Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: Preliminary Results from the LIPICOR Cohort Study
Abstract
1. Introduction
2. Results
2.1. Study Population
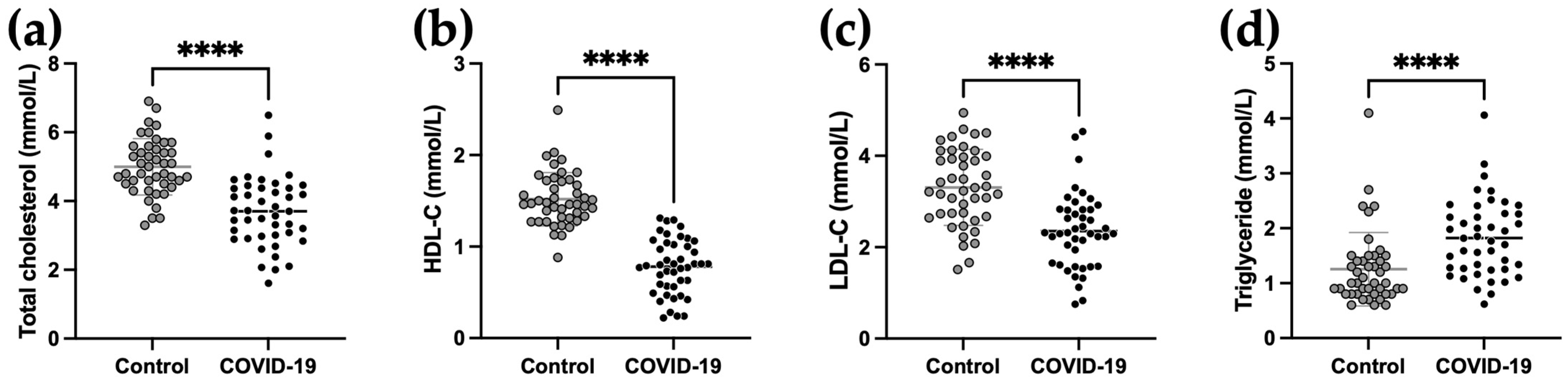
2.2. Lipid Parameters of COVID-19 Patients at Admission Compared to Control
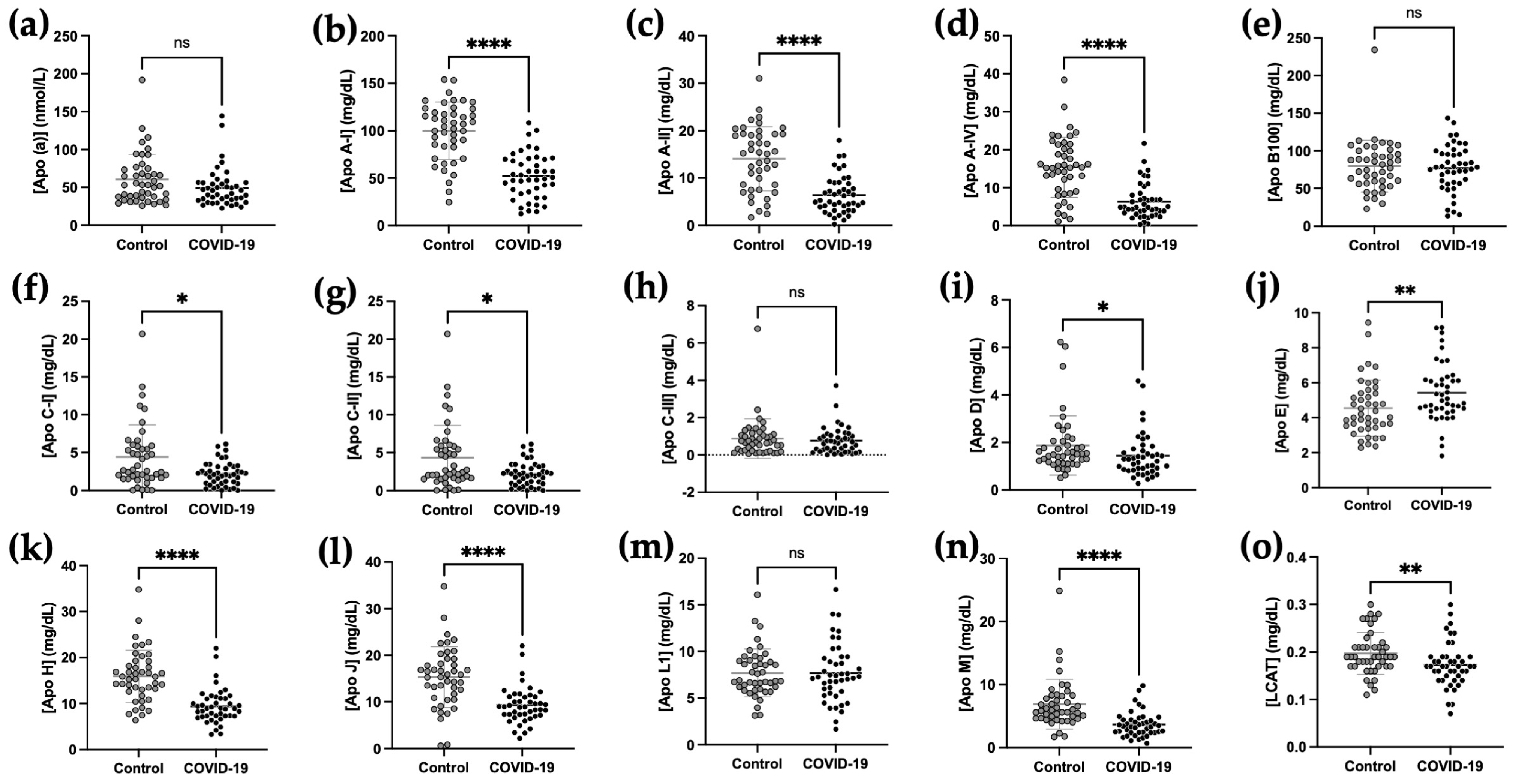
2.3. Comparison of Plasma Apolipoprotein Concentrations between COVID-19 and Control Subjects
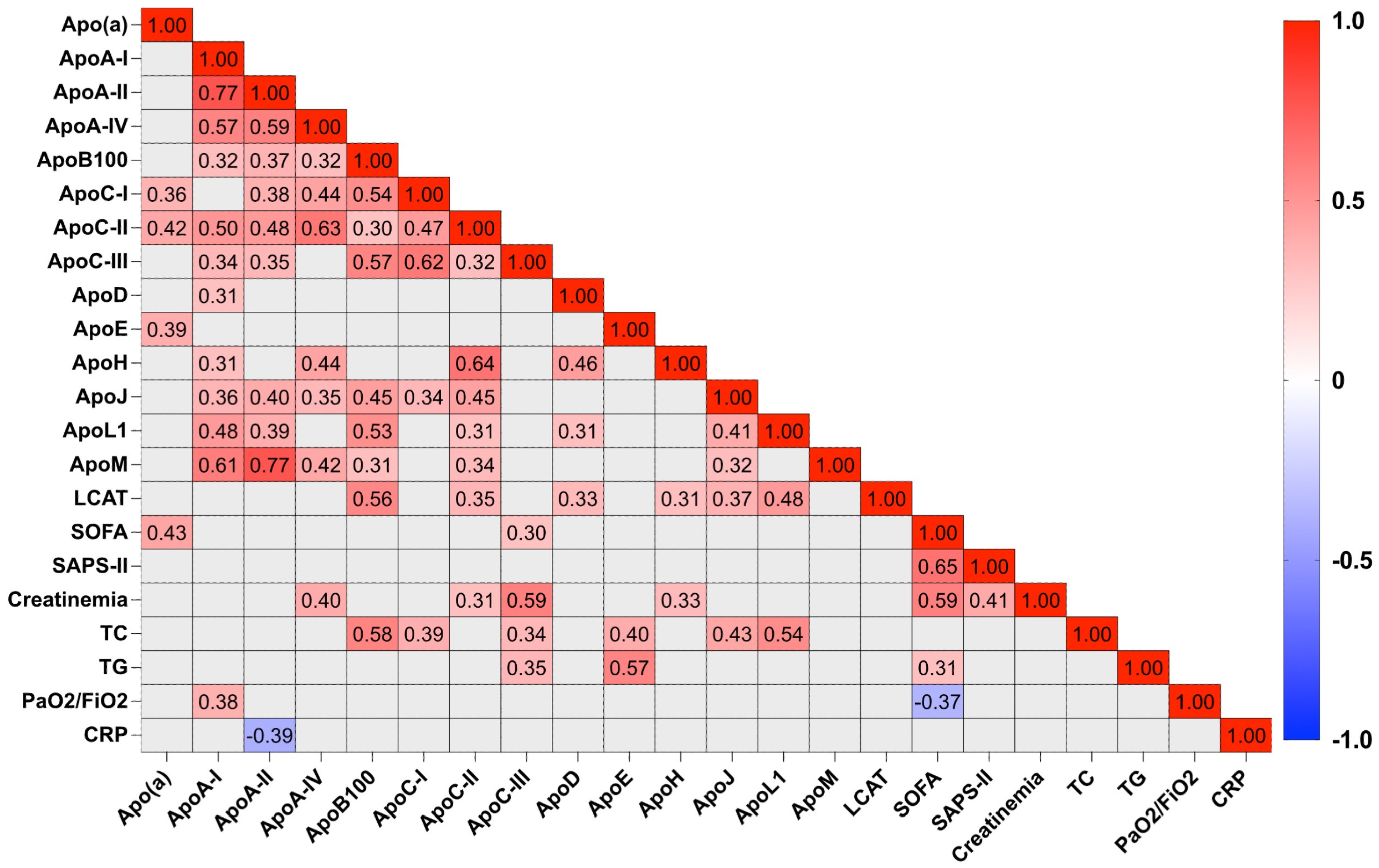
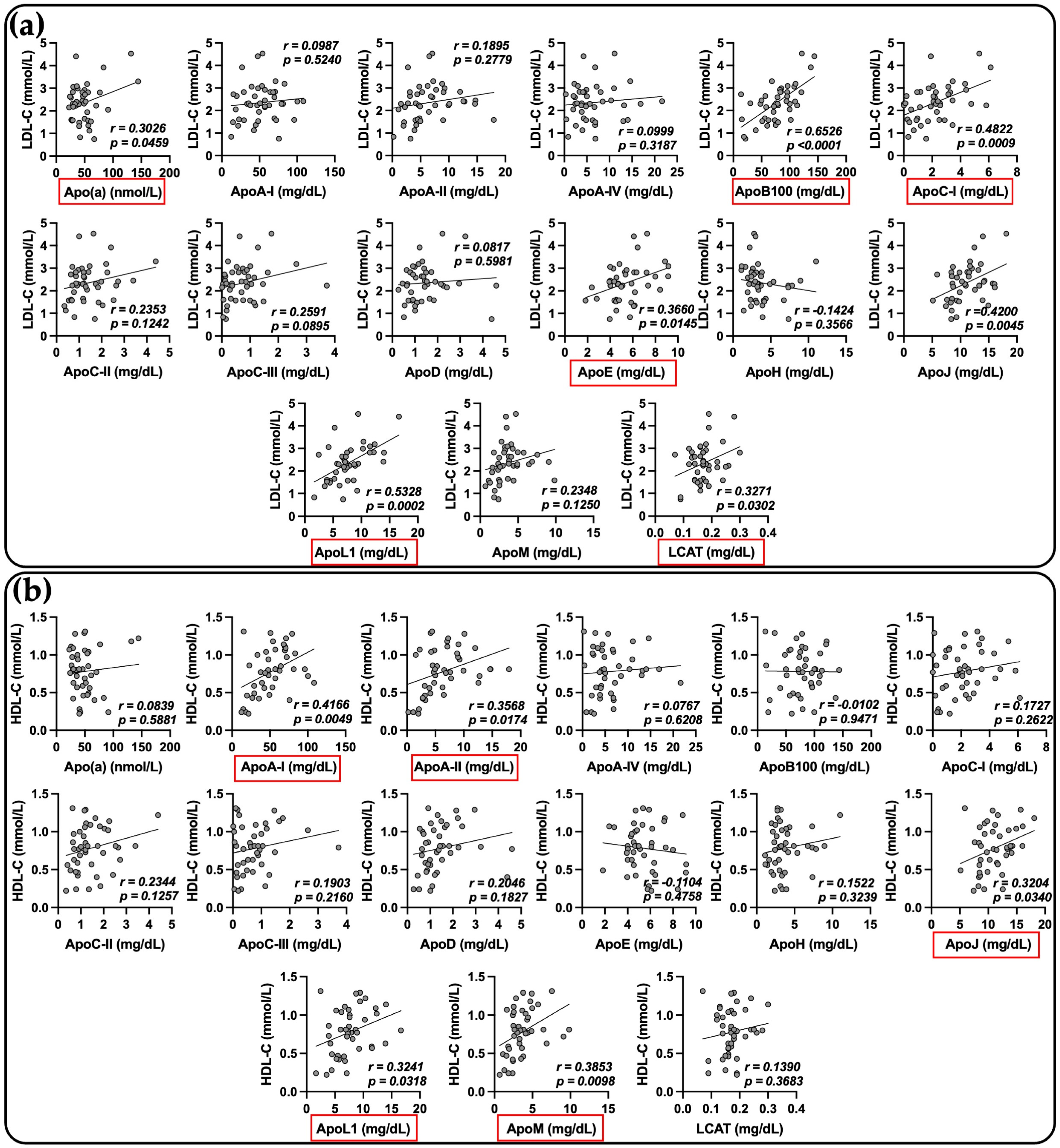
2.4. Relationship between Apolipoprotein Concentrations and Biological Markers in COVID-19 Patients
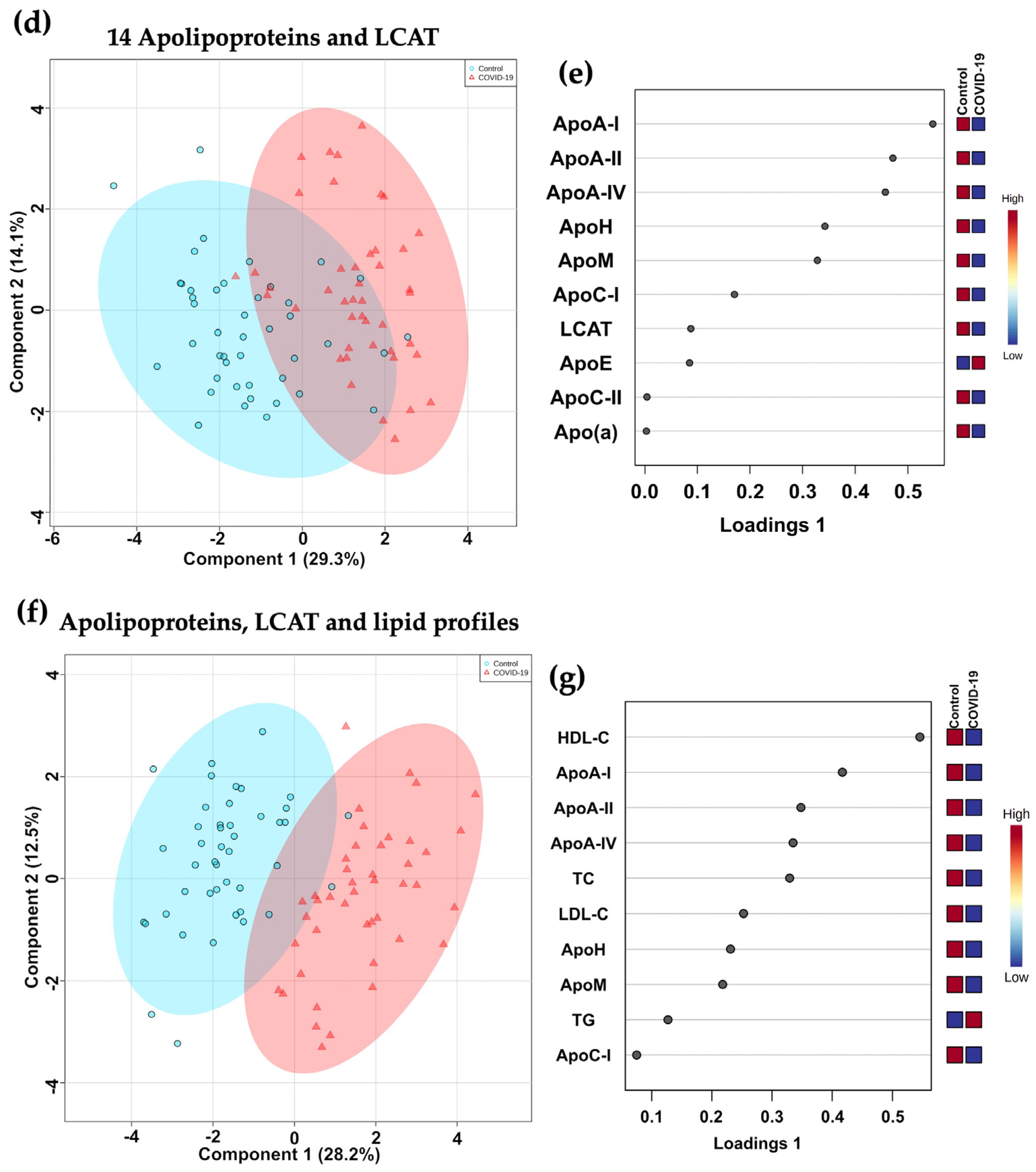
2.5. Principal Component Analysis (PCA) and Sparse Partial Least Squares Regression Discriminant Analysis (sPLS-DA)
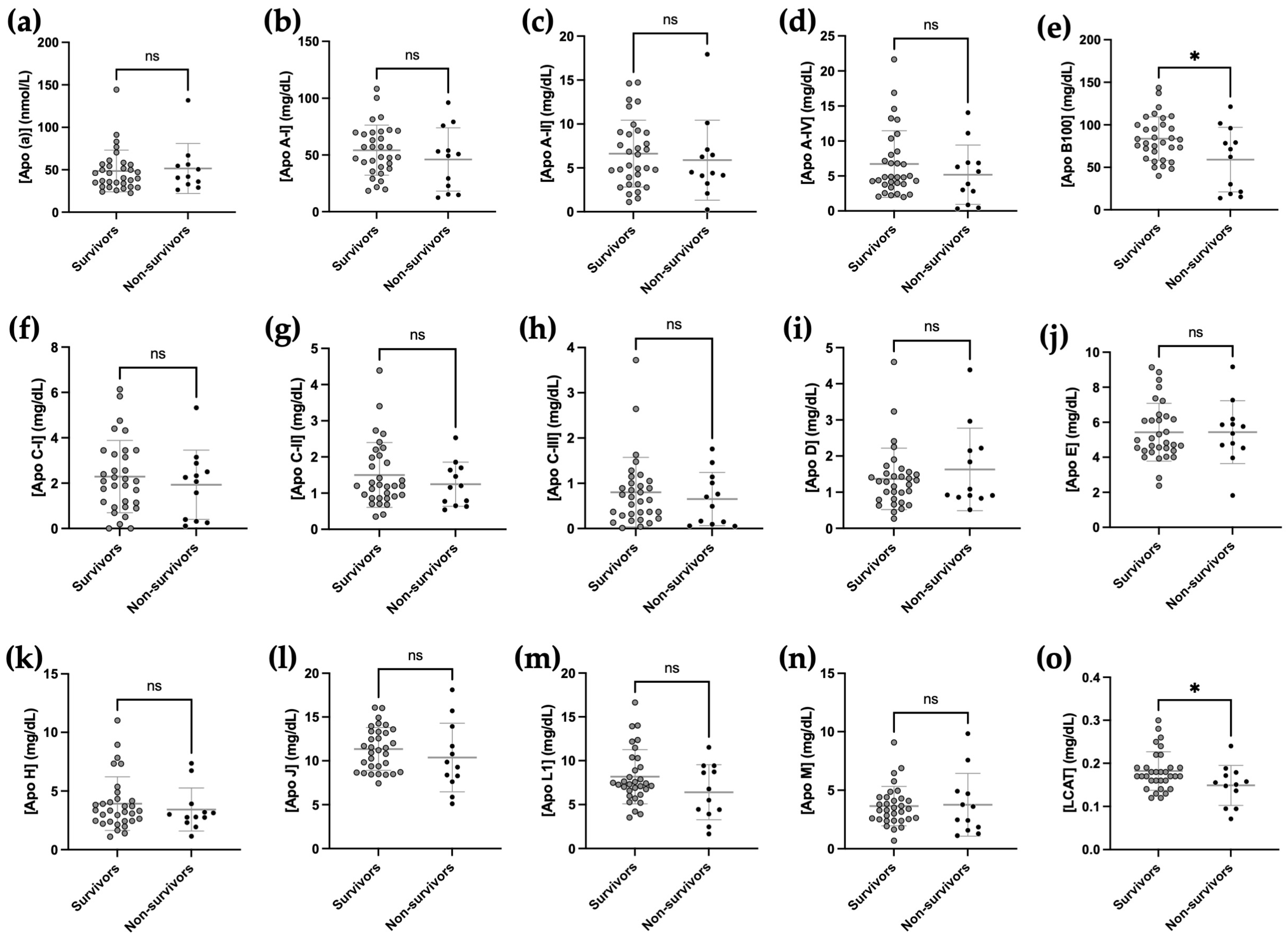
2.6. Relationship between Apolipoprotein Concentrations at Admission and Mortality
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Quantification of Plasma Apolipoprotein Concentration
4.2.1. Plasma Preparation for LC-MS/MS Analysis
4.2.2. Mass Spectrometry Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Apo | apolipoprotein |
| COVID-19 | coronavirus disease 2019 |
| ECMO | extracorporeal membrane oxygenation |
| HDL-C | high-density lipoprotein cholesterol |
| HDL | high-density lipoprotein |
| ICU | intensive care unit |
| LCAT | lecithin-cholesterol acyltransferase |
| LDL-C | low-density lipoprotein cholesterol |
| LDL | low-density lipoproteins |
| LOS | length of stay |
| PCA | principal component analysis |
| RRT | renal replacement therapy |
| SAPS-II | simplified acute physiology score-II |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SOFA | Sepsis-related Organ Failure Assessment |
| sPLS-DA | sparse partial least squares discriminant analysis |
| TC | total cholesterol |
| TG | triglycerides |
