Endocrine-Disrupting Chemicals and Disease Endpoints
Abstract
1. Introduction
2. Brief Properties of EDCs
2.1. Complex Mechanisms of EDCs
2.2. Transgenerational Effects
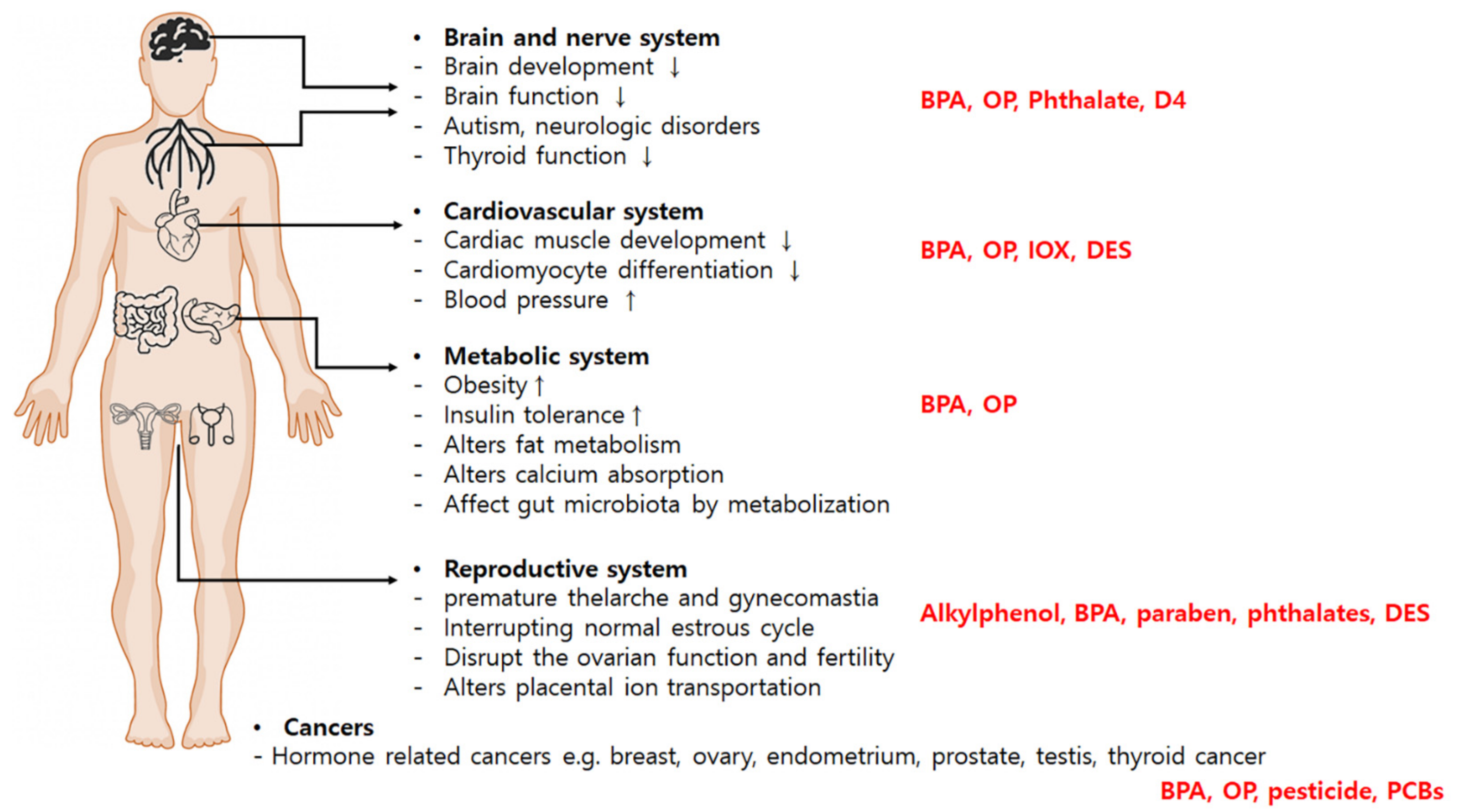
3. Disease Endpoints in Relation to EDCs
3.1. Reproductive Disorders
3.2. Metabolic Disorders
3.3. Neurologic Disorders
3.4. Cardiovascular Development
3.5. Cancers
4. Cohort Studies: Impact of EDCs on Disease Endpoints
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Combarnous, Y.; Nguyen, T.M.D. Comparative overview of the mechanisms of action of hormones and endocrine disruptor compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Cooke, P.S. Endocrine disruption through membrane estrogen receptors and novel pathways leading to rapid toxicological and epigenetic effects. J. Steroid Biochem. Mol. Biol. 2019, 187, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ai, W.; Lin, W.; Fang, F.; Wang, X.; Huang, H.; Dahlgren, R.A.; Wang, H. Identification of receptors for eight endocrine disrupting chemicals and their underlying mechanisms using zebrafish as a model organism. Ecotoxicol. Environ. Saf. 2020, 204, 111068. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Palioura, E.; Kandarakis, S.A.; Koutsilieris, M. The impact of endocrine disruptors on endocrine targets. Horm. Metab. Res. 2010, 42, 543–552. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, D.; Franssen, D.; Bakker, J.; Lomniczi, A.; Parent, A.-S. Cellular and molecular features of EDC exposure: Consequences for the GnRH network. Nat. Rev. Endocrinol. 2021, 17, 83–96. [Google Scholar] [CrossRef]
- Biemann, R.; Blüher, M.; Isermann, B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546. [Google Scholar] [CrossRef]
- Kusunoki, T.; Shimoke, K.; Komatsubara, S.; Kishi, S.; Ikeuchi, T. p-Nonylphenol induces endoplasmic reticulum stress-mediated apoptosis in neuronally differentiated PC12 cells. Neurosci. Lett. 2008, 431, 256–261. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Lieu, C.V.; Belsham, D.D. Bisphenol A induces miR-708-5p through an ER stress-mediated mechanism altering neuronatin and neuropeptide Y expression in hypothalamic neuronal models. Mol. Cell. Endocrinol. 2022, 539, 111480. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Qu, Z.; Qian, H.; Zhang, J.; Wang, H.; Xu, X.; Liu, S. Intrauterine exposure to low-dose DBP in the mice induces obesity in offspring via suppression of UCP1 mediated ER stress. Sci. Rep. 2020, 10, 16360. [Google Scholar] [CrossRef]
- Figueiredo, L.S.; Oliveira, K.M.; Freitas, I.N.; Silva, J.A., Jr.; Silva, J.N.; Favero-Santos, B.C.; Bonfleur, M.L.; Carneiro, E.M.; Ribeiro, R.A. Bisphenol-A exposure worsens hepatic steatosis in ovariectomized mice fed on a high-fat diet: Role of endoplasmic reticulum stress and fibrogenic pathways. Life Sci. 2020, 256, 118012. [Google Scholar] [CrossRef]
- Pan, J.; Yao, Y.; Guo, X.; Kong, F.; Zhou, J.; Meng, X. Endoplasmic reticulum stress, a novel significant mechanism responsible for DEHP-induced increased distance between seminiferous tubule of mouse testis. J. Cell. Physiol. 2019, 234, 19807–19823. [Google Scholar] [CrossRef]
- Ahn, C.; Kang, H.S.; Lee, J.H.; Hong, E.J.; Jung, E.M.; Yoo, Y.M.; Jeung, E.B. Bisphenol A and octylphenol exacerbate type 1 diabetes mellitus by disrupting calcium homeostasis in mouse pancreas. Toxicol. Lett. 2018, 295, 162–172. [Google Scholar] [CrossRef]
- Ozkemahli, G.; Erkekoglu, P.; Ercan, A.; Zeybek, N.D.; Yersal, N.; Kocer-Gumusel, B. Effects of single or combined exposure to bisphenol A and mono (2-ethylhexyl) phthalate on oxidant/antioxidant status, endoplasmic reticulum stress, and apoptosis in HepG2 cell line. Environ. Sci. Pollut. Res. 2023, 30, 12189–12206. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Uptake of endocrine-disrupting chemicals by quagga mussels (Dreissena bugensis) in an urban-impacted aquatic ecosystem. Environ. Sci. Pollut. Res. Int. 2019, 26, 250–258. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Yadav, S.; Panuwet, P.; Kumar, S.; Rajacharya, G.H.; Johnson, C.; Rawal, I.; Mohan, D.; Mohan, V.; Tandon, N. Metabolite of the pesticide DDT and incident type 2 diabetes in urban India. Environ. Int. 2019, 133, 105089. [Google Scholar] [CrossRef]
- Jeung, E.B.; Choi, K.C. Toxicological mechanism of endocrine disrupting chemicals:is estrogen receptor involved? Toxicol. Res. 2010, 26, 237–243. [Google Scholar] [CrossRef]
- Cimmino, I.; D’Esposito, V.; Liguoro, D.; Liguoro, P.; Ambrosio, M.R.; Cabaro, S.; D’Andrea, F.; Beguinot, F.; Formisano, P.; Valentino, R. Low-dose Bisphenol-A regulates inflammatory cytokines through GPR30 in mammary adipose cells. J. Mol. Endocrinol. 2019, 63, 273–283. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. ERβ and NFκB—Modulators of zearalenone-induced oxidative stress in human prostate cancer cells. Toxins 2020, 12, 199. [Google Scholar] [CrossRef]
- Xie, M.; Liang, J.-L.; Huang, H.-D.; Wang, M.-J.; Zhang, T.; Yang, X.-F. Low Doses of Nonylphenol Promote Growth of Colon Cancer Cells through Activation of ERK1/2 via G Protein-Coupled Receptor 30. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 1620. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Evaluating the Effects of PCBs in Non-Alcoholic Fatty Liver Disease & Diabetes and the Role of AhR in Regulating the Hepatic Proteome and Lipid Metabolism. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2020. [Google Scholar]
