Kinase Inhibitors in Genetic Diseases
Abstract
1. Introduction
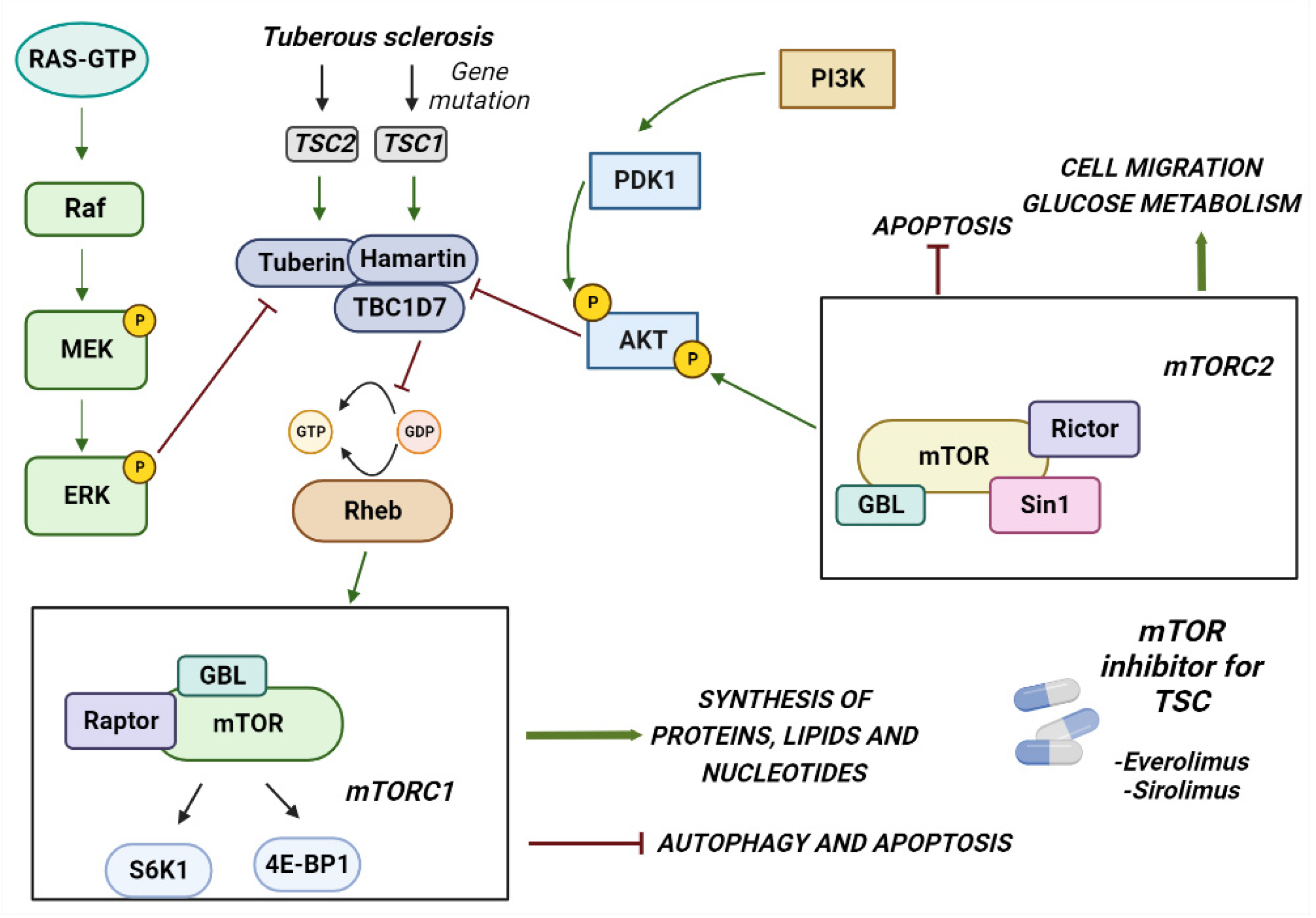
2. Tuberous Sclerosis Complex
2.1. Clinical Manifestation
2.2. Genetics of TSC
2.3. Inhibition of mTOR
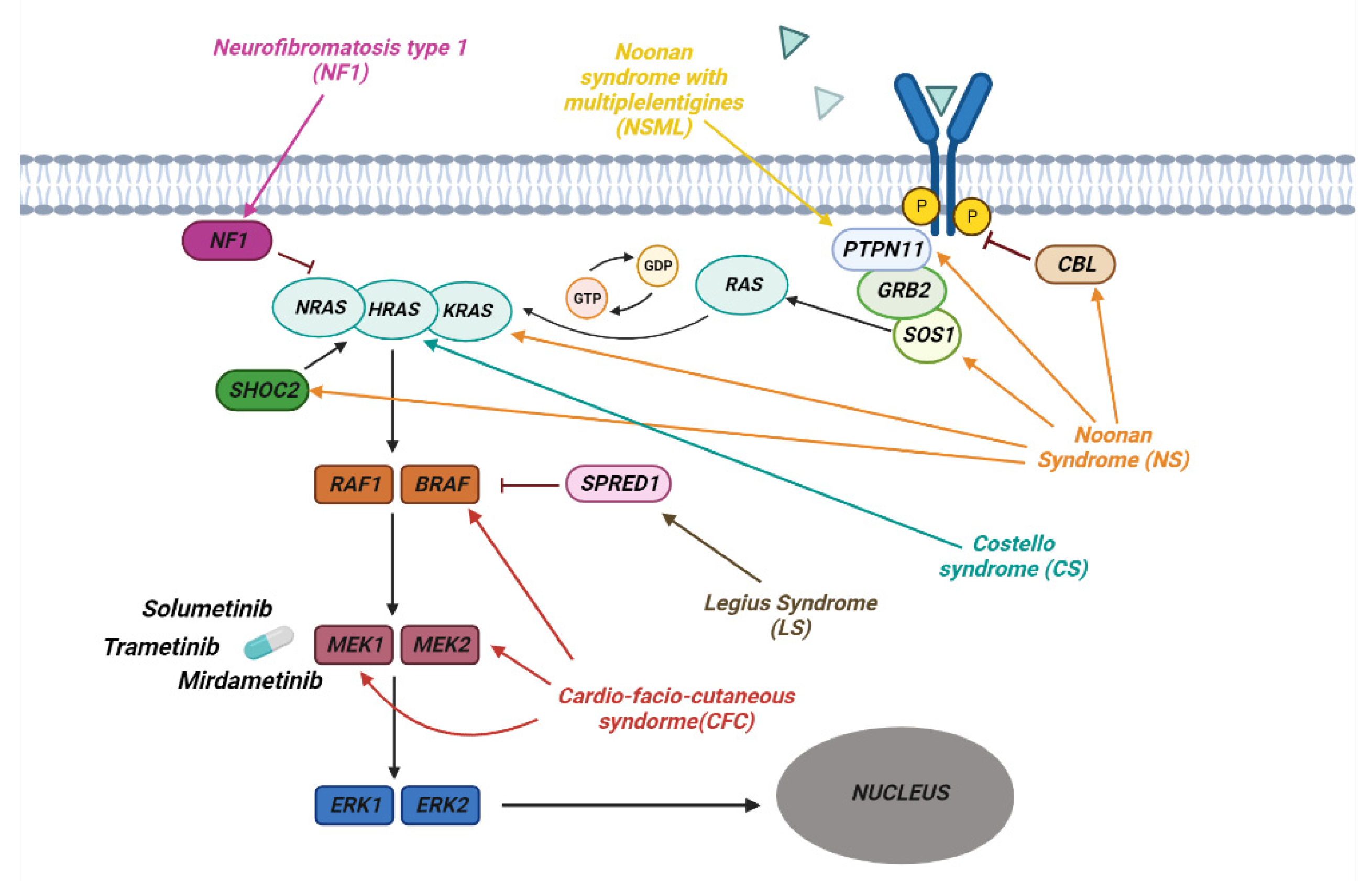
3. RASopathies
3.1. Clinical Manifestation
3.2. Neurofibromatosis
3.3. Noonan Syndrome
3.4. Genetics of RASopathies
| Disease | Mutated Genes |
|---|---|
| Neurofibromatosis type 1 (NF1) | NF1 [98,99,100,101] |
| Noonan syndrome (NS) | PTPN11 [81,82] SOS1 [84,85] KRAS [86,87] BRAF [88] SHOC2 [89] CBL [90] |
| Noonan syndrome with multiple lentigines (NSML) | PTPN11 [102] |
| Costello syndrome (CS) | HRAS [92,93] |
| Cardio-facio-cutaneous syndorme (CFC) | BRAF [94] MAP2K1(MEK1) [95] MAP2K2(MEK2) [95] |
| Legius Syndrome | SPRED1 [97] |
3.5. Inibition of RAS Downstream Targets
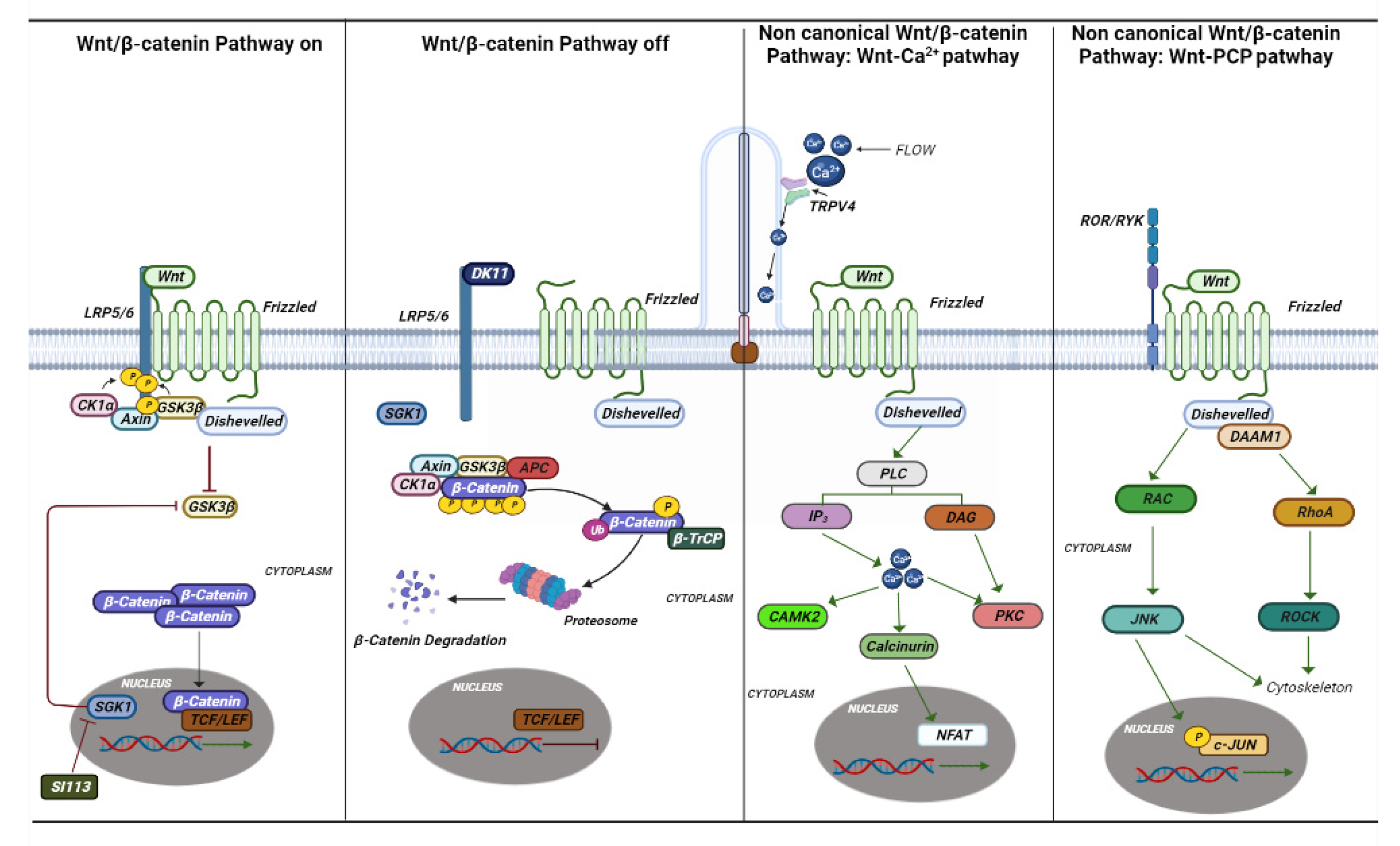
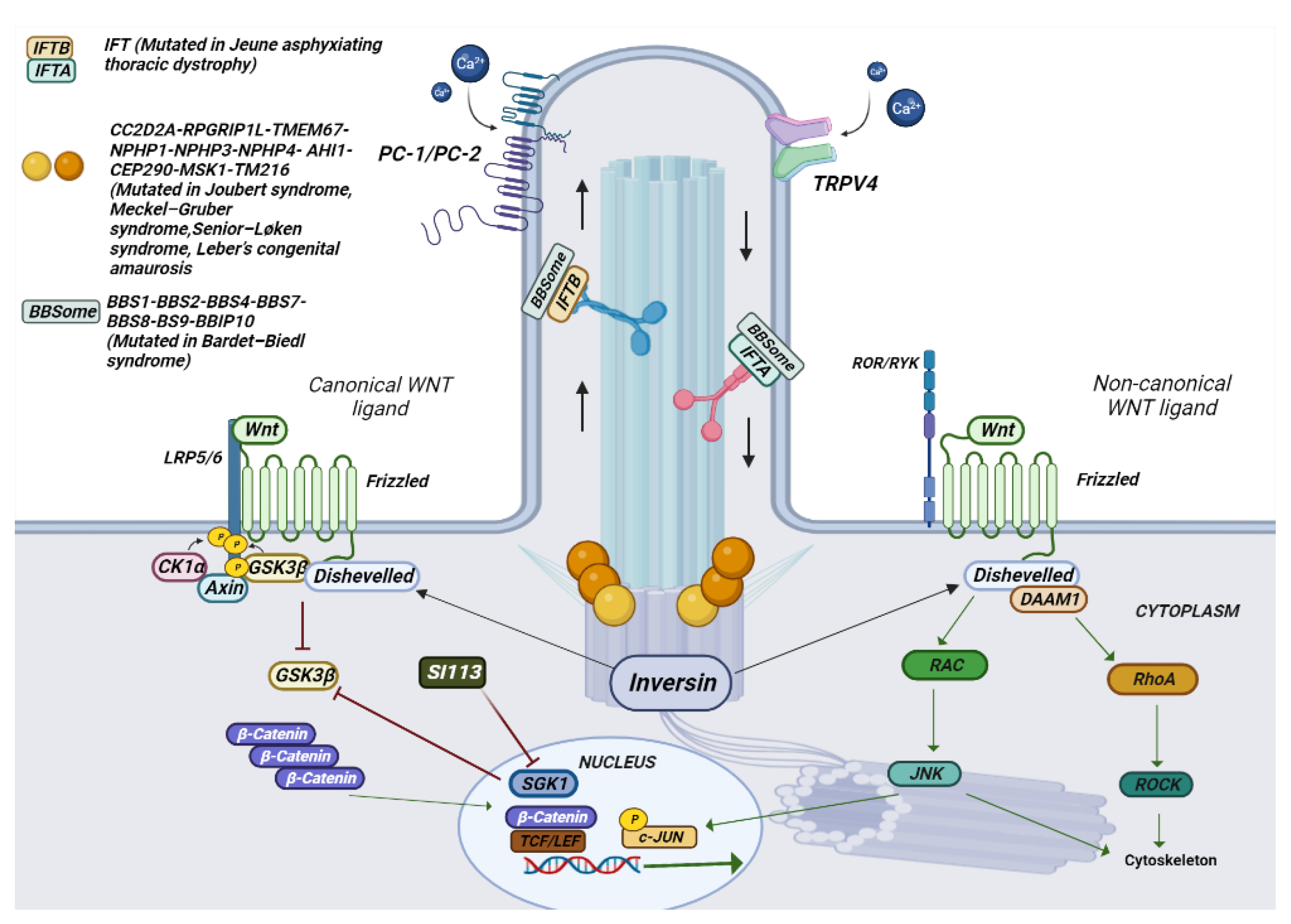
4. Ciliopathies
4.1. Clinical Manifestation
| Syndrome | Gene Involved |
|---|---|
| Joubert syndrome | INPP5E [133], ARL13B [134], CC2D2A [135], RPGRIP1L [136], TMEM67 [137], NPHP1 [138], AHI1 [139], CEP290 [140], CXORF5 [141], and TMEM216 [142]. |
| Meckel–Gruber syndrome | MKS1 [143], MKS3 (TMEM67) [144], CEP290 [137], RPGRIP1L [136], CC2D2A [145], and TMEM216 [142] |
| Senior–Løken syndrome | CEP290 (also known as NPHP6 and MKS4) [140], NPHP1 [146], NPHP3 [147], NPHP4 [148], and NPHP5 (also known as IQCB1) [149]. |
| Type 1 orofacial syndrome | OFD1 [150] |
| Leber’s congenital amaurosis | GUCY2D [151], RPE65 [152], SPATA7 [153], AIPL1 [154], LCA5 [155], RPGRIPL1 [156], CRX [157], CRB1 [158], IMPD1 [159], RD3 [160], CEP290 [140], NPHP5 [149], and RDH12 [161]. |
| Bardet–Biedl syndrome | BBS1 [162], BBS2 [163], ARL6/BBS3 [164], BBS4 [165], BBS5 [166], MKKS/BBS6 [167], BBS7 [168], TTC8/BBS8 [169], B1/BBS9 [170], BBS10 [171], TRIM32/BBS11 [172], BBS12 [173], MKS1/BBS13 [174], CEP290/BBS14 [174], C2ORF86/FRITZ/BBS15 [175], and SDCCAG8/BBS16 [176] |
| Alström syndrome | ALMS1 [177] |
| Jeune asphyxiating thoracic dystrophy | IFT [178] |
| Rhyns syndrome | TMEM67 [132] |
4.2. The Genetics of Ciliopathies
4.3. Inibition of TRPV4 and SGK1
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Li, Y.; Zhang, Y.; Li, X.; Yi, S.; Xu, J. Gain-of-Function Mutations: An Emerging Advantage for Cancer Biology. Trends Biochem. Sci. 2019, 44, 659–674. [Google Scholar] [CrossRef]
- Downward, J.; Yarden, Y.; Mayes, E.; Scrace, G.; Totty, N.; Stockwell, P.; Ullrich, A.; Schlessinger, J.; Waterfield, M.D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature 1984, 307, 521–527. [Google Scholar] [CrossRef]
