Abstract
Spodoptera litura is a damaging and notorious insect pest of agricultural crops that has developed resistance to various insecticides. Broflanilide is a novel pesticide with a unique mode of action that displays high efficiency against lepidopterous larvae. We here determined the baseline susceptibility of a laboratory strain of S. litura to broflanilide and 10 other popular insecticides. Furthermore, we measured susceptibility and cross-resistance using three common insecticides in 11 field-collected S. litura populations. Broflanilide caused the highest toxicity among all tested insecticides, with the laboratory strain and all field-collected populations showing high susceptibility. Moreover, no cross-resistance was detected between broflanilide and the other tested insecticides. We subsequently evaluated the sublethal effects of broflanilide and found that treatment with the 25% lethal concentration (LC25) prolonged the development duration in the larvae, reduced the pupation rate and pupae weight, and decreased egg hatchability. Finally, the activities of three detoxifying enzymes were measured in S. litura after treatment with the LC25 dose. The results suggested that enhanced cytochrome P450 monooxygenase (P450) activity could be involved in broflanilide detoxification. Overall, these findings demonstrate the strong toxicity and significant sublethal effects of broflanilide in S. litura and suggest that increased P450 activity may be associated with broflanilide detoxification.
1. Introduction
Broflanilide is a new meta-diamide pesticide that targets the γ-aminobutyric acid receptor (GABAR) in insect pests through a novel mechanism of action [1]. The Insecticide Resistance Action Committee has classified it in a novel group (group 30) based on its action as an allosteric modulator of GABAR [2]. It not only exhibits excellent lethal effects against a host of lepidopteran pests, such as Plutella xylostella, Helicoverpa armigera, Spodoptera frugiperda, Spodoptera litura, and Spodoptera exigua [3,4,5], but also shows highly lethal pesticidal activity against other insect pests including thrips, cotton aphids, and two-spotted spider mites [6,7,8]. Broflanilide was registered as a commercialized pesticide in China in 2020 and is considered a promising chemical agent for field application to control agricultural pests [9]. Moreover, it displays minimal non-target effects on various natural crop enemies such as Cyrtorhinus lividipennis, Singa pygmaea, Pirata subpiraticus, Erigonidium graminicolum, and Theridion octomaculatum [10]. Broflanilide could be effective for controlling herbivores that are resistant to other pesticides, and this compound has broad potential applications in insecticide-resistance management both locally and abroad. Although, on the basis of previous publications and in view of its unique mode of action, broflanilide is considered a very promising chemical agent for controlling pests that are resistant to other insecticides, it is important to study characteristics such as toxicity, baseline field susceptibility, and cross-resistance to establish efficient integrated pest management programs and inform safe usage practices.
Chemical insecticides gradually degrade after field spraying, and the target insect pests are often exposed to low concentrations of them in the field [11,12,13]. Apart from the lethal effects of pesticides, the low residual concentrations can exert sublethal effects, impacting biological, physiological, and biochemical processes, immunological function, development, reproduction of pests and even community ecology [12]. For instance, in Bemisia tabaci, sublethal concentrations of various chemical agents (such as afidopyropen, cycloxaprid, cyantraniliprole, clothianidin, and dinotefuran) shorten the duration of insect development and decrease the number of oviposition days, female fecundity, and egg hatchability [14,15,16,17,18]. Similarly, sublethal concentrations of chlorantraniliprole not only extend the duration of larval development and decrease egg hatchability in beet armyworm, but also reduce viability and reproduction in diamondback moth [19]. In contrast, exposure to sublethal pesticide concentrations can stimulate the development and reproduction of some insect pests. These stimulatory effects, referred to as hormesis, occur in many insect pests that are exposed to various pesticides [20,21]. The demonstration of hormesis is critical to management strategies of insect pests, and because hormesis results from sublethal concentrations of pesticides, extensive pesticide application can cause insect pest resurgences [21,22,23].
