Models of Congenital Adrenal Hyperplasia for Gene Therapies Testing
Abstract
1. Introduction
Adrenal Insufficiency Treatment Approaches
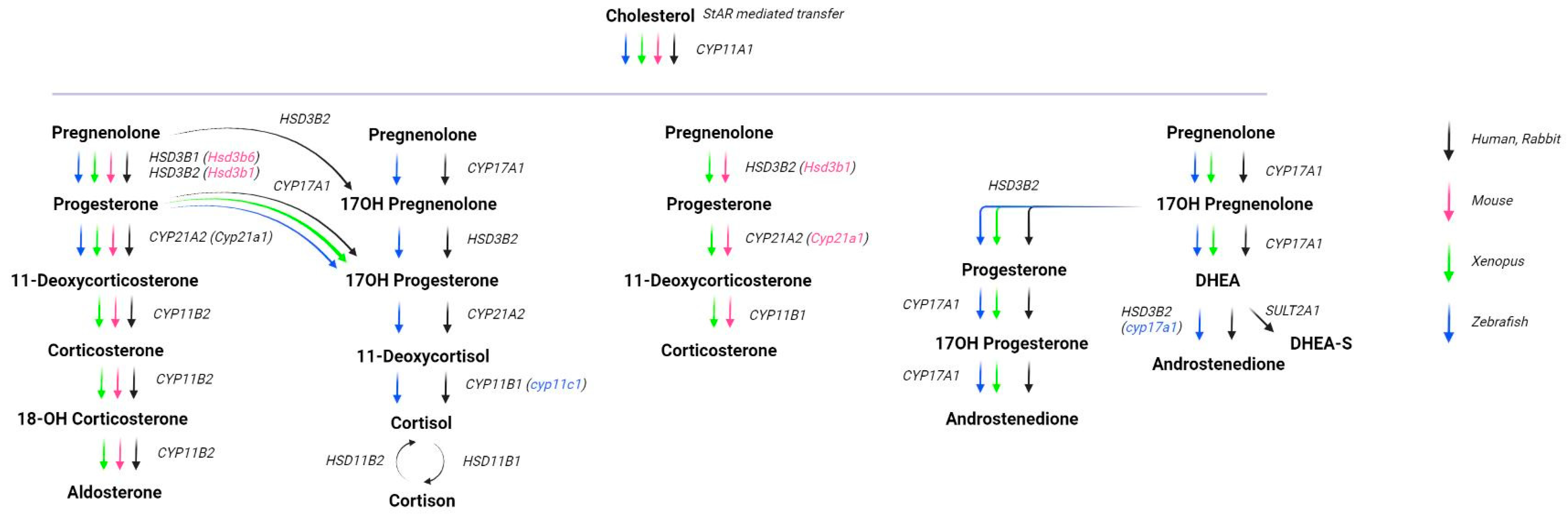
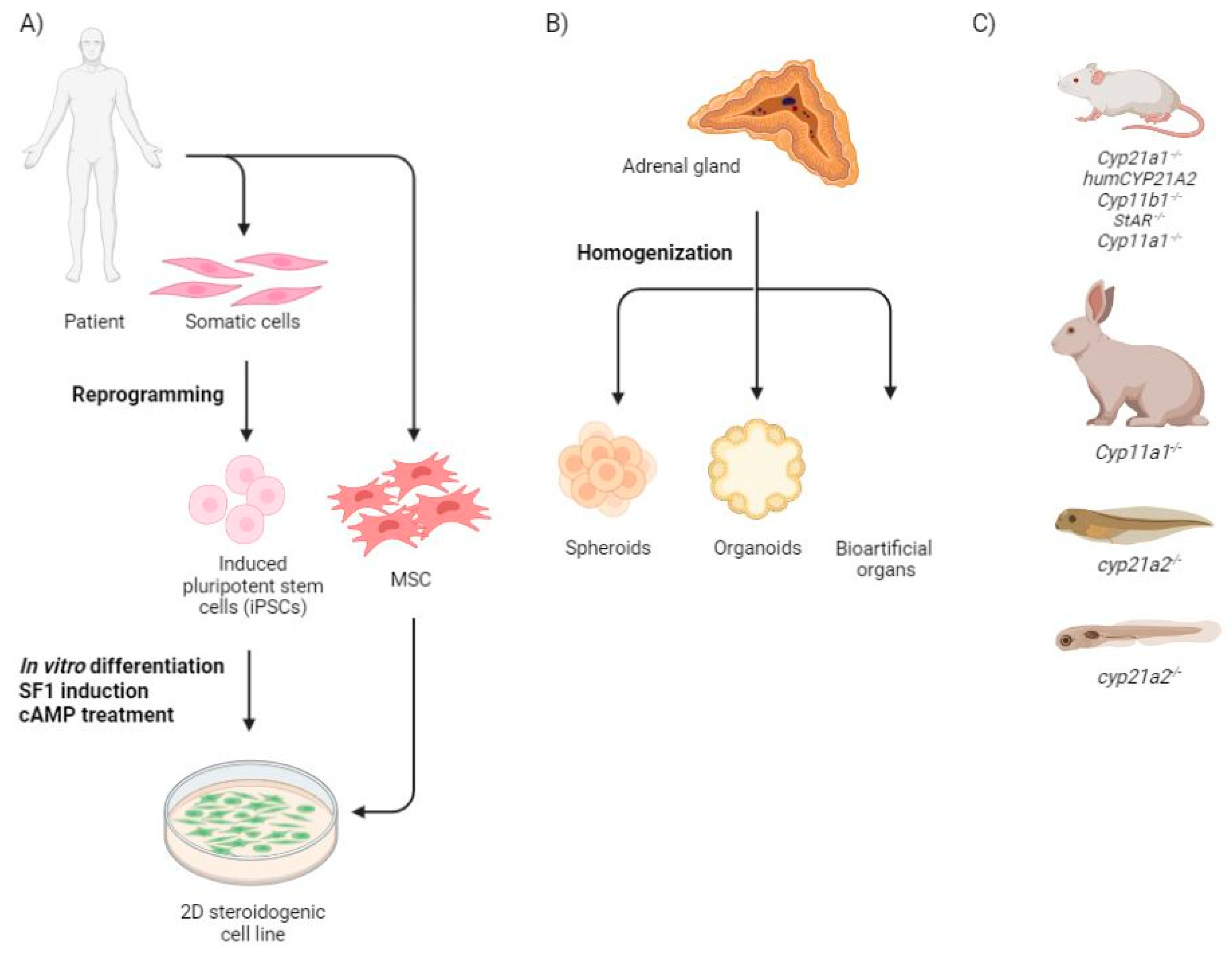
2. Congenital Adrenal Hyperplasia Models
2.1. Monolayer Cells
2.2. Three Dimensional Structures
2.3. Animal Models
3. Transcriptomics of the Adrenal Gland for Improvement Differentiation Strategies
4. Challenges in the Artificial Cellular Differentiation to the Adrenal Gland
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammer, G.D.; Basham, K.J. Stem cell function and plasticity in the normal physiology of the adrenal cortex. Mol. Cell. Endocrinol. 2021, 519, 111043. [Google Scholar]
- Schimmer, B.P.; White, P.C. Minireview: Steroidogenic Factor 1: Its Roles in Differentiation, Development, and Disease. Mol. Endocrinol. 2010, 24, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Parker, K.L.; Morohashi, K. Developmental Links between the Fetal and Adult Zones of the Adrenal Cortex Revealed by Lineage Tracing. Mol. Cell. Biol. 2008, 28, 7030–7040. [Google Scholar] [CrossRef]
- Ward, R.D.; Raetzman, L.T.; Suh, H.; Stone, B.M.; Nasonkin, I.O.; Camper, S.A. Role of PROP1 in Pituitary Gland Growth. Mol. Endocrinol. 2005, 19, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Mesiano, S.; Jaffe, R.B. Developmental and Functional Biology of the Primate Fetal Adrenal Cortex. Endocr. Rev. 1997, 18, 378–403. [Google Scholar] [CrossRef] [PubMed]
- Mokrysheva, N.G.; Melnichenko, G.A.; Adamyan, L.V.; Troshina, E.A.; Molashenko, N.V.; Sazonova, A.I.; Uvarova, E.V.; Esayan, R.M.; Andreeva, E.N.; Uzhegova, Z.A.; et al. Russian clinical practice guidelines «congenital adrenal hyperplasia». Obes. Metab. 2021, 18, 345–382. [Google Scholar] [CrossRef]
- Prasad, R.; Deswal, S. New Horizons: Molecular Basis and Novel Therapeutics in Congenital Adrenal Hyperplasia. Indian J. Clin. Biochem. 2022, 37, 1–2. [Google Scholar] [CrossRef]
- Buonocore, F.; Achermann, J.C. Primary adrenal insufficiency: New genetic causes and their long-term consequences. Clin. Endocrinol. 2020, 92, 11. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Loechner, K.J.; McLaughlin, J.T.; Calikoglu, A.S. Alternative Strategies for the Treatment of Classical Congenital Adrenal Hyperplasia: Pitfalls and Promises. Int. J. Pediatr. Endocrinol. 2010, 2010, 670960. [Google Scholar] [CrossRef]