| UPLC-MS/MS | ultra-high-performance liquid chromatography tandem mass spectrometry |
| ARA-II | angiotensin II receptor antagonist |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Suffiotti, M.; Marques-Vidal, P.; Wiedemann, A.; Levy, Y.; Laouénan, C.; Ghosn, J.; Fenwick, C.; Comte, D.; Roger, T.; et al. The cytokines HGF and CXCL13 predict the severity and the mortality in COVID-19 patients. Nat. Commun. 2021, 12, 4888. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Alaupovic, P. The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr. Atheroscler. Rep. 2003, 5, 459–467. [Google Scholar] [CrossRef]
- Kastelein, J.J.P.; van der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef]
- Alaupovic, P. [2] Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1996; Volume 263, pp. 32–60. ISBN 978-0-12-182164-7. [Google Scholar]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Dashti, N. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 2001, 42, 1346–1367. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C. High-Density Lipoproteins and Apolipoprotein A1. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Subcellular Biochemistry; Springer: Cham, Switzerland, 2020; Volume 94, pp. 399–420. ISBN 978-3-030-41768-0. [Google Scholar]
- Tanaka, S.; Diallo, D.; Delbosc, S.; Genève, C.; Zappella, N.; Yong-Sang, J.; Patche, J.; Harrois, A.; Hamada, S.; Denamur, E.; et al. High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann. Intensive Care 2019, 9, 68. [Google Scholar] [CrossRef]
- Tanaka, S.; Stern, J.; Bouzid, D.; Robert, T.; Dehoux, M.; Snauwaert, A.; Zappella, N.; Cournot, M.; Lortat-Jacob, B.; Augustin, P.; et al. Relationship between lipoprotein concentrations and short-term and 1-year mortality in intensive care unit septic patients: Results from the HIGHSEPS study. Ann. Intensive Care 2021, 11, 11. [Google Scholar] [CrossRef]
