Macrophage-Specific Coxsackievirus and Adenovirus Receptor Deletion Enhances Macrophage M1 Polarity in CVB3-Induced Myocarditis
Abstract
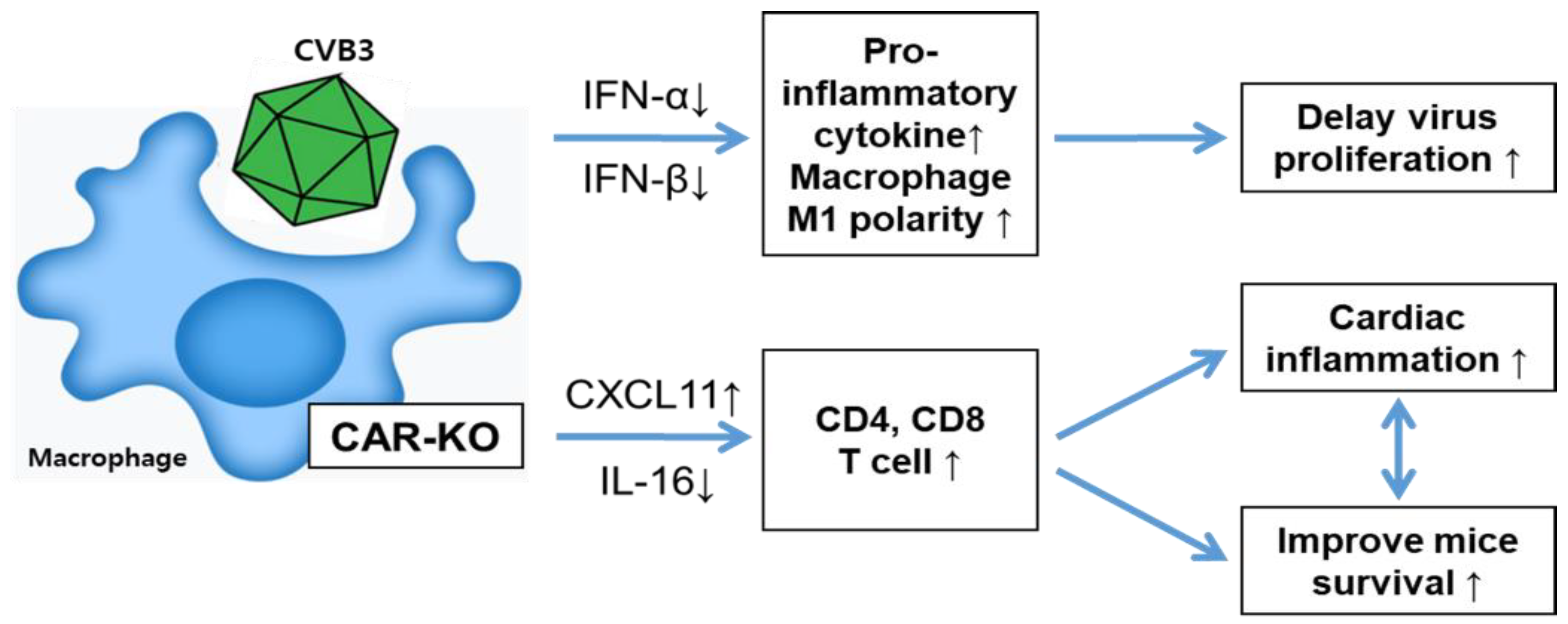
1. Introduction
2. Results
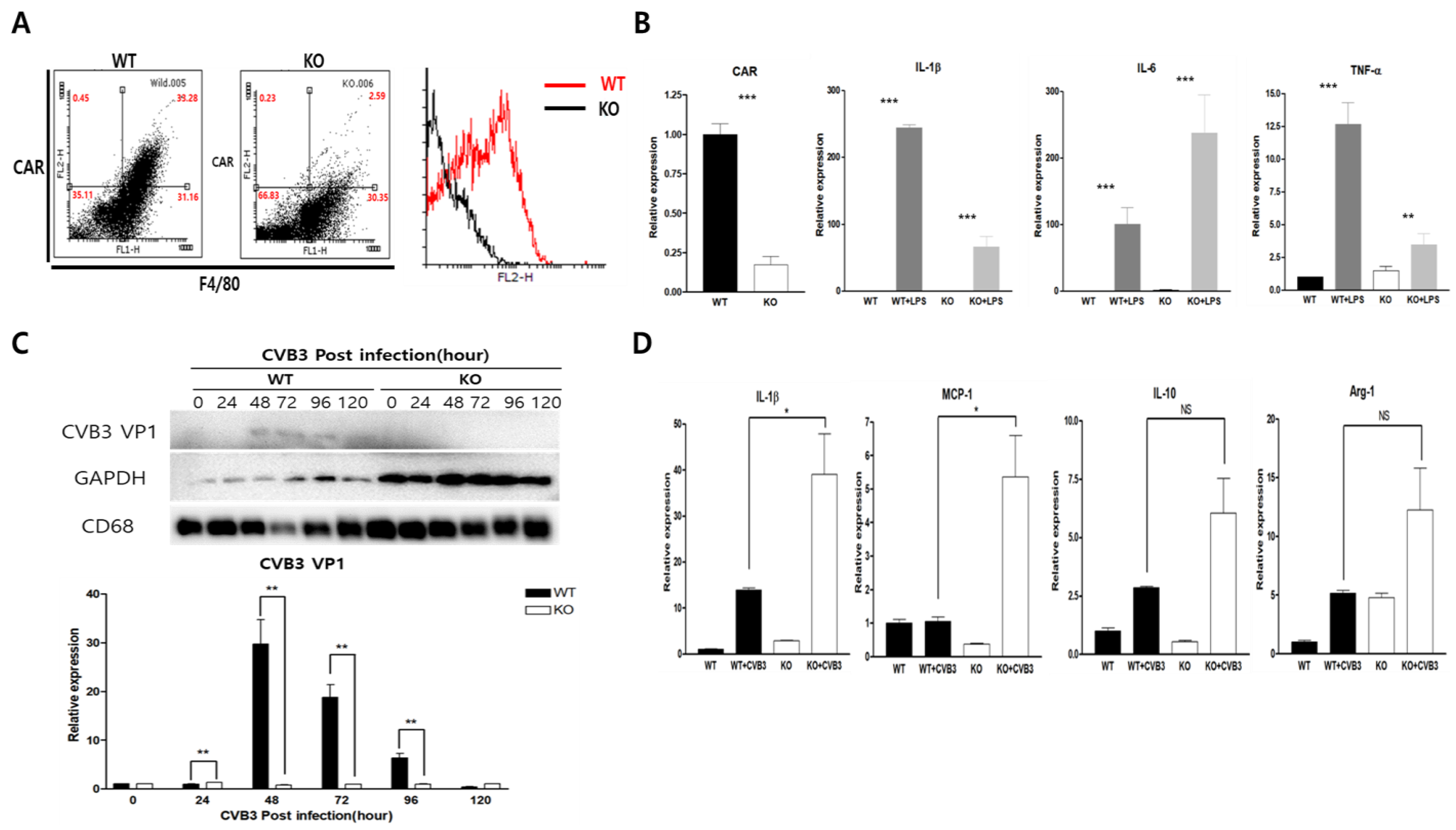
2.1. CAR Expression Was Increased in LPS and TNF-α Stimulated Macrophages
2.2. CAR Deficiency Increased Macrophage Inflammatory Cytokine Expression in CVB3 Infection
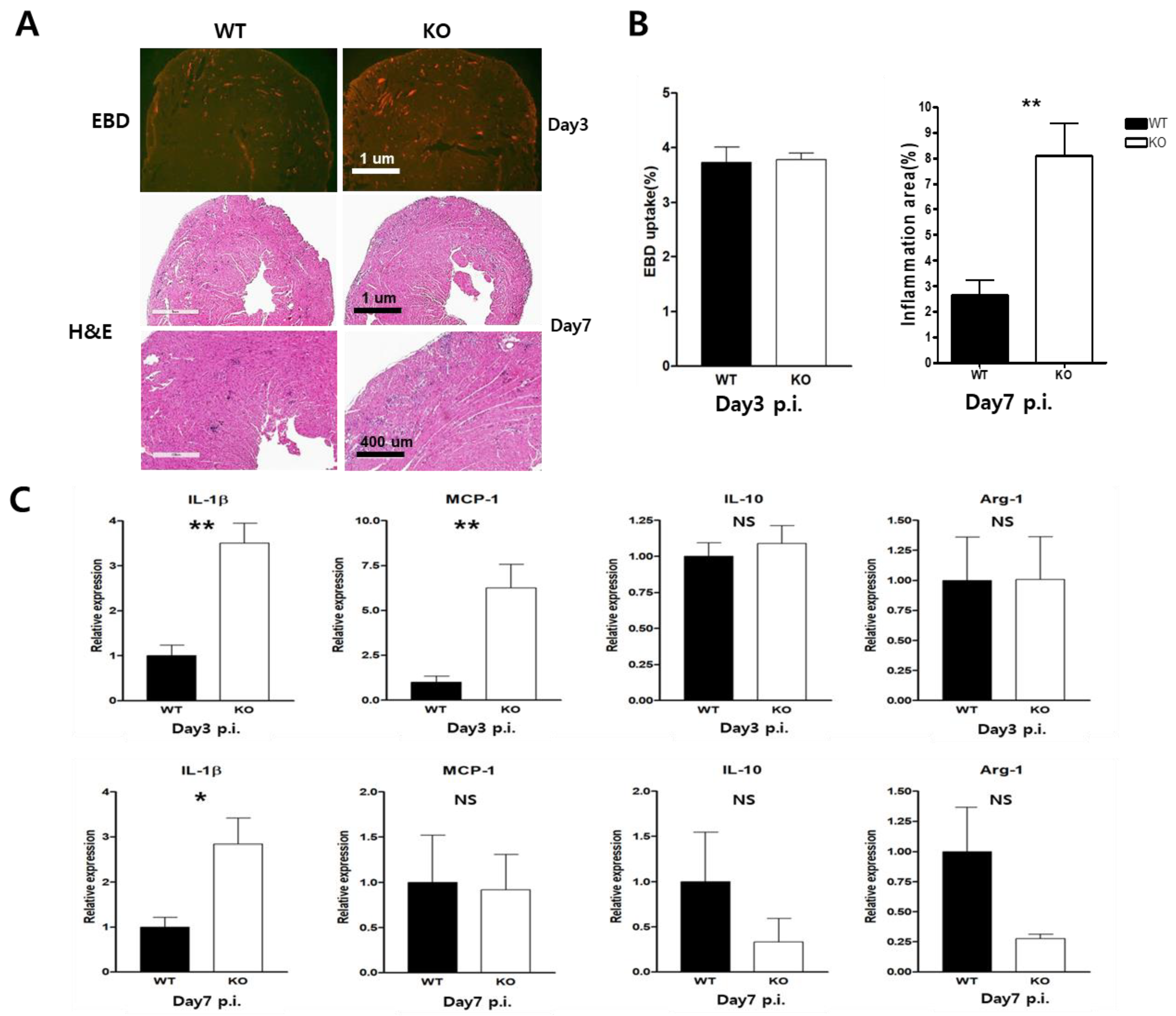
2.3. Macrophage CAR Deficiency Improves Mice Survival in CVB3-Induced Myocarditis
2.4. Heart Inflammation Increased in CAR Knockout Mice
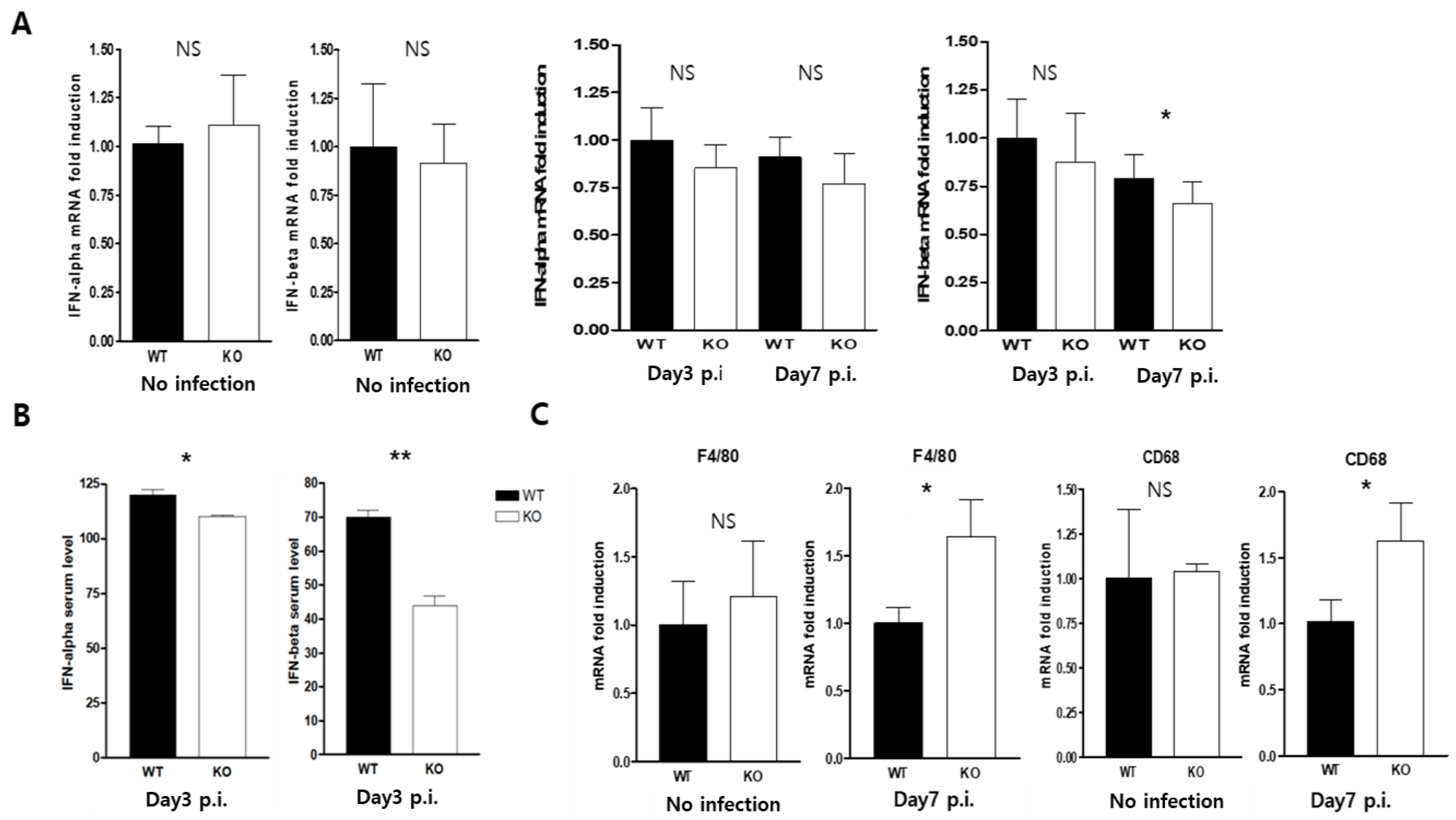
2.5. CAR Deficiency Attenuated the Expression of Type1 Interferon
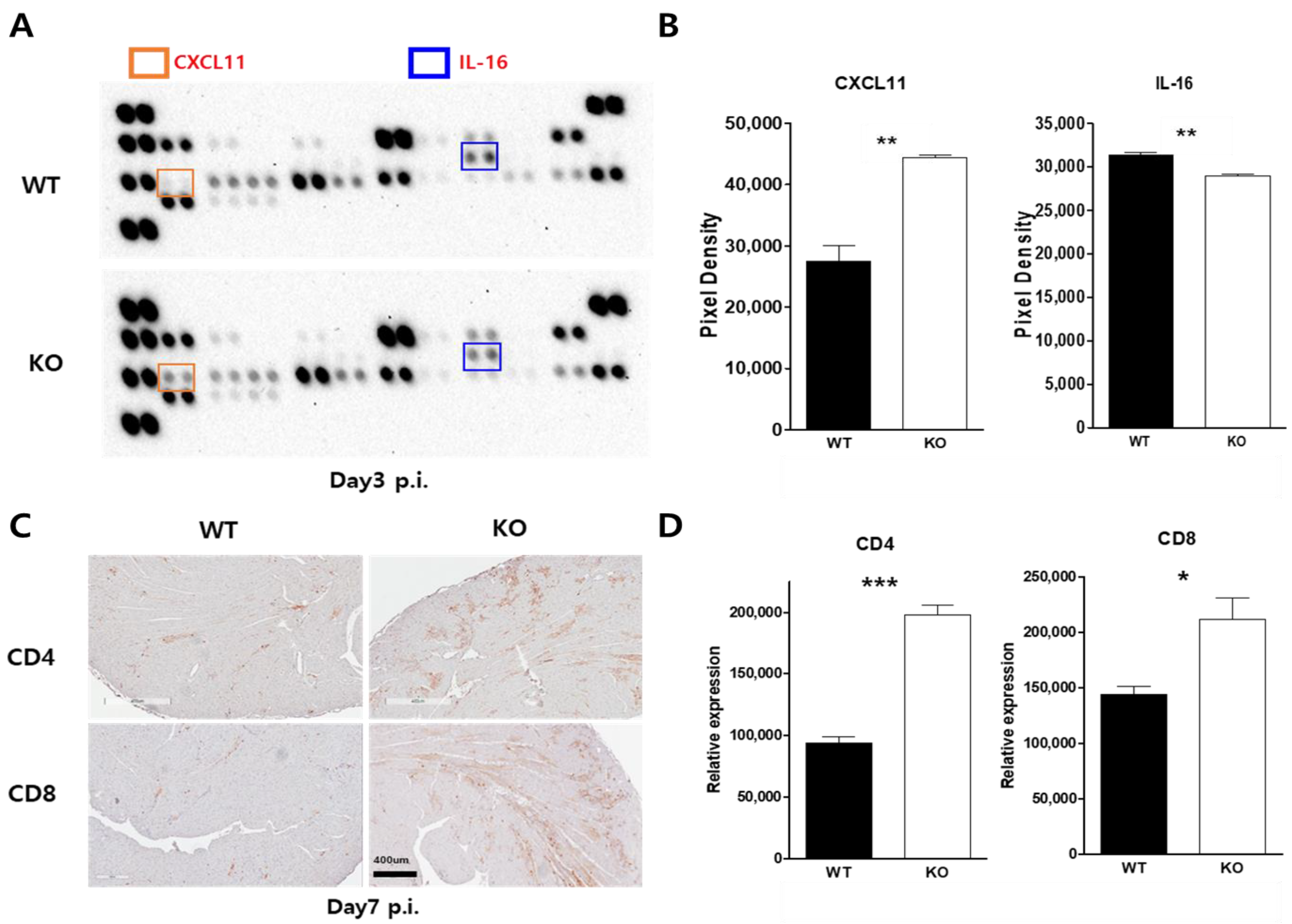
2.6. Macrophage CAR Ablation Induced T Cell Activation in Case of CVB3 Infection
3. Discussion
4. Materials and Methods
4.1. Viruses and Cells
4.2. Isolation of Peritoneal Macrophage Cells (PMCs)
4.3. Generation of Macrophage-Specific CAR Knockout Mice and CVB3 Infection
4.4. Total RNA Extraction and Quantitative Real-Time PCR
4.5. Western Blot Analysis
4.6. Histopathology and Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, B.K.; Xiong, D.; Dorner, A.; Youn, T.J.; Yung, A.; Liu, T.I.; Gu, Y.; Dalton, N.D.; Wright, A.T.; Evans, S.M.; et al. