Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates
Abstract
1. Introduction
2. Immune Response: Evolutionary Overview
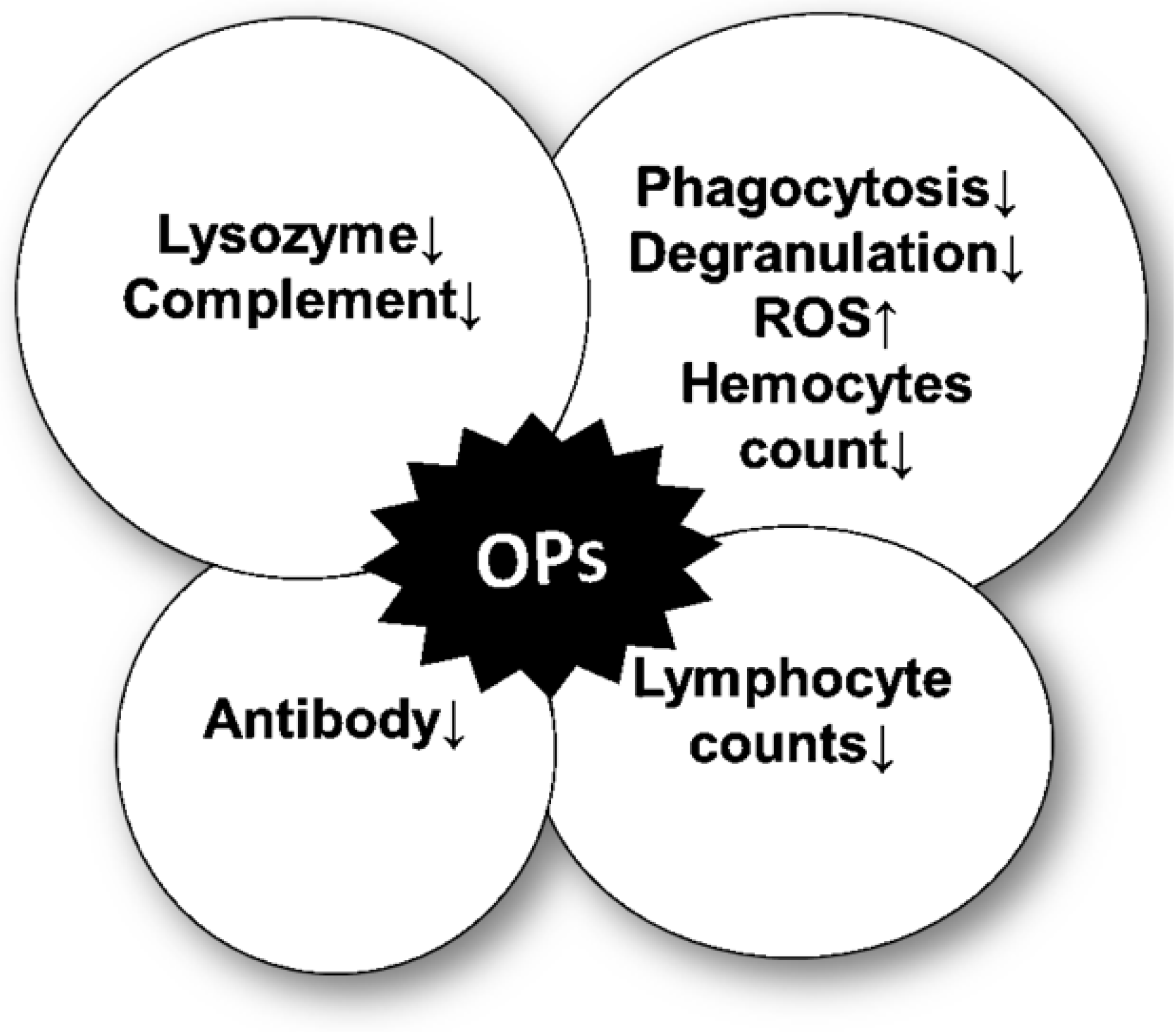
3. OP-Mediated Modulation of the Immune Response
3.1. OP-Mediated Modulation of the Innate Immune Response in Invertebrates
3.2. OP-Mediated Modulation of the Innate Immune Response of Vertebrates
3.2.1. Fish
3.2.2. Amphibia
3.2.3. Reptilia
3.2.4. Birds
3.2.5. Mammals
3.3. OP-Mediated Modulation of the Adaptive Immune Response in Vertebrates
3.3.1. Fish
3.3.2. Amphibia
3.3.3. Reptilia
3.3.4. Birds
3.3.5. Mammals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Al-Ghanim, K.A. Acude toxicity and effects of sub-lethal malathion exposure on biochemical and haematological parameters of Oreochromis niloticus. Sci. Res. Essays 2012, 7, 1674–1680. [Google Scholar]
- Derbalah, A.; Chidya, R.; Jadoon, W.; Sakugawa, H. Temporal trends in organophosphorus pesticides use and concentrations in river water in Japan, and risk assessment. J. Environ. Sci. 2019, 79, 135–152. [Google Scholar] [CrossRef]
- Robb, E.L.; Baker, M.B. Organophosphate Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Saborío, C.; Ishtar, E.; Mora Valverde, M.; Durán Monge, M.P. Organophospate poisoning. Med. Leg. Costa Rica 2019, 36, 110–117. [Google Scholar]
- Kwong, T.C. Organophosphate pesticides: Biochemistry and clinical toxicology. Ther. Drug Monit. 2002, 24, 144–149. [Google Scholar] [CrossRef]
- Camacho-Pérez, M.R.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Mercado-Salgado, U.; Ponce-Regalado, M.D.; Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Organophosphorus pesticides as modulating substances of inflammation through the cholinergic pathway. Int. J. Mol. Sci. 2022, 23, 4523. [Google Scholar] [CrossRef]
- Mulla, S.I.; Ameen, F.; Talwar, M.P.; Egani, S.A.M.A.S.; Bharagaya, R.N.; Saxena, G.; Tallur, P.N.; Ninnekar, H.Z. Organophosphate pesticides: Impact on environment, toxicity, and their degradation. Bioremediat. Ind. Waste Environ. Saf. 2020, 1, 265–290. [Google Scholar]
- Fanta, E.; Rios, F.S.A.; Romão, S.; Vianna, A.C.C.; Freiberger, S. Histopatología del pez Corydoras paleatus contaminado con niveles subletales de organofosforados en agua y alimentos. Ecotoxicol. Segur. Ambient. 2003, 54, 119–130. [Google Scholar]
- Furnes, B.; Schlenk, D. Metabolismo extrahepático de carbamatos y compuestos de tioéter organofosforados por los sistemas de monooxigenasa y citocromo P450 que contienen flavina. Metab. Disposición Fármacos 2005, 33, 214–218. [Google Scholar]
- Burkina, V.; Rasmussen, M.K.; Pilipenko, N.; Zamaratskaia, G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology 2017, 375, 10–27. [Google Scholar] [CrossRef]
- Mahajan, R.; Verma, S.; Chandel, S.; Chatterjee, S. Organophosphate pesticide: Usage, environmental exposure, health effects, and microbial bioremediation. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 473–490. [Google Scholar]
- Díaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Girón-Pérez, M.I. Modulation of immune response by organophosphorus pesticides: Fishes as a potential model in immunotoxicology. J. Immunol. Res. 2015, 2015, 213836. [Google Scholar] [CrossRef] [PubMed]
- Vittozzi, L.; Fabrizi, L.; Di Consiglio, E.; Testai, E. Mechanistic aspects of organophosphorothionate toxicity in fish and humans. Environ. Int. 2001, 26, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Santed, F.; Colomina, M.T.; Hernández, E.H. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016, 74, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Jett, D.A.; Lein, P.J. Noncholinesterase Mechanisms of Central and Peripheral Neurotoxicity: Muscarinic Receptors and Other Targets; Elsevier Inc.: Amsterdam, The Netherlands, 2005; ISBN 9780080543109. [Google Scholar]
