Xerostomia and Its Cellular Targets
Abstract
1. Introduction
2. Unidirectional Movement of Fluid in the Salivary Glands
2.1. Structure of the Salivary Glands
2.2. Polarized Acinar Cells and Support Cells
3. Salivation by G-Protein-Coupled Receptor (GPCR)-Mediated Intracellular Calcium (Ca2+) Signaling
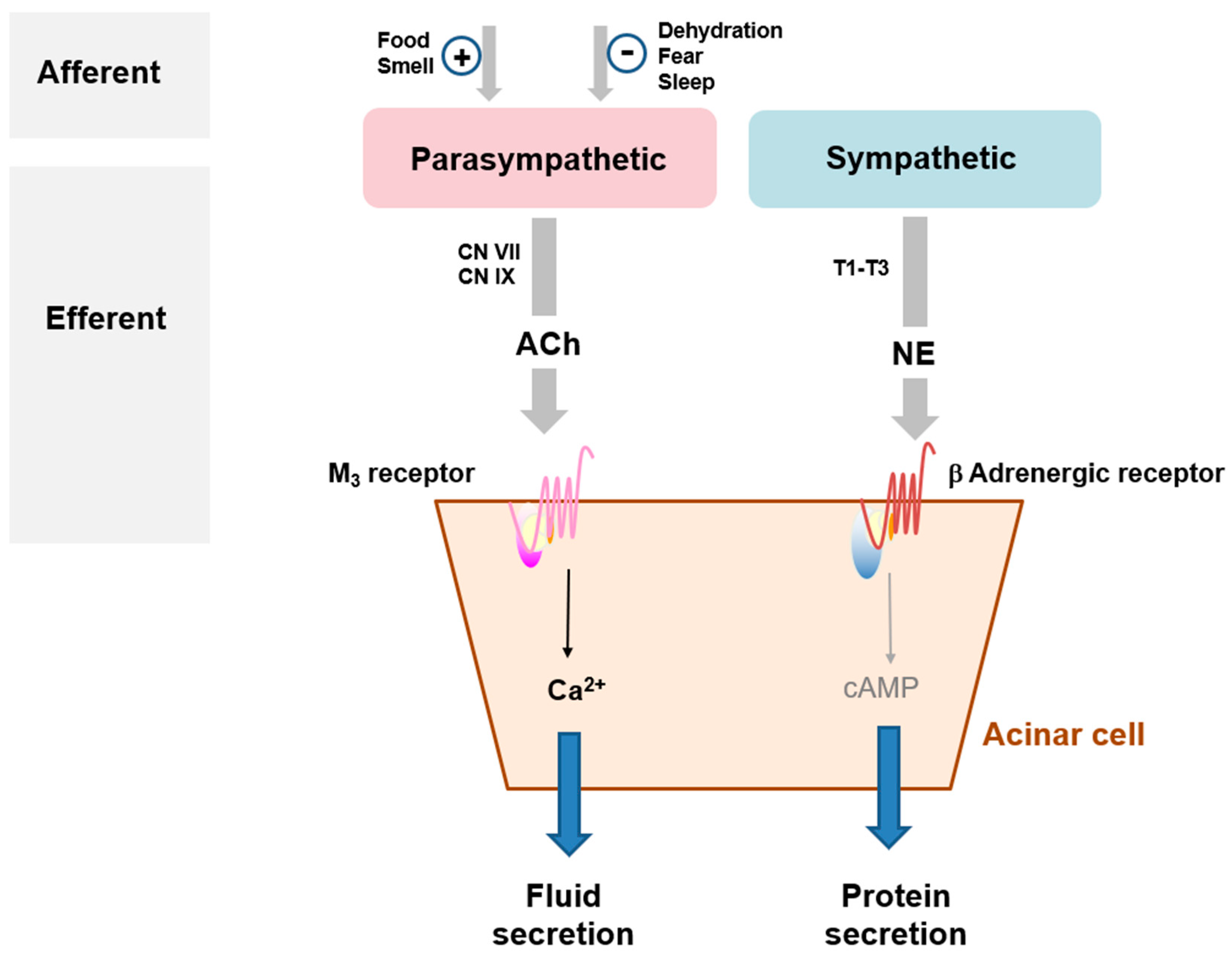
3.1. Mode of Action of Salivation
3.2. GPCRs as Keys for Cell-to-Cell Communication
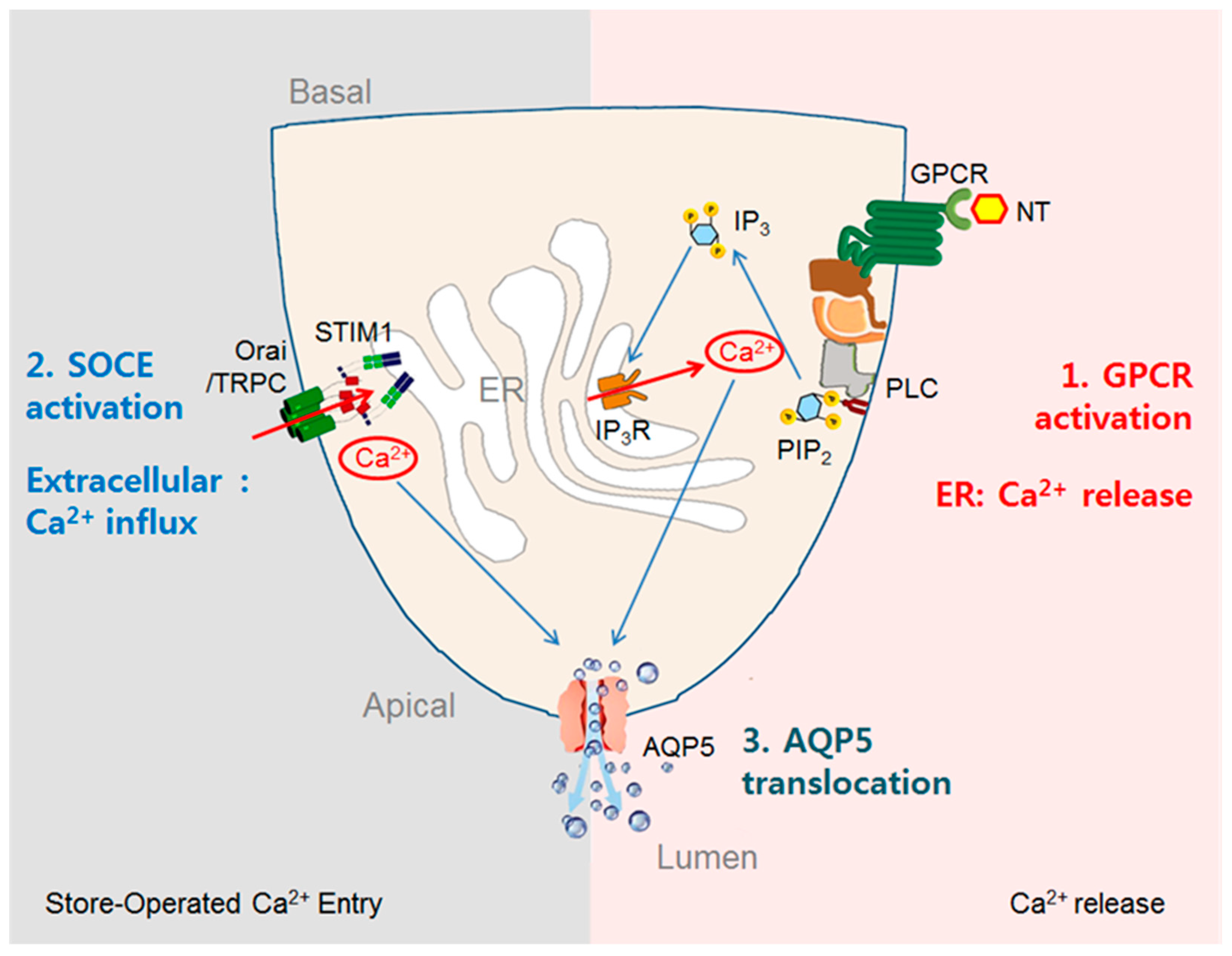
3.3. Stimulation of Fluid Secretion by GPCR-Mediated Increases in [Ca2+]i in Acinar Cells
4. Increasing Prevalence of Xerostomia
4.1. Xerogenic Drugs as the Most Common Cause of Xerostomia
4.2. Systemic Diseases and Salivary Gland Disorders That Compromise Glandular Tissue Integrity
4.3. Current Palliative Care and Pharmacological Therapies
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scully, C. 8—Dry mouth (xerostomia and hyposalivation). In Oral and Maxillofacial Medicine, 3rd ed.; Scully, C., Ed.; Churchill Livingstone: London, UK, 2013; pp. 91–97. [Google Scholar]
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the management of dry mouth: Topical therapies. Cochrane Database Syst. Rev. 2011, 7, CD008934. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Fox, P.C.; Baum, B.J. How much saliva is enough? ‘Normal’ function defined. J. Am. Dent. Assoc. 1991, 122, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Orellana, M.; Lagravère, M.; Boychuk, D.; Major, P.; Flores-Mir, C.; Ortho, C. Prevalence of xerostomia in population-based samples: A systematic review. J. Public Health Dent. 2006, 66, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Abati, S. Risk factors and symptoms associated with xerostomia: A cross-sectional study. Aust. Dent. J. 2011, 56, 290–295. [Google Scholar] [CrossRef]
- Hopcraft, M.S.; Tan, C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010, 55, 238–244; quiz 353. [Google Scholar] [CrossRef]
- Pajukoski, H.; Meurman, J.; Halonen, P.; Sulkava, R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 641–649. [Google Scholar] [CrossRef]
- Thomson, W.M.; Smith, M.B.; Ferguson, C.A.; Moses, G. The Challenge of Medication-Induced Dry Mouth in Residential Aged Care. Pharmacy 2021, 9, 162. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Kamnerdsupaphon, P.; Tharavichitkul, E.; Sumitsawan, Y.; Sittitrai, P.; Pattarasakulchai, T.; Lorvidhaya, V.; Sukthomya, V.; Pukanhaphan, N.; Traisatit, P. Effect of oral pilocarpine on post-irradiation xerostomia in head and neck cancer patients: A single-center, single-blind clinical trial. J. Med. Assoc. Thail. 2008, 91, 1410–1415. [Google Scholar]
- Cifuentes, M.; Del Barrio-Díaz, P.; Vera-Kellet, C. Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: A double-blind randomized controlled trial. Br. J. Dermatol. 2018, 179, 1056–1061. [Google Scholar] [CrossRef]
- Cheng, C.Q.; Xu, H.; Liu, L.; Wang, R.-N.; Liu, Y.-T.; Li, J.; Zhou, X.-K. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 236–243. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontology 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Mandel, I.D. The functions of saliva. J. Dent. Res. 1987, 66, 623–627. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef]