- Balaguer, P.; Delfosse, V.; Bourguet, W. Mechanisms of endocrine disruption through nuclear receptors and related pathways. Curr. Opin. Endocr. Metab. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Moche, H.; Chentouf, A.; Neves, S.; Corpart, J.-M.; Nesslany, F. Comparison of in vitro endocrine activity of phthalates and alternative plasticizers. J. Toxicol. 2021, 2021, 8815202. [Google Scholar] [CrossRef]
- Kim, D.; Cho, H.; Eggers, R.; Kim, S.K.; Ryu, C.S.; Kim, Y.J. Development of a liquid chromatography/mass spectrometry-based inhibition assay for the screening of steroid 5-α reductase in human and fish cell lines. Molecules 2021, 26, 893. [Google Scholar] [CrossRef]
- Svingen, T.; Schwartz, C.L.; Rosenmai, A.K.; Ramhøj, L.; Johansson, H.K.L.; Hass, U.; Draskau, M.K.; Davidsen, N.; Christiansen, S.; Ballegaar, A.-S.R. Using alternative test methods to predict endocrine disruption and reproductive adverse outcomes: Do we have enough knowledge? Environ. Pollut. 2022, 304, 119242. [Google Scholar] [CrossRef]
- Salehi, A.; Loganathan, N.; Belsham, D.D. Bisphenol A induces Pomc gene expression through neuroinflammatory and PPARγ nuclear receptor-mediated mechanisms in POMC-expressing hypothalamic neuronal models. Mol. Cell. Endocrinol. 2019, 479, 12–19. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, J.S.; Ahn, C.; Jeung, E.B. Endocrine-Disrupting Chemicals and Their Adverse Effects on the Endoplasmic Reticulum. Int. J. Mol. Sci. 2022, 23, 1581. [Google Scholar] [CrossRef]
- Alavian-Ghavanini, A.; Ruegg, J. Understanding Epigenetic Effects of Endocrine Disrupting Chemicals: From Mechanisms to Novel Test Methods. Basic Clin. Pharmacol. Toxicol. 2018, 122, 38–45. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Jacobs, M.N.; Gant, T.W. Environmentally induced epigenetic toxicity: Potential public health concerns. Crit. Rev. Toxicol. 2016, 46, 676–700. [Google Scholar] [CrossRef]
- Skinner, M.K. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 2016, 12, 68–70. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- López-Rodríguez, D.; Aylwin, C.F.; Delli, V.; Sevrin, E.; Campanile, M.; Martin, M.; Franssen, D.; Gérard, A.; Blacher, S.; Tirelli, E. Multi-and transgenerational disruption of maternal behavior and female puberty by Endocrine Disrupting Chemical (EDC) mixture exposure. bioRxiv 2020. [Google Scholar] [CrossRef]
- Den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Alboghobeish, S.; Mahdavinia, M.; Zeidooni, L.; Samimi, A.; Oroojan, A.A.; Alizadeh, S.; Dehghani, M.A.; Ahangarpour, A.; Khorsandi, L. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran. J. Basic Med. Sci. 2019, 22, 315. [Google Scholar]
- Klenov, V.; Flor, S.; Ganesan, S.; Adur, M.; Eti, N.; Iqbal, K.; Soares, M.J.; Ludewig, G.; Ross, J.W.; Robertson, L.W. The Aryl hydrocarbon receptor mediates reproductive toxicity of polychlorinated biphenyl congener 126 in rats. Toxicol. Appl. Pharmacol. 2021, 426, 115639. [Google Scholar] [CrossRef]
- Johnson, K.J.; Passage, J.; Lin, H.; Sriram, S.; Budinsky, R.A. Dioxin male rat reproductive toxicity mode of action and relative potency of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and 2,3,7,8-tetrachlorodibenzofuran characterized by fetal pituitary and testis transcriptome profiling. Reprod. Toxicol. 2020, 93, 146–162. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, L.; Flaws, J.A. Exposure to an Environmentally Relevant Phthalate Mixture Causes Transgenerational Effects on Female Reproduction in Mice. Endocrinology 2017, 158, 1739–1754. [Google Scholar] [CrossRef]
- Gore, A.C.; Moore, T.; Groom, M.J.; Thompson, L.M. Prenatal Exposure to an EDC Mixture, NeuroMix: Effects on Brain, Behavior, and Stress Responsiveness in Rats. Toxics 2022, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.K.; Vandenberg, L.N. REPRODUCTIVE TOXICOLOGY: The male mammary gland: A novel target of endocrine-disrupting chemicals. Reproduction 2021, 162, F79–F89. [Google Scholar] [CrossRef] [PubMed]
- Castiello, F.; Freire, C. Exposure to non-persistent pesticides and puberty timing: A systematic review of the epidemiological evidence. Eur. J. Endocrinol. 2021, 184, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Gea, M.; Toso, A.; Bentivegna, G.N.; Buganza, R.; Abrigo, E.; De Sanctis, L.; Schilirò, T. Oestrogenic Activity in Girls with Signs of Precocious Puberty as Exposure Biomarker to Endocrine Disrupting Chemicals: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-S.; Lee, J.D.; Song, S.-W.; Shin, H.-C.; Choi, Y.-K.; Shin, C.Y.; Lee, B.-M.; Kim, K.-B. Thirteen-week subcutaneous repeated dose toxicity study of butylparaben and its toxicokinetics in rats. Arch. Toxicol. 2021, 95, 2037–2050. [Google Scholar] [CrossRef]
- Mohammadi, L.; Parandin, R.; Pournaghi, P. Effects of Methyl paraben neonatal treatment on puberty onset, estrus cycle, and development of ovarian follicles in female mice (Balb/c). J. Shahid Sadoughi Univ. Med. Sci. 2019, 27, 1556–1567. [Google Scholar] [CrossRef]