- Bomser, J.A.; Casida, J.E. Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol. Lett. 2001, 119, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Huff, R.A.; Corcoran, J.J.; Anderson, J.K.; Abou-Donia, M.B. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J. Pharmacol. Exp. Ther. 1994, 269, 329–335. [Google Scholar]
- Esquivel-Sentíes, M.S.; Barrera, I.; Ortega, A.; Vega, L. Organophosphorous pesticide metabolite (DEDTP) induces changes in the activation status of human lymphocytes by modulating the interleukin 2 receptor signal transduction pathway. Toxicol. Appl. Pharmacol. 2010, 248, 122–133. [Google Scholar] [CrossRef]
- Perry, J.; Cotton, J.; Rahman, M.; Brumby, S. Organophosphate exposure and the chronic effects on farmers: A narrative review. Rural. Remote Health 2020, 20, 4508. [Google Scholar] [CrossRef]
- Thrasher, J.D.; Heuser, G.; Broughton, A. Immunological Abnormalities in Humans Chronically Exposed to Chlorpyrifos. Arch. Environ. Health Int. J. 2002, 57, 181–187. [Google Scholar] [CrossRef]
- Yang, K.J.; Lee, J.; Park, H.L. Organophosphate pesticide exposure and breast cancer risk: A rapid review of human, animal, and cell-based studies. Int. J. Environ. Res. Public Health 2020, 17, 5030. [Google Scholar] [CrossRef]
- Moretto, A. Testing for Organophosphate-Induced Delayed Polyneuropathy. Curr. Protoc. Toxicol. 1999, 1, 11–15. [Google Scholar] [CrossRef]
- Esa, A.H.; Warr, G.A.; Newcombe, D.S. Immunotoxicity of organophosphorus compounds: Modulation of cell-mediated immune responses by inhibition of monocyte accessory functions. Clin. Immunol. Immunopathol. 1988, 49, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Yang, C.C.; Deng, J.F.; Wu, M.L.; Ger, J.; Lin, H.C.; Chang, F.Y.; Lee, S.D. The clinical significance of hyperamylasemia in organophosphate poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.; Handy, R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 2003, 12, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M. A comprehensive review of pesticides and the immune dysregulation:mechanisms, evidence and consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Díaz-Reséndiz, K.J.G.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Ortiz-Lazareno, P.C.; Girón-Pérez, M.I. Phagocytosis and ROS production as biomarkers in Nile tilapia (Oreochromis niloticus) leukocytes by exposure to organophosphorus pesticides. Fish Shellfish Immunol. 2019, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.J.G.; Ortiz-Lazareno, P.C.; Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Girón-Pérez, M.I. Effect of diazinon, an organophosphate pesticide, on signal transduction and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 89, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibarra, G.A.; Giron-Perez, M.I.; Covantes-Rosales, C.E.; Ventura-Ramon, G.H.; Pérez-Sánchez, G.; López-Torres, A.; Díaz-Resendiz, K.J.G.; Becerril-Villanueva, E.; Pavón, L. Alterations in the non-neuronal cholinergic system induced by in-vitro exposure to diazoxon in spleen mononuclear cells of Nile tilapia (O. niloticus). Fish Shellfish Immunol. 2021, 108, 134–141. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Bernal-Ortega, J.A.; Covantes-Rosales, C.E.; Ortiz-Lazareno, P.C.; Toledo-Ibarra, G.A.; Ventura-Ramon, G.H.; Girón-Pérez, M.I. In-vitro effect of diazoxon, a metabolite of diazinon, on proliferation, signal transduction, and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 105, 8–15. [Google Scholar] [CrossRef]
