.071 |
| V max (CD/min/mg LDL) | 4.3 ± 0.1 | 5.5 ± 0.6 * | 4.7 ± 0.3 | 0.095 |
| Time to half-maximum (min) | 69.3 ± 1.0 | 61.0 ± 2.3 * | 59.3 ± 1.7 * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, A.; Muñoz-García, N.; Padró, T.; Vilahur, G.; Bertomeu-González, V.; Escribano, D.; Flores, E.; Zuazola, P.; Badimon, L. HDL Function and Size in Patients with On-Target LDL Plasma Levels and a First-Onset ACS. Int. J. Mol. Sci. 2023, 24, 5391. https://doi.org/10.3390/ijms24065391
Cordero A, Muñoz-García N, Padró T, Vilahur G, Bertomeu-González V, Escribano D, Flores E, Zuazola P, Badimon L. HDL Function and Size in Patients with On-Target LDL Plasma Levels and a First-Onset ACS. International Journal of Molecular Sciences. 2023; 24(6):5391. https://doi.org/10.3390/ijms24065391
Chicago/Turabian StyleCordero, Alberto, Natàlia Muñoz-García, Teresa Padró, Gemma Vilahur, Vicente Bertomeu-González, David Escribano, Emilio Flores, Pilar Zuazola, and Lina Badimon. 2023. "HDL Function and Size in Patients with On-Target LDL Plasma Levels and a First-Onset ACS" International Journal of Molecular Sciences 24, no. 6: 5391. https://doi.org/10.3390/ijms24065391
APA StyleCordero, A., Muñoz-García, N., Padró, T., Vilahur, G., Bertomeu-González, V., Escribano, D., Flores, E., Zuazola, P., & Badimon, L. (2023). HDL Function and Size in Patients with On-Target LDL Plasma Levels and a First-Onset ACS. International Journal of Molecular Sciences, 24(6), 5391. https://doi.org/10.3390/ijms24065391