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J. Clin. Investig. 2008, 118, 2758–2770. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Bergelson, J.M. CAR: A virus receptor within the tight junction. Adv. Drug Deliv. Rev. 2005, 57, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Zhou, B.; Yu, Q.C.; Shin, S.J.; Jiao, K.; Schneider, M.D.; Baldwin, H.S.; Bergelson, J.M. Cardiomyocyte-specific deletion of the coxsackievirus and adenovirus receptor results in hyperplasia of the embryonic left ventricle and abnormalities of sinuatrial valves. Circ. Res. 2006, 98, 923–930. [Google Scholar] [CrossRef]
- Cohen, C.J.; Shieh, J.T.; Pickles, R.J.; Okegawa, T.; Hsieh, J.T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef]
- Asher, D.R.; Cerny, A.M.; Weiler, S.R.; Horner, J.W.; Keeler, M.L.; Neptune, M.A.; Jones, S.N.; Bronson, R.T.; Depinho, R.A.; Finberg, R.W. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis 2005, 42, 77–85. [Google Scholar] [CrossRef]
- Dorner, A.A.; Wegmann, F.; Butz, S.; Wolburg-Buchholz, K.; Wolburg, H.; Mack, A.; Nasdala, I.; August, B.; Westermann, J.; Rathjen, F.G.; et al. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J. Cell Sci. 2005, 118 Pt 15, 3509–3521. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.H.; Rhyu, H.S.; Kim, S.H.; Jeon, E.S.; Lim, B.K. Vascular Endothelial Integrity Affects the Severity of Enterovirus-Mediated Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 3053. [Google Scholar] [CrossRef]