- Chibly, A.M.; Aure, M.; Patel, V.; Hoffman, M. Salivary gland function, development, and regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef]
- Saitou, M.; Gaylord, E.A.; Xu, E.; May, A.J.; Neznanova, L.; Nathan, S.; Grawe, A.; Chang, J.; Ryan, W.; Ruhl, S.; et al. Functional Specialization of Human Salivary Glands and Origins of Proteins Intrinsic to Human Saliva. Cell Rep. 2020, 33, 108402. [Google Scholar] [CrossRef] [PubMed]
- Valstar, M.H.; de Bakker, B.S.; Steenbakkers, R.J.; de Jong, K.H.; Smit, L.A.; Nulent, T.J.K.; van Es, R.J.; Hofland, I.; de Keizer, B.; Jasperse, B.; et al. The tubarial salivary glands: A potential new organ at risk for radiotherapy. Radiother. Oncol. 2021, 154, 292–298. [Google Scholar] [CrossRef]
- Ghannam, M.G.; Singh, P.V. Anatomy, Head and Neck, Salivary Glands. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis and regeneration of salivary glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pin, C.L.; Bonvissuto, A.C.; Konieczny, S.F. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat. Rec. 2000, 259, 157–167. [Google Scholar] [CrossRef]
- Larsen, H.S.; Aure, M.H.; Peters, S.; Larsen, M.; Messelt, E.B.; Galtung, H.K. Localization of AQP5 during development of the mouse submandibular salivary gland. J. Mol. Histol. 2011, 42, 71–81. [Google Scholar] [CrossRef]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Coutinho-Camillo, C.M.; Nico, M.M.S.; Lourenco, S.V. The expression of water channel proteins during human salivary gland development: A topographic study of aquaporins 1, 3 and 5. J. Mol. Histol. 2017, 48, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Jeng, Y.M.; Lee, Y.H. Mist1: A novel nuclear marker for acinic cell carcinoma of the salivary gland. Virchows Arch. 2019, 475, 617–624. [Google Scholar] [CrossRef]
- Garrett, J.R.; Ekström, J.; Anderson, L.C. Glandular Mechanisms of Salivary Secretion; Karger Medical and Scientific Publishers: Basel, Switzerland, 1998; Volume 10. [Google Scholar]
- Berkovitz, B.K.; Holland, G.R.; Moxham, B.J. A Color Atlas and Text of Oral Anatomy, Histology and Embryology; Mosby Elsevier Health Science: St. Louis, MO, USA, 1992. [Google Scholar]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef]
- Furuse, M.; Tsukita, S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006, 16, 181–188. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 2005, 57, 815–855. [Google Scholar] [CrossRef] [PubMed]
- Baker, O.J. Tight junctions in salivary epithelium. J. Biomed. Biotechnol. 2010, 2010, 278948. [Google Scholar] [CrossRef]
- Kondo, Y.; Nakamoto, T.; Jaramillo, Y.; Choi, S.; Catalan, M.; Melvin, J. Functional differences in the acinar cells of the murine major salivary glands. J. Dent. Res. 2015, 94, 715–721. [Google Scholar] [CrossRef]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.A.; Nakamoto, T.; Gonzalez-Begne, M.; Camden, J.M.; Wall, S.M.; Clarke, L.L.; Melvin, J.E. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J. Physiol. 2010, 588, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef]
- Tandler, B.; Gresik, E.W.; Nagato, T.; Phillips, C.J. Secretion by striated ducts of mammalian major salivary glands: Review from an ultrastructural, functional, and evolutionary perspective. Anat. Rec. 2001, 264, 121–145. [Google Scholar] [CrossRef]