- Corsini, E.; Sokooti, M.; Galli, C.L.; Moretto, A.; Colosio, C. Pesticide induced immunotoxicity in humans: A comprehensive review of the existing evidence. Toxicology 2013, 307, 123–135. [Google Scholar] [CrossRef]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Boehm, T.; Swann, J.B. Origin and evolution of adaptive immunity. Annu. Rev. Anim. Biosci. 2014, 2, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Somamoto, T.; Nakanishi, T. Chapter 6—Fish Immunology; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.-M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 95–119. ISBN 978-0-12-812211-2. [Google Scholar]
- Kordon, A.O.; Pinchuk, L.; Karsi, A. Adaptive Immune System in Fish. Turk. J. Fish. Aquat. Sci. 2021, 22, 4. [Google Scholar] [CrossRef]
- Makesh, M.; Bedekar, M.K.; Rajendran, K.V. Overview of Fish Immune System BT—Fish Immune System and Vaccines; Makesh, M., Rajendran, K.V., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 1–16. ISBN 978-981-19-1268-9. [Google Scholar]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Garate, O.F.; Gazzaniga, S.; Cochón, A.C. A comparative study of enzymatic and immunological parameters in Planorbarius corneus and Biomphalaria glabrata exposed to the organophosphate chlorpyrifos. Aquat. Toxicol. 2020, 225, 105544. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T cells in fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef]
- Rajak, P.; Dutta, M.; Roy, S. Effect of acute exposure of acephate on hemocyte abundance in a non-target victim Drosophila melanogaster. Toxicol. Environ. Chem. 2014, 96, 768–776. [Google Scholar] [CrossRef]
- Rajak, P.; Dutta, M.; Roy, S. Altered differential hemocyte count in 3rd instar larvae of Drosophila melanogaster as a response to chronic exposure of Acephate. Interdiscip. Toxicol. 2015, 8, 84–88. [Google Scholar] [CrossRef]
- Rajak, P.; Khatun, S.; Dutta, M.; Mandi, M.; Roy, S. Chronic exposure to acephate triggers ROS-mediated injuries at organismal and sub-organismal levels of Drosophila melanogaster. Toxicol. Res. 2018, 7, 874–887. [Google Scholar] [CrossRef]
- Edward-George, P.J.; Ambrose, D.P. Impact of insecticides on the haemogram of Rhynocoris kumarii Ambrose and Livingstone (Hem., Reduviidae). J. Appl. Entomol. 2004, 128, 600–604. [Google Scholar] [CrossRef]
- Bautista-Covarrubias, J.C.; Aguilar-Juárez, M.; Voltolina, D.; Navarro-Nava, R.G.; Aranda-Morales, S.A.; Arreola-Hernández, J.O.; Soto-Jiménez, M.F.; Frías-Espericueta, M.G. Immunological response of white shrimp (Litopenaeus vannamei) to sublethal concentrations of malathion and endosulfan, and their mixture. Ecotoxicol. Environ. Saf. 2020, 188, 109893. [Google Scholar] [CrossRef] [PubMed]
- Dolar, A.; Selonen, S.; Van Gestel, C.A.; Perc, V.; Drobne, D.; Kokalj, A.J. Microplastics, chlorpyrifos and their mixtures modulate immune processes in the terrestrial crustacean Porcellio scaber. Sci. Total Environ. 2021, 772, 144900. [Google Scholar] [CrossRef] [PubMed]
- Kalita, M.K.; Devi, D. Immunomodulatory effect of chlorpyrifos formulation (Pyrifos-20 EC) on Philosamia ricini (Lepidoptera: Saturniidae). J. Entomol. Zool. Stud. 2016, 4, 26–31. [Google Scholar]
- Canty, M.N.; Hagger, J.A.; Moore, R.T.B.; Cooper, L.; Galloway, T.S. Sublethal impact of short term exposure to the organophosphate pesticide azamethiphos in the marine mollusc Mytilus edulis. Mar. Pollut. Bull. 2007, 54, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Yaqin, K.; Lay, B.W.; Riani, E.; Masud, Z.A.; Hansen, P.D. The use of selected biomarkers, phagocytic and cholinesterase activity to detect the effects of dimethoate on marine mussel (Mytilus edulis). HAYATI J. Biosci. 2008, 15, 32–38. [Google Scholar] [CrossRef]