- Chung, J.; Kim, K.H.; An, S.H.; Lee, S.; Lim, B.K.; Kang, S.W.; Kwon, K. Coxsackievirus and adenovirus receptor mediates the responses of endothelial cells to fluid shear stress. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Lim, B.K.; Choi, J.H.; Nam, J.H.; Gil, C.O.; Shin, J.O.; Yun, S.H.; Kim, D.K.; Jeon, E.S. Virus receptor trap neutralizes coxsackievirus in experimental murine viral myocarditis. Cardiovasc. Res. 2006, 71, 517–526. [Google Scholar] [CrossRef]
- Baboonian, C.; Davies, M.J.; Booth, J.C.; McKenna, W.J. Coxsackie B viruses and human heart disease. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1997; Volume 223, pp. 31–52. [Google Scholar]
- Huber, S.A.; Lodge, P.A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am. J. Pathol. 1986, 122, 284–291. [Google Scholar]
- Woodruff, J.F. Viral myocarditis. A review. Am. J. Pathol. 1980, 101, 425–484. [Google Scholar] [PubMed]
- Knowlton, K.U.; Badorff, C. The immune system in viral myocarditis: Maintaining the balance. Circ. Res. 1999, 85, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lim, B.K.; Ho, S.H.; Yun, S.H.; Shin, J.O.; Park, E.M.; Kim, D.K.; Kim, S.; Jeon, E.S. TNFR-Fc fusion protein expressed by in vivo electroporation improves survival rates and myocardial injury in coxsackievirus induced murine myocarditis. Biochem. Biophys. Res. Commun. 