Spodoptera litura (Fabricius), commonly known as tobacco cutworm, is an extensively distributed and notoriously devastating agricultural insect pest. S. litura is distributed worldwide in temperate and subtropical zones and attacks hundreds of different crop species [24,25], especially in subtropical and tropical Asian countries such as China, Japan, Pakistan, and India [26,27,28]. Owing to its great capacity of reproduction, over-reliance on insecticides against S. litura has contributed to the development of resistance to various insecticides applied around the world, and over the past 10 years in China, excessive reliance on chemical insecticides for crop management has caused S. litura to develop significant resistance to different types of pesticides such as carbamates, organophosphates, pyrethroids, and benzoylurea, and novel pesticides such as indoxacarb, metaflumizone, chlorantraniliprole, and pyridalyl [28,29,30,31,32,33]. Continuous over-utilization of these pesticides is unlikely to efficiently control S. litura. It is therefore urgent to identify a novel chemical agent for use in rotation with existing pesticides. In the present study, we firstly confirmed the toxicity of broflanilide to S. litura and then determined the baseline susceptibility of field-sampled S. litura populations and assessed pesticide cross-resistance with other three popular chemical agents. With this work, we found that all field-sampled populations were highly susceptible to broflanilide, and no cross-resistance to the other tested pesticides was observed. Moreover, we assessed the sublethal effects of broflanilide on S. litura and then illustrated the biochemical mechanisms associated with these sublethal effects by measuring the activities of esterase (EST), glutathione S-transferase (GST), and cytochrome P450 monooxygenase (P450). In summary, this study describes the optimal use of broflanilide against S. litura and lays the foundation for future research and the development of broflanilide as a novel pesticide.
2. Results
2.1. Toxicity and Baseline Susceptibility of S. litura to Broflanilide
The LC50 values were calculated for broflanilide and 10 other popular insecticides using S. litura larvae (Table 1). Broflanilide showed the highest toxicity against S. litura (LC50 = 0.08 mg/L), followed by abamectin (0.10 mg/L), tetraniliprole (0.19 mg/L), spinetoram (0.46 mg/L), chlorfenapyr (0.88 mg/L), chromafenozide (0.91 mg/L), pyridalyl (1.22 mg/L), cyantraniliprole (1.32 mg/L), chlorantraniliprole (2.21 mg/L), metaflumizone (3.61 mg/L), and flubendiamide (9.95 mg/L); these compounds were 1.3, 2.4, 5.8, 11.0, 11.4, 15.3, 16.5, 27.6, 45.1, and 124.4 times less toxic than broflanilide, respectively. The baseline broflanilide susceptibility was then determined in S. litura populations collected from 11 Chinese provinces (Figure 1) and compared to that of the susceptible Lab-S strain. Little broflanilide resistance was observed in any of the field populations (Figure 2).

Table 1.
Toxicitiy of broflanilide and 10 other popular insecticides in the susceptible Spodoptera litura strain Lab-S.
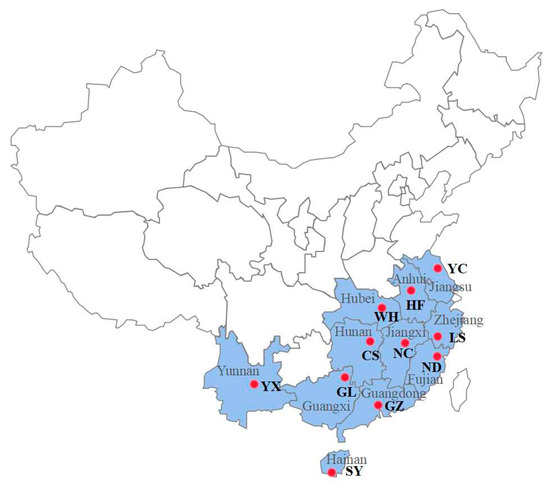
Figure 1.
Spodoptera litura field populations of Yunnan (Yuxi, YX), Anhui (Hefei, HF), Hubei (Wuhan, WH), Jiangsu (Yancheng, YC), Jiangxi (Nanchang, NC), Zhejiang (Lishui, LS), Fujian (Ningde, ND), Hunan (Changsha, CS), Guangdong (Guangzhou, GZ), Guangxi (Guilin, GL), and Hainan (Sanya, SY) sampling sites in China. Samples were collected in 2021.
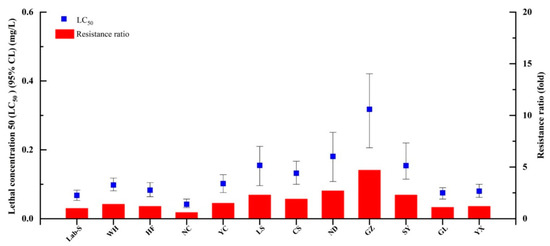
Figure 2.
Susceptibility of field-collected Spodoptera litura populations to broflanilide. LC50, median lethal concentration.
2.2. Cross-Resistance to Broflanilide and Three Other Popular Insecticides
Three field-collected populations (GZ, YX, and ND) were used to establish the cross-resistance patterns between broflanilide and three other popular insecticides (metaflumizone, chlorantraniliprole, and pyridalyl) as previously described by our lab [33]. Compared to the reference strain Lab-S, the GZ, YX, and ND populations displayed 80.4-, 64.7-, and 51.8-fold higher resistance, respectively, to metaflumizone; 86.4-, 56.4-, and 59.7-fold higher resistance, respectively, to chlorantraniliprole; and 48.8-, 78.3-, and 40.5-fold higher resistance, respectively, to pyridalyl (Table 2). Compared to the Lab-S strain, the GZ, YX, and ND populations showed 3.3-, 1.8-, 2.1-fold higher resistance, respectively, to broflanilide. Thus, broflanilide displayed little cross-resistance with metaflumizone, chlorantraniliprole, or pyridalyl.