- Redman, R.S. Myoepithelium of salivary glands. Microsc. Res. Technol. 1994, 27, 25–45. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Dartt, D.A. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr. Mol. Biol. Rep. 2015, 1, 115–123. [Google Scholar] [CrossRef]
- Hawley, D.; Tang, X.; Zyrianova, T.; Shah, M.; Janga, S.; Letourneau, A.; Schicht, M.; Paulsen, F.; Hamm-Alvarez, S.; Makarenkova, H.P.; et al. Myoepithelial cell-driven acini contraction in response to oxytocin receptor stimulation is impaired in lacrimal glands of Sjögren’s syndrome animal models. Sci. Rep. 2018, 8, 9919. [Google Scholar] [CrossRef]
- Emmelin, N.; Gjörstrup, P. On the function of myoepithelial cells in salivary glands. J. Physiol. 1973, 230, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Lung, M.A. Autonomic nervous control of myoepithelial cells and secretion in submandibular gland of anaesthetized dogs. J. Physiol. 2003, 546, 837–850. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Dissection of calcium signaling events in exocrine secretion. Neurochem. Res. 2011, 36, 1212–1221. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jo, Y.; Lee, Y.-H.; Park, K.; Park, H.-K.; Choi, S.-Y. Zn2+ stimulates salivary secretions via metabotropic zinc receptor ZnR/GPR39 in human salivary gland cells. Sci. Rep. 2019, 9, 17648. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsui, M.; Uchida, K.; Futatsugi, A.; Kusakawa, S.; Matsumoto, N.; Nakamura, K.; Manabe, T.; Taketo, M.M.; Mikoshiba, K. M3muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J. Physiol. 2004, 558, 561–575. [Google Scholar] [CrossRef]
- Jin, M.; Hwang, S.-M.; Koo, N.-Y.; Kim, B.; Kho, H.-S.; Choi, S.-Y.; Song, Y.; Park, K. Autoantibodies in Sjögren’s syndrome patients acutely inhibit muscarinic receptor function. Oral Dis. 2012, 18, 132–139. [Google Scholar] [CrossRef]
- Gautam, D.; Heard, T.S.; Cui, Y.; Miller, G.; Bloodworth, L.; Wess, J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004, 66, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, A.T.; Warfvinge, G.; Axelsson, L.; Soukup, O.; Götrick, B.; Tobin, G. Expression of muscarinic receptor subtypes in salivary glands of rats, sheep and man. Arch. Oral Biol. 2008, 53, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Beroukas, D.; Goodfellow, R.; Hiscock, J.; Jonsson, R.; Gordon, T.; Waterman, S.A. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjögren’s syndrome. Lab Investig. 2002, 82, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, M.G.; Woods, L.T.; Jasmer, K.; Forti, K.M.; Camden, J.M.; Jensen, J.L.; Limesand, K.H.; Galtung, H.K.; Weisman, G.A. P2 Receptors as Therapeutic Targets in the Salivary Gland: From Physiology to Dysfunction. Front. Pharmacol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.-H.; Moon, Y.W.; Hwang, S.; Kim, D.; Jo, S.-H.; Oh, S.B.; Kim, J.S.; Jahng, J.W.; Lee, J.-H.; et al. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J. Pharmacol. Exp. Ther. 2009, 330, 403–412. [Google Scholar] [CrossRef]