- Herbert, L.T.; Castro, J.M.; Bianchi, V.A.; Cossi, P.F.; Luquet, C.M.; Kristoff, G. Effects of azinphos-methyl on enzymatic activity and cellular immune response in the hemolymph of the freshwater snail Chilina gibbosa. Pestic. Biochem. Physiol. 2018, 150, 71–77. [Google Scholar] [CrossRef]
- Castro, J.M.; Bianchi, V.A.; Pascual, M.; Venturino, A.; Luquet, C.M. Modulation of immune and antioxidant responses by azinphos-methyl in the freshwater mussel Diplodon chilensis challenged with Escherichia coli. Environ. Toxicol. Chem. 2017, 36, 1785–1794. [Google Scholar] [CrossRef]
- Patetsini, E.; Dimitriadis, V.K.; Kaloyianni, M. Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat. Toxicol. 2013, 126, 338–345. [Google Scholar] [CrossRef]
- De Guise, S.; Maratea, J.; Perkins, C. Malathion immunotoxicity in the American lobster (Homarus americanus) upon experimental exposure. Aquat. Toxicol. 2004, 66, 419–425. [Google Scholar] [CrossRef]
- Yeh, S.P.; Sung, T.G.; Chang, C.C.; Cheng, W.; Kuo, C.M. Effects of an organophosphorus insecticide, trichlorfon, on hematological parameters of the giant freshwater prawn, Macrobrachium rosenbergii (de Man). Aquaculture 2005, 243, 383–392. [Google Scholar] [CrossRef]
- Chang, C.C.; Rahmawaty, A.; Chang, Z.W. Molecular and immunological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to the organophosphorus insecticide, trichlorfon. Aquat. Toxicol. 2013, 130, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Jayesh, P.; Mohandas, A.; Philip, R.; Singh, I.B. Application of primary haemocyte culture of Penaeus monodon in the assessment of cytotoxicity and genotoxicity of heavy metals and pesticides. Mar. Environ. Res. 2011, 71, 169–177. [Google Scholar] [CrossRef]
- Lewis, J.A.; Szilagyi, M.; Gehman, E.; Dennis, W.E.; Jackson, D.A. Distinct patterns of gene and protein expression elicited by organophosphorus pesticides in Caenorhabditis elegans. BMC Genom. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Viñuela, A.; Snoek, L.B.; Riksen, J.A.; Kammenga, J.E. Genome-wide gene expression analysis in response to organophosphorus pesticide chlorpyrifos and diazinon in C. elegans. PLoS ONE 2010, 5, e12145. [Google Scholar] [CrossRef] [PubMed]
- Christin, M.S.; Menard, L.; Gendron, A.D.; Ruby, S.; Cyr, D.; Marcogliese, D.J.; Rollins-Smith, L.; Fournier, M. Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aquat. Toxicol. 2004, 67, 33–43. [Google Scholar] [CrossRef]
- Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Wate 2023, 15, 585. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in agricultural pest management and their environmental risks: A review. Int. J. Environ. Sci. Technol. 2023, 1–26. [Google Scholar] [CrossRef]
- Ural, M.Ş. Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio carpio: Ameliorative effect of lycopene. Chemosphere 2013, 90, 2059–2064. [Google Scholar] [CrossRef]
- Ahmadi, K.; Mirvaghefei, A.R.; Banaee, M.; Vosoghei, A.R. Effects of long-term diazinon exposure on some immunological and haematological parameters in rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Toxicol. Environ. Health Sci. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Hedayati, A.; Tarkhani, R. Hematological and gill histopathological changes in iridescent shark, Pangasius hypophthalmus (Sauvage, 1878) exposed to sublethal diazinon and deltamethrin concentrations. Fish Physiol. Biochem. 2014, 40, 715–720. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Elshopakey, G.E.; Aziza, A.E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hajirezaee, S.; Rafieepour, A.; Shafiei, S.; Rahimi, R. Immunostimulating effects of Ginkgo biloba extract against toxicity induced by organophosphate pesticide, diazinon in rainbow trout, Oncorhynchus mykiss: Innate immunity components and immune-related genes. Environ. Sci. Pollut. Res. 2019, 26, 8798–8807. [Google Scholar] [CrossRef] [PubMed]