- Chenaud, C.; Merlani, P.G.; Roux-Lombard, P.; Burger, D.; Harbarth, S.; Luyasu, S.; Graf, J.-D.; Dayer, J.-M.; Ricou, B. Low apolipoprotein A-I level at intensive care unit admission and systemic inflammatory response syndrome exacerbation. Crit. Care Med. 2004, 32, 632–637. [Google Scholar] [CrossRef]
- Barlage, S.; Gnewuch, C.; Liebisch, G.; Wolf, Z.; Audebert, F.X.; Gluck, T.; Frohlich, D.; Kramer, B.K.; Rothe, G.; Schmitz, G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009, 35, 1877–1885. [Google Scholar] [CrossRef]
- Chien, J.Y.; Jerng, J.S.; Yu, C.J.; Yang, P.C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005, 33, 1688–1693. [Google Scholar] [CrossRef]
- Guirgis, F.W.; Dodani, S.; Leeuwenburgh, C.; Moldawer, L.; Bowman, J.; Kalynych, C.; Grijalva, V.; Reddy, S.T.; Jones, A.E.; Moore, F.A. HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS ONE 2018, 13, e0203813. [Google Scholar] [CrossRef]
- Tanaka, S.; Couret, D.; Tran-Dinh, A.; Duranteau, J.; Montravers, P.; Schwendeman, A.; Meilhac, O. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 2020, 24, 134. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Ye, G.; Cao, X.; Xu, X.; Tan, W.; Zhang, Y. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metab. Clin. Exp. 2020, 107, 154243. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta 2020, 510, 105–110. [Google Scholar] [CrossRef]
- Feingold, K.R. Lipid and Lipoprotein Levels in Patients with COVID-19 Infections. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jericó, C.; Guerrero, C.; la Miret, M.; Näf, S.; Pardo, A.; et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef]