- Perrotti, N.; Taylor, S.I.; Richert, N.D.; Rapp, U.R.; Pastan, I.H.; Roth, J. Immunoprecipitation of Insulin Receptors from Cultured Human Lymphocytes (IM-9 Cells) by Antibodies to pp60 src. Science 1985, 227, 761–763. [Google Scholar] [CrossRef]
- Davies, H.; Hunter, C.; Smith, R.; Stephens, P.; Greenman, C.; Bignell, G.; Teague, J.; Butler, A.; Edkins, S.; Stevens, C.; et al. Somatic Mutations of the Protein Kinase Gene Family in Human Lung Cancer. Cancer Res 2005, 65, 7591–7595. [Google Scholar] [CrossRef]
- Stephens, P.; Edkins, S.; Davies, H.; Greenman, C.; Cox, C.; Hunter, C.; Bignell, G.; Teague, J.; Smith, R.; Stevens, C.; et al. A screen of the complete protein kinase gene family identifies diverse patterns of somatic mutations in human breast cancer. Nat. Genet. 2005, 37, 590–592. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL Family Kinases in Cancer: From Leukemia to Solid Tumors. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Impli-cations. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar]
- Leicht, D.T.; Balan, V.; Kaplun, A.; Singh-Gupta, V.; Kaplun, L.; Dobson, M.; Tzivion, G. Raf kinases: Function, regulation and role in human cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1196–1212. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Mahadevan, D. A comprehensive review of protein kinase inhibitors for cancer therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Grant, S.K. Therapeutic Protein Kinase Inhibitors. Cell. Mol. Life Sci. 2008, 66, 1163–1177. [Google Scholar] [CrossRef]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small Molecule Tyrosine Kinase Inhibitors of ErbB2/HER2/Neu in the Treatment of Aggressive Breast Cancer. Molecules 2014, 19, 15196–15212. [Google Scholar]
- Schlam, I.; Swain, S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer 2021, 7, 56. [Google Scholar] [CrossRef]
- Fanotto, V.; Ongaro, E.; Rihawi, K.; Avallone, A.; Silvestris, N.; Fornaro, L.; Vasile, E.; Antonuzzo, L.; Leone, F.; Rosati, G.; et al. HER-2 inhibition in gastric and colorectal cancers: Tangible achievements, novel acquisitions and future perspectives. Oncotarget 2016, 7, 69060–69074. [Google Scholar] [CrossRef]
- Son, J.; Jang, J.; Beyett, T.S.; Eum, Y.; Haikala, H.M.; Verano, A.; Lin, M.; Hatcher, J.M.; Kwiatkowski, N.P.; Eser, P.Ö.; et al. A Novel HER2-Selective Kinase Inhibitor Is Effective in HER2 Mutant and Amplified Non-Small Cell Lung Cancer. Cancer Res. 2022, 82, 1633–1645. [Google Scholar] [CrossRef]
- Mitani, S.; Kawakami, H. Emerging Targeted Therapies for HER2 Positive Gastric Cancer That Can Overcome Trastuzumab Resistance. Cancers 2020, 12, 400. [Google Scholar] [CrossRef]