- Raibeemol, K.P.; Chitra, K.C. Induction of immunological, hormonal and histological alterations after sublethal exposure of chlorpyrifos in the freshwater fish, Pseudetroplus maculatus (Bloch, 1795). Fish Shellfish Immunol. 2020, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Cossarini-Dunier, M.; Studnicka, M.; Demael, A. In vivo effect of the organophosphorus insecticide trichlorphon on immune response of carp (Cyprinus carpio): II. Effect of high doses of trichlorphon on nonspecific immune response. Ecotoxicol. Environ. Saf. 1990, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dunier, M.; Siwicki, A.K.; Demaël, A. Effects of organophosphorus insecticides: Effects of trichlorfon and dichlorvos on the immune response of carp (Cyprinus carpio): III. In Vitro effects on lymphocyte proliferation and phagocytosis and in vivo effects on humoral response. Ecotoxicol. Environ. Saf. 1991, 22, 79–87. [Google Scholar] [CrossRef]
- Khoshbavar-Rostami, H.A.; Soltani, M.; Hassan, H.M.D. Immune response of great sturgeon (Huso huso) subjected to long-term exposure to sublethal concentration of the organophosphate, diazinon. Aquaculture 2006, 256, 88–94. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Effect of chlorpyrifos on the immune response of Nile tilapia (Oreochromis niloticus). Conacyt 2014, 3, 59–64. [Google Scholar]
- Soltani, M.; Pourgholam, R. Lysozyme activity of grass carp (Ctenopharingodon idella) following exposure to sublethal concentrations of organophosphate, diazinon. J. Vet. Res. 2007, 62, 49–52. [Google Scholar]
- Li, X.; Liu, L.; Zhang, Y.; Fang, Q.; Li, Y.; Li, Y. Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio L.). Chemosphere 2013, 93, 428–433. [Google Scholar] [CrossRef]
- Yonar, S.M.; Ural, M.Ş.; Silici, S.; Yonar, M.E. Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: Protective role of propolis. Ecotoxicol. Environ. Saf. 2014, 102, 202–209. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Barcelos-Garcia, R.; Vidal-Chavez, Z.G.; Romero-Bañuelos, C.A.; Robledo-Marenco, M.L. Effect of chlorpyrifos on the hematology and phagocytic activity of Nile tilapia cells (Oreochromis niloticus). Toxicol. Mech. Methods 2006, 16, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Holladay, S.D.; Smith, S.A.; El-Habback, H.; Caceci, T. Influence of chlorpyrifos, an organophosphate insecticide, on the immune system of Nile tilapia. J. Aquat. Anim. Health 1996, 8, 104–110. [Google Scholar] [CrossRef]
- El-Bouhy, Z.; El-Nobi, G.; Reda, R.M.; Ibrahim, R. Effect of Insecticide. Zagazig Vet. J. 2016, 44, 196–204. [Google Scholar] [CrossRef]
- Harford, A.J.; O’Halloran, K.; Wright, P.F. The effects of in vitro pesticide exposures on the phagocytic function of four native Australian freshwater fish. Aquat. Toxicol. 2005, 75, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.G. Ecotoxicología de Anfibios en Agroecosistemas del Noreste de la Región Pampeana. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2013. [Google Scholar]
- Mahananda, M.R.; Mohanty, B.P. Toxicity on Biochemical and Hematological Parameters in Bufo melanostictus (Schneider) (Common Indian Toad) Exposed to Malathion. Pestic. Adv. Chem. Bot. Pestic. 2012, 2, 24–30. [Google Scholar]
- Christin, M.S.; Ménard, L.; Giroux, I.; Marcogliese, D.J.; Ruby, S.; Cyr, D.; Fournier, M.; Brousseau, P. Effects of agricultural pesticides on the health of Rana pipiens frogs sampled from the field. Environ. Sci. Pollut. Res. 2013, 20, 601–611. [Google Scholar] [CrossRef]
- Gromysz-Kałkowska, K.; Szubartowska, E. Toxicity of tetrachlorvinphos to Rana temporaria L. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1993, 105, 285–290. [Google Scholar] [CrossRef]