- Gu, P.; Goodwin, B.; Chung, A.C.-K.; Xu, X.; Wheeler, D.A.; Price, R.R.; Galardi, C.; Peng, L.; Latour, A.M.; Koller, B.H.; et al. Orphan Nuclear Receptor LRH-1 Is Required to Maintain Oct4 Expression at the Epiblast Stage of Embryonic Development. Mol. Cell. Biol. 2005, 25, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Perdomini, M.; Santos, D.; Goumeaux, C.; Blouin, V.; Bougnères, P. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21−/− mouse model. Gene Ther. 2017, 24, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, T.; Sone, M.; Honda, K.; Taura, D.; Kojima, K.; Inuzuka, M.; Kanamoto, N.; Tamura, N.; Nakao, K. Differentiation of Human Embryonic Stem Cells and Human Induced Pluripotent Stem Cells into Steroid-Producing Cells. Endocrinology 2012, 153, 4336–4345. [Google Scholar] [CrossRef]
- Yazawa, T.; Kawabe, S.; Inaoka, Y.; Okada, R.; Mizutani, T.; Imamichi, Y.; Ju, Y.; Yamazaki, Y.; Usami, Y.; Kuribayashi, M.; et al. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol. Cell. Endocrinol. 2011, 336, 127–132. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Gorskaja, U.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Tanaka, T.; Gondo, S.; Okabe, T.; Ohe, K.; Shirohzu, H.; Morinaga, H.; Nomura, M.; Tani, K.; Takayanagi, R.; Nawata, H.; et al. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J. Mol. Endocrinol. 2007, 39, 343–350. [Google Scholar] [CrossRef]
- Gondo, S.; Yanase, T.; Okabe, T.; Tanaka, T.; Morinaga, H.; Nomura, M.; Goto, K.; Nawata, H. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 2004, 9, 1239–1247. [Google Scholar] [CrossRef]
- Gondo, S.; Okabe, T.; Tanaka, T.; Morinaga, H.; Nomura, M.; Takayanagi, R.; Nawata, H.; Yanase, T. Adipose Tissue-Derived and Bone Marrow-Derived Mesenchymal Cells Develop into Different Lineage of Steroidogenic Cells by Forced Expression of Steroidogenic Factor 1. Endocrinology 2008, 149, 4717–4725. [Google Scholar] [CrossRef]
- Yazawa, T.; Mizutani, T.; Yamada, K.; Kawata, H.; Sekiguchi, T.; Yoshino, M.; Kajitani, T.; Shou, Z.; Umezawa, A.; Miyamoto, K. Differentiation of Adult Stem Cells Derived from Bone Marrow Stroma into Leydig or Adrenocortical Cells. Endocrinology 2006, 147, 4104–4111. [Google Scholar] [CrossRef]
- Yazawa, T.; Inaoka, Y.; Okada, R.; Mizutani, T.; Yamazaki, Y.; Usami, Y.; Kuribayashi, M.; Orisaka, M.; Umezawa, A.; Miyamoto, K. PPAR-γ Coactivator-1α Regulates Progesterone Production in Ovarian Granulosa Cells with SF-1 and LRH-1. Mol. Endocrinol. 2010, 24, 485–496. [Google Scholar] [CrossRef]
- Wei, X.; Peng, G.; Zheng, S.; Wu, X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012, 45, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [PubMed]