- Duchnowska, R.; Loibl, S.; Jassem, J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 2018, 67, 71–77. [Google Scholar] [CrossRef]
- Ling, Y.; Xie, Q.; Zhang, Z.; Zhang, H. Protein kinase inhibitors for acute leukemia. Biomark. Res. 2018, 6, 8. [Google Scholar] [CrossRef]
- An, X.; Tiwari, A.K.; Sun, Y.; Ding, P.-R.; Ashby, C.R.; Chen, Z.-S. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk. Res. 2010, 34, 1255–1268. [Google Scholar] [CrossRef]
- Lee, H.; Basso, I.N.; Kim, D.D.H. Target spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia. Int. J. Hematol. 2021, 113, 632–641. [Google Scholar] [CrossRef]
- Osborne, J.P.; Fryer, A.; Webb, D. Epidemiology of Tuberous Sclerosis. Ann. New York Acad. Sci. 1991, 615, 125–127. [Google Scholar] [CrossRef]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous sclerosis. Lancet 2008, 372, 657–668. [Google Scholar]
- DiMario, F.J. Brain Abnormalities in Tuberous Sclerosis Complex. J. Child Neurol. 2004, 19, 650–657. [Google Scholar] [CrossRef]
- Luat, A.F.; Makki, M.; Chugani, H.T. Neuroimaging in tuberous sclerosis complex. Curr. Opin. Neurol. 2007, 20, 142–150. [Google Scholar] [CrossRef]
- Islam, M.P.; Roach, E.S. Tuberous sclerosis complex. Handb. Clin. Neurol. 2015, 132, 97–109. [Google Scholar]
- Kaczorowska, M.; Jurkiewicz, E.; Domańska-Pakieła, D.; Syczewska, M.; Lojszczyk, B.; Chmielewski, D.; Kotulska, K.; Kuczyński, D.; Kmieć, T.; Dunin-Wąsowicz, D.; et al. Cerebral tuber count and its impact on mental outcome of patients with tuberous sclerosis complex. Epilepsia 2011, 52, 22–27. [Google Scholar] [CrossRef]
- Capal, J.K.; Bernardino-Cuesta, B.; Horn, P.S.; Murray, D.; Byars, A.W.; Bing, N.M.; Kent, B.; Pearson, D.A.; Sahin, M.; Krueger, D.A. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017, 70, 245–252. [Google Scholar] [CrossRef]
- Jansen, F.E.; Vincken, K.L.; Algra, A.; Anbeek, P.; Braams, O.; Nellist, M.; Zonnenberg, B.A.; Jennekens-Schinkel, A.; Ouweland, A.V.D.; Halley, D.; et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 2007, 70, 916–923. [Google Scholar] [CrossRef]
- Ehninger, D.; Sano, Y.; De Vries, P.J.; Dies, K.; Franz, D.; Geschwind, D.H.; Kaur, M.; Lee, Y.-S.; Li, W.; Lowe, J.K.; et al. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol. Psychiatry 2010, 17, 62–70. [Google Scholar] [CrossRef]
- DiMario, F.J.; Sahin, M.; Ebrahimi-Fakhari, D. Tuberous sclerosis complex. Pediatr. Clin. N. Am. 2015, 62, 633–648. [Google Scholar]
- Franz, D.N. Non-Neurologic Manifestations of Tuberous Sclerosis Complex. J. Child Neurol. 2004, 19, 690–698. [Google Scholar] [CrossRef]