- Latorre, M.A.; López González, E.C.; Larriera, A.; Poletta, G.L.; Siroski, P.A. Effects of in vivo exposure to Roundup® on immune system of Caiman latirostris. J. Immunotoxicol. 2013, 10, 349–354. [Google Scholar] [CrossRef]
- Soltanian, S.; Fallahi, R.; Fereidouni, M.S. Effects of diazinon on some innate resistance parameters in the Caspian pond turtle (Mauremys caspica caspica). Bulg. J. Vet. Med. 2018, 21, 212–223. [Google Scholar] [CrossRef]
- Siroski, P.A.; Poletta, G.L.; Latorre, M.A.; Merchant, M.E.; Ortega, H.H.; Mudry, M.D. Immunotoxicity of commercial-mixed glyphosate in broad snouted caiman (Caiman latirostris). Chem. Biol. Interact. 2016, 244, 64–70. [Google Scholar] [CrossRef]
- Mestre, A.P.; Amavet, P.S.; Van der Sloot, I.S.; Carletti, J.V.; Poletta, G.L.; Siroski, P.A. Effects of glyphosate, cypermethrin, and chlorpyrifos on hematological parameters of the tegu lizard (Salvator merianae) in different embryo stages. Chemosphere 2020, 252, 126433. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, C.; Mandal, F.B. Synthetic chemical pesticides and their effects on birds. Res. J. Environ. Toxicol. 2011, 5, 81–96. [Google Scholar] [CrossRef]
- Garg, U.K.; Pal, A.K.; Jha, G.J.; Jadhao, S.B. Haemato-biochemical and immuno-pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. Int. Immunopharmacol. 2004, 4, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Khan, A.; Khan, M.Z.; Mahmood, F.; Gul, S.T.; Saleemi, M.K. Immuno-pathologic effects of oral administration of chlorpyrifos in broiler chicks. J. Immunotoxicol. 2015, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nain, S.; Bour, A.; Chalmers, C.; Smits, J.E.G. Immunotoxicity and disease resistance in Japanese quail (Corturnix coturnix japonica) exposed to malathion. Ecotoxicology 2011, 20, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Gowri, U.K.; Patel, P.V.; Suresh, B. Evaluation of pesticide induced developmental immunotoxicity in rir chicks. J. Cell Tissue Res. 2010, 10, 2229–2234. [Google Scholar]
- Khan, N.A.; Uggini, G.K.; Patel, S.; Balakrishnan, S. In-ovo treatment of chlorpyrifos and cypermethrin in combination altered the haematological parameters in two generations of domestic hen. Ind. J. Fundam. Appl. Life Sci. 2015, 5, 120–126. [Google Scholar]
- Banerjee, B.D.; Pasha, S.T.; Hussain, Q.Z.; Koner, B.C.; Ray, A. A comparative evaluation of immunotoxicity of malathion after subchronic exposure in experimental animals. Ind. J. Exp. Biol. 1998, 36, 273–282. [Google Scholar]
- Suke, S.G.; Ahmed, R.S.; Tripathi, A.K.; Chakraborti, A.; Banerjee, B.D. Immunotoxicity of phosphamidon following subchronic exposure in albino rats. Experiment 2006, 44, 316–320. [Google Scholar]
- Ayub, S.; Verma, J.; Das, N. Effect of endosulfan and malathion on lipid peroxidation, nitrite and TNF-α release by rat peritoneal macrophages. Int. Immunopharmacol. 2003, 3, 1819–1828. [Google Scholar] [CrossRef]
- Singh, A.K.; Jiang, Y. Lipopolysaccharide (LPS) induced activation of the immune system in control rats and rats chronically exposed to a low level of the organothiophosphate insecticide, acephate. Toxicol. Ind. Health 2003, 19, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.; Ellefson, D. Mechanism of the modulation of murine peritoneal cell function and mast cell degranulation by low doses of malathion. Agents Actions 1992, 35, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, W.; Yoshida, H.; Hirose, A.; Akashi, T.; Takeshita, T.; Kuroki, N.; Shibata, A.; Hongo, A.; Hashiguchi, S.; Konno, K.; et al. Perinatal exposure to insecticide methamidophos suppressed production of proinflammatory cytokines responding to virus infection in lung tissues in mice. BioMed Res. Int. 2013, 2013, 151807. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nakadai, A.; Takeda, K.; Kawada, T. Dimethyl 2, 2-dichlorovinyl phosphate (DDVP) markedly inhibits activities of natural killer cells, cytotoxic T lymphocytes and lymphokine-activated killer cells via the Fas-ligand/Fas pathway in perforin-knockout (PKO) mice. Toxicology 2004, 204, 41–50. [Google Scholar] [CrossRef]