- Zhao, M.; Luo, Z.; He, H.; Shen, B.; Liang, J.; Zhang, J.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; et al. Decreased Low-Density Lipoprotein Cholesterol Level Indicates Poor Prognosis of Severe and Critical COVID-19 Patients: A Retrospective, Single-Center Study. Front. Med. 2021, 8, 585851. [Google Scholar] [CrossRef]
- Tanaka, S.; De Tymowski, C.; Assadi, M.; Zappella, N.; Jean-Baptiste, S.; Robert, T.; Peoc’h, K.; Lortat-Jacob, B.; Fontaine, L.; Bouzid, D.; et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: Results from the ApoCOVID study. PLoS ONE 2020, 15, e0239573. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chen, Y.; Jie, Y.; Tan, M.; Jiang, Z.; Yuan, L.; Cao, J.; Li, G.; Chong, Y.; Qu, J.; et al. U-Shaped Relationship of Low-Density Lipoprotein Cholesterol With Risk of Severe COVID-19 From a Multicenter Pooled Analysis. Front. Cardiovasc. Med. 2021, 8, 604736. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H. Importance of Apolipoprotein A-I and A-II Composition in HDL and Its Potential for Studying COVID-19 and SARS-CoV-2. Medicines 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, Y.; Fan, L.; Ye, S.; Lou, K.; Hua, X.; Huang, Z.; Shi, Q.; Gao, G. Low serum level of apolipoprotein A1 may predict the severity of COVID-19: A retrospective study. J. Clin. Lab. Anal. 2021, 35, e23911. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Deckmyn, O.; Rudler, M.; Peta, V.; Ngo, Y.; Vautier, M.; Akhavan, S.; Calvez, V.; Franc, C.; Castille, J.M.; et al. Performance of serum apolipoprotein-A1 as a sentinel of COVID-19. PLoS ONE 2020, 15, e0242306. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lu, R.; Dai, M.; Shen, M.; Zhang, J.; Cui, Y.; Liu, B.; Lin, F.; Chen, L.; et al. Lipid metabolism changes in patients with severe COVID-19. Clin. Chim. Acta 2021, 517, 66–73. [Google Scholar] [CrossRef]