- Józwiak, S.; Schwartz, R.A.; Janniger, C.K.; Michałowicz, R.; Chmielik, J. Skin lesions in children with tuberous sclerosis complex: Their prevalence, natural course, and diagnostic significance. Int. J. Dermatol. 1998, 37, 911–917. [Google Scholar] [CrossRef]
- Webb, D.W.; Clarke, A.; Fryer, A.; Osborne, J.P. The cutaneous features of tuberous sclerosis: A population study. Br. J. Dermatol. 1996, 135, 1–5. [Google Scholar]
- Hyman, M.H.; Whittemore, V.H. National Institutes of Health Consensus Conference: Tuberous Sclerosis Complex. Arch. Neurol. 2000, 57, 662–665. [Google Scholar] [CrossRef]
- Cudzilo, C.J.; Szczesniak, R.D.; Brody, A.S.; Rattan, M.S.; Krueger, D.A.; Bissler, J.J.; Franz, D.N.; McCormack, F.X.; Young, L.R. Lymphangioleiomyomatosis Screening in Women with Tuberous Sclerosis. Chest 2013, 144, 578–585. [Google Scholar] [CrossRef]
- Wu, S.; Wu, F.; Jiang, Z. Identification of hub genes, key miRNAs and potential molecular mechanisms of colorectal cancer. Oncol. Rep. 2017, 38, 2043–2050. [Google Scholar] [CrossRef]
- Van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; van den Ouweland, A.; Halley, D.; Young, J.; et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef]
- Sancak, O.; Nellist, M.; Goedbloed, M.; Elfferich, P.; Wouters, C.; Maat-Kievit, A.; Zonnenberg, B.; Verhoef, S.; Halley, D.; Ouweland, A.V.D. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: Genotype—Phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur. J. Hum. Genet. 2005, 13, 731–741. [Google Scholar] [CrossRef]
- Cheadle, J.P.; Reeve, M.P.; Sampson, J.R.; Kwiatkowski, D.J. Molecular genetic advances in tuberous sclerosis. Hum. Genet. 2000, 107, 97–114. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Manning, B.D. Molecular Basis of Giant Cells in Tuberous Sclerosis Complex. N. Engl. J. Med. 2014, 371, 778–780. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Tee, A.R.; Fingar, D.C.; Manning, B.D.; Kwiatkowski, D.J.; Cantley, L.C.; Blenis, J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13571–13576. [Google Scholar] [CrossRef]
- Crino, P.B. mTOR: A pathogenic signaling pathway in developmental brain malformations. Trends Mol. Med. 2011, 17, 734–742. [Google Scholar] [CrossRef]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar]
- Huang, J.; Manning, B.D. The TSC1–TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Erdjument-Bromage, H.; Tempst, P.; Pandolfi, P.P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 2005, 121, 179–193. [Google Scholar] [CrossRef]
- Carson, R.P.; Fu, C.; Winzenburger, P.; Ess, K.C. Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Hum. Mol. Genet. 2012, 22, 140–152. [Google Scholar] [CrossRef]