2006, 344, 765–771. [Google Scholar] [CrossRef]
- Lim, B.K.; Choe, S.C.; Shin, J.O.; Ho, S.H.; Kim, J.M.; Yu, S.S.; Kim, S.; Jeon, E.S. Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation 2002, 105, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yue, Y.; Xiong, S. NK-derived IFN-gamma/IL-4 triggers the sexually disparate polarization of macrophages in CVB3-induced myocarditis. J. Mol. Cell. Cardiol. 2014, 76, 15–25. [Google Scholar] [CrossRef]
- Martino, T.A.; Liu, P.; Sole, M.J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 1994, 74, 182–188. [Google Scholar] [CrossRef]
- Liu, P.; Martino, T.; Opavsky, M.A.; Penninger, J. Viral myocarditis: Balance between viral infection and immune response. Can. J. Cardiol. 1996, 12, 935–943. [Google Scholar] [PubMed]
- Alidjinou, E.K.; Sane, F.; Trauet, J.; Copin, M.C.; Hober, D. Coxsackievirus B4 Can Infect Human Peripheral Blood-Derived Macrophages. Viruses 2015, 7, 6067–6079. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Matveeva, O.; Nechipurenko, Y.; Lagutkin, D.; Yegorov, Y.E.; Kzhyshkowska, J. SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry. Front. Immunol. 2022, 13, 1050478. [Google Scholar] [CrossRef]