- Hermanowicz, A.; Kossman, S. Neutrophil function and infectious disease in workers occupationally exposed to phosphoorganic pesticides: Role of mononuclear-derived chemotactic factor for neutrophils. Clin. Immunol. Immunopathol. 1984, 33, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Rodgers, K. Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J. Toxicol. Environ. Health 1997, 51, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Casale, G.P.; Bavari, S.; Connolly, J.J. Inhibition of human serum complement activity by diisopropylfluorophosphate and selected anticholinesterase insecticides. Fundam. Appl. Toxicol. 1989, 12, 460–468. [Google Scholar] [CrossRef]
- Schäfer, M.; Koppe, F.; Stenger, B.; Brochhausen, C.; Schmidt, A.; Steinritz, D.; Thiermann, H.; Kirkpatrick, C.J.; Pohl, C. Influence of organophosphate poisoning on human dendritic cells. Chem. Biol. Interact. 2013, 206, 472–478. [Google Scholar] [CrossRef]
- Ohnishi, T.; Yoshida, T.; Igarashi, A.; Muroi, M.; Tanamoto, K.I. Effects of possible endocrine disruptors on MyD88-independent TLR4 signaling. FEMS Immunol. Med. Microbiol. 2008, 52, 293–295. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Santerre, A.; Gonzalez-Jaime, F.; Casas-Solis, J.; Hernández-Coronado, M.; Peregrina-Sandoval, J.; Akiro, T.; Zaitseva, G. Immunotoxicity and hepatic function evaluation in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Fish Shellfish Immunol. 2007, 23, 760–769. [Google Scholar] [CrossRef]
- Peillex, C.; Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, K.S.; Aly, N.M.; El-Sebae, A.H. Effects of edifenphos and glyphosate on the immune response and protein biosynthesis of bolti fish (Tilapia nilotica). J. Environ. Sci. Health Part B 1998, 33, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.B.D.; Fraga, R.E.; Nishiyama, P.B.; Silva, I.S.S.D.; Costa, N.L.B.; De Oliveira, L.A.A.; Rocha, M.A.; Juncá, F.A. Leukocyte profiles in Odontophrynus carvalhoi (Amphibia: Odontophrynidae) tadpoles exposed to organophosphate chlorpyrifos pesticides. Water Air Soil Pollut. 2020, 231, 372. [Google Scholar] [CrossRef]
- Rooney, A.A.; Bermudez, D.S.; Guillette, L.J., Jr. Altered histology of the thymus and spleen in contaminant-exposed juvenile American alligators. J. Morphol. 2003, 256, 349–359. [Google Scholar] [CrossRef]
- Garg, S.; Singh, B.P.; Chauhan, R.S. Immunopathological effects of quinalphos on humoral immune response in chickens. J. Immunol. Immunopathol. 2002, 4, 97–100. [Google Scholar]
- Crisol-Martínez, E.; Moreno-Moyano, L.T.; Wilkinson, N.; Prasai, T.; Brown, P.H.; Moore, R.J.; Stanley, D. A low dose of an organophosphate insecticide causes dysbiosis and sex-dependent responses in the intestinal microbiota of the Japanese quail (Coturnix japonica). PeerJ 2002, 4, e2002. [Google Scholar] [CrossRef]
- Ȕndeger, U.; Institoris, L.; Siroki, O.; Nehéz, M.; Desi, I. Simultaneous geno-and immunotoxicological investigations for early detection of organophosphate toxicity in rats. Ecotoxicol. Environ. Saf. 2000, 45, 43–48. [Google Scholar] [CrossRef]
- Essa, S.S.; El-Saied, E.M.; El-Tawil, O.S.; Gamal, I.M.; Abd El-Rahman, S.S. Nanoparticles of zinc oxide defeat chlorpyrifos-induced immunotoxic effects and histopathological alterations. Vet. World 2019, 12, 440. [Google Scholar] [CrossRef]