Table 2.
Cross-resistance between broflanilide and three popular insecticides in Spodoptera litura.
2.3. Sublethal Effects of Broflanilide in S. litura
To determine potential sublethal effects of broflanilide, several biological parameters were measured in 3rd-instar larvae treated with the LC25 dose: development duration, weight of pupae and larvae, pupation and emergence rate, female fecundity and oviposition duration, and egg hatchability (Figure 3, Figure 4 and Figure 5). In comparison with control individuals, the development duration from 3rd- to 6th-instar larvae was greatly extended (by 1.95 d) in individuals treated with the LC25 dose, although the duration of the pre-pupa and pupa stages was not significantly different (Figure 3A). The successful pupation rate was decreased in the LC25-treated group compared to the control, yet a small significant difference was observed in the emergence rate (Figure 3B). The mean pupal weight was significantly decreased (by 55.76 mg) in the LC25 treatment group compared to the control group, whereas there were no significant differences in weight at any of the other four tested stages (Figure 4). The mean fecundity per female and oviposition duration were not significantly different between the LC25 and the control groups, although egg hatchability was significantly reduced (by 9.09%) in the treatment group (Figure 5).
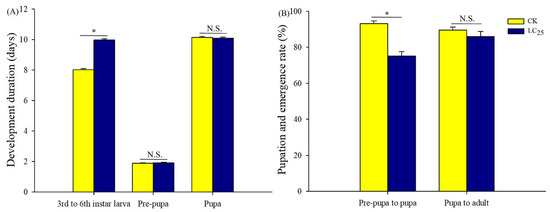
Figure 3.
Development duration (A) and pupation and emergence rates (B) of Spodoptera litura. Yellow, control (CK) individuals. Dark blue, individuals treated with the 25% lethal concentration (LC25) of broflanilide. Values are presented as the mean ± standard error. * p < 0.05 (Student’s t-test) and N.S. indicates not significant.
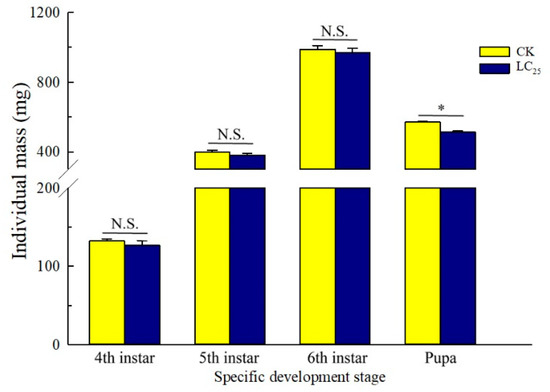
Figure 4.
Spodoptera litura larval weight at selected developmental stages. Yellow, control (CK) individuals. Dark blue, individuals treated with the 25% lethal concentration (LC25) of broflanilide. Values are presented as the mean ± standard error. * p < 0.05 (Student’s t-test) and N.S. indicates not significant.
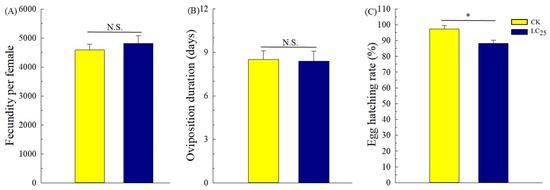
Figure 5.
Fecundity (A), oviposition duration (B), and egg hatching rate (C) of Spodoptera litura. Yellow, control (CK) individuals. Dark blue, individuals treated with the 25% lethal concentration (LC25) of broflanilide. Values are presented as the mean ± standard error. * p < 0.05 (Student’s t-test) N.S. indicates not significant.
2.4. Detoxifying Enzyme Activity in LC25-Treated Insects
To confirm the potential functions of S. litura detoxifying enzymes in response to sublethal concentrations of broflanilide, P450, GST, and EST activities were assayed in control and LC25-treated insects (Table 3). In comparison with the control individuals, those treated with LC25 dose showed significantly enhanced P450 activity (1.6-fold higher). Similarly, the LC25 treatment group showed significantly increased GST activity, 1.7-fold higher than in the control group. EST activity was little increased in the LC25 treatment group compared to the control, but the difference was not significant.

Table 3.
Detoxification enzyme activities in Spodoptera litura in the control (CK) and 25% lethal concentration (LC25) broflanilide treatment groups a.