- Chau, Y.M.; Crawford, P.A.; Woodson, K.G.; Polish, J.A.; Olson, L.M.; Sadovsky, Y. Role of Steroidogenic-Factor 1 in Basal and 3′,5′-Cyclic Adenosine Monophosphate-Mediated Regulation of Cytochrome P450 Side-Chain Cleavage Enzyme in the Mouse. Biol. Reprod. 1997, 57, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Lund, J. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3′,5′-monophosphate-responsive sequence in the bovine CYP17 gene. Mol. Endocrinol. 1995, 9, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sewer, M.B.; Waterman, M.R. Transcriptional complexes at the CYP17 CRS. Endocr. Res. 2009, 28, 551–558. [Google Scholar]
- Lee, S.L.; Sadovsky, Y.; Swirnoff, A.H.; Polish, J.A.; Goda, P.; Gavrilina, G.; Milbrandt, J. Luteinizing Hormone Deficiency and Female Infertility in Mice Lacking the Transcription Factor NGFI-A (Egr-1). Science 1996, 273, 1219–1221. [Google Scholar] [CrossRef]
- Morohashi, K.I.; Zanger, U.M.; Honda, S.I.; Hara, M.; Waterman, M.R.; Omura, T. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol. Endocrinol. 1993, 7, 1196–1204. [Google Scholar] [CrossRef]
- Leers-Sucheta, S.; Morohashi, K.I.; Mason, J.I.; Melner, M.H. Synergistic Activation of the Human Type II 3β-Hydroxysteroid Dehydrogenase/Δ5-Δ4 Isomerase Promoter by the Transcription Factor Steroidogenic Factor-1/Adrenal 4-binding Protein and Phorbol Ester. J. Biol. Chem. 1997, 272, 7960–7967. [Google Scholar]
- Kawabe, K.; Shikayama, T.; Tsuboi, H.; Oka, S.; Oba, K.; Yanase, T.; Nawata, H.; Morohashi, K.I. Dax-1 as One of the Target Genes of Ad4BP/SF-1. Mol. Endocrinol. 1999, 13, 1267–1284. [Google Scholar] [CrossRef]
- De Santa Barbara, P.; Bonneaud, N.; Boizet, B.; Desclozeaux, M.; Moniot, B.; Sudbeck, P.; Scherer, G.; Poulat, F.; Berta, P. Direct Interaction of SRY-Related Protein SOX9 and Steroidogenic Factor 1 Regulates Transcription of the Human Anti-Müllerian Hormone Gene. Mol. Cell. Biol. 1998, 18, 6653–6665. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008, 453, 930–934. [Google Scholar] [CrossRef] [PubMed]
- De Santa Barbara, P.; Méjean, C.; Moniot, B.; Malclès, M.H.; Berta, P.; Boizet-Bonhoure, B. Steroidogenic Factor-1 Contributes to the Cyclic-Adenosine Monophosphate Down-Regulation of Human SRY Gene Expression. Biol. Reprod. 2001, 64, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, T.; Imamichi, Y.; Miyamoto, K.; Khan, M.R.I.; Uwada, J.; Umezawa, A.; Taniguchi, T. Regulation of Steroidogenesis, Development, and Cell Differentiation by Steroidogenic Factor-1 and Liver Receptor Homolog-1. Zool. Sci. 2015, 32, 323–330. [Google Scholar] [CrossRef]