- Lee, H.R.; Jeung, E.B.; Cho, M.H.; Kim, T.H.; Leung, P.C.; Choi, K.C. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J. Cell. Mol. Med. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Polak, G.; Banaszewska, B.; Filip, M.; Radwan, M.; Wdowiak, A. Environmental Factors and Endometriosis. Int. J. Environ. Res. Public Health 2021, 18, 11025. [Google Scholar] [CrossRef]
- Kawa, I.A.; Akbar, M.; Fatima, Q.; Mir, S.A.; Jeelani, H.; Manzoor, S.; Rashid, F. Endocrine disrupting chemical Bisphenol A and its potential effects on female health. Diabetes Metab. Syndr. 2021, 15, 803–811. [Google Scholar] [CrossRef]
- Gao, X.; Yu, L.; Castro, L.; Moore, A.B.; Hermon, T.; Bortner, C.; Sifre, M.; Dixon, D. An endocrine-disrupting chemical, fenvalerate, induces cell cycle progression and collagen type I expression in human uterine leiomyoma and myometrial cells. Toxicol. Lett. 2010, 196, 133–141. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.M.; Yoo, Y.M.; Ahn, C.; Kang, H.Y.; Choi, K.C.; Hyun, S.H.; Dang, V.H.; Pham, T.N.; Jeung, E.B. Depletion of follicles accelerated by combined exposure to phthalates and 4-vinylcyclohexene diepoxide, leading to premature ovarian failure in rats. Reprod. Toxicol. 2018, 80, 60–67. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.M.; Ahn, C.; Lee, J.H.; Yoo, Y.M.; Jeung, E.B. Effects of Bisphenol A and 4-tert-Octylphenol on Embryo Implantation Failure in Mouse. Int. J. Environ. Res. Public Health 2018, 15, 1614. [Google Scholar] [CrossRef]
- Hanson, H.A.; Anderson, R.E.; Aston, K.I.; Carrell, D.T.; Smith, K.R.; Hotaling, J.M. Subfertility increases risk of testicular cancer: Evidence from population-based semen samples. Fertil. Steril. 2016, 105, 322–328.e321. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Bayen, S.; Castaneda-Cortes, D.; Delbes, G.; Grigorova, P.; Langlois, V.S.; Martyniuk, C.J.; Metcalfe, C.D.; Parent, L.; Rwigemera, A.; et al. Impacts of endocrine disrupting chemicals on reproduction in wildlife and humans. Environ. Res. 2022, 208, 112584. [Google Scholar] [CrossRef]
- Spinder, N.; Bergman, J.E.; van Tongeren, M.; Boezen, H.M.; Kromhout, H.; de Walle, H.E. Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum. Reprod. 2022, 37, 142–151. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Lahlou, N.; Coquillard, P.; Wagner-Mahler, K.; Pugeat, M.; Panaia-Ferrari, P.; Brucker-Davis, F. Endocrine Disrupting Chemicals Interfere With Leydig Cell Hormone Pathways During Testicular Descent in Idiopathic Cryptorchidism. Front. Endocrinol. 2018, 9, 786. [Google Scholar] [CrossRef]
- Sharma, A.; Mollier, J.; Brocklesby, R.W.; Caves, C.; Jayasena, C.N.; Minhas, S. Endocrine-disrupting chemicals and male reproductive health. Reprod. Med. Biol. 2020, 19, 243–253. [Google Scholar] [CrossRef]
- Cannarella, R.; Gül, M.; Rambhatla, A.; Agarwal, A. Temporal decline of sperm concentration: Role of endocrine disruptors. Endocrine 2022, 79, 1–16. [Google Scholar] [CrossRef]
- Walker, C.; Ghazisaeidi, S.; Collet, B.; Boisvert, A.; Culty, M. In utero exposure to low doses of genistein and di-(2-ethylhexyl) phthalate (DEHP) alters innate immune cells in neonatal and adult rat testes. Andrology 2020, 8, 943–964. [Google Scholar] [CrossRef]
- Jager, C.d.; Patrick, S.; Aneck-Hahn, N.; Bornman, M. Environmental Toxicants and Sperm Production in Men and Animals. In XIIIth International Symposium on Spermatology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 47–59. [Google Scholar]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef] [PubMed]
- Amorello, D.; Indelicato, R.; Barreca, S.; Orecchio, S.; Orecchio, S. Analytical Method for Quantification of Several Phthalate Acid Esters by Gas Chromatography-Mass Spectrometry in Coffee Brew Samples. ChemistryOpen 2022, 11, e202200082. [Google Scholar] [CrossRef] [PubMed]
- Bornman, M.S.; Aneck-Hahn, N.H. EDCs and male urogenital cancers. Adv. Pharmacol. 2021, 92, 521–553. [Google Scholar] [PubMed]
- Lee, J.H.; Ahn, C.; Kang, H.Y.; Hong, E.J.; Hyun, S.H.; Choi, K.C.; Jeung, E.B. Effects of Octylphenol and Bisphenol A on the Metal Cation Transporter Channels of Mouse Placentas. Int. J. Environ. Res. Public Health 2016, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fang, R.; Wang, H.; Xu, D.X.; Yang, J.; Huang, X.; Cozzolino, D.; Fang, M.; Huang, Y. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ. Int. 2022, 158, 106941. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Tabb, M.M.; Blumberg, B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006, 20, 475–482. [Google Scholar] [CrossRef]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288. [Google Scholar] [CrossRef]
- Ranciere, F.; Lyons, J.G.; Loh, V.H.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef]
- Mallhi, T.H.; Khokhar, A.; Khan, Y.H.; Alotaibi, N.H.; Khan, A. Endocrine Disrupting Chemicals Induced Childhood Obesity. In Endocrine Disrupting Chemicals-Induced Metabolic Disorders and Treatment Strategies; Springer: Berlin/Heidelberg, Germany, 2021; pp. 147–160. [Google Scholar]