- Seo, J.; Koo, N.Y.; Choi, W.Y.; Kang, J.A.; Min, J.H.; Jo, S.H.; Lee, S.J.; Oh, S.B.; Kim, J.S.; Lee, J.H.; et al. Sphingosine-1-phosphate signaling in human submandibular cells. J. Dent. Res. 2010, 89, 1148–1153. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.-J.; Choi, L.-M.; Choi, S.; Nam, H.; Ko, H.-Y.; Chung, G.; Lee, J.-H.; Jo, S.-H.; Lee, G.; et al. Human salivary gland cells express bradykinin receptors that modulate the expression of proinflammatory cytokines. Eur. J. Oral Sci. 2017, 125, 18–27. [Google Scholar] [CrossRef]
- Turner, J.; Landon, L.A.; Gibbons, S.J.; Talamo, B.R. Salivary gland P2 nucleotide receptors. Crit. Rev. Oral Biol. Med. 1999, 10, 210–224. [Google Scholar] [CrossRef]
- Lee, M.G.; Zeng, W.; Muallem, S. Characterization and localization of P2 receptors in rat submandibular gland acinar and duct cells. J. Biol. Chem. 1997, 272, 32951–32955. [Google Scholar] [CrossRef]
- Park, M.K.; Garrad, R.C.; Weisman, G.A.; Turner, J.T. Changes in P2Y1 nucleotide receptor activity during the development of rat salivary glands. Am. J. Physiol. Physiol. 1997, 272, C1388–C1393. [Google Scholar] [CrossRef]
- Baker, O.J.; Camden, J.M.; Rome, D.E.; Seye, C.I.; Weisman, G.A. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 [corrected] expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol. Immunol. 2008, 45, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.; Davitt, W.F.; Bokosky, J.E.; Nichols, K.K.; Yerxa, B.R.; Schaberg, A.E.; LaVange, L.M.; Mills-Wilson, M.C.; Kellerman, D.J. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 2004, 23, 784–792. [Google Scholar] [CrossRef]
- Koh, S. Clinical utility of 3% diquafosol ophthalmic solution in the treatment of dry eyes. Clin. Ophthalmol. 2015, 9, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Takamura, E.; Tsubota, K.; Watanabe, H.; Ohashi, Y.; For the Diquafosol Ophthalmic Solution Phase 3 Study Group. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 2012, 96, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Kiselyov, K.; Wang, X.; Shin, D.M.; Zang, W.; Muallem, S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium 2006, 40, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, X.; Muallem, S. Functional Mapping of Ca2+ Signaling Complexes in Plasma Membrane Microdomains of Polarized Cells. J. Biol. Chem. 2004, 279, 27837–27840. [Google Scholar] [CrossRef]
- Mikoshiba, K.; Hisatsune, C.; Futatsugi, A.; Mizutani, A.; Nakamura, T.; Miyachi, K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea 2008, 27, S3–S8. [Google Scholar] [CrossRef]
- Yule, D.I. Subtype-specific regulation of inositol 1,4,5-trisphosphate receptors: Controlling calcium signals in time and space. J. Gen. Physiol. 2001, 117, 431–434. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Polarization of calcium signaling and fluid secretion in salivary gland cells. Curr. Med. Chem. 2012, 19, 5774–5781. [Google Scholar] [CrossRef]
- Futatsugi, A.; Nakamura, T.; Yamada, M.K.; Ebisui, E.; Nakamura, K.; Uchida, K.; Kitaguchi, T.; Takahashi-Iwanaga, H.; Noda, T.; Aruga, J.; et al. IP 3 Receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 2005, 309, 2232–2234. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, K.T.; Bandyopadhyay, B.; Pani, B.; Dietrich, A.; Paria, B.; Swaim, W.; Beech, D.; Yildrim, E.; Singh, B.; et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc. Natl. Acad. Sci. USA 2007, 104, 17542–17547. [Google Scholar]