- Yazawa, T.; Inanoka, Y.; Mizutani, T.; Kuribayashi, M.; Umezawa, A.; Miyamoto, K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology 2009, 150, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Babot, G.; Balyura, M.; Hadjidemetriou, I.; Ajodha, S.J.; Taylor, D.R.; Ghataore, L.; Taylor, N.F.; Schubert, U.; Ziegler, C.G.; Storr, H.L.; et al. Modeling Congenital Adrenal Hyperplasia and Testing Interventions for Adrenal Insufficiency Using Donor-Specific Reprogrammed Cells. Cell Rep. 2018, 22, 1236–1249. [Google Scholar] [PubMed]
- Naiki, Y.; Miyado, M.; Shindo, M.; Horikawa, R.; Hasegawa, Y.; Katsumata, N.; Takada, S.; Akutsu, H.; Onodera, M.; Fukami, M. Adeno-Associated Virus-Mediated Gene Therapy for Patients’ Fibroblasts, Induced Pluripotent Stem Cells, and a Mouse Model of Congenital Adrenal Hyperplasia. Hum. Gene Ther. 2022, 33, 801–809. [Google Scholar] [CrossRef]
- Markmann, S.; De, B.P.; Reid, J.; Jose, C.L.; Rosenberg, J.B.; Leopold, P.L.; Kaminsky, S.M.; Sondhi, D.; Pagovich, O.; Crystal, R.G. Biology of the Adrenal Gland Cortex Obviates Effective Use of Adeno-Associated Virus Vectors to Treat Hereditary Adrenal Disorders. Hum. Gene Ther. 2018, 29, 403–412. [Google Scholar] [CrossRef]
- Schubert, T.; Reisch, N.; Naumann, R.; Reichardt, I.; Landgraf, D.; Quitter, F.; Thirumalasetty, S.R.; Heninger, A.K.; Sarov, M.; Peitzsch, M.; et al. CYP21A2 Gene Expression in a Humanized 21-Hydroxylase Mouse Model Does Not Affect Adrenocortical Morphology and Function. J. Endocr. Soc. 2022, 6, bvac062. [Google Scholar] [CrossRef]
- Hasegawa, T.; Zhao, L.; Caron, K.M.; Majdic, G.; Suzuki, T.; Shizawa, S.; Sasano, H.; Parker, K.L. Developmental Roles of the Steroidogenic Acute Regulatory Protein (StAR) as Revealed by StAR Knockout Mice. Mol. Endocrinol. 2000, 14, 1462–1471. [Google Scholar] [CrossRef]
- Hu, M.C.; Hsu, N.C.; El Hadj, N.B.; Pai, C.I.; Chu, H.P.; Wang, C.K.L.; Chung, B.C. Steroid Deficiency Syndromes in Mice with Targeted Disruption of Cyp11a1. Mol. Endocrinol. 2002, 16, 1943–1950. [Google Scholar] [CrossRef]
- Yang, X.; Iwamoto, K.; Wang, M.; Artwohl, J.; Mason, J.I.; Pang, S. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology 1993, 132, 1977–1982. [Google Scholar] [CrossRef]
- Paul, B.; Shewade, L.H.; Buchholz, D.R. cyp21a2 Knockout Tadpoles Survive Metamorphosis Despite Low Corticosterone. Endocrinology 2022, 164, bqac182. [Google Scholar] [CrossRef]
- Eachus, H.; Zaucker, A.; Oakes, J.A.; Griffin, A.; Weger, M.; Güran, T.; Taylor, A.; Harris, A.; Greenfield, A.; Quanson, J.L.; et al. Genetic Disruption of 21-Hydroxylase in Zebrafish Causes Interrenal Hyperplasia. Endocrinology 2017, 158, 4165–4173. [Google Scholar] [CrossRef]
- Mariniello, K.; Guasti, L. Towards novel treatments for adrenal diseases: Cell- and gene therapy-based approaches. Mol. Cell. Endocrinol. 2021, 524, 111160. [Google Scholar]