- Famulski, K.S.; Kayser, D.; Einecke, G.; Allanach, K.; Badr, D.; Venner, J.; Sis, B.; Halloran, P.F. Alternative macrophage activation-associated transcripts in T-cell-mediated rejection of mouse kidney allografts. Am. J. Transplant. 2010, 10, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2016, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Tse, M.J.; Read, E.L.; Liu, W.F. Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. 2016, 8, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszynski, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Yu, S.; Ge, H.; Li, S.; Qiu, H.J. Modulation of Macrophage Polarization by Viruses: Turning Off/On Host Antiviral Responses. Front. Microbiol. 2022, 13, 839585. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, S.; Chen, X.; Cheng, S.; Zhang, Z.; Li, F.; Huang, L.; Yang, Y.; Zhou, B.; Yue, D.; et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J. Immunother. Cancer 2019, 7, 42. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, W.G.; Kim, Y.C.; Ju, E.S.; Lim, B.K.; Choi, J.O.; Kim, D.K.; Jeon, E.S. Antiviral activity of coxsackievirus B3 3C protease inhibitor in experimental murine myocarditis. J. Infect. Dis. 2012, 205, 491–497. [Google Scholar] [CrossRef]
- Babaev, V.R.; Yancey, P.G.; Ryzhov, S.V.; Kon, V.; Breyer, M.D.; Magnuson, M.A.; Fazio, S.; Linton, M.F. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1647–1653. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, H.H.; Kim, J.H.; Park, J.H.; Jeon, E.S.; Lim, B.K. Protein Kinase B2 (PKB2/AKT2) Is Essential for Host Protection in CVB3-Induced Acute Viral Myocarditis. Int. J. Mol. Sci. 2022, 23, 1489. [Google Scholar] [CrossRef]


| Sense (5′→3′) | Antisense (5′→3′) | |
|---|---|---|
| CAR | GGA CTA CTT GCA CTC CGA GAA G | CAT AGT GGC ACC GTC CTT GAT C |
| MCP-1 | ACC TGG ATC GGA ACC AAA TG | CCT TAG GGC AGA TGC AGT TTT AA |
| IL-1β | TTG ACG GAC CCC AAA GAG TG | ACT CCT GTA CTC GTG GAA GA |
| IL-6 | GTA CTC CAG AAG ACC AGA GG | TGC TGG TGA CAA CCA CGG CC |
| TNF-α | TTG ACC TCA GCG CTG AGT TG | CCT GTA GCC CAC GTC GTA GC |
| IFN-α | GCA ATG ACCATCC ATC AGC AGC T | GTG GAA GTA TGT CCT CAC AGC C |
| IFN-β | GCC TTT GCC ATC CAA GAG ATG C | ACA CTG TCT GCT GGT GGA GTT C |
| IL-10 | AGT GAA CTG CGC TGT CAA TG | TTC AGG GTC AAG GCA AAC TT |
| Arg-1 | CGC CTT TCT CAA AAG GAC AG | CGC CTT TCT CAA AAG GAC AG |
| CD4 | GTT CAG GAC AGC GAC TTC TGG A | GAA GGA GAA CTC CGC TGA CTC T |
| CD8 | ACT ACC AAG CCA GTG CTG CGA A | ATC ACA GGC GAA GTC CAA TCC G |
| F4/80 | GGA AAG CAC CAT GTT AGC TG | CCT CTG GCT GCC AAG TTA AT |