- Hong, J.H.; Li, Q.; Kim, M.S.; Shin, D.M.; Feske, S.; Birnbaumer, L.; Cheng, K.T.; Ambudkar, I.S.; Muallem, S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic 2011, 12, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Ong, H.L.; Liu, X.; Ambudkar, I.S. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. In Current Topics in Membranes; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 71, pp. 149–179. [Google Scholar]
- Yuan, J.P.; Zeng, W.; Dorwart, M.R.; Choi, Y.-J.; Worley, P.F.; Muallem, S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 2009, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M. The molecular physiology of CRAC channels. Immunol. Rev. 2009, 231, 88–98. [Google Scholar] [CrossRef]
- Birnbaumer, L.; Zhu, X.; Jiang, M.; Boulay, G.; Peyton, M.; Vannier, B.; Brown, D.; Platano, D.; Sadeghi, H.; Stefani, E.; et al. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15195–15202. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.-J.; Cho, Y.Y.; Chung, S.; Jo, S.-H.; Choi, S.-Y. Polychlorinated biphenyl 19 blocks the most common form of store-operated Ca2+ entry through Orai. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, C.; Elkashty, O.; Chivasso, C.; Perret, J.; Tran, S.; Delporte, C. Insight into Salivary Gland Aquaporins. Cells 2020, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The Evolutionary Aspects of Aquaporin Family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [Google Scholar] [CrossRef]
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef]
- Stamboni, M.B.; Gomes, N.D.M.; de Souza, M.M.; Oliveira, K.K.; Arruda, C.F.J.; de Paula, F.; Bettim, B.B.; Marques, M.M.; Kowalski, L.P.; Pinto, C.A.L.; et al. Aquaporin 1, 3, and 5 Patterns in Salivary Gland Mucoepidermoid Carcinoma: Expression in Surgical Specimens and an In Vitro Pilot Study. Int. J. Mol. Sci. 2020, 21, 1287. [Google Scholar] [CrossRef]
- Delporte, C. Aquaporins in salivary glands and pancreas. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Nico, B.; Ribatti, D.; Ruggieri, S.; Lofrumento, D.D.; Lisi, S. Abnormal distribution of AQP4 in minor salivary glands of primary Sjögren’s syndrome patients. Autoimmunity 2017, 50, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, K.; Yao, C.; Hasegawa, T.; Yoshimura, H.; Akamatsu, T. Dynamics of Salivary Gland AQP5 under Normal and Pathologic Conditions. Int. J. Mol. Sci. 2020, 21, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Tada, J.; Sawa, T.; Yamanaka, N.; Shono, M.; Akamatsu, T.; Tsumura, K.; Parvin, M.N.; Kanamori, N.; Hosoi, K. Involvement of Vesicle–Cytoskeleton Interaction in AQP5 Trafficking in AQP5-Gene-Transfected HSG Cells. Biochem. Biophys. Res. Commun. 1999, 266, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.-V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef]
- Li, X.; Azlina, A.; Karabasil, M.R.; Purwanti, N.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Degradation of submandibular gland AQP5 by parasympathetic denervation of chorda tympani and its recovery by cevimeline, an M3 muscarinic receptor agonist. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G112–G123. [Google Scholar] [CrossRef] [PubMed]