- Tanaka, T.; Aoyagi, C.; Mukai, K.; Nishimoto, K.; Kodama, S.; Yanase, T. Extension of Survival in Bilaterally Adrenalectomized Mice by Implantation of SF-1/Ad4BP-Induced Steroidogenic Cells. Endocrinology 2020, 161, 1–11. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M.; Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. Cell Cult. 2018, 2, 1–22. [Google Scholar] [CrossRef]
- Werdermann, M.; Berger, I.; Scriba, L.D.; Santambrogio, A.; Schlinkert, P.; Brendel, H.; Morawietz, H.; Schedl, A.; Peitzsch, M.; King, A.J.F.; et al. Insulin and obesity transform hypothalamic-pituitary-adrenal axis stemness and function in a hyperactive state. Mol. Metab. 2021, 43, 101112. [Google Scholar]
- Poli, G.; Sarchielli, E.; Guasti, D.; Benvenuti, S.; Ballerini, L.; Mazzanti, B.; Armignacco, R.; Cantini, G.; Lulli, M.; Chortis, V.; et al. Human fetal adrenal cells retain age-related stem- and endocrine-differentiation potential in culture. FASEB J. 2019, 33, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.A.; Seltz, L.M.; Jiang, H.; Kasick, R.T.; Sellaro, T.L.; Badylak, S.F.; Ogilvie, J.B. Adrenal Extracellular Matrix Scaffolds Support Adrenocortical Cell Proliferation and Function In Vitro. Tissue Eng. Part A 2010, 16, 3363–3374. [Google Scholar] [CrossRef]
- Balyura, M.; Gelfgat, E.; Ehrhart-Bornstein, M.; Ludwig, B.; Gendlerc, Z.; Barkai, U.; Zimerman, B.; Rotem, A.; Block, N.L.; Schally, A.V.; et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc. Natl. Acad. Sci. USA 2015, 112, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Balyura, M.; Gelfgat, E.; Steenblock, C.; Androutsellis-Theotokis, A.; Ruiz-Babot, G.; Guasti, L.; Werdermann, M.; Ludwig, B.; Bornstein, T.; Schally, A.V.; et al. Expression of progenitor markers is associated with the functionality of a bioartificial adrenal cortex. PLoS ONE 2018, 13, e0194643. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Wang, B.; Ye, T. C-17-heteroaryl steroidal compounds as inhibitors of cyp11b, cyp17, and/or cyp21. WO2012083112A3, 8 November 2012. [Google Scholar]
- Lee, J.S.; Jeon, Y.J.; Park, S.Y.; Son, C.G. An Adrenalectomy Mouse Model Reflecting Clinical Features for Chronic Fatigue Syndrome. Biomolecules 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Makimura, H.; Mizuno, T.M.; Beasley, J.; Silverstein, J.H.; Mobbs, C.V. Adrenalectomy stimulates hypothalamic proopiomelanocortin expression but does not correct diet-induced obesity. BMC Physiol. 2003, 3, 4. [Google Scholar] [CrossRef]
- van der Sluis, R.J.; van Puijvelde, G.H.; Van Berkel, T.J.C.; Hoekstra, M. Adrenalectomy stimulates the formation of initial atherosclerotic lesions: Reversal by adrenal transplantation. Atherosclerosis 2012, 221, 76–83. [Google Scholar] [CrossRef]