3. Discussion
S. litura is an economically damaging insect pest that is notorious for its ability to develop pesticide resistance [34]. Due to its notable history of evolving resistance, it is essential to determine S. litura baseline susceptibility to novel pesticides before they are applied in the field. We here established the baseline susceptibility of several field-sampled populations of S. litura to 10 popular insecticides using our previously published method [33]. This is the first report about the baseline susceptibility of this pest to broflanilide in China. The data of the current research displayed that the novel pesticide broflanilide was greatly effective against S. litura. Moreover, we found a narrow range of geographical variation in broflanilide susceptibility between populations (less than five-fold resistance ratio). Another study revealed that field populations of the insect pests P. xylostella, H. armigera, and S. frugiperda in China are highly susceptible to broflanilide [5]. This novel insecticide could thus be a powerful tool to control the four lepidopteran species of the most common and highly damaging insect pests in China. In other orders of agricultural insect pests in China, an increasing number of species have exhibited baseline susceptibility to broflanilide; it has been reported that broflanilide is potentially useful against cotton aphids and several thrip species [7,8]. The utilization of novel pesticides is considered a critical strategy to avoid or delay the development of resistance to common pesticides in agricultural herbivores. Our results, therefore, serve as a valuable reference when monitoring broflanilide resistance in S. litura, contributing to the improvement of resistance management measures in China henceforth.
Three field-collected resistant strains of S. litura were used to determine the cross-resistance between broflanilide and three other popular insecticides (metaflumizone, chlorantraniliprole, and pyridalyl). These comparisons indicated a little significant cross-resistance, meaning that it is highly feasible to rotate broflanilide with metaflumizone, chlorantraniliprole, and pyridalyl in the field to combat S. litura. Similarly, significant cross-resistance to broflanilide was not observed in three diamide-resistant populations of diamondback moth and one spinosyns-resistant population of fall armyworm [5]. Earlier reports indicated that broflanilide displays excellent efficiency against fipronil- and dieldrin-resistant housefly, fipronil-resistant Sogatella furcifera and Oulema oryzae, diamide-resistant diamondback moth [1,35], and dieldrin- and pyrethroid-resistant Anopheles gambiae [36,37]. All things considered, our results suggest that there is minimal or no cross-resistance between broflanilide and other common pesticides that are associated with diverse mechanisms of resistance. Broflanilide can thus be a helpful tool to complete the management of pests that are already resistant to popular chemical agents.
In addition to killing insects at lethal concentrations, sublethal concentrations of chemical agents can exert significant effects on insect behavior, physiology, and even community ecology. These effects must be studied as part of an integrated evaluation of pesticide effects [12]. We here found that the LC25 dose of broflanilide greatly slowed larval development, decreased the pupation rate and pupae weight, and reduced egg hatchability in S. litura. Other studies previously showed that a variety of chemical agents exert sublethal effects on S. litura, interfering with its development and reproduction [38,39,40]. A recent study of broflanilide effects on S. frugiperda showed that sublethal doses were associated with decreased larval body length, prolonged larval and pupal duration, and malformed development of pupae and adults [41]. In Tetranychus urticae, sublethal concentrations of broflanilide not only reduced the total insect’s life span, but decreased the fecundity of adult females, causing a population decline [6]. Although hormesis has been reported in several insect species as a result of exposure to various insecticides [21], such effects have not been reported for broflanilide. Furthermore, we found that the activities of GST and P450, two major detoxifying enzymes in insects, were significantly increased in S. litura after exposure to the LC25 dose of broflanilide. In B. tabaci, treatment with the LC25 dose of β-asarone (a plant-derived potential insecticide) significantly induces P450 activity, and a sublethal concentration of afidopyropen enhances GST activity [18,42]; in contrast, GST activity is significantly inhibited in Panonychus citri treated with sublethal concentrations of the acaricides fenazaquin and acequinocyl [43]. Based on the RNA-seq technology, recent studies have suggested that the mechanisms of insecticide sublethal effects are strongly associated with detoxifying gene expression, and those results indicate that cytochrome P450 monooxygenases, esterases, glutathione S-transferases, and ATP-binding cassette transporters could be up- or down-regulated with exposure to sublethal concentrations of insecticides [44,45]. Transcriptomic analyses will therefore be carried out in S. litura treated with sublethal doses of broflanilide to identify related transcriptional changes, understand the functions of detoxifying genes, and finally delineate the mechanisms of action of this insecticide.