- Azlina, A.; Javkhlan, P.; Hiroshima, Y.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Roles of lysosomal proteolytic systems in AQP5 degradation in the submandibular gland of rats following chorda tympani parasympathetic denervation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1106–G1117. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kawedia, J.D.; Menon, A.G. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J. Biol. Chem. 2003, 278, 32173–32180. [Google Scholar] [CrossRef]
- Chen, G.; Yao, C.; Hasegawa, T.; Akamatsu, T.; Yoshimura, H.; Hosoi, K. Effects of isoproterenol on aquaporin 5 levels in the parotid gland of mice in vivo. Am. J. Physiol. Metab. 2014, 306, E100–E108. [Google Scholar] [CrossRef]
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, recognition and treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Talha, B.; Swarnkar, S.A. Xerostomia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Turner, M.D. Hyposalivation and Xerostomia: Etiology, Complications, and Medical Management. Dent. Clin. N. Am. 2016, 60, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C.; Hegarty, A. An update of the etiology and management of xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 28–46. [Google Scholar] [CrossRef]
- Gupta, A.; Epstein, J.B.; Sroussi, H. Hyposalivation in elderly patients. J. Can. Dent. Assoc. 2006, 72, 841–846. [Google Scholar]
- Toan, N.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. [Google Scholar] [CrossRef]
- Chibly, A.M.; Nguyen, T.; Limesand, K.H. Palliative Care for Salivary Gland Dysfunction Highlights the Need for Regenerative Therapies: A Review on Radiation and Salivary Gland Stem Cells. J. Palliat. Care Med. 2014, 4, 1000180. [Google Scholar] [PubMed]
- Chambers, M.S.; Jones, C.U.; Biel, M.A.; Weber, R.S.; Hodge, K.M.; Chen, Y.; Holland, J.M.; Ship, J.A.; Vitti, R.; Armstrong, I.; et al. Open-label, long-term safety study of cevimeline in the treatment of postirradiation xerostomia. Int. J. Radiat. Oncol. 2007, 69, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.; Moreira, T.S.; Laitano, S.; De Luca, L.; Renzi, A.; Menani, J.V. Central muscarinic receptors signal pilocarpine-induced salivation. J. Dent. Res. 2003, 82, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef]
- Loesche, W.J.; Bromberg, J.; Terpenning, M.S.; Bretz, W.A.; Dominguez, B.L.; Grossman, N.S.; Langmore, S.E. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J. Am. Geriatr. Soc. 1995, 43, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y. Medications That Cause Dry Mouth as an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef]
- Kapourani, A.; Kontogiannopoulos, K.N.; Manioudaki, A.-E.; Poulopoulos, A.K.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers 2022, 14, 850. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M.; Movahhedian, A.; Mohammadi, M.; Khodadoustan, A. Xerostomia due to systemic disease: A review of 20 conditions and mechanisms. Ann. Med. Health Sci. Res. 2014, 4, 503–510. [Google Scholar] [CrossRef]
- Stewart, C.M.; Berg, K.M.; Cha, S.; Reeves, W.H. Salivary dysfunction and quality of life in Sjögren syndrome: A critical oral-systemic connection. J. Am. Dent. Assoc. 2008, 139, 291–299; quiz 358–359. [Google Scholar] [CrossRef]