- Castonguay, T.W.; Beaulieu, S.; Eskay, R.L.; Barden, N.; Kamara, K.; Khozin, S.; Lustberg, L.; Brown, L. The effects of adrenalectomy and aldosterone replacement in transgenic mice expressing antisense RNA to the type 2 glucocorticoid receptor. Physiol. Behav. 2002, 77, 417–423. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Hawley, J.; Birt, D.F. Adrenalectomy Abrogates Reduction of 12-O-Tetradecanoylphorbol-13-acetate-induced Extracellular Signal-regulated Protein Kinase Activity in the Epidermis of Dietary Energy-restricted SENCAR Mice. Cancer Epidemiol. Biomark. Prevention 2002, 11, 299–304. [Google Scholar]
- Papers, P.; Mach, K.E. The In Vivo Effect of ACTH on CYP17 mRNA in the Rabbit Adrenal. Ph.D. Thesis, The University of Montana, Missoula, MT, USA, 1993. [Google Scholar]
- Owens, S.L.; Downey, M.E.; Pressler, B.M.; Birkenheuer, A.J.; Chandler, D.W.; Scott-Moncrieff, J.C. Congenital Adrenal Hyperplasia Associated with Mutation in an 11β-Hydroxylase-Like Gene in a Cat. J. Vet. Intern. Med. 2012, 26, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Hammes, S.R. Xenopus laevis CYP17 regulates androgen biosynthesis independent of the cofactor cytochrome b5. J. Biol. Chem. 2005, 280, 10196–10201. [Google Scholar] [CrossRef]
- Paul, B.; Sterner, Z.R.; Buchholz, D.R.; Shi, Y.B.; Sachs, L.M. Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells 2022, 11, 1595. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; Hrabě De Angelis, M.; Adamski, J. Zebrafish and steroids: What do we know and what do we need to know? J. Steroid Biochem. Mol. Biol. 2013, 137, 165–173. [Google Scholar]
- Rege, J.; Nakamura, Y.; Wang, T.; Merchen, T.D.; Sasano, H.; Rainey, W.E. Transcriptome Profiling Reveals Differentially Expressed Transcripts Between the Human Adrenal Zona Fasciculata and Zona Reticularis. J. Clin. Endocrinol. Metab. 2014, 99, E518–E527. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.H.T.; Lin, L.; Qi, C.Y.; Cha, M.; Gifford, D.K.; Sherwood, R.I. A Multiplexed Barcodelet Single-Cell RNA-Seq Approach Elucidates Combinatorial Signaling Pathways that Drive ESC Differentiation. Cell Stem Cell 2020, 26, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, J.; Chen, Y.; Zou, C.; Zhang, H.; Yang, X.; Zhang, Q.; Li, T.; Mo, L.; Zeng, Y.; et al. Single-cell transcriptomes reveal characteristic features of cell types within the human adrenal microenvironment. J. Cell. Physiol. 2021, 236, 7308–7321. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Khoo, W.H.; Moran, I.; Croucher, P.I.; Phan, T.G. Single cell RNA sequencing of rare immune cell populations. Front. Immunol. 2018, 9, 1553. [Google Scholar] [CrossRef]
