Abstract
Cholic acid is a trihydroxy bile acid with a nice peculiarity: the average distance between the oxygen atoms (O7 and O12) of the hydroxy groups located at C7 and C12 carbon atoms is 4.5 Å, a value which perfectly matches with the O/O tetrahedral edge distance in Ih ice. In the solid phase, they are involved in the formation of hydrogen bonds with other cholic acid units and solvents. This fact was satisfactorily used for designing a cholic dimer which encapsulates one single water molecule between two cholic residues, its oxygen atom (Ow) being exactly located at the centroid of a distorted tetrahedron formed by the four steroid hydroxy groups. The water molecule participates in four hydrogen bonds, with the water simultaneously being an acceptor from the 2 O12 (hydrogen lengths are 2.177 Å and 2.114 Å) and a donor towards the 2 O7 (hydrogen bond lengths are 1.866 Å and 1.920 Å). These facts suggest that this system can be a nice model for the theoretical study of the formation of ice-like structures. These are frequently proposed to describe the water structure found in a plethora of systems (water interfaces, metal complexes, solubilized hydrophobic species, proteins, and confined carbon nanotubes). The above tetrahedral structure is proposed as a reference model for those systems, and the results obtained from the application of the atoms in molecules theory are presented here. Furthermore, the structure of the whole system allows a division into two interesting subsystems in which water is the acceptor of one hydrogen bond and the donor of another. The analysis of the calculated electron density is performed through its gradient vector and the Laplacian. The calculation of the complexation energy used correction of the basis set superposition error (BSSE) with the counterpoise method. As expected, four critical points located in the H…O bond paths were identified. All calculated parameters obey the proposed criteria for hydrogen bonds. The total energy for the interaction in the tetrahedral structure is 54.29 kJ/mol, while the summation obtained of the two independent subsystems and the one between the alkyl rings without water is only 2.5 kJ/mol higher. This concordance, together with the calculated values for the electron density, the Laplacian of the electron density, and the lengths of the oxygen atom and the hydrogen atom (involved in the formation of each hydrogen bond) to the hydrogen bond critical point, suggests that each pair of hydrogen bonds can be considered independent of each other.
1. Introduction
During evolution, nature has learned to distinguish what works from what does not. Consequently, all living beings have adopted successful mechanisms and molecules for solving the challenges they must face to achieve a particular purpose. The knowledge of the involved processes provides the scientific community with strategies for designing new molecules with specific properties to reach a desired target. According to Menger [], the design of a molecule from the beginning with a list of optimal functionalities is not an easy task, although it may be facilitated by emulating the biological mechanisms. Among many other molecules, bile acids fulfill the steps described by Lenh [] involved in the evolution of matter. Hofmann [] has discussed their structural variation and its possible evolutionary significance. The steroid nucleus has been implied in a key evolutionary step as it is ubiquitous in animals (as hormones, cholesterol, and bile acids) and plants (as brassinosteroids).
All the above information led us to use natural bile acids as raw materials for synthesizing derivatives that self-organize into new supramolecular structures [,,,]. Among the designs, we studied a cholic dimer, which encapsulates one single water molecule between two cholic residues [].
Bile acids (BAs) have a bifacial polarity since the hydroxy groups (up to three at C3, C7, and C12 carbon atoms) lie beneath the plane of the steroid nucleus (hydrophilic α-side). The characterization of the crystal structures of BA and their derivatives by X-ray analysis has been a topic of interest for years [,,,,,,,,]. Common to all crystal structures is that the hydroxy groups are always involved in the formation of hydrogen bonds (HB) with other BA molecules, the solvent, or both species. In cholic acid (Figure 1), the average hydrogen bond distances formed by the C7-OH and C12-OH hydroxy groups (from here these oxygen atoms will be identified as O7 and O12) with water have been recompiled with values of 2.79 ± 0.09 Å and 2.86 ± 0.10 Å, respectively [], the O7/O12 distance being 4.5 Å, a value which perfectly matches with the O/O tetrahedral edge distance in Ih ice, respectively [,]. These facts suggest the design of the cholic dimer mentioned previously []. In this complex, the water oxygen atom (Ow) is exactly located at the centroid of a distorted tetrahedron formed by the four steroid hydroxy groups (Figure 2, left). Both O12-H are hydrogen bond donors towards Ow, while both O7-H are acceptors from Ow. Figure 2 (right) shows the values for the four hydrogen bonds. It may be noticed that the O7…Ow distances are close to the one measured in Ih ice while the O12…Ow distances match the O…O distance observed in the water dimer in gas phase (see below). In his review on the hydrogen bond in the solid state, Steiner [] has indicated average values of 1.880(2) Å and 2.825(2) Å for H…OH2 and O…O distances, respectively, for the dimer HO-H…OH2.
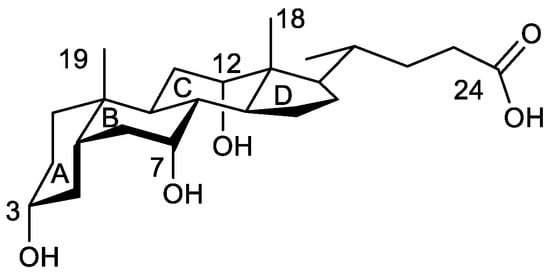
Figure 1.
Structure of cholic acid. Significant carbon atoms are numbered as well as the four rings of the steroid nucleus. In the text, the numbers of oxygen atoms are those of the carbon atoms to which they are bonded.