- Pérez, P.; Kwon, Y.-J.; Alliende, C.; Leyton, L.; Aguilera, S.; Molina, C.; Labra, C.; Julio, M.; Leyton, C.; González, M.-J. Increased acinar damage of salivary glands of patients with Sjögren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis Rheum. 2005, 52, 2751–2760. [Google Scholar]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Ferro, F.; Izzetti, R.; Vitali, S.; Aringhieri, G.; Fonzetti, S.; Donati, V.; Dini, V.; Mosca, M.; Gabriele, M.; Caramella, D.; et al. Ultra-high frequency ultrasonography of labial glands is a highly sensitive tool for the diagnosis of Sjögren’s syndrome: A preliminary study. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 210–215. [Google Scholar] [PubMed]
- Izzetti, R.; Ferro, F.; Vitali, S.; Nisi, M.; Fonzetti, S.; Oranges, T.; Donati, V.; Caramella, D.; Baldini, C.; Gabriele, M. Ultra-high frequency ultrasonography (UHFUS)-guided minor salivary gland biopsy: A promising procedure to optimize labial salivary gland biopsy in Sjögren’s syndrome. J. Oral Pathol. Med. 2021, 50, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ortholan, C.; Benezery, K.; Bensadoun, R.J. Normal tissue tolerance to external beam radiation therapy: Salivary glands. Cancer Radiother. 2010, 14, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.L.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz016. [Google Scholar] [CrossRef] [PubMed]
- Riantiningtyas, R.R.; Carrouel, F.; Bruyas, A.; Bredie, W.L.; Kwiecien, C.; Giboreau, A.; Dougkas, A. Oral Somatosensory Alterations in Head and Neck Cancer Patients—An Overview of the Evidence and Causes. Cancers 2023, 15, 718. [Google Scholar] [CrossRef]
- Sun, E.C.; Curtis, R.; Melbye, M.; Goedert, J.J. Salivary gland cancer in the United States. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1095–1100. [Google Scholar]
- Chuong, M.; Bryant, J.; Hartsell, W.; Larson, G.; Badiyan, S.; Laramore, G.E.; Katz, S.; Tsai, H.; Vargas, C. Minimal acute toxicity from proton beam therapy for major salivary gland cancer. Acta Oncol. 2020, 59, 196–200. [Google Scholar] [CrossRef]
- Yeh, C.K.; Johnson, D.A.; Dodds, M.W.J. Impact of aging on human salivary gland function: A community-based study. Aging Clin. Exp. Res. 1998, 10, 421–428. [Google Scholar] [CrossRef]
- Choi, J.-S.; Park, I.S.; Kim, S.-K.; Lim, J.-Y.; Kim, Y.-M. Analysis of age-related changes in the functional morphologies of salivary glands in mice. Arch. Oral Biol. 2013, 58, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Gorsky, M.; Epstein, J.B.; Parry, J.; Epstein, M.S.; Le, N.D.; Silverman, S. The efficacy of pilocarpine and bethanechol upon saliva production in cancer patients with hyposalivation following radiation therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 97, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Güneri, P.; Alpöz, E.; Epstein, J.B.; Çankaya, H.; Ates, M. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec. Care Dent. 2011, 31, 123–128. [Google Scholar] [CrossRef]
- Takesh, T.; Ho, J.; Firmalino, M.V.; Islip, D.; Anbarani, A.; Wilder-Smith, P. Effects of a Novel Formulation on Oral Biofilm, pH Buffering, and Gingival Health in Patients with Dry Mouth. Int. J. Dent. 2018, 2018, 2748274. [Google Scholar] [CrossRef] [PubMed]
| Classification | Drugs |
|---|---|
| Analgesics | Opioids, pregabalin, tramadol. |
| Anticonvulsants | Carbamazepine, gabapentin, lamotrigine. |