- Cuomo, A.S.E.; Seaton, D.D.; McCarthy, D.J.; Martinez, I.; Bonder, M.J.; Garcia-Bernardo, J.; Amatya, S.; Madrigal, P.; Isaacson, A.; Buettner, F.; et al. Single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun. 2020, 11, 810. [Google Scholar] [CrossRef]
- Michki, N.S.; Li, Y.; Sanjasaz, K.; Zhao, Y.; Shen, F.Y.; Walker, L.A.; Cao, W.; Lee, C.Y.; Cai, D. The molecular landscape of neural differentiation in the developing Drosophila brain revealed by targeted scRNA-seq and multi-informatic analysis. Cell Rep. 2021, 35, 109039. [Google Scholar] [CrossRef]
- Chu, L.F.; Leng, N.; Zhang, J.; Hou, Z.; Mamott, D.; Vereide, D.T.; Choi, J.; Kendziorski, C.; Stewart, R.; Thomson, J.A. Single-cell RNA-seq reveals novel regulators of human embryonic stem cell differentiation to definitive endoderm. Genome Biol. 2016, 17, 173. [Google Scholar] [CrossRef]
- Ruan, H.; Liao, Y.; E, W.; Ren, Z.; Mao, L.; Yao, F.; Yu, P.; Ye, Y.; Zhang, Z.; Li, S.; et al. Single-cell reconstruction of differentiation trajectory reveals a critical role of ETS1 in human cardiac lineage commitment. BMC Biol. 2019, 17, 89. [Google Scholar] [CrossRef]
- Lai, S.; Ma, L.; Ye, F.; Chen, H.; Han, X.; Guo, G. Mapping a mammalian adult adrenal gland hierarchy across species by microwell-seq. Cell Regen. 2020, 9, 11. [Google Scholar] [CrossRef]
- Lopez, J.P.; Brivio, E.; Santambrogio, A.; De Donno, C.; Kos, A.; Peters, M.; Rost, N.; Czamara, D.; Brückl, T.M.; Roeh, S.; et al. Single-cell molecular profiling of all three components of the HPA axis reveals adrenal ABCB1 as a regulator of stress adaptation. Sci. Adv. 2021, 7, 4497–4524. [Google Scholar] [CrossRef]
- Hanemaaijer, E.S.; Margaritis, T.; Sanders, K.; Bos, F.L.; Candelli, T.; Al-Saati, H.; van Noesel, M.M.; Meyer-Wentrup, F.A.G.; van de Wetering, M.; Holstege, F.C.P.; et al. Single-cell atlas of developing murine adrenal gland reveals relation of Schwann cell precursor signature to neuroblastoma phenotype. Proc. Natl. Acad. Sci. USA 2021, 118, e2022350118. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, Z.; Fei, L.; Sun, H.; Wang, R.; Chen, Y.; Chen, H.; Wang, J.; Tang, H.; Ge, W.; et al. Construction of a human cell landscape at single-cell level. Nature 2020, 581, 303–309. [Google Scholar] [CrossRef]
- Human Cell Atlas–To Create Comprehensive Reference Maps of All Human Cells—The Fundamental Units of Life—As a Basis for Both Understanding Human Health and Diagnosing, Monitoring, and Treating Disease. Available online: https://www.humancellatlas.org/ (accessed on 2 November 2022).