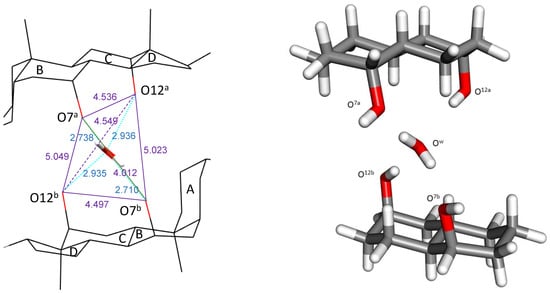
Figure 2.
Left: Oxygen–Oxygen distances (lines and data in amethyst color) of the tetrahedron formed by the O7-H and O12-H hydroxy atoms of the two steroid residues encapsulating a water molecule located at their centroid []. The four hydrogen bonds are indicated with blue lines, as are the hydrogen bond distance values (Ow-H…O7 and O12-H…Ow). All data in Å. Right: Simplified system model.
The term ice-like is frequently used to describe the structure of water found in a plethora of systems. Among them, we can mention water clusters (H2O)n in compounds as metal-organic networks in the solid state [], liquid water solubilizing hydrophobic species [,,,] or proteins [,], and in water interfaces []. However, Bonn et al. [] have concluded that the vibrational spectrum of water at both water-lipid and water-protein interfaces is inconsistent with the presence of “ice-like” structures. Ice-like behavior is also recognized in carbon nanotubes (CNTs) [,,,] and in sub-nanometer carbon slit pores [], but it can be suppressed in supercooled water in tight confinements [].
Weissmann et al. [] self-limited their study on the hydrogen bond in an ice-like structure to “the interactions of one water molecule with its four nearest neighbors” somehow accepting that a water molecule should form four hydrogen bonds, the oxygen atom simultaneously being a hydrogen bond donor and acceptor (two of each). Therefore, in the analysis of published structures that we have carried out, only tetrahedral water and the interaction with neutral oxygen atoms have been considered. Different O…O hydrogen bond distances are observed in water clusters in metal-organic complexes, depending on the role of the oxygen atom as acceptor or donor of a hydrogen bond [,,]. This difference can be as high as 0.17 Å (measured from cif files) []. In our opinion, this distinction has not been sufficiently analyzed in the literature. Obviously, such a distinction cannot be made in Ih ice, as all O…O hydrogen bond distances have the same value.
All the previous facts, together with the perfect distinction between hydrogen bond donors and acceptors for water linked to a tetrahedral hydroxy structure, surrounded by apolar alkyl skeletons, constitute a unique model to pursue a theoretical study. Keeping this in mind, the “atoms in molecules” (AIM) theory [,] has been applied to a model system derived from this ice-like structure. On the other hand, when analyzing BA crystals for the acceptance of the formation of a hydrogen bond, the geometric criteria (bond lengths and angles) [] have been used exclusively. This is the first time that the AIM theory has been applied to a BA crystal.
2. Results and Discussion
2.1. Complex O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b
The electron density, ρ, is the starting point of the AIM theory. Its topology is easily deduced from the gradient vector, ∇ρ, and the Laplacian, ∇2ρ. The electron density is usually visualized by drawing contour lines connecting electron density points with the same value. Figure 3 and Figure 4 show two examples for the present system. In Figure 3, the plane is defined by the oxygen nuclei O7a, O7b, and Ow, while in Figure 4, the plane is defined by oxygen atoms O12a, O12b, and Ow. The thin gray lines are defined by infinitesimal gradient vectors, which describe gradient paths.
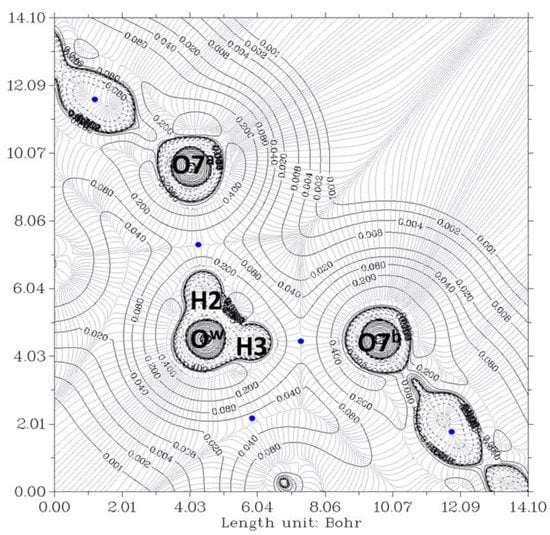
Figure 3.
The electron density contour of the O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b complex (thin black lines) and BCP (3,-1) (blue dots). The plane is defined by O7a, O7b, and Ow oxygen atoms of the pseudo-bile acid residues and water, respectively. Thin gray lines correspond to the gradient of the electron density.
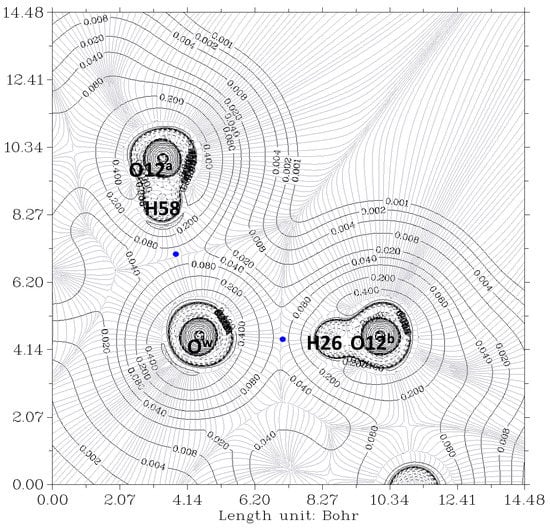
Figure 4.