- Zabrodskii, P.F. The effect of chronic intoxication by organophosphate insecticides on the parameters of innate and adaptive immunity and realization of the cholinergic anti-inflammatory pathway. Pharm. Pharm. Int. J. 2018, 6, 418–420. [Google Scholar] [CrossRef]
- Hassouna, I.; Ibrahim, H.; Abdel Gaffar, F.; El-Elaimy, I.; Abdel Latif, H. Simultaneous administration of hesperidin or garlic oil modulates diazinon-induced hemato-and immunotoxicity in rats. Immunopharmacol. Immunotoxicol. 2015, 37, 442–449. [Google Scholar] [CrossRef]
- Fukuyama, T.; Tajima, Y.; Ueda, H.; Hayashi, K.; Kosaka, T. Prior exposure to immunosuppressive organophosphorus or organochlorine compounds aggravates the TH1-and TH2-type allergy caused by topical sensitization to 2, 4-dinitrochlorobenzene and trimellitic anhydride. J. Immunotoxicol. 2011, 8, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Tajima, Y.; Ueda, H.; Hayashi, K.; Shutoh, Y.; Harada, T.; Kosaka, T. Apoptosis in immunocytes induced by several types of pesticides. J. Immunotoxicol. 2010, 7, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.T.; Imam, T.S.; Abo-Elmaaty, A.M.; Arisha, A.H. Amelioration of fenitrothion induced oxidative DNA damage and inactivation of caspase-3 in the brain and spleen tissues of male rats by N-acetylcysteine. Life Sci. 2019, 231, 116534. [Google Scholar] [CrossRef] [PubMed]
- Ola-Davies, O.E.; Azeez, O.I.; Oyagbemi, A.A.; Abatan, M.O. Acute coumaphos organophosphate exposure in the domestic dogs: Its implication on haematology and liver functions. Int. J. Vet. Sci. Med. 2018, 6, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Caloni, F.; Cortinovis, C.; Rivolta, M.; Davanzo, F. Suspected poisoning of domestic animals by pesticides. Sci. Total Environ. 2016, 539, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Chauhan, R.S. Immunopathological effects of monocrotophos on cell mediated immune response in sheep. J. Immunol. Immunopathol. 2001, 3, 50–53. [Google Scholar]
- Lee, G.H.; Choi, K.C. Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-González, K.G.; Covantes-Rosales, C.E.; Camacho-Pérez, M.R.; Mercado-Salgado, U.; Barajas-Carrillo, V.W.; Girón-Pérez, D.A.; Montoya-Hidalgo, A.C.; Díaz-Resendiz, K.J.G.; Barcelos-García, R.G.; Toledo-Ibarra, G.A.; et al. Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates. Int. J. Mol. Sci. 2023, 24, 5360. https://doi.org/10.3390/ijms24065360
Bernal-González KG, Covantes-Rosales CE, Camacho-Pérez MR, Mercado-Salgado U, Barajas-Carrillo VW, Girón-Pérez DA, Montoya-Hidalgo AC, Díaz-Resendiz KJG, Barcelos-García RG, Toledo-Ibarra GA, et al. Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates. International Journal of Molecular Sciences. 2023; 24(6):5360. https://doi.org/10.3390/ijms24065360
Chicago/Turabian StyleBernal-González, Karime Guadalupe, Carlos Eduardo Covantes-Rosales, Milton Rafael Camacho-Pérez, Ulises Mercado-Salgado, Victor Wagner Barajas-Carrillo, Daniel Alberto Girón-Pérez, Ashley Carolina Montoya-Hidalgo, Karina Janice Guadalupe Díaz-Resendiz, Rocío Guadalupe Barcelos-García, Gladys Alejandra Toledo-Ibarra, and et al. 2023. "Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates" International Journal of Molecular Sciences 24, no. 6: 5360. https://doi.org/10.3390/ijms24065360
APA StyleBernal-González, K. G., Covantes-Rosales, C. E., Camacho-Pérez, M. R., Mercado-Salgado, U., Barajas-Carrillo, V. W., Girón-Pérez, D. A., Montoya-Hidalgo, A. C., Díaz-Resendiz, K. J. G., Barcelos-García, R. G., Toledo-Ibarra, G. A., & Girón-Pérez, M. I. (2023). Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates. International Journal of Molecular Sciences, 24(6), 5360. https://doi.org/10.3390/ijms24065360