- Jääskeläinen, J. Molecular biology of androgen insensitivity. Mol. Cell. Endocrinol. 2012, 352, 4–12. [Google Scholar] [CrossRef] [PubMed]
| Authors | Object | Mutated Genes | Mutations | Treatment | DOI |
|---|---|---|---|---|---|
| Ruiz-Babot et al., 2018 [35] | Steroidogenic cells differentiated from human urine-derived stem cells (USCs) | CYP21A2 | c.515T > A, p.(Ile172Asn) | Lentiviruses | 10.1016/j.celrep.2018.01.003 |
| c.955C > T, p.(Gln319Stop) | |||||
| STAR | c.666delC, p.(Thr223Leufs × 98) | ||||
| HSD3B | NA | ||||
| CYP11A1 | c.940G > A, p.(Glu314Lys) | ||||
| Naiki et al., 2022 [36] | Steroidogenic cells differentiated from human iPSc | CYP21A2 | p.I172N/p.I172N | AAV2 | 10.1089/hum.2022.005 |
| p.R356W/IVS2–13A/C>G | AAV2 | ||||
| N.A. | AAV2 | ||||
| IVS2–13A/C > GR483delInt, CGG>CC | AAV2 | ||||
| CYP17A1 | DF54/Y329KfsX418 | AAV2 | |||
| CYP11B1 | p.W116X/p.W116X | AAV9 | |||
| Markmann et al., 2017 [37] | mice C57Bl/10SnSlc-H-2aw18 | Cyp21a1 | Cyp21a1-Cyp21a2ps chimera | AAVrh10 | 10.1089/hum.2017.203 |
| Naiki et al. et al., 2022 [36] | mice C57BL/6-DBA/2/Cyp11b1-/- | Cyp11b1 | The 4th exons disruped | AAV9 | 10.1089/hum.2022.005 |
| Schubert et al., 2022 [38] | C57Bl/6NCrl-Cyp21a1tg(CYP21A2)Koe | Cyp21a2 | replacing the 2620-bp mCyp21a1with the 2713-bp hCYP21A2 | – | 10.1210/jendso/bvac062 |
| Hasegawa et al., 2000 [39] | mice C57BL/6/ StAR-/- | StAR | disrupted the StAR gene by deleting part of exon 2 and all of exon 3 | – | 10.1073/pnas.94.21.11540 |
| Hu et al., 2002 [40] | mice C57BL/6/Cyp11a1-/- | Cyp11a1 | neo gene was inserted within exon 1 | – | 10.1210/me.2002–0055 |
| Yang et al., 1993 [41] | rabbit | deletion | – | 10.1210/endo.132.5.7682938 | |
| Paul et al., 2022 [42] | Xenopus tropicalis | cyp21a2 | 11-base pair deletion | – | 10.1210/endocr/bqac182 |
| Eachus et al., 2017 [43] | Zebrafish | cyp21a2 | c.del211–224, p.P70 fs26X | – | 10.1210/en.2017–00549 |
| c.del212–224, p.P70fs13X | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazova, O.; Bastrich, A.; Deviatkin, A.; Onyanov, N.; Kaziakhmedova, S.; Shevkova, L.; Sakr, N.; Petrova, D.; Vorontsova, M.V.; Volchkov, P. Models of Congenital Adrenal Hyperplasia for Gene Therapies Testing. Int. J. Mol. Sci. 2023, 24, 5365. https://doi.org/10.3390/ijms24065365
Glazova O, Bastrich A, Deviatkin A, Onyanov N, Kaziakhmedova S, Shevkova L, Sakr N, Petrova D, Vorontsova MV, Volchkov P. Models of Congenital Adrenal Hyperplasia for Gene Therapies Testing. International Journal of Molecular Sciences. 2023; 24(6):5365. https://doi.org/10.3390/ijms24065365
Chicago/Turabian StyleGlazova, Olga, Asya Bastrich, Andrei Deviatkin, Nikita Onyanov, Samira Kaziakhmedova, Liudmila Shevkova, Nawar Sakr, Daria Petrova, Maria V. Vorontsova, and Pavel Volchkov. 2023. "Models of Congenital Adrenal Hyperplasia for Gene Therapies Testing" International Journal of Molecular Sciences 24, no. 6: 5365. https://doi.org/10.3390/ijms24065365
APA StyleGlazova, O., Bastrich, A., Deviatkin, A., Onyanov, N., Kaziakhmedova, S., Shevkova, L., Sakr, N., Petrova, D., Vorontsova, M. V., & Volchkov, P. (2023). Models of Congenital Adrenal Hyperplasia for Gene Therapies Testing. International Journal of Molecular Sciences, 24(6), 5365. https://doi.org/10.3390/ijms24065365