4. Materials and Methods
4.1. Insects
The lab-raised susceptible S. litura strain Lab-S was reared as previously described [33] with no pesticide exposure for over five years. Eleven field populations of S. litura were collected from southern China (Figure 1) and named Yunnan (Yuxi, YX), Anhui (Hefei, HF), Hubei (Wuhan, WH), Jiangsu (Yancheng, YC), Jiangxi (Nanchang, NC), Zhejiang (Lishui, LS), Fujian (Ningde, ND), Hunan (Changsha, CS), Guangdong (Guangzhou, GZ), Guangxi (Guilin, GL), and Hainan (Sanya, SY). Among the above field-collected populations of S. litura, the GZ, YX, and ND populations displayed middle to high levels of resistance to the three insecticides metaflumizone, chlorantraniliprole, and pyridalyl, respectively, according to our previous work [33]. The GZ, YX, and ND populations and the Lab-S strain were used to establish the cross-resistance patterns. All populations were maintained in a well-controlled growth chamber at 26 ± 2 °C with 65 ± 5% relative humidity and a 16/8 h light/dark photoperiod. All larval populations were fed an artificial diet, and adults were reared on a 10% sugar solution.
4.2. Insecticides and Chemicals
The insecticides and chemicals utilized for this study were analytical-grade standards. Broflanilide (Chemical Abstracts Service [CAS] #1207727-04-5), tetraniliprole (CAS #1229654-66-3), chlorantraniliprole (CAS #500008-45-7), chromafenozide (CAS #143807-66-3), and spinetoram (CAS #187166-40-1) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Cyantraniliprole (CAS #736994-63-1), flubendiamide (CAS #272451-65-7), pyridalyl (CAS #179101-81-6), metaflumizone (CAS #139968-49-3), chlorfenapyr (CAS #122453-73-0), abamectin (CAS #71751-41-2), dimethyl sulfoxide (DMSO) (CAS #67-68-5), and Triton X-100 (CAS #9002-93-1) were purchased from Sigma Aldrich (Shanghai, China).
4.3. Bioassays
All bioassays in this study were carried out with the use of a previously published leaf-dip method [33] with slight changes. Third-instar larvae were randomly sampled, and working concentrations of the pesticides to be tested were generated by dilution in DMSO and sterile water with 0.1% Triton X-100. Leaf discs (4.5 cm in diameter) were cut from Brassica oleracea (cabbage), dipped into a working concentration of pesticide for 20 s, dried at room temperature in the growth chamber, then put into a Petri dish (5 cm in diameter). Ten 3rd-instar larvae were placed onto each leaf disc to form one replication. There were four replicates for each working concentration of each pesticide. All larvae were maintained in a well-controlled growth chamber under the conditions described above.
4.4. Evaluation of Sublethal Broflanilide Effects on S. litura
To assess the sublethal effects of broflanilide on S. litura, leaf discs were prepared with the 25% lethal concentration (LC25) of broflanilide (0.03 mg/L) using the leaf-dip method described above. The leaf discs were then incubated with 150 12-h-old 3rd-instar larvae for 48 h to generate the LC25 treatment group. The control group comprised an additional 150 untreated third-instar larvae. The larvae in each treatment group were randomly divided into 15 biological replicate groups containing 10 larvae each. After pupation, the deformed pupae were counted, and the pupation rate was recorded. After the adults emerged, the rate of emergence, male/female ratio, and deformed adult rate were recorded. Fifteen pairs of female and male adults were coupled within 12 h and put in a plastic cup (4 × 8 cm in diameter × height) containing a 10% (w/v) honey solution, which was replaced daily. Longevity was measured daily for male and female adults; for female adults, the duration of oviposition and the number of eggs were also recorded every day.
4.5. Detoxifying Enzyme Assays in LC25-Treated Insects
Fifteen 3rd-instar larvae were selected and homogenized in 20 mL of homogenization buffer (0.1 M phosphate buffer at pH 7.6 with 1 mM EDTA, 1 mM PTU, 1 mM DTT, 20% glycerol, and 1 mM PMSF). The samples were centrifuged at 4 °C and 12,000× g for 20 min. The supernatant was removed and transferred to a new Eppendorf tube on ice, then immediately assayed for protein content and P450, EST, and GST activity using a previously published method [46] with slight changes. P450 activity was determined using p-nitroanisole as the substrate; for the p-nitroanisole O-demethylation (PNOD) assay, the activity was measured in nmol p-nitrophenol min−1 mg−1 protein. GST activity was measured using 1-chloro-2, 4-dinitrobenzene (CDNB) as the substrate and computed using an extinction coefficient of 9.6 mM−1 cm−1 for CDNB [47]. EST activity was assayed using α-naphthyl acetate (α-NA) as the substrate and measured in nmol α-naphthol min−1 mg−1 protein. The total protein content was measured using bovine serum albumin (BSA) as the standard, as described by Bradford [48]. There were three replicates per treatment group for each assay.