| Antidepressants | Tricyclics (e.g., amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, trimipramine), selective serotonin reuptake inhibitors (e.g., citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline), serotonin and noradrenaline reuptake inhibitors (e.g., venlafaxine), and atypical antidepressants (e.g., bupropion, duloxetine, mirtazapine, trazodone). |
| Antiemetics | Buclizine, cyclizine, dimenhydrinate, meclizine, metocloopramide, prochloperazine, scopolamine, thiethylperazine, trimethobenzamide. |
| Antihistamines | First-generation antihistamines (carbinoxamine, clemastine dexchlorpheniramine, dimenhydranate, diphenhydramine, hydroxyzine, meclizine, promethazine), and second-generation antihistamines (cetirizine, desloratadine, fexofenadine, levocetirizine loratadine). |
| Antihypertensives | α-agonists (clonidine, guanabenz, guanfacine, methldopa), β-blockers (acebutolol, atenolol, bebivolol, betaxolol, bisoprolol, carvedilol, esmolol, labetalol, metoprolol, nadolol, penbutolol, pindolol, propranolol, stalol, timolol), diuretics (bumetanide, furosemide, torsemide), Ca2+ channel blockers (amlodipine, diltiazem, felodipine, isradipine, nifedipine, nimodipine, verapamil), and angiotensin-converting enzyme inhibitors (benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril). |
| Antiparkinsonian | amantadine, benztropine, bromocriptine, carbidopa, entcapone, levodopa, pramipexole, rasagiline, ropinirole, selegiline, trihexyphenidyl. |
| Antipsychotics | Typical antipsychotics (e.g., chlorpromazine, fluphenazine, haloperidol, loxapine, perphenazine, pimozide, trifluoperazine) and atypical antipsychotics (e.g., aripiprazole, amisulpiride, clozapine, olanzapine). |
| Appetite suppressants/stimulants | Benzphetamine, diethylpropion, phentermine, phendimetrazine, sibutramine. |
| Anxiolytics | Alprazolam, chlordiazepoxide, clorazepate, diazepam, doxepin, hydroxyzine, lorazepam, meprobamate, oxazepam, prazepam. |
| Bronchodilators | Albuterol, eformoterol, ipratropium, metaproterenol, pirbuterol, salbutamol, salmeterol, tiotropium, umeclidinium. |
| Cardiovascular agents | Atenolol, clonidine, metoprolol, prazosin. |
| Muscle relaxants | Baclofen, cyclobenzaprine, orphenadrine. |
| Systemic Diseases |
|---|
| Sjögren’s syndrome |
| Systemic lupus erythematosus |
| Diabetes (type 1 and type 2) |
| Viral infection (e.g., human immunodeficiency virus, hepatitis C virus, and human T-lymphotropic virus type 1) |
| End-stage renal disease |
| Primary biliary cirrhosis |
| Ectodermal dysplasia |
| Graft-versus-host disease |
| Sarcoidosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J. Xerostomia and Its Cellular Targets. Int. J. Mol. Sci. 2023, 24, 5358. https://doi.org/10.3390/ijms24065358
Kim Y-J. Xerostomia and Its Cellular Targets. International Journal of Molecular Sciences. 2023; 24(6):5358. https://doi.org/10.3390/ijms24065358
Chicago/Turabian StyleKim, Yoon-Jung. 2023. "Xerostomia and Its Cellular Targets" International Journal of Molecular Sciences 24, no. 6: 5358. https://doi.org/10.3390/ijms24065358
APA StyleKim, Y.-J. (2023). Xerostomia and Its Cellular Targets. International Journal of Molecular Sciences, 24(6), 5358. https://doi.org/10.3390/ijms24065358