| CD68 | CTT CCC ACA GGC AGC ACA G | AAT GAT GAG AGG CAG CAA GAG G |
| GAPDH | ATC AAC GAC CCC TTC ATT GAC C | CCA GTA GAC TCC ACG ACA TAC TCA GC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.-H.; Jeon, E.-S.; Lim, B.-K. Macrophage-Specific Coxsackievirus and Adenovirus Receptor Deletion Enhances Macrophage M1 Polarity in CVB3-Induced Myocarditis. Int. J. Mol. Sci. 2023, 24, 5309. https://doi.org/10.3390/ijms24065309
Shin H-H, Jeon E-S, Lim B-K. Macrophage-Specific Coxsackievirus and Adenovirus Receptor Deletion Enhances Macrophage M1 Polarity in CVB3-Induced Myocarditis. International Journal of Molecular Sciences. 2023; 24(6):5309. https://doi.org/10.3390/ijms24065309
Chicago/Turabian StyleShin, Ha-Hyeon, Eun-Seok Jeon, and Byung-Kwan Lim. 2023. "Macrophage-Specific Coxsackievirus and Adenovirus Receptor Deletion Enhances Macrophage M1 Polarity in CVB3-Induced Myocarditis" International Journal of Molecular Sciences 24, no. 6: 5309. https://doi.org/10.3390/ijms24065309
APA StyleShin, H.-H., Jeon, E.-S., & Lim, B.-K. (2023). Macrophage-Specific Coxsackievirus and Adenovirus Receptor Deletion Enhances Macrophage M1 Polarity in CVB3-Induced Myocarditis. International Journal of Molecular Sciences, 24(6), 5309. https://doi.org/10.3390/ijms24065309