- Masuda, R.; Lodge, S.; Nitschke, P.; Spraul, M.; Schaefer, H.; Bong, S.-H.; Kimhofer, T.; Hall, D.; Loo, R.L.; Bizkarguenaga, M.; et al. Integrative Modeling of Plasma Metabolic and Lipoprotein Biomarkers of SARS-CoV-2 Infection in Spanish and Australian COVID-19 Patient Cohorts. J. Proteome Res. 2021, 20, 4139–4152. [Google Scholar] [CrossRef]
- Ballout, R.A.; Kong, H.; Sampson, M.; Otvos, J.D.; Cox, A.L.; Agbor-Enoh, S.; Remaley, A.T. The NIH Lipo-COVID Study: A Pilot NMR Investigation of Lipoprotein Subfractions and Other Metabolites in Patients with Severe COVID-19. Biomedicines 2021, 9, 1090. [Google Scholar] [CrossRef]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef]
- Souza, D.R., Jr.; Silva, A.R.M.; Rosa-Fernandes, L.; Reis, L.R.; Alexandria, G.; Bhosale, S.D.; Ghilardi, F.d.R.; Dalçóquio, T.F.; Bertolin, A.J.; Nicolau, J.C.; et al. HDL proteome remodeling associates with COVID-19 severity. J. Clin. Lipidol. 2021, 15, 796–804. [Google Scholar] [CrossRef]
- Dominiczak, M.H.; Caslake, M.J. Apolipoproteins: Metabolic role and clinical biochemistry applications. Ann. Clin. Biochem. 2011, 48, 498–515. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Karathanasis, S.K.; Yang, Z.-H.; Freeman, L.; Kotani, K.; Remaley, A.T. COVID-19—Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020, 34, 9843–9853. [Google Scholar] [CrossRef]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 2022, 19, 168–179. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Shishkova, E.; Miller, I.J.; Balnis, J.; Bernstein, M.N.; Peters-Clarke, T.M.; Meyer, J.G.; Quan, Q.; Muehlbauer, L.K.; Trujillo, E.A.; et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst. 2021, 12, 23–40.e7. [Google Scholar] [CrossRef]
- Messner, C.B.; Demichev, V.; Wendisch, D.; Michalick, L.; White, M.; Freiwald, A.; Textoris-Taube, K.; Vernardis, S.I.; Egger, A.-S.; Kreidl, M.; et al. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020, 11, 11–24.E4. [Google Scholar] [CrossRef]