4.6. Statistical Analysis
Probit analysis was conducted to confirm the significance of the death rate statistics in the samples treated with a series of working concentrations of chemical agents. The concentration–mortality response, median lethal concentration (LC50), 95% fiducial limit (FL), and slope value were calculated for each compound with PoloPlus [49]. The resistance ratio (RR) was estimated as LC50 (field-collected population)/LC50 (Lab-S), and the levels of insecticide resistance are published by our previous work [33]. Specifically, susceptibility corresponded to the RR less than 5-fold higher than the reference value, low level of resistance corresponded to the RR from 5- to 10-fold higher, middle level of resistance corresponded to the RR from 10- to 40-fold higher, high level of resistance corresponded to the RR from 40- to 160-fold higher, and very high level of resistance corresponded to the RR over 160-fold higher. Student’s t-test was performed to determine the statistical significance of the differences in growth duration, viability, fecundity, oviposition time, and egg hatchability of S. litura between the LC25-treated and the control groups. Student’s t-test was also used to assess differences in detoxifying enzyme activity between the LC25-treated and the control groups. All statistical analyses were conducted in SPSS [50].
5. Conclusions
In the current work, firstly, we found that the novel meta-diamide pesticide, broflanilide, is the most toxic to larvae of S. litura among eleven popular commercialized chemical agents which are commonly used against S. litura. After that, we monitored the status of resistance to broflanilide by using eleven populations of S. litura field-collected across southern China and established for the first time the baseline susceptibility to broflanilide of S. litura in China. We showed that the susceptibility was very high, and no significant resistance was detected in China. After that, the cross-resistance patterns with the three common insecticides metaflumizone, chlorantraniliprole, and pyridalyl were established using three field-evolved resistant populations of S. litura, and no cross-resistance between broflanilide and the three tested insecticides was observed. Then, the sublethal effects of broflanilide were evaluated, and after treatment with the 25% lethal concentration (LC25) of the third-instar larvae, we found the development duration of the larvae was prolonged, the pupation rate and pupae weight were reduced, and egg hatchability was decreased. Based on the LC25 treatment, the activities of the three main detoxifying enzymes cytochrome P450 monooxygenase (P450), glutathione S-transferase (GST), and esterase (EST) were estimated in S. litura after the treatment, and the results indicated that increased P450 activity could contribute to the detoxification of broflanilide. Overall, all the above findings illustrated the high toxicity and significant sublethal effects of broflanilide in S. litura and showed that increased P450 activity could be related ti the detoxification of broflanilide.
Author Contributions
Conceptualization, Y.L., L.Z. and R.W.; methodology, Y.L., L.Z. and R.W.; software, Y.L.; validation, C.Q.; formal analysis, Y.L.; investigation, Y.L., L.Z. and R.W.; resources, Q.Z.; data curation, Q.Z.; writing—original draft preparation, Y.L. and R.W.; writing—review and editing, R.W.; visualization, R.W.; supervision, L.Z., C.L. and R.W.; project administration, C.L. and R.W.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the China Agriculture Research System of MOF and MARA (CARS-24-C-03), the Scientific and Technological Innovation Capacity Construction Special Funds of Beijing Academy of Agriculture and Forestry Sciences, Beijing, China (KJCX20210437).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are available from the corresponding author on request (wangran@ipepbaafs.cn).
Acknowledgments
We acknowledge the excellent technical assistance and collection of field populations by Ziyi Zhang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification–a tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Hu, F.; Wang, P.; Fu, W.; Liu, X. Broflanilide effectively controls Helicoverpa armigera and Spodoptera exigua exhibiting diverse susceptibilities to chlorantraniliprole and emamectin benzoate. Pest Manag. Sci. 2021, 77, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Liu, H.; Mou, T.; Ma, Y.; Li, Y.; Song, Z.; Tang, T.; Han, Z.; Zhao, C. Novel meta-diamide insecticide, broflanilide, suppresses the population of common cutworm Spodoptera litura through its lethal and sublethal effects. Pest Manag. Sci. 2022, 78, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, T.; Tang, P.; Liu, S.; Hou, B.; Jiang, D.; Lu, J.; Yang, Y.; Carrière, Y.; Wu, Y. Baseline susceptibility of Helicoverpa armigera, Plutella xylostella, and Spodoptera frugiperda to the meta-diamide insecticide broflanilide. Insect Sci. 2022. [Google Scholar] [CrossRef]
- Shen, N.; Li, Y.; Leviticus, K.; Chang, X.; Tang, T.; Cui, L.; Han, Z.; Zhao, C. Effect of broflanilide on the phytophagous mite Tetranychus urticae and the predatory mite Typhlodromips swirskii. Pest Manag. Sci. 2021, 77, 2964–2970. [Google Scholar] [CrossRef]