- Hong, S.H.; Sung, Y.A.; Hong, Y.S.; Ha, E.; Jeong, K.; Chung, H.; Lee, H. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin. Endocrinol. 2017, 86, 506–512. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Minguez-Alarcon, L.; Ford, J.B.; Keller, M.; Seely, E.W.; Messerlian, C.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M.; et al. Trimester-Specific Urinary Bisphenol A Concentrations and Blood Glucose Levels Among Pregnant Women From a Fertility Clinic. J. Clin. Endocrinol. Metab. 2017, 102, 1350–1357. [Google Scholar] [CrossRef]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef]
- Wade, M.; Delawder, V.; Reneau, P.; Dos Santos, J.M. The effect of BPA exposure on insulin resistance and type 2 diabetes–The impact of muscle contraction. Med. Hypotheses 2020, 140, 109675. [Google Scholar] [CrossRef]
- Kim, J.; Kang, E.J.; Park, M.N.; Kim, J.E.; Kim, S.C.; Jeung, E.B.; Lee, G.S.; Hwang, D.Y.; An, B.S. The adverse effect of 4-tert-octylphenol on fat metabolism in pregnant rats via regulation of lipogenic proteins. Environ. Toxicol. Pharmacol. 2015, 40, 284–291. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Yuan, Y.Z.; Feng, Q.P.; Hu, M.Y.; Li, W.J.; Wu, X.; Xiang, S.Y.; Yu, S.Q. Food emulsifier polysorbate 80 promotes the intestinal absorption of mono-2-ethylhexyl phthalate by disturbing intestinal barrier. Toxicol. Appl. Pharmacol. 2021, 414, 115411. [Google Scholar] [CrossRef]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef]
- Foulds, C.E.; Trevino, L.S.; York, B.; Walker, C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef]
- Yi, D.; Kim, K.; Lee, M.; Jung, E.-m.; Jeung, E.-B. Effects of Maternal Exposure to Decamethylcyclopentasiloxane on the Alternations in Offspring Behaviors in Mice. Biomedicines 2022, 11, 35. [Google Scholar] [CrossRef]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Morris, E.A.R.; Miranda-Alves, L.; Denicol, A.C. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Heck, A.L.; De Guzman, R.M.; Handa, R.J. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol. Sex Differ. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Park, S.-M.; Jung, E.-M.; Jeung, E.-B. Prenatal Octamethylcyclotetrasiloxane Exposure Impaired Proliferation of Neuronal Progenitor, Leading to Motor, Cognition, Social and Behavioral Functions. Int. J. Mol. Sci. 2021, 22, 12949. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Rouget, F.; Warembourg, C.; Monfort, C.; Limon, G.; Cordier, S.; Chevrier, C. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: The PELAGIE mother-child cohort. Occup. Environ. Med. 2017, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Limon, G.; Rouget, F.; Monfort, C.; Durand, G.; Cordier, S.; Chevrier, C. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother-child cohort. Environ. Int. 2015, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Clark-Reyna, S.E.; Grineski, S.E.; Collins, T.W. Ambient Concentrations of Metabolic Disrupting Chemicals and Children’s Academic Achievement in El Paso, Texas. Int. J. Environ. Res. Public Health 2016, 13, 874. [Google Scholar] [CrossRef]
- Lamb, J.C.t.; Boffetta, P.; Foster, W.G.; Goodman, J.E.; Hentz, K.L.; Rhomberg, L.R.; Staveley, J.; Swaen, G.; Van Der Kraak, G.; Williams, A.L. Critical comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals-2012. Regul. Toxicol. Pharmacol. 2014, 69, 22–40. [Google Scholar] [CrossRef]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.M.; Yoo, Y.M.; Jeung, E.B. 4-tert-Octylphenol Exposure Disrupts Brain Development and Subsequent Motor, Cognition, Social, and Behavioral Functions. Oxid. Med. Cell. Longev. 2020, 2020, 8875604. [Google Scholar] [CrossRef]
- Atolani, O.; Baker, M.T.; Adeyemi, O.S.; Olanrewaju, I.R.; Hamid, A.A.; Ameen, O.M.; Oguntoye, S.O.; Usman, L.A. COVID-19: Critical discussion on the applications and implications of chemicals in sanitizers and disinfectants. EXCLI J. 2020, 19, 785–799. [Google Scholar]
- Lewis, D.S.M.; Ho, J.; Wills, S.; Kawall, A.; Sharma, A.; Chavada, K.; Ebert, M.; Evoli, S.; Singh, A.; Rayalam, S.; et al. Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 in vitro. Sci. Rep. 2022, 12, 2145. [Google Scholar] [CrossRef]
- Maksymowicz, M.; Machowiec, P.A.; Ręka, G.; Korzeniowska, A.; Leszczyk, P.; Piecewicz Szczęsna, H. Mechanism of action of triclosan as an endocrine-disrupting chemical with its impact on human health–literature review. J. Pre-Clin. Clin. Res. 2021, 15, 169–175. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.M.; Yoo, Y.M.; Lee, J.H.; Jeung, E.B. Perinatal Exposure to Triclosan Results in Abnormal Brain Development and Behavior in Mice. Int. J. Mol. Sci. 2020, 21, 4009. [Google Scholar] [CrossRef]
- Baud, O.; Berkane, N. Hormonal changes associated with intra-uterine growth restriction: Impact on the developing brain and future neurodevelopment. Front. Endocrinol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Köhrle, J.; Frädrich, C. Thyroid hormone system disrupting chemicals. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101562. [Google Scholar] [CrossRef]