- Di Maio, S.; Lamina, C.; Coassin, S.; Forer, L.; Würzner, R.; Schönherr, S.; Kronenberg, F. Lipoprotein(a) and SARS-CoV-2 infections: Susceptibility to infections, ischemic heart disease and thromboembolic events. J. Intern. Med. 2022, 291, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dib, I.; Khalil, A.; Chouaib, R.; El-Makhour, Y.; Noureddine, H. Apolipoprotein C-III and cardiovascular diseases: When genetics meet molecular pathologies. Mol. Biol. Rep. 2021, 48, 875–886. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Blanchard, V.; Garçon, D.; Jaunet, C.; Chemello, K.; Billon-Crossouard, S.; Aguesse, A.; Garfa, A.; Famchon, G.; Torres, A.; Le May, C.; et al. A high-throughput mass spectrometry-based assay for large-scale profiling of circulating human apolipoproteins. J. Lipid Res. 2020, 61, 1128–1139. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | ICU COVID-19 (n = 44) | Controls (n = 44) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 62 (51–68) | 50 (45–57) | <0.0001 |
| Male sex, n (%) | 33 (75) | 33 (75) | >0.9999 |
| BMI, kg/m2, median (IQR) | 30 (27–32) | 23 (26–32) | <0.0001 |
| Presence of comorbidities | |||
| High blood pressure, n (%) | 17 (39) | 0 (0) | <0.0001 |
| Diabetes mellitus, n (%) | 6 (14) | 0 (0) | <0.0001 |
| Medications | |||
| Statin, n (%) | 0 (0) | ||
| ARA- II, n (%) | 8 (18) | ||
| Timing of hospitalization | |||
| Between first symptoms and hospitalization (days) | 6 (4–8) | ||
| Between hospitalization and ICU admission (days) | 0 (0–2) | ||
| Severity scores at admission | |||
| SAPS-II, median (IQR) | 34 (26–46) | ||
| SOFA score, median (IQR) | 4 (3–4) | ||
| Treatments during ICU stay | |||
| Norepinephrine, n (%) | 5 (11) | ||
| Mechanical ventilation, n (%) | 13 (30) | ||