- Chen, J.; Cao, L.; Sun, L.; Gao, Y.; Cao, H.; Ma, Z.; Ma, L.; Shen, X.; Wang, J.; Gong, Y.; et al. Variation in the toxicity of a novel meta-diamide insecticide, broflanilide, among thrips pest species and developmental stages. Pest Manag. Sci. 2022, 78, 5090–5096. [Google Scholar] [CrossRef]
- Li, R.; Cheng, S.; Chen, Z.; Guo, T.; Liang, P.; Zhen, C.; Wang, J.; Zhang, L.; Liang, P.; Gao, X. Establishment of toxicity and susceptibility baseline of broflanilide for Aphis gossypii Glove. Insects 2022, 13, 1033. [Google Scholar] [CrossRef]
- Sun, X.; Wei, R.; Li, L.; Zhu, B.; Liang, P.; Gao, X. Resistance and fitness costs in diamondback moths after selection using broflanilide, a novel meta-diamide insecticide. Insect Sci. 2022, 29, 188–198. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Li, B.; Shi, X.; Xiong, Z. Toxicity of broflanilide on major rice pests and its influence on natural enemies in paddy fields. J. Plant Prot. 2019, 46, 574–581. [Google Scholar]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations following applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.R.; Istchuk, A.N.; Foresti, J.; Hunt, T.E.; de Araújo, T.A.; Fernandes, F.L.; de Alencar, E.R.; Bastos, C.S. Economic injury levels and economic thresholds for Diceraeus (Dichelops) melacanthus (Hemiptera: Pentatomidae) in vegetative maize. Crop Prot. 2021, 143, 105476. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, H.; Qu, C.; Wang, Z.; Kong, Z.; Luo, C. Lethal and sublethal effects of a novel cis-nitromethylene neonicotinoid insecticide, cycloxaprid, on Bemisia tabaci. Crop Prot. 2016, 83, 15–19. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Che, W.; Qu, C.; Li, F.; Desneux, N.; Luo, C. Lethal and sublethal effects of cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci (Hemiptera: Aleyrodidae) MED. Crop Prot. 2017, 91, 108–113. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Luo, C.; Wang, R. Lethal and sublethal effects of clothianidin on the development and reproduction of Bemisia tabaci (Hemiptera: Aleyrodidae) MED and MEAM1. J. Insect Sci. 2018, 18, 37. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, W.; Li, F.; Tetreau, G.; Luo, C.; Wang, R. Lethal and sublethal effects of dinotefuran on two invasive whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae) J. Asia-Pac. Entomol. 2017, 20, 325–330. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Zheng, H.; Zhang, Q.; Gong, J.; Li, C.; Wang, R. Physiological and biochemical responses to sublethal concentrations of the novel pyropene insecticide, afidopyropen, in whitefly Bemisia tabaci MED. Agronomy 2021, 11, 2260. [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Shen, F.; Liu, M.; Ren, C.; Gao, X. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1184–1190. [Google Scholar] [CrossRef]
- Cutler, G.C. Insects, insecticides and hormesis: Evidence and considerations for study. Dose-Response 2013, 11, 154–177. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 823, 153899. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide induced stress in Arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2019, 65, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, J.; Okuno, S.; Nakai, M.; Kunimi, Y. Genetic and phenotypic comparisons of viral genotypes from two nucleopolyhedroviruses interacting with a common host species, Spodoptera litura (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2016, 139, 42–49. [Google Scholar] [CrossRef]
- Gong, J.; Cheng, T.; Wu, Y.; Yang, X.; Feng, Q.; Mita, K. Genome-wide patterns of copy number variations in Spodoptera litura. Genomics 2019, 111, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sayyed, A.H.; Saleem, M.A.; Ahmad, M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008, 27, 1367–1372. [Google Scholar] [CrossRef]
- Shad, S.A.; Sayyed, A.H.; Fazal, S.; Saleem, M.A.; Zaka, S.M.; Ali, M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J. Pest. Sci. 2012, 85, 153–162. [Google Scholar] [CrossRef]
- Tong, H.; Su, Q.; Zhou, X.; Bai, L. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J. Pest. Sci. 2013, 86, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Q.; Hao, Q.; Ran, S.; Wu, Y.; Cui, P.; Yang, J.; Jiang, C.; Yang, Q. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 2018, 106, 110–116. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.; Yao, X.; Gong, C.; Shen, L. Effects of bistrifluron resistance on the biological traits of Spodoptera litura (Fab.) (Noctuidae: Lepidoptera). Ecotoxicology 2019, 28, 323–332. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Liu, H.; Chen, X.; Zhou, L. Metabolism and antioxidant activity of SlGSTD1 in Spodoptera litura as a detoxification enzyme to pyrethroids. Sci. Rep. 2022, 12, 10108. [Google Scholar] [CrossRef]
- Hou, W.; Staehelin, C.; Elzaki, M.E.A.; Hafeez, M.; Luo, Y.; Wang, R. Functional analysis of CYP6AE68, a cytochrome P450 gene associated with indoxacarb resistance in Spodoptera litura (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2021, 178, 104946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, B.; Qu, C.; Gong, J.; Li, W.; Luo, C.; Wang, R. Resistance monitoring for six insecticides in vegetable field-collected populations of Spodoptera litura from China. Horticulturae 2022, 8, 255. [Google Scholar] [CrossRef]
- APRD. Arthropod Pesticide Resistance Database. 2022. Available online: https://www.pesticideresistance.org/ (accessed on 21 May 2022).