- El-Hashemite, N.; Zhang, H.; Henske, E.P.; Kwiatkowski, D.J. Mutation in TSC2 and activation of mammalian target of ra-pamycin signalling pathway in renal angiomyolipoma. Lancet 2003, 361, 1348–1349. [Google Scholar] [CrossRef]
- Musa, J.; Orth, M.F.; Dallmayer, M.; Baldauf, M.; Pardo, C.; Rotblat, B.; Kirchner, T.; Leprivier, G.; Grünewald, T.G.P. Eu-karyotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 2016, 35, 4675–4688. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Saucedo, L.J.; Ru, B.; Edgar, B.A.; Pan, D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nature 2003, 5, 578–581. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976, Erratum in Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef]
- Jossin, Y.; Goffinet, A.M. Reelin Signals through Phosphatidylinositol 3-Kinase and Akt To Control Cortical Development and through mTor To Regulate Dendritic Growth. Mol. Cell. Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alvarez, V.A.; Ridenour, D.A.; Kwiatkowski, D.J.; Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005, 8, 1727–1734. [Google Scholar] [CrossRef]
- Chévere-Torres, I.; Kaphzan, H.; Bhattacharya, A.; Kang, A.; Maki, J.M.; Gambello, M.J.; Arbiser, J.L.; Santini, E.; Klann, E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2012, 45, 1101–1110. [Google Scholar] [CrossRef]
- Ho, H.; Kapadia, R.; Al-Tahan, S.; Ahmad, S.; Ganesan, A.K. WIPI1 Coordinates Melanogenic Gene Transcription and Mel-anosome Formation via TORC1 Inhibition. J. Biol. Chem. 2011, 286, 12509–12523. [Google Scholar] [CrossRef]
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef]
- Franz, D.N.; Krueger, D.A. mTOR inhibitor therapy as a disease modifying therapy for tuberous sclerosis complex. Am. J. Med. Genet. Part C Semin. Med. Genet. 2018, 178, 365–373. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014, 15, 1513–1520. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Bissler, J.J.; McCormack, F.X.; Young, L.R.; Elwing, J.M.; Chuck, G.; Leonard, J.M.; Schmithorst, V.J.; Laor, T.; Brody, A.S.; Bean, J.; et al. Sirolimus for Angiomyolipoma in Tuberous Sclerosis Complex or Lymphangioleiomyomatosis. N. Engl. J. Med. 2008, 358, 140–151. [Google Scholar] [CrossRef]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and Safety of Sirolimus in Lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef]
- Sugalska, M.; Tomik, A.; Jóźwiak, S.; Werner, B. Treatment of Cardiac Rhabdomyomas with mTOR Inhibitors in Children with Tuberous Sclerosis Complex—A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4907. [Google Scholar] [CrossRef]