- Sokal, A.; Jarmakiewicz-Czaja, S.; Tabarkiewicz, J.; Filip, R. Dietary intake of endocrine disrupting substances presents in environment and their impact on thyroid function. Nutrients 2021, 13, 867. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by stealth-Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Oulhote, Y.; Chevrier, J.; Bouchard, M.F. Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Hypothyroidism in Canadian Women. J. Clin. Endocrinol. Metab. 2016, 101, 590–598. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, Y.M.; Jung, E.M.; Ahn, C.H.; Jeung, E.B. Inhibitory effect of octyl-phenol and bisphenol A on calcium signaling in cardiomyocyte differentiation of mouse embryonic stem cells. J. Physiol. Pharmacol. 2019, 70, 435–442. [Google Scholar]
- Kurokawa, J.; Furukawa, T. Non-genomic action of sex steroid hormones and cardiac repolarization. Biol. Pharm. Bull. 2013, 36, 8–12. [Google Scholar] [CrossRef]
- Bae, S.; Lim, Y.H.; Lee, Y.A.; Shin, C.H.; Oh, S.Y.; Hong, Y.C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension 2017, 69, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cenciarini, M.E.; Proietti, C.J. Molecular mechanisms underlying progesterone receptor action in breast cancer: Insights into cell proliferation and stem cell regulation. Steroids 2019, 152, 108503. [Google Scholar] [CrossRef] [PubMed]
- Solís-Pérez, J.; Gómez-Aguilar, J.; Atangana, A. A fractional mathematical model of breast cancer competition model. Chaos Solitons Fractals 2019, 127, 38–54. [Google Scholar] [CrossRef]
- Cohn, B.A.; La Merrill, M.; Krigbaum, N.Y.; Yeh, G.; Park, J.S.; Zimmermann, L.; Cirillo, P.M. DDT Exposure in Utero and Breast Cancer. J. Clin. Endocrinol. Metab. 2015, 100, 2865–2872. [Google Scholar] [CrossRef]
- Eberle, C.E.; Sandler, D.P.; Taylor, K.W.; White, A.J. Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. Int. J. Cancer 2020, 147, 383–391. [Google Scholar] [CrossRef]
- Ilozumba, M.N.; Shelver, W.L.; Hong, C.-C.; Ambrosone, C.B.; Cheng, T.-Y.D. Urinary Concentrations of Triclosan, Bisphenol A, and Brominated Flame Retardants and the Association of Triclosan with Demographic Characteristics and Body Fatness among Women with Newly Diagnosed Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 4681. [Google Scholar] [CrossRef]
- Lee, G.A.; Hwang, K.A.; Choi, K.C. Inhibitory effects of 3,3’-diindolylmethane on epithelial-mesenchymal transition induced by endocrine disrupting chemicals in cellular and xenograft mouse models of breast cancer. Food Chem. Toxicol. 2017, 109, 284–295. [Google Scholar] [CrossRef]
- Vasdev, N.; Moon, A.; Thorpe, A.C. Classification, epidemiology and therapies for testicular germ cell tumours. Int. J. Dev. Biol. 2013, 57, 133–139. [Google Scholar] [CrossRef]
- Hardell, L.; van Bavel, B.; Lindstrom, G.; Carlberg, M.; Dreifaldt, A.C.; Wijkstrom, H.; Starkhammar, H.; Eriksson, M.; Hallquist, A.; Kolmert, T. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ. Health Perspect. 2003, 111, 930–934. [Google Scholar] [CrossRef]
- Hardell, L.; Bavel, B.; Lindstrom, G.; Eriksson, M.; Carlberg, M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int. J. Androl. 2006, 29, 228–234. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Quraishi, S.M.; Graubard, B.I.; Weber, J.P.; Rubertone, M.V.; Erickson, R.L. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J. Natl. Cancer Inst. 2008, 100, 663–671. [Google Scholar] [CrossRef]
- Li, K.; Pang, J.; Cheng, H.; Liu, W.P.; Di, J.M.; Xiao, H.J.; Luo, Y.; Zhang, H.; Huang, W.T.; Chen, M.K.; et al. Manipulation of prostate cancer metastasis by locus-specific modification of the CRMP4 promoter region using chimeric TALE DNA methyltransferase and demethylase. Oncotarget 2015, 6, 10030–10044. [Google Scholar] [CrossRef]
- Alavanja, M.C.; Samanic, C.; Dosemeci, M.; Lubin, J.; Tarone, R.; Lynch, C.F.; Knott, C.; Thomas, K.; Hoppin, J.A.; Barker, J.; et al. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol. 2003, 157, 800–814. [Google Scholar] [CrossRef]
- Mahajan, R.; Bonner, M.R.; Hoppin, J.A.; Alavanja, M.C. Phorate exposure and incidence of cancer in the agricultural health study. Environ. Health Perspect. 2006, 114, 1205–1209. [Google Scholar] [CrossRef]
- Prins, G.S. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 2008, 15, 649–656. [Google Scholar] [CrossRef]
- Prins, G.S.; Calderon-Gierszal, E.L.; Hu, W.Y. Stem Cells as Hormone Targets That Lead to Increased Cancer Susceptibility. Endocrinology 2015, 156, 3451–3457. [Google Scholar] [CrossRef]
- Prins, G.S.; Hu, W.Y.; Shi, G.B.; Hu, D.P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef]
- Cheong, A.; Zhang, X.; Cheung, Y.Y.; Tang, W.Y.; Chen, J.; Ye, S.H.; Medvedovic, M.; Leung, Y.K.; Prins, G.S.; Ho, S.M. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics 2016, 11, 674–689. [Google Scholar] [CrossRef]
- Wang, Q.; Trevino, L.S.; Wong, R.L.; Medvedovic, M.; Chen, J.; Ho, S.M.; Shen, J.; Foulds, C.E.; Coarfa, C.; O’Malley, B.W.; et al. Reprogramming of the Epigenome by MLL1 Links Early-Life Environmental Exposures to Prostate Cancer Risk. Mol. Endocrinol. 2016, 30, 856–871. [Google Scholar] [CrossRef]
- Modugno, F.; Weissfeld, J.L.; Trump, D.L.; Zmuda, J.M.; Shea, P.; Cauley, J.A.; Ferrell, R.E. Allelic variants of aromatase and the androgen and estrogen receptors: Toward a multigenic model of prostate cancer risk. Clin. Cancer Res. 2001, 7, 3092–3096. [Google Scholar]