| Length of mechanical ventilation, median (IQR) | 23 (17–32) | ||
| Days alive without MV at day 28, median (IQR) | 28 (8–28) | ||
| Prone positioning, n (%) | 10 (23) | ||
| Tracheostomy, n (%) | 3 (7) | ||
| ECMO, n (%) | 2 (5) | ||
| RRT, n (%) | 3 (7) | ||
| COVID-19-specific treatments | |||
| Tocilizumab, n (%) | 3 (7) | ||
| Remdesivir, n (%) | 1 (2.5) | ||
| Corticosteroids, n (%) | 44 (100) | ||
| Outcome | |||
| ICU LOS (days), median (IQR) | 7 (5–18) | ||
| Hospital LOS (days), median (IQR) | 11 (7–25) | ||
| Mortality at day-28, n (%) | 10 (23) |
| Characteristics | Survivors (n = 32) | Non-Survivors (n = 12) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 59 (49–67) | 68 (65–69) | 0.0129 |
| Male sex, n (%) | 22 (69) | 11 (92) | 0.2397 |
| BMI, kg/m2, median (IQR) | 31 (26–34) | 30 (27–31) | 0.3821 |
| Presence of comorbidities | |||
| High blood pressure, n (%) | 11 (34) | 6 (50) | 0.4889 |
| Diabetes mellitus, n (%) | 5 (16) | 1 (8) | >0.9999 |
| Medications | |||
| Statin, n (%) | 0 (0) | 0 (0) | >0.9999 |
| ARA-II, n (%) | 4 (13) | 4 (33) | 0.1847 |
| Severity scores at admission | |||
| SAPS-II, median (IQR) | 31 (25–40) | 43 (36–47) | 0.0152 |
| SOFA score, median (IQR) | 3 (3–4) | 4 (4–5) | 0.0303 |
| Treatments during ICU stay | |||
| Norepinephrine, n (%) | 0 (0) | 1 (8) | 0.2727 |
| Mechanical ventilation, n (%) | 7 (22) | 6 (50) | 0.1347 |
| Length of mechanical ventilation, median [IQR] | 0 (0–0) | 0 (0–15) | 0.2138 |
| Days alive without MV at day 28, median [IQR] | 28 (28–28) | 5 (3–7) | <0.0001 |
| Tracheostomy, n (%) | 4 (13) | 1 (8) | >0.9999 |
| ECMO, n (%) | 1 (3) | 1 (8) | 0.4757 |
| RRT, n (%) | 3 (9) | 0 (0) | 0.5506 |
| COVID-19-specific treatments | |||
| Tocilizumab, n (%) | 3 (9) | 0 (0) | 0.5506 |
| Remdesivir, n (%) | 0 (0) | 1 (8) | 0.2727 |
| Corticosteroids, n (%) | 32 (100) | 12 (100) | >0.9999 |