The electron density contour of the O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b complex (thin black lines) and BCP (3,-1) (blue dots). The plane is defined by the O12a, O12b, and Ow oxygen atoms of the bile acid residues and water, respectively. Thin gray lines correspond to the gradient of the electron density.
When ∇2ρ < 0, the electronic charge is locally concentrated, as in the case of covalent bonds []. When ∇2ρ > 0, the electronic charge is locally depleted [], resulting in what are called closed-shell interactions. This happens in hydrogen bonds (HB), in which the charge concentrations are separately localized in the basins of the neighboring atoms []. Figure 5 shows bond critical points where the gradient ∇ρ vanishes. Numbers 17, 43, 50, and 59, located between hydrogen and oxygen atoms, correspond to hydrogen bond critical points (HBCP), which are (3,−1) saddle points. Other numbers correspond to covalent bonds (located, for instance, between two carbon atoms).
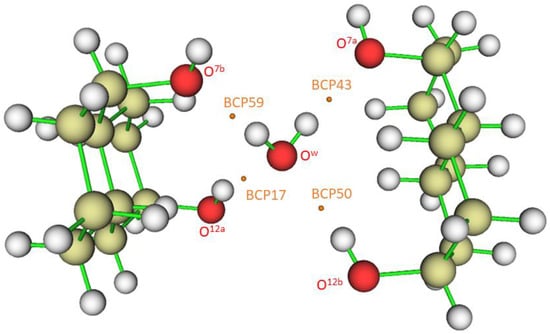
Figure 5.
Bond critical points (BCP) and hydrogen bond critical points (HBCP) obtained for the complex O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b. HBCPs are identified with numbers 17, 43, 50, and 59.
In Figure 3, the contour lines of the electronic density around the water oxygen (Ow) basin resemble a Mickey Mouse profile. This is a consequence of the fact that the two hydrogen atoms of water (named H2 and H3 in the Figure) form covalent bonds with Ow. In other words, Ow is behaving as a HB donor, while from this perspective, the basin of the O7 oxygen atom has a circle shape. Similarly, Figure 4 shows the contour lines of the hydrogen bonds between Ow and the two O12-H hydroxy groups. The plane in the Figure is defined by these three oxygen atoms. Now the contour around the Ow resembles a basin, while the profiles around the O12-H groups resemble peanuts. The two O12 are donors, and Ow is the acceptor. Furthermore, the bond paths of the four hydrogen bonds link the expected two atoms, the hydrogen and the acceptor. It is evident that the first condition of the criteria to characterize a hydrogen bond published by Popelier [,] is fulfilled. Furthermore, according to Popelier [], the ρ values at the HBCP, ρb, should be in the range 0.002−0.035 au, Table 1 showing that this is the case for the four HB. These values are about one order of magnitude smaller than those found for a covalent bond (ρb = 0.391 au, for O-H in H2O) []. On the other hand, it may be noticed that the values when water is a donor (towards O7) are almost double than when it behaves as an acceptor (from O12).

Table 1.
Lengths involved in the formation of hydrogen bonds were determined from the crystal structure and calculated along with electron density and Laplacian values.
The correlation between the O–O length and ρb has been published [,]. The shorter the former, the higher the latter. The values obtained here differ by less than ±0.004 au with those obtained from the equation , ( in Å) []. They also match values recompiled by Steiner [] (see Figure 3 of this reference).
A third criterion proposed by Popelier refers to the Laplacian of the charge density evaluated at the bond critical point, where charge density is a local minimum along the bond path, i.e., ρb is locally depleted with respect to neighboring points along the bond path. The range values (Table 1) are also within the proposed range of 0.024–0.139 au. ∇2ρb follows an analogous dependence with the O-O length than ρb.
Previous ρb and ∇2ρb values may be compared with those for the water dimer, H-O-H…OH2 in the gas phase. The water dimer is a system of two water molecules bound by a single hydrogen bond, often used as the paradigmatic system [,]. Its equilibrium geometry is well-known, as is the dissociation energy. The dimer has a “trans-linear” structure, and the O…O distance was first measured by Dyke et al. from the microwave spectrum [,,], the value being rO…O = 2.98 ± 0.04 Å. Lane [] has calculated a value of rO…O = 2.91 Å (truncated value to the hundredth of Å) as the best estimation. The O-H distances depend on the role of the water molecules, with values of rOH = 0.958 pm Å and rOH = 0.95 Å when water is a donor or acceptor, respectively []. Bader et al. [] have obtained that ρb and ∇2ρb are 0.0199 and 0.0624 (data in au), respectively. Other values can be found elsewhere [,]. From the four values of ρb (Table 1), an average value of 0.020 au is obtained, which matches the one for the water dimer. The ∇2ρb value for H-O-H…OH2 is closer to those in which the oxygens (O12) of hydroxy groups are donors and Ow is an acceptor. It should be noticed that in these last two cases, the rOO lengths are also closer to that of the H-O-H…OH2 dimer.