- Nakao, T.; Banba, S.; Nomura, M.; Hirase, K. Metadiamide insecticides acting on distinct sites of RDL GABA receptor from those for conventional noncompetitive antagonists. Insect Biochem. Mol. Biol. 2013, 43, 366–375. [Google Scholar] [CrossRef]
- Lees, R.S.; Ambrose, P.; Williams, J.; Morgan, J.; Praulins, G.; Ingham, V.A.; Williams, C.T.; Logan, R.A.E.; Ismail, H.M.; Malone, D. Tenebenal: A meta-diamide with potential for use as a novel mode of action insecticide for public health. Malar. J. 2020, 19, 398. [Google Scholar] [CrossRef] [PubMed]
- Ngufor, C.; Govoetchan, R.; Fongnikin, A.; Vigninou, E.; Syme, T.; Akogbeto, M.; Rowland, M. Efficacy of broflanilide (VECTRON T500), a new meta-diamide insecticide, for indoor residual spraying against pyrethroid-resistant malaria vectors. Sci. Rep. 2021, 11, 7976. [Google Scholar] [CrossRef]
- Zhu, Q.; He, Y.; Yao, J.; Liu, Y.; Tao, L.; Huang, Q. Effects of sublethal concentrations of the chitin synthesis inhibitor, hexaflumuron, on the development and hemolymph physiology of the cutworm, Spodoptera litura. J. Insect Sci. 2012, 12, 27. [Google Scholar] [CrossRef]
- Liu, D.; Jia, Z.; Peng, Y.; Sheng, C.; Tao, T.; Xu, L.; Han, Z.; Zhao, C. Toxicity and sublethal effects of fluralaner on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2018, 152, 8–16. [Google Scholar] [CrossRef]
- Kong, F.; Song, Y.; Zhang, Q.; Wang, Z.; Liu, Y. Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) moth: Implication for attract-and-kill strategy. Toxics 2021, 9, 20. [Google Scholar] [CrossRef]
- Jia, Z.; Zhan, E.; Zhang, S.; Jones, A.K.; Zhu, L.; Wang, Y.; Huang, Q.T.; Han, Z.; Zhao, C. Sublethal doses of broflanilide prevents molting in the fall armyworm, Spodoptera frugiperda via altering molting hormone biosynthesis. Pestic. Biochem. Physiol. 2022, 181, 105017. [Google Scholar] [CrossRef]
- Wang, R.; Fang, Y.; Che, W.; Zhang, Q.; Wang, J.; Luo, C. The toxicity, sublethal effects, and biochemical mechanism of β-asarone, a potential plant-derived insecticide, against Bemisia tabaci. Int. J. Mol. Sci. 2022, 23, 10462. [Google Scholar] [CrossRef] [PubMed]
- Raoufi, H.; Jafari, S.; Ghadamyari, M.; Arbabi, M. Lethal and sublethal effects of fenazaquin and acequinocyl on demographic and some biochemical parameters of Panonychus citri (McGregor) (Acari: Tetranychidae). Int. J. Acarol. 2021, 48, 27–35. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, H.; Zhu, J.; Liu, F. Transcriptome analysis to identify responsive genes under sublethal concentration of bifenazate in the diamondback moth, Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae). Int. J. Mol. Sci. 2022, 23, 13173. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, J.; Xu, D.; Xu, G.; Gu, Z.; Xiao, Z.; Dewer, Y.; Zhang, Y. Application of transcriptomic analysis to unveil the toxicity mechanisms of fall armyworm response after exposure to sublethal chlorantraniliprole. Ecotoxicol. Environ. Saf. 2022, 230, 113145. [Google Scholar] [CrossRef]
- Su, J.; Lai, T.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Pu, X.; Yang, Y.; Wu, S.; Wu, Y. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag. Sci. 2010, 66, 371–378. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- LeOra Software. Polo Plus; A User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2002.
- SPSS. Release 13.0 Version for Windows; SPSS: Chicago, IL, USA, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).