- Mlczoch, E.; Hanslik, A.; Luckner, D.; Kitzmüller, E.; Prayer, D.; Michel-Behnke, I. Prenatal diagnosis of giant cardiac rhab-domyoma in tuberous sclerosis complex: A new therapeutic option with everolimus. Ultrasound Obstet. Gynecol. 2014, 45, 618–621. [Google Scholar] [CrossRef]
- Wienecke, R.; Fackler, I.; Linsenmaier, U.; Mayer, K.; Licht, T.; Kretzler, M. Antitumoral Activity of Rapamycin in Renal An-giomyolipoma Associated With Tuberous Sclerosis Complex. Am. J. Kidney Dis. 2006, 48, e27–e29. [Google Scholar] [CrossRef]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EX-IST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef]
- Siroky, B.J.; Towbin, A.J.; Trout, A.T.; Schäfer, H.; Thamann, A.R.; Agricola, K.D.; Tudor, C.; Capal, J.; Dixon, B.P.; Krueger, D.A.; et al. Improvement in Renal Cystic Disease of Tuberous Sclerosis Complex After Treatment with Mammalian Target of Rapamycin Inhibitor. J. Pediatr. 2017, 187, 318–322.e2. [Google Scholar] [CrossRef]
- Rodrik-Outmezguine, V.S.; Okaniwa, M.; Yao, Z.; Novotny, C.J.; McWhirter, C.; Banaji, A.; Won, H.; Wong, W.; Berger, M.; De Stanchina, E.; et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016, 534, 272–276. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hauschild, A. Melanoma in 2013: Melanoma--the run of success continues. Nat. Rev. Clin. Oncol. 2014, 11, 75–76. [Google Scholar] [CrossRef]
- Shao, Z.; Bao, Q.; Jiang, F.; Qian, H.; Fang, Q.; Hu, X. VS-5584, a Novel PI3K-mTOR Dual Inhibitor, Inhibits Melanoma Cell Growth In Vitro and In Vivo. PLoS ONE 2015, 10, e0132655. [Google Scholar] [CrossRef]
- Webster, R.M.; Mentzer, S.E. The malignant melanoma landscape. Nat. Rev. Drug Discov. 2014, 13, 491–492. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Wen, J.; Ma, F.; Wang, F.; Yu, K.; Tang, M.; Wu, W.; Dong, Y.; Cheng, X.; et al. SKLB-M8 induces apoptosis through the AKT/mTOR signaling pathway in melanoma models and inhibits angiogenesis with de-crease of ERK1/2 phosphorylation. J. Pharmacol. Sci. 2014, 126, 198–207. [Google Scholar] [CrossRef]
- Rauen, K.A. The RASopathies. Annu. Rev. Genom. Hum. Genet. 2013, 14, 355–369. [Google Scholar] [CrossRef]
- Bergqvist, C.; Network, N.F.; Servy, A.; Valeyrie-Allanore, L.; Ferkal, S.; Combemale, P.; Wolkenstein, P. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J. Rare Dis. 2020, 15, 37. [Google Scholar] [CrossRef]
- Darrigo, L.G.; Geller, M.; Bonalumi Filho, A.; Azulay, D.R. Prevalence of plexiform neurofibroma in children and ado-lescents with type I neurofibromatosis. J. Pediatr. (Rio J.) 2007, 83, 571–573. [Google Scholar]