The mutual penetration of the hydrogen (H) and acceptor atoms (A, oxygen) is another criterion of hydrogen bond formation. This criterion is often considered a necessary and sufficient condition for the classification of an intermolecular interaction as hydrogen bonding []. It is estimated as (i, are the atoms involved in the hydrogen bond, A or H), ri being the bonded radius of each atom and the corresponding nonbonded radius []. The nonbonded radius is the distance of a nucleus from a given electron density contour (usually 0.001 au) in the absence of interaction. This value is taken because it yields atomic diameters in good agreement with van der Waals radii in the gas phase []. The bonded radius is the distance from a nucleus to the bond critical point (HBCP). Numbers 17, 43, 50, and 59 identify these HBCPs in Figure 6. Table 1 shows the HBCP…O and HBCP…H lengths calculated for the complex. Obviously, the sum of both lengths should coincide with the imposed one from the crystal (r1+ r2 = rO…H length in Table 1). The HBCP…H length, r2, for the hydrogen bonds with Ow as acceptor is larger (>0.1 Å) than those for Ow being the donor. All of them are considerable smaller than this distance for the water dimer in the gas phase (=1.34 Å). Accepting that = 1.1 Å [], < 0 in all cases. Similarly, if = 1.58 Å [], then < 0. These data provide evidence of a mutual penetration of hydrogen and oxygen atoms, a conclusion which may be raised from checking the contour electron density values of Figure 4 and Figure 5. It should be noted that Isaev has defined [] , i.e., and . In all cases, , meaning that the hydrogen atom is more penetrated than the acceptor one.
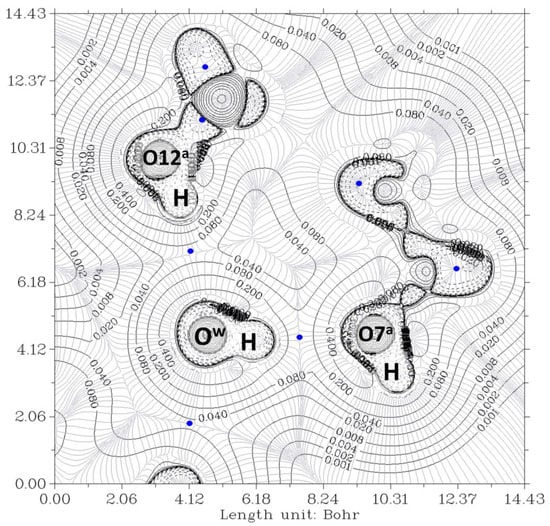
Figure 6.
The electron density contour of the O12a-H…Ow-H…O7a complex (thin black lines) and BCP (3,-1) (blue dots). O12a, O7a, and Ow are oxygen atoms from the pseudo-bile acid residues and water, respectively. Thin gray lines correspond to the gradient of the electron density.
2.2. Complexes O12a-H…Ow-H…O7a, O12b-H…Ow-H…O7b and O12a-H/H…O7a//O12b-H/H…O7b
Without changing the coordinates of the atoms, the whole complex O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b can be divided into two independent complexes, O12a-H…Ow-H…O7a and O12b-H…Ow-H…O7b, in which the water molecule only interacts with one of the pseudo-steroid residues of the original complex. It must be noticed that in both hemicomplexes, the water molecule is participating in the formation of two hydrogen bonds, being an acceptor and donor towards the O12 and O7 oxygen atoms, respectively. Thus, in each complex, only one O-H bond of water participates as a donor.
Figure 6 and Figure 7 show the contour lines at the planes defined by O7a-Ow-O12a and O7b-Ow-O12b, respectively. In both cases, Ow and O12 exhibit peanut profiles directed towards the associated acceptor atoms O7 and Ow, respectively. In all cases, the bond paths of the four hydrogen bonds link the expected two atoms, and the HBCP is indicated with a blue color point. The analysis of the data was carried out as previously. The HBCPs are identified by numbers (Figure not shown), and, for association purposes, the HBCPs of the full complex are shown in brackets. Table 2 shows the obtained results.
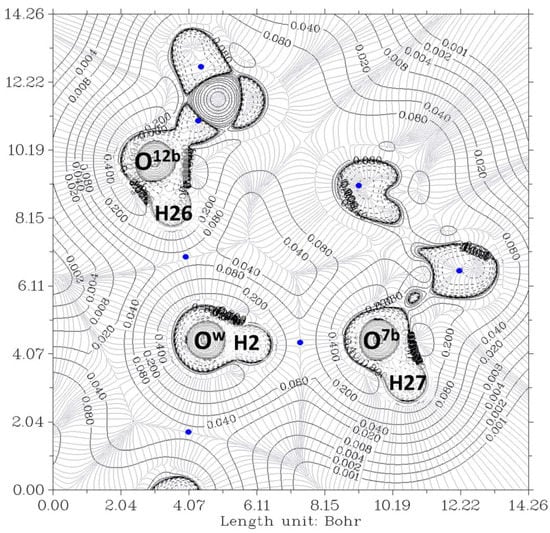
Figure 7.
The electron density contour of the O7b-Ow-O12b complex (thin black lines) and BCP (3,-1) (blue dots). O12b, O7b, and Ow are oxygen atoms from the pseudo-bile acid residues and water, respectively. Thin gray lines correspond to the gradient of the electron density.

Table 2.
Electron density, Laplacian of the electron density, and lengths involved in the formation of hydrogen bonds of the two semi complexes: O12a-H…Ow-H…O7a and O12a-H…Ow-H…O7a.
It may be observed that all the values in Table 2 perfectly match those in Table 1. This is partially due to the fact that the original geometric parameters of the C-H2O-C crystal are kept constant. Because of previous agreements, the mutual penetration of hydrogen and oxygen atoms is not discussed.