- Margel, D.; Fleshner, N.E. Oral contraceptive use is associated with prostate cancer: An ecological study. BMJ Open 2011, 1, e000311. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Hanke, W. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2011, 24, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Asci, A.; Erkekoglu, P.; Akcurin, S.; Gumusel, B.K.; Bircan, I. Urinary bisphenol a levels in girls with idiopathic central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cragin, L.A.; Kesner, J.S.; Bachand, A.M.; Barr, D.B.; Meadows, J.W.; Krieg, E.F.; Reif, J.S. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ. Res. 2011, 111, 1293–1301. [Google Scholar] [CrossRef]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Berry, K.F.; Calafat, A.M.; Ye, X.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health Perspect. 2012, 120, 978–983. [Google Scholar] [CrossRef]
- Smarr, M.M.; Mirzaei Salehabadi, S.; Boyd Barr, D.; Buck Louis, G.M.; Sundaram, R. A multi-pollutant assessment of preconception persistent endocrine disrupting chemicals and incident pregnancy loss. Environ. Int. 2021, 157, 106788. [Google Scholar] [CrossRef]
- Perry, M.J. Effects of environmental and occupational pesticide exposure on human sperm: A systematic review. Hum. Reprod. Update 2008, 14, 233–242. [Google Scholar] [CrossRef]
- Martenies, S.E.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 2013, 307, 66–73. [Google Scholar] [CrossRef]
- Baillie-Hamilton, P.F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement Med. 2002, 8, 185–192. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Xu, Q. The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 7605. [Google Scholar] [CrossRef]
- Li, R.; Yang, S.; Gao, R.; Deng, Y.; Liu, J.; Yuan, C.; Yao, Q.; Lv, X.; Wang, K.; Ye, X.; et al. Relationship between the Environmental Endocrine Disruptor Bisphenol a and Dyslipidemia: A Five-Year Prospective Study. Endocr. Pract. 2020, 26, 399–406. [Google Scholar] [CrossRef]
- Cardenas, A.; Hivert, M.F.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Lin, P.D.; Fleisch, A.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; et al. Associations of Perfluoroalkyl and Polyfluoroalkyl Substances With Incident Diabetes and Microvascular Disease. Diabetes Care 2019, 42, 1824–1832. [Google Scholar] [CrossRef]
- Gutierrez-Torres, D.S.; Barraza-Villarreal, A.; Hernandez-Cadena, L.; Escamilla-Nunez, C.; Romieu, I. Prenatal Exposure to Endocrine Disruptors and Cardiometabolic Risk in Preschoolers: A Systematic Review Based on Cohort Studies. Ann. Glob. Health 2018, 84, 239–249. [Google Scholar] [CrossRef]
- Song, Y.; Chou, E.L.; Baecker, A.; You, N.C.; Song, Y.; Sun, Q.; Liu, S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J. Diabetes 2016, 8, 516–532. [Google Scholar] [CrossRef]
- Valvi, D.; Casas, M.; Romaguera, D.; Monfort, N.; Ventura, R.; Martinez, D.; Sunyer, J.; Vrijheid, M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ. Health Perspect. 2015, 123, 1022–1029. [Google Scholar] [CrossRef]
- Troisi, R.; Titus, L.; Hatch, E.E.; Palmer, J.R.; Huo, D.; Strohsnitter, W.C.; Adam, E.; Ricker, W.; Hyer, M.; Hoover, R.N. A Prospective Cohort Study of Prenatal Diethylstilbestrol Exposure and Cardiovascular Disease Risk. J. Clin. Endocrinol. Metab. 2018, 103, 206–212. [Google Scholar] [CrossRef]
- Fu, X.; Xu, J.; Zhang, R.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef]
- Shoaff, J.R.; Calafat, A.M.; Schantz, S.L.; Korrick, S.A. Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ. Res. 2019, 172, 231–241. [Google Scholar] [CrossRef]
- Weng, J.C.; Hong, C.I.; Tasi, J.D.; Shen, C.Y.; Su, P.H.; Wang, S.L. The association between prenatal endocrine-disrupting chemical exposure and altered resting-state brain fMRI in teenagers. Brain Struct. Funct. 2020, 225, 1669–1684. [Google Scholar] [CrossRef]
- O’Shaughnessy, K.L.; Fischer, F.; Zenclussen, A.C. Perinatal exposure to endocrine disrupting chemicals and neurodevelopment: How articles of daily use influence the development of our children. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101568. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Endocrine disrupting chemicals and breast cancer: A systematic review of epidemiological studies. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Brureau, L.; Emeville, E.; Helissey, C.; Thome, J.P.; Multigner, L.; Blanchet, P. Endocrine disrupting-chemicals and biochemical recurrence of prostate cancer after prostatectomy: A cohort study in Guadeloupe (French West Indies). Int. J. Cancer 2020, 146, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, T.; Yuan, J.M.; Koh, W.P.; Jin, A.; Odegaard, A. Occupational exposure to endocrine disrupting substances and the risk of breast Cancer: The Singapore Chinese health study. BMC Public Health 2018, 18, 929. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.W.; Diaz Santana, M.; Manson, J.E.; Hankinson, S.E.; Zoeller, R.T.; Bigelow, C.; Sturgeon, S.R.; Spiegelman, D.; Tinker, L.; Luo, J.; et al. Urinary Phthalate Biomarker Concentrations and Postmenopausal Breast Cancer Risk. J. Natl. Cancer Inst. 2019, 111, 1059–1067. [Google Scholar] [CrossRef]
- Morgan, M.; Deoraj, A.; Felty, Q.; Roy, D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol. Cell. Endocrinol. 2017, 457, 89–102. [Google Scholar] [CrossRef]