| Outcome | |||
| ICU LOS (days), median (IQR) | 7 (5–17) | 8 (3–25) | 0.7294 |
| Hospital LOS (days), median (IQR) | 12 (7–29) | 10 (3–25) | 0.0004 |
| Mortality at day-28, n (%) | 0 (0) | 10 (23) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begue, F.; Chemello, K.; Veeren, B.; Lortat-Jacob, B.; Tran-Dinh, A.; Zappella, N.; Snauwaert, A.; Robert, T.; Rondeau, P.; Lagrange-Xelot, M.; et al. Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: Preliminary Results from the LIPICOR Cohort Study. Int. J. Mol. Sci. 2023, 24, 4605. https://doi.org/10.3390/ijms24054605
Begue F, Chemello K, Veeren B, Lortat-Jacob B, Tran-Dinh A, Zappella N, Snauwaert A, Robert T, Rondeau P, Lagrange-Xelot M, et al. Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: Preliminary Results from the LIPICOR Cohort Study. International Journal of Molecular Sciences. 2023; 24(5):4605. https://doi.org/10.3390/ijms24054605
Chicago/Turabian StyleBegue, Floran, Kévin Chemello, Bryan Veeren, Brice Lortat-Jacob, Alexy Tran-Dinh, Nathalie Zappella, Aurelie Snauwaert, Tiphaine Robert, Philippe Rondeau, Marie Lagrange-Xelot, and et al. 2023. "Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: Preliminary Results from the LIPICOR Cohort Study" International Journal of Molecular Sciences 24, no. 5: 4605. https://doi.org/10.3390/ijms24054605
APA StyleBegue, F., Chemello, K., Veeren, B., Lortat-Jacob, B., Tran-Dinh, A., Zappella, N., Snauwaert, A., Robert, T., Rondeau, P., Lagrange-Xelot, M., Montravers, P., Couret, D., Tanaka, S., & Meilhac, O. (2023). Plasma Apolipoprotein Concentrations Are Highly Altered in Severe Intensive Care Unit COVID-19 Patients: Preliminary Results from the LIPICOR Cohort Study. International Journal of Molecular Sciences, 24(5), 4605. https://doi.org/10.3390/ijms24054605