Finally, the electron density of the complex without water, O12a-H/H…O7a//O12b-H/H…O7b, has also been studied. Figure 8 shows the contour map of the two halves of the complex. As it was expected, the contour lines strongly differ from previous ones, and HBCP are not observed.
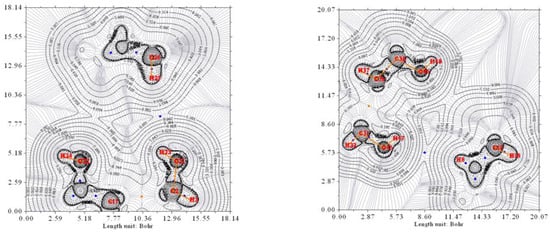
Figure 8.
The electron density contour of the O12a-H/H…O7a//O12b-H/H…O7b complex (thin black lines). O7a and O7b are oxygen atoms from the pseudo-bile acid residues. Thin gray lines correspond to the gradient of the electron density.
2.3. Energy of Hydrogen Bonds
The interaction energy for the formation of the complex O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b is −54.29 kJ mol−1. This value cannot be exclusively ascribed to the formation of the four hydrogen bonds. In fact, for the complex O12a-H/H…O7a//O12b-H/H…O7b (in which the water molecule has been removed), a value of −4.60 kJ/mol has been calculated. Because of the length differences between the hydrogen bonds in which Ow is the donor and those in which it is the acceptor (2.7 Å vs. 2.9 Å), the energy of each hydrogen bond is expected to be different []. Having these considerations in mind, the average energy of the hydrogen bonds is −13.57 kJ/mol. Such a value indicates that they are moderate hydrogen bonds according to Jeffrey’s categories [] or weak to medium according to the ranges proposed by Emamian et al. []. Rocher-Casterline et al. [] have determined a value of 13.2 ± 0.5 kJ mol−1 for the bond dissociation energy (Do) of the water dimer. Ruscic [], from a new partition function for water, has obtained dissociation enthalpy values for the water dimer, the values being 13.220 ± 0.096 kJ mol−1 and 15.454 ± 0.074 kJ mol−1 at 0 K and 298.15 K, respectively, and Feyereisen et al. [], from the thermal conductivity of the vapor, measured a value of −15.07 ± 2.1 kJ mol−1. Most of the values calculated theoretically for this dimer are within the interval −13.4/−23.1 kJ mol−1 [,,,,,,,,].
For the formation of hydrogen bonds between water and methanol, in gas phase, Moin et al. [] have obtained values of 1.96–2.04 Å (OmethH…Ow) and 1.94–2.02 Å (OwH…Ometh), for the H…O distances, while the hydrogen bond energies were in the ranges of −20.45/−27.04 kJ mol−1 (OmethH…Ow) and −21.24/−29.39 kJ mol−1 (OwH…Ometh), the values depending on the level of the theory. These values are in line with the different behavior of water depending on whether it is a donor or acceptor, as observed previously.
For a series of hydrogen-bonded complexes between nitrites and hydrogen chloride, Boyd and Choi [] have noticed a correlation between the electron density at the HBCP ρb and the energy of the hydrogen bond. The energies ranged from 10 kJ/mol to 38 kJmol, while the range of ρb was 0.01103−0.02391. Many other equations have been published; the subject is being reviewed by Rozenberg []. There is no objective reason to choose one or another equation for the present system, and as an orientation, we will use the following relationship that Rozenberg obtained from 24 equations:
E(kJ/mol) = −(6.6 ± 8.0) + (1215 ± 440)ρ
After its application to each hydrogen bond of the present system, the summation of the individual values gives a total energy of −72 ± 24 kJ/mol, with a high standard deviation.
As indicated above, the nature of the complex O12a-H/H…O7a//O12b-H/H…O7b allows the calculation of the interaction of two subsystems, O12a-H…Ow-H…O7a and O12b-H…Ow-H…O7b, both having two hydrogen bonds with water acting as donor and acceptor. The calculated values are −25.91 kJ/mol and −21.18 kJ/mol for the “a” and “b” subsystems, respectively. By considering the interaction energy between the two pseudosteroids) (see above) and the previous values, the difference between the calculated energy of the whole system and that resulting from the sum of the three subsystems is only 2.5 kJ/mol.
3. Materials and Methods
Crystal Structure and Computational Details
The crystal structure of the reference system (C-H2O-C) was previously published []. In this reference, a complete image of Figure 2, is shown on the left. The Cif files (CCDC 867499) contain the supplementary crystallographic data for the C-suc-C crystal (the acronym given in that paper). These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Given the high number of atoms involved in the two bile acid dimers, to analyze the interaction with the water molecule, we have simplified the system by reducing the number of atoms in the bile acid unit while keeping the same geometric parameters of the remaining atoms. Thus, A and D rings were suppressed, and the carbon atoms linking them to B and C rings were replaced by hydrogen atoms. This system will be referred to as O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b, where the superscripts “a” and “b” refer to the upper and lower pseudo-bile acid residues, respectively (see Figure 2). This complex is later divided into two independent subsystems, named O12a-H…Ow-H…O7a and O12b-H…Ow-H, which allow the calculation of the interaction of the water molecule with only one of the pseudo-steroid residues. The interaction between the two pseudo-bile acid residues, without water complexed between them, O12a-H/H…O7a//O12b-H/H…O7b, has also been studied.