- Warner, M.; Mocarelli, P.; Samuels, S.; Needham, L.; Brambilla, P.; Eskenazi, B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ. Health Perspect. 2011, 119, 1700–1705. [Google Scholar] [CrossRef]
- Arrebola, J.P.; Fernandez-Rodriguez, M.; Artacho-Cordon, F.; Garde, C.; Perez-Carrascosa, F.; Linares, I.; Tovar, I.; Gonzalez-Alzaga, B.; Exposito, J.; Torne, P.; et al. Associations of persistent organic pollutants in serum and adipose tissue with breast cancer prognostic markers. Sci. Total Environ. 2016, 566–567, 41–49. [Google Scholar] [CrossRef]
- Biggs, M.L.; Davis, M.D.; Eaton, D.L.; Weiss, N.S.; Barr, D.B.; Doody, D.R.; Fish, S.; Needham, L.L.; Chen, C.; Schwartz, S.M. Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2012–2018. [Google Scholar] [CrossRef]
- Swartz, S.J.; Morimoto, L.M.; Whitehead, T.P.; DeRouen, M.C.; Ma, X.; Wang, R.; Wiemels, J.L.; McGlynn, K.A.; Gunier, R.; Metayer, C. Proximity to endocrine-disrupting pesticides and risk of testicular germ cell tumors (TGCT) among adolescents: A population-based case-control study in California. Int. J. Hyg. Environ. Health 2022, 239, 113881. [Google Scholar] [CrossRef]
- Bariani, M.V.; Rangaswamy, R.; Siblini, H.; Yang, Q.; Al-Hendy, A.; Zota, A.R. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 380–387. [Google Scholar] [CrossRef]
- Wen, X.; Xiong, Y.; Qu, X.; Jin, L.; Zhou, C.; Zhang, M.; Zhang, Y. The risk of endometriosis after exposure to endocrine-disrupting chemicals: A meta-analysis of 30 epidemiology studies. Gynecol. Endocrinol. 2019, 35, 645–650. [Google Scholar] [CrossRef]
- Smarr, M.M.; Kannan, K.; Buck Louis, G.M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 2016, 106, 959–966. [Google Scholar] [CrossRef]
- Williams, K.E.; Miroshnychenko, O.; Johansen, E.B.; Niles, R.K.; Sundaram, R.; Kannan, K.; Albertolle, M.; Zhou, Y.; Prasad, N.; Drake, P.M.; et al. Urine, peritoneal fluid and omental fat proteomes of reproductive age women: Endometriosis-related changes and associations with endocrine disrupting chemicals. J. Proteom. 2015, 113, 194–205. [Google Scholar] [CrossRef]
- Vos, J.G.; Dybing, E.; Greim, H.A.; Ladefoged, O.; Lambre, C.; Tarazona, J.V.; Brandt, I.; Vethaak, A.D. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. 2000, 30, 71–133. [Google Scholar] [CrossRef]
- Barrett, E.S.; Sobolewski, M. Polycystic ovary syndrome: Do endocrine-disrupting chemicals play a role? Semin. Reprod. Med. 2014, 32, 166–176. [Google Scholar]
- Lee, H.R.; Hwang, K.A.; Nam, K.H.; Kim, H.C.; Choi, K.C. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem. Res. Toxicol. 2014, 27, 834–842. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zaha, H.; Nagano, R.; Yoshinaga, J.; Yonemoto, J.; Sone, H. Xenoestrogens down-regulate aryl-hydrocarbon receptor nuclear translocator 2 mRNA expression in human breast cancer cells via an estrogen receptor alpha-dependent mechanism. Toxicol. Lett. 2011, 206, 152–157. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Ding, T.; Yeoman, K.B.; Archibong, A.; Arosh, J.A.; Osteen, K.G. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS ONE 2014, 9, e105084. [Google Scholar] [CrossRef]
- Sirohi, D.; Al Ramadhani, R.; Knibbs, L.D. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: A systematic literature review. Rev. Environ. Health 2021, 36, 101–115. [Google Scholar] [CrossRef]
- Murro, I.; Lisco, G.; Di Noia, C.; Lampignano, L.; Zupo, R.; Giagulli, V.A.; Guastamacchia, E.; Triggiani, V.; De Pergola, G. Endocrine Disruptors and Obesity: An Overview. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 798–806. [Google Scholar] [PubMed]
- Wieczorek, K.; Szczesna, D.; Jurewicz, J. Environmental Exposure to Non-Persistent Endocrine Disrupting Chemicals and Endometriosis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5608. [Google Scholar] [CrossRef] [PubMed]
- Aubert, M.L.; Nef, S.; Soto, A.M. Special issue on the topic: Role of endocrine disruptors from the environment in the aetiology of obesity and diabetes. Mol. Cell. Endocrinol. 2009, 304, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Endocrine disruptors: A missing link in the pandemy of type 2 diabetes and obesity? Presse Med. 2016, 45, 88–97. [Google Scholar] [CrossRef]
- Shafei, A.E.; Nabih, E.S.; Shehata, K.A.; Abd Elfatah, E.S.M.; Sanad, A.B.A.; Marey, M.Y.; Hammouda, A.; Mohammed, M.M.M.; Mostafa, R.; Ali, M.A. Prenatal Exposure to Endocrine Disruptors and Reprogramming of Adipogenesis: An Early-Life Risk Factor for Childhood Obesity. Child. Obes. 2018, 14, 18–25. [Google Scholar] [CrossRef]
- Gonzalez-Casanova, J.E.; Pertuz-Cruz, S.L.; Caicedo-Ortega, N.H.; Rojas-Gomez, D.M. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. Biomed Res. Int. 2020, 2020, 7453786. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef]
- Taylor, P.N.; Okosieme, O.E.; Murphy, R.; Hales, C.; Chiusano, E.; Maina, A.; Joomun, M.; Bestwick, J.P.; Smyth, P.; Paradice, R.; et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: Data from the Controlled Antenatal Thyroid Study. J. Clin. Endocrinol. Metab. 2014, 99, 4291–4298. [Google Scholar] [CrossRef]
- Trichopoulos, D. Is breast cancer initiated in utero? Epidemiology 1990, 1, 95–96. [Google Scholar]
- Ekbom, A.; Akre, O. Increasing incidence of testicular cancer--birth cohort effects. APMIS 1998, 106, 225–229, discussion 229–231. [Google Scholar] [CrossRef]