- Gross, A.M.; Singh, G.; Akshintala, S.; Baldwin, A.; Dombi, E.; Ukwuani, S.; Goodwin, A.; Liewehr, D.J.; Steinberg, S.M.; Widemann, B.C. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro-Oncology 2018, 20, 1643–1651. [Google Scholar] [CrossRef]
- Nguyen, R.; Kluwe, L.; Fuensterer, C.; Kentsch, M.; Friedrich, R.E.; Mautner, V.-F. Plexiform neurofibromas in children with neurofibromatosis type 1: Frequency and associated clinical deficits. J. Pediatr. 2011, 159, 652–655.e2. [Google Scholar] [CrossRef]
- Weiss, B.; Bollag, G.; Shannon, K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am. J. Med. Genet. 1999, 89, 14–22. [Google Scholar] [CrossRef]
- Tartaglia, M.; Zampino, G.; Gelb, B. Noonan Syndrome: Clinical Aspects and Molecular Pathogenesis. Mol. Syndr. 2010, 1, 2–26. [Google Scholar] [CrossRef]
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; Van Der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef]
- Matozaki, T.; Murata, Y.; Saito, Y.; Okazawa, H.; Ohnishi, H. Protein tyrosine phosphatase SHP-2: A proto-oncogene product that promotes Ras activation. Cancer Sci. 2009, 100, 1786–1793. [Google Scholar] [CrossRef]
- Quilliam, L.A.; Rebhun, J.F.; Castro, A.F. A growing family of guanine nucleotide exchange factors is responsible for activation of ras-family GTPases. In Progress in Nucleic Acid Research and Molecular Biology; Academic Press: Cambridge, MA, USA, 2002; Volume 71, pp. 391–444. [Google Scholar]
- Tartaglia, M.; Pennacchio, L.; Zhao, C.; Yadav, K.K.; Fodale, V.; Sarkozy, A.; Pandit, B.; Oishi, K.; Martinelli, S.; Schackwitz, W.; et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat. Genet. 2006, 39, 75–79. [Google Scholar] [CrossRef]
- Gremer, L.; Merbitz-Zahradnik, T.; Dvorsky, R.; Cirstea, I.C.; Kratz, C.P.; Zenker, M.; Wittinghofer, A.; Ahmadian, M.R. Germline KRAS mutations cause aberrant biochemical and physical properties leading to developmental disorders. Hum. Mutat. 2010, 32, 33–43. [Google Scholar] [CrossRef]
- Schubbert, S.; Bollag, G.; Lyubynska, N.; Nguyen, H.; Kratz, C.P.; Zenker, M.; Niemeyer, C.M.; Molven, A.; Shannon, K. Bi-ochemical and Functional Characterization of Germ Line KRAS Mutations. Mol. Cell. Biol. 2007, 27, 7765–7770. [Google Scholar] [CrossRef]
- Pandit, B.; Sarkozy, A.; Pennacchio, L.A.; Carta, C.; Oishi, K.; Martinelli, S.; Pogna, E.A.; Schackwitz, W.; Ustaszewska, A.; Landstrom, A.; et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyo-pathy. Nat. Genet. 2007, 39, 1007–1012. [Google Scholar] [CrossRef]
- Mutation Analysis of the SHOC2 Gene in Noonan-Like Syndrome and in Hematologic Malignancies|Journal of Human Ge-netics. Available online: https://www.nature.com/articles/jhg2010116 (accessed on 13 January 2023).