We have maintained the original interatomic distances obtained from the x-ray resolution of the C-H2O-C complex, and no minimization of the energy of the complex was carried out. Calculations of the complexation energy used for correction of the basis set superposition error (BSSE) with the counterpoise method implemented in Gaussian 19 []. Laplacian of electronic density and critical points (AIM) were calculated using the Multiwfn_3.8_dev software [].
4. Conclusions
There are two main oxygen-oxygen (rOO) distances when a hydrogen bond is formed between water molecules: the one observed in the gas phase in the formation of a dimer (rOO = 2.98 Å) and the one in ice (rOO = 2.75 Å). Both lengths are observed in the C-succ-C crystal, in which a water molecule is encapsulated by four hydroxy groups belonging to two cholic acid dimers. The shorter one corresponds to hydrogen bonds in which the water oxygen is donor and the larger one when it is the acceptor. The application of the AIM theory to a simplified system O12a-H…Ow-H…O7a//O12b-H…Ow-H…O7b confirms the existence of saddle critical points (HBCP) in all four of these hydrogen bonds. The estimated interaction energy in the formation of the complex, −54.29 kJ mol−1, is in acceptable agreement with the summation of the energies of the two hemicomplexes, O12a-H…Ow-H…O7a and O12b-H…Ow-H…O7b, in which the water molecule forms two hydrogen bonds (acting as donor and acceptor) and the interaction energy of the two pseudo-steroid nucleus O12a-H/H…O7a//O12b-H/H…O7b (i.e., without complexed water). This fact and the calculated values for the electron density, the Laplacian of the electron density, and the lengths of the oxygen atom and the hydrogen atom (involved in the formation of each hydrogen bond) to the HBCP suggest that each pair of hydrogen bonds can be considered independent of each other.
Author Contributions
Conceptualization, J.A.S. and J.V.T.; methodology, J.A.S. and J.V.T.; formal analysis, M.P.V.-T., F.M., J.A.S. and J.V.T.; investigation, M.P.V.-T., F.M. and S.d.F.; writing—original draft preparation, writing—review and editing, M.P.V.-T., F.M., J.A.S. and J.V.T.; funding acquisition, M.P.V.-T., F.M., J.A.S. and J.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministerio de Ciencia y Tecnología, Spain (Project MAT201786109P).
Acknowledgments
Part of this paper was presented at the 26th International Electronic Conference on Synthetic Organic Chemistry. 15–30 November 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menger, F.M. Supramolecular chemistry and self-assembly. Proc. Natl. Acad. Sci. USA 2002, 99, 4818–4822. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef]
- Soto, V.H.; Jover, A.; Meijide, F.; Vázquez Tato, J.; Galantini, L.; Pavel, N.V. Supramolecular structures generated by a p-tert-butylphenyl-amide derivative of cholic acid. From vesicles to molecular tubes. Adv. Mater. 2007, 19, 1752–1756. [Google Scholar] [CrossRef]
- Miragaya, J.; Jover, A.; Fraga, F.; Meijide, F.; Tato, J.V. Enantioresolution and Chameleonic Mimicry of 2-Butanol with an Adamantylacetyl Derivative of Cholic Acid. Cryst. Growth Des. 2010, 10, 1124–1129. [Google Scholar] [CrossRef]
- Manghisi, N.; Leggio, C.; Jover, A.; Meijide, F.; Pavel, N.V.; Tellini, V.H.S.; Tato, J.V.; Agostino, R.G.; Galantini, L. Catanionic Tubules with Tunable Charge. Angew. Chem. Int. Ed. 2010, 49, 6604–6607. [Google Scholar] [CrossRef]
- Galantini, L.; di Gregorio, M.C.; Gubitosi, M.; Travaglini, L.; Vázquez Tato, J.; Jover, A.; Meijbe, F.; Tellini, V.H.S.; Pavel, N.V. Bile salts and derivatives: Rigid un-conventional amphiphiles as dispersants, carriers and superstructure building blocks. Curr. Opin. Colloid Interface Sci. 2015, 20, 170–182. [Google Scholar] [CrossRef]
- Soto, V.H.; Alvarez, M.; Meijide, F.; Trillo, J.V.; Antelo, A.; Jover, A.; Galantini, L.; Tato, J.V. Ice-like encapsulated water by two cholic acid moieties. Steroids 2012, 77, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Meijide, F.; de Frutos, S.; Soto, V.H.; Jover, A.; Seijas, J.A.; Vázquez-Tato, M.P.; Fraga, F.; Tato, J.V. A Standard Structure for Bile Acids and Derivatives. Crystals 2018, 8, 86. [Google Scholar] [CrossRef]
- Giglio, E. Structural aspect of inclusion compounds formed by organic host lattices. In Inclusion Compounds of Deoxycholic Acid; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, UK, 1984; pp. 207–229. [Google Scholar]
- Miyata, M.; Sada, K. Deoxycholic acid and related hosts. In Comprehensive Supra-Molecular Chemistry 6; MacNicol, D.D.T.F., Bishop, R., Eds.; Elsevier: Oxford, UK, 1996; pp. 147–176. [Google Scholar]
- Campanelli, A.R.; De Sanctis, S.C.; Giglio, E.; Pavel, N.V.; Quagliata, C. From crystal to micelle: A new approach to the micellar structure. J. Incl. Phenom. Macrocycl. Chem. 1989, 7, 391–400. [Google Scholar] [CrossRef]