- Huyghe, E.; Matsuda, T.; Thonneau, P. Increasing incidence of testicular cancer worldwide: A review. J. Urol. 2003, 170, 5–11. [Google Scholar] [CrossRef]
- Bray, F.; Richiardi, L.; Ekbom, A.; Pukkala, E.; Cuninkova, M.; Moller, H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int. J. Cancer 2006, 118, 3099–3111. [Google Scholar] [CrossRef]
- Buterin, T.; Koch, C.; Naegeli, H. Convergent transcriptional profiles induced by endogenous estrogen and distinct xenoestrogens in breast cancer cells. Carcinogenesis 2006, 27, 1567–1578. [Google Scholar] [CrossRef]
- Cargouet, M.; Bimbot, M.; Levi, Y.; Perdiz, D. Xenoestrogens modulate genotoxic (UVB)-induced cellular responses in estrogen receptors positive human breast cancer cells. Environ. Toxicol. Pharmacol. 2006, 22, 104–112. [Google Scholar] [CrossRef]
- Wu, F.; Safe, S. Differential activation of wild-type estrogen receptor alpha and C-terminal deletion mutants by estrogens, antiestrogens and xenoestrogens in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2007, 103, 1–9. [Google Scholar] [CrossRef]
- Buteau-Lozano, H.; Velasco, G.; Cristofari, M.; Balaguer, P.; Perrot-Applanat, M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J. Endocrinol. 2008, 196, 399–412. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Russo, J. Estrogen and xenoestrogens in breast cancer. Toxicol. Pathol. 2010, 38, 110–122. [Google Scholar] [CrossRef]
- Hwang, K.A.; Park, S.H.; Yi, B.R.; Choi, K.C. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab. Anim. Res. 2011, 27, 99–107. [Google Scholar] [CrossRef]
| System | Organ | Disease | Chemicals | Ref |
|---|---|---|---|---|
| Reproductive system | Uterus | Uterine fibroid | Bisphenols, phthalates, pesticides (DDT, DDE, endosulfans, DES, etc.) | [53,153] |
| Endometrium | Endometriosis | Bisphenols, phthalates, pesticides (chlorpyrifos, HCB), dioxin, PCBs | [154,155,156] | |
| Ovary | Infertility, subfertility | DES, phthalates, bisphenols, parabens, heavy metals, dioxin, PCBs, pesticides (DDT, DDE), triclosan | [5,157] | |
| Irregular reproductive cycles, Early menopause, PCOS | Phthalates, bisphenols, dioxins, PCBs, pesticides (DDT, DDE), parabens, DES, OP, NP, triclosan | [50,51,52,158] | ||
| Breast | Breast cancer | Bisphenols, phytoestrogens, DES, TCDD, PCBs, DDT, DDE, pesticides (vinclozolin) | [108,159,160] | |
| Testis | Infertility, subfertility | Phthalates, PCBs, Pesticides, Pesticides (vinclozolin, ethylene dibromide) | [35,161,162] | |
| Cryptorchidism | Bisphenols, Phthalates, dioxins, PCBs, pesticides (vinclozolin), parabens, DES | [5,58,157] | ||
| Hypospadias | Phthalates, DES, progestin, loratadine, clomiphene, pesticides (vinclozolin, DDT, atrazine) | [163,164,165] | ||
| Metabolic system | Pancreas | Pancreatic β cell damage, | Bisphenols, DDT, OP, nonylphenol, rodenticide (pyrinuron) | [14,70,75,166] |
| Intestine | Type II diabetes | Bisphenols, DDT, octylphenol, NP, phthalates | [14,166,167] | |
| Changes in Gut microbiota | Dioxins, pesticides, pyrethroids, PCBs, flame retardants, triclosan | [79] | ||
| Adipose tissue | Alters fat metabolism, hypertrophy or hyperplasia of adipocytes | Bisphenols, DES, PCBs, Tributyltin, OP | [168,169] | |
| Nervous system | Brain | psychiatric, cognitive, and behavioral disorders, Autism Spectrum Disorders | Bisphenols, Diethylstilbestrol, phthalates, pesticides, octamethylcyclotetrasiloxane, OP, triclosan | [91,95] |
| Neuroendocrine system | Thyroid | Decrease in hormone biosynthesis, transport, metabolism, and thyroid hormone receptor (TR) activity | Bisphenols, PBDEs, TBP, PCBs, phthalates, perchlorate | [101,170,171] |
| Cardiovascular system | Heart | Abnormal cardiovascular development, interrupted calcium signaling, increasing the probability of cardiovascular disease, | Bisphenols, OP, ioxynil, DES, TCDD, DDT | [71,102] |
| - | Cancers | Hormone-related cancers or hormone-sensitive cancers, such as breast, endometrium, ovary, prostate, testis, and thyroid cancer Parkinson’s disease | Bisphenol, DDT, triclosan, OP, TCDD, DES, phthalates (Breast cancer) Bisphenols, DDT, PCBs, phthalates (Uterine cancer) Bisphenols, Herbicides (chlorotriazine), DES, TCDD (Ovarian cancer) DDE, benzenes, (Testicular cancer) Bisphenols, DES, pesticides (endosulfans, malathion, vinclozolin), Agricultural chemicals, Dioxin, arsenic, cadmium, PCBs (Prostate cancer) | [65,107,108,110,112,113,115,116,117,118,119,121,122,123,124,159,160,172,173,174,175,176,177,178,179,180,181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. https://doi.org/10.3390/ijms24065342
Ahn C, Jeung E-B. Endocrine-Disrupting Chemicals and Disease Endpoints. International Journal of Molecular Sciences. 2023; 24(6):5342. https://doi.org/10.3390/ijms24065342
Chicago/Turabian StyleAhn, Changhwan, and Eui-Bae Jeung. 2023. "Endocrine-Disrupting Chemicals and Disease Endpoints" International Journal of Molecular Sciences 24, no. 6: 5342. https://doi.org/10.3390/ijms24065342
APA StyleAhn, C., & Jeung, E.-B. (2023). Endocrine-Disrupting Chemicals and Disease Endpoints. International Journal of Molecular Sciences, 24(6), 5342. https://doi.org/10.3390/ijms24065342