- Sada, K.; Sugahara, M.; Kato, K.; Miyata, M. Controlled Expansion of a Molecular Cavity in a Steroid Host Compound. J. Am. Chem. Soc. 2001, 123, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Sada, K.; Miyata, M. A robust structural motif in inclusion crystals of nor-bile acids. Chem. Commun. 1999, 3, 293–294. [Google Scholar] [CrossRef]
- Sugahara, M.; Hirose, J.; Sada, K.; Miyata, M. Inclusion Abilities of Bile Acids with Different Side Chain Length. Mol. Cryst. Liq. Cryst. 2001, 356, 155–162. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Aoki, Y.; Sada, K.; Miyata, M. Selective Inclusion Phenomena in Lithocholamide Crystal Lattices; Design of Bilayered Assemblies through Ladder-type Hydrogen Bonding Network. Chem. Lett. 1998, 27, 1289–1290. [Google Scholar] [CrossRef]
- Miyata, M.; Tohnai, N.; Hisaki, I. Supramolecular Chirality in Crystalline Assemblies of Bile Acids and Their Derivatives; Three-Axial, Tilt, Helical, and Bundle Chirality. Molecules 2007, 12, 1973–2000. [Google Scholar] [CrossRef]
- Fletcher, N.H. The Chemical Physics of Ice. The Chemical Physics of Ice; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar]
- Bergmann, U.; Di Cicco, A.; Wernet, P.; Principi, E.; Glatzel, P.; Nilsson, A. Nearest-neighbor oxygen distances in liquid water and ice observed by x-ray Raman based extended x-ray absorption fine structure. J. Chem. Phys. 2007, 127, 174504. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Atwood, J.L.; Barbour, L.J.; Ness, T.J.; Raston, C.L.; Raston, P.L. A Well-Resolved Ice-like (H2O)8 Cluster in an Organic Supramolecular Complex. J. Am. Chem. Soc. 2001, 123, 7192–7193. [Google Scholar] [CrossRef]
- Barbour, L.J.; Orr, G.W.; Atwood, J.L. An intermolecular (H2O)10 cluster in a solid-state supramolecular complex. Nature 1998, 393, 671–673. [Google Scholar] [CrossRef]
- Barbour, L.; Orr, G.; Atwood, J. CCDC 145404: Experimental Crystal Structure Determination. Chem. Commun. 2000, 859. [Google Scholar] [CrossRef]
- Hoelzl, C.; Horinek, D. Pressure increases the ice-like order of water at hydrophobic interfaces. Phys. Chem. Chem. Phys. 2018, 20, 21257–21261. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A. First example of an ice-like water hexamer boat tape structure in a supramolecular organic host. Chem. Commun. 2006, 12, 1825–1827. [Google Scholar] [CrossRef]
- Smolin, N.; Daggett, V. Formation of Ice-like Water Structure on the Surface of an Antifreeze Protein. J. Phys. Chem. B 2008, 112, 6193–6202. [Google Scholar] [CrossRef] [PubMed]
- Mahatabuddin, S.; Fukami, D.; Arai, T.; Nishimiya, Y.; Shimizu, R.; Shibazaki, C.; Kondo, H.; Adachi, M.; Tsuda, S. Polypentagonal ice-like water networks emerge solely in an activity-improved variant of ice-binding protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5456–5461. [Google Scholar] [CrossRef] [PubMed]
- Odendahl, N.L.; Geissler, P.L. Local Ice-like Structure at the Liquid Water Surface. J. Am. Chem. Soc. 2022, 144, 11178–11188. [Google Scholar] [CrossRef] [PubMed]
- Bonn, M.; Bakker, H.J.; Tong, Y.; Backus, E.H.G. No Ice-Like Water at Aqueous Biological Interfaces. Biointerphases 2012, 7, 20. [Google Scholar] [CrossRef]
- He, Z.; Zhou, J.; Lu, X.; Corry, B. Ice-like Water Structure in Carbon Nanotube (8,8) Induces Cationic Hydration Enhancement. J. Phys. Chem. C 2013, 117, 11412–11420. [Google Scholar] [CrossRef]
- Takaiwa, D.; Hatano, I.; Koga, K.; Tanaka, H. Phase diagram of water in carbon nanotubes. Proc. Natl. Acad. Sci. USA 2008, 105, 39–43. [Google Scholar] [CrossRef]
- Pascal, T.A.; Goddard, W.A.; Jung, Y. Entropy and the driving force for the filling of carbon nanotubes with water. Proc. Natl. Acad. Sci. USA 2011, 108, 11794–11798. [Google Scholar] [CrossRef]
- Agrawal, K.V.; Shimizu, S.; Drahushuk, L.W.; Kilcoyne, D.; Strano, M.S. Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes. Nat. Nanotechnol. 2017, 12, 267–273. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Futamura, R.; Iiyama, T. Ice-like Structure of Water Confined in Hydrophobic Sub-nanometer Spaces at Room Temperature. Chem. Lett. 2022, 51, 760–764. [Google Scholar] [CrossRef]
- Banerjee, D.; Bhat, S.N.; Bhat, S.V.; Leporini, D. Molecular probe dynamics reveals suppression of ice-like regions in strongly confined supercooled water. PLoS ONE 2012, 7, e44382. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, M.; Blum, L.; Cohan, N.V. On the hydrogen bond in an ice-like structure. Chem. Phys. Lett. 1967, 1, 95–98. [Google Scholar] [CrossRef]
- Sun, D.; Xu, H.-R.; Yang, C.-F.; Wei, Z.-H.; Zhang, N.; Huang, R.-B.; Zheng, L.-S. Encapsulated Diverse Water Aggregates in Two Ag(I)/4,4’-Bipyridine/Dicarboxylate Hosts: 1D Water Tape and Chain. Crystal Growth Des. 2010, 10, 4642–4649. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Sun, H.-L.; Gao, S. Cyclic water pentamer in a tape-like structure. Chem. Commun. 2004, 19, 2220–2221. [Google Scholar] [CrossRef]
- Popelier, P.L.A. On the full topology of the Laplacian of the electron densityOn the full topology of the Laplacian of the electron density. Coord. Chem Rev. 2000, 197, 169–189. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen Bond—Definitions, Criteria of Existence and Various Types. In Understanding Hydrogen Bonds: Theoretical and Experimental Views; From Book Series: Theoretical and Computational Chemistry Series; Royal Society of Chemistry: London, UK, 2020; Chapter 1; pp. 1–40. [Google Scholar] [CrossRef]
- Popelier, P.L.A. Characterization of a Dihydrogen Bond on the Basis of the Electron Density. J. Phys. Chem. A 1998, 102, 1873–1878. [Google Scholar] [CrossRef]
- Carroll, M.T.; Chang, C.; Bader, R.F. Prediction of the structures of hydrogen-bonded complexes using the laplacian of the charge density. Mol. Phys. 1988, 63, 387–405. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, I.; Elguero, J. Bond Length–Electron Density Relationships: From Covalent Bonds to Hydrogen Bond Interac-tions. Struct. Chem. 1998, 9, 243–247. [Google Scholar] [CrossRef]
- Tang, T.-H.; Deretey, E.; Jensen, S.J.K.; Csizmadia, I.G. Hydrogen bonds: Relation between lengths and electron densities at bond critical points. Eur. Phys. J. D 2005, 37, 217–222. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Cole, W.T.; Saykally, R.J. The water dimer I: Experimental characterization. Chem. Phys. Lett. 2015, 633, 13–26. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Xantheas, S.S.; Saykally, R.J. The water dimer II: Theoretical investigations. Chem. Phys. Lett. 2018, 700, 163–175. [Google Scholar] [CrossRef]
- Dyke, T.R.; Muenter, J.S. Microwave spectrum and structure of hydrogen bonded water dimer. J. Chem. Phys. 1974, 60, 2929–2930. [Google Scholar] [CrossRef]
- Dyke, T.R.; Mack, K.M.; Muenter, J.S. The structure of water dimer from molecular beam electric resonance spectroscopy. J. Chem. Phys. 1977, 66, 498–510. [Google Scholar] [CrossRef]
- Odutola, J.A.; Dyke, T.R. Partially deuterated water dimers: Microwave spectra and structure. J. Chem. Phys. 1980, 72, 5062–5070. [Google Scholar] [CrossRef]
- Lane, J.R. CCSDTQ Optimized Geometry of Water Dimer. J. Chem. Theory Comput. 2013, 9, 316–323. [Google Scholar] [CrossRef]
- Grabowski, S.J. Ab Initio Calculations on Conventional and Unconventional Hydrogen BondsStudy of the Hydrogen Bond Strength. J. Phys. Chem. A 2001, 105, 10739–10746. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Isaev, A.N. Ammonia and phosphine complexes with proton donors. Hydrogen bonding from the backside of the N(P) lone pair. Comput. Theor. Chem. 2018, 1142, 28–38. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Wendler, K.; Thar, J.; Zahn, S.; Kirchner, B. Estimating the Hydrogen Bond Energy. J. Phys. Chem. A 2010, 114, 9529–9536. [Google Scholar] [CrossRef]
- Jeffrey, G.J. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997; p. 12. [Google Scholar]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Rocher-Casterline, B.E.; Ch’Ng, L.C.; Mollner, A.K.; Reisler, H. Communication: Determination of the bond dissociation energy (D0) of the water dimer, (H2O)2, by velocity map imaging. J. Chem. Phys. 2011, 134, 211101. [Google Scholar] [CrossRef]
- Ruscic, B. Active Thermochemical Tables: Water and Water Dimer. J. Phys. Chem. A 2013, 117, 11940–11953. [Google Scholar] [CrossRef]
- Feyereisen, M.W.; Feller, D.; Dixon, D.A. Hydrogen Bond Energy of the Water Dimer. J. Phys. Chem. 1996, 100, 2993–2997. [Google Scholar] [CrossRef]
- Grabowski, S.J. High-Level Ab Initio Calculations of Dihydrogen-Bonded Complexes. J. Phys. Chem. A 2000, 104, 5551–5557. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental Properties of the CH···O Interaction: Is It a True Hydrogen Bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Shank, A.; Wang, Y.; Kaledin, A.; Braams, B.J.; Bowman, J.M. Accurate ab initio and “hybrid” potential energy surfaces, intramolecular vibrational energies, and classical ir spectrum of the water dimer. J. Chem. Phys. 2009, 130, 144314. [Google Scholar] [CrossRef] [PubMed]
- Moin, S.T.; Hofer, T.S.; Randolf, B.R.; Rode, B.M. Structure and dynamics of methanol in water: A quantum mechanical charge field molecular dynamics study. J. Comput. Chem. 2011, 32, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.; Choi, S.C. Hydrogen bonding between nitriles and hydrogen halides and the topological properties of molecular charge distributions. Chem. Phys. Lett. 1986, 129, 62–65. [Google Scholar] [CrossRef]
- Rozenberg, M. The hydrogen bond—Practice and QTAIM theory. RSC Adv. 2014, 4, 26928–26931. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 RC; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).