- Martinelli, S.; De Luca, A.; Stellacci, E.; Rossi, C.; Checquolo, S.; Lepri, F.; Caputo, V.; Silvano, M.; Buscherini, F.; Consoli, F.; et al. Heterozygous Germline Mutations in the CBL Tumor-Suppressor Gene Cause a Noonan Syndrome-like Phenotype. Am. J. Hum. Genet. 2010, 87, 250–257. [Google Scholar] [CrossRef]
- Sarkozy, A.; Digilio, M.C.; Dallapiccola, B. Leopard syndrome. Orphanet. J. Rare Dis. 2008, 3, 13. [Google Scholar] [CrossRef]
- Gripp, K.W.; Morse, L.A.; Axelrad, M.; Chatfield, K.C.; Chidekel, A.; Dobyns, W.; Doyle, D.; Kerr, B.; Lin, A.E.; Schwartz, D.D.; et al. Costello syn-drome: Clinical phenotype, genotype, and management guidelines. Am. J. Med. Genet. Part A 2019, 179, 1725–1744. [Google Scholar] [CrossRef]
- Zenker, M. Noonan Syndrome and Related Disorders: A Matter of Deregulated Ras Signaling; Karger Medical and Scientific Publishers: Basel, Switzerland, 2009. [Google Scholar]
- Nava, C.; Hanna, N.; Michot, C.; Pereira, S.; Pouvreau, N.; Niihori, T.; Aoki, Y.; Matsubara, Y.; Arveiler, B.; Lacombe, D.; et al. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: Genotype phenotype relationships and overlap with Costello syndrome. J. Med. Genet. 2007, 44, 763–771. [Google Scholar] [CrossRef]
- Ordan, M.; Pallara, C.; Maik-Rachline, G.; Hanoch, T.; Gervasio, F.L.; Glaser, F.; Fernandez-Recio, J.; Seger, R. Intrinsically active MEK variants are differentially regulated by proteinases and phosphatases. Sci. Rep. 2018, 8, 11830. [Google Scholar] [CrossRef]
- Eerola, I.; Boon, L.M.; Mulliken, J.B.; Burrows, P.E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary Malformation–Arteriovenous Malformation, a New Clinical and Genetic Disorder Caused by RASA1 Mutations. Am. J. Hum. Genet. 2003, 73, 1240–1249. [Google Scholar] [CrossRef]
- Pasmant, E.; Sabbagh, A.; Hanna, N.; Masliah-Planchon, J.; Jolly, E.; Goussard, P.; Ballerini, P.; Cartault, F.; Barbarot, S.; Landman-Parker, J.; et al. SPRED1 germline mutations caused a neurofibromatosis type 1 overlapping phenotype. J. Med. Genet. 2009, 46, 425–430. [Google Scholar] [CrossRef]
- Shannon, K.M.; O’Connell, P.; Martin, G.A.; Paderanga, D.; Olson, K.; Dinndorf, P.; McCormick, F. Loss of The Normal NF1 Allele from the Bone Marrow of Children with Type 1 Neurofibromatosis and Malignant Myeloid Disorders. N. Engl. J. Med. 1994, 330, 597–601. [Google Scholar] [CrossRef]
- Wallace, M.R.; Marchuk, D.A.; Andersen, L.B.; Letcher, R.; Odeh, H.M.; Saulino, A.M.; Fountain, J.W.; Brereton, A.; Nicholson, J.; Mitchell, A.L.; et al. Type 1 neurofibromatosis gene: Identification of a large transcript disrupted in three NF1 patients. Science 1990, 249, 181–186. [Google Scholar] [CrossRef]
- Viskochil, D.; Buchberg, A.M.; Xu, G.; Cawthon, R.M.; Stevens, J.; Wolff, R.K.; Culver, M.; Carey, J.C.; Copeland, N.G.; Jenkins, N.A.; et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 1990, 62, 187–192. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Prim. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Sarkozy, A.; Conti, E.; Digilio, M.C.; Marino, B.; Morini, E.; Pacileo, G.; Wilson, M.; Calabrò, R.; Pizzuti, A.; Dallapiccola, B. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J. Med. Genet. 2004, 41, e68. [Google Scholar] [CrossRef]
- Widemann, B.C.; Babovic-Vuksanovic, D.; Dombi, E.; Wolters, P.L.; Goldman, S.; Martin, S.; Goodwin, A.; Goodspeed, W.; Kieran, M.W.; Cohen, B.; et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr. Blood Cancer 2014, 61, 1598–1602. [Google Scholar] [CrossRef]
- Widemann, B.C.; Salzer, W.L.; Arceci, R.J.; Blaney, S.M.; Fox, E.; End, D.; Gillespie, A.; Whitcomb, P.; Palumbo, J.S.; Pitney, A.; et al. Phase I Trial and Pharmacokinetic Study of the Farnesyltransferase Inhibitor Tipifarnib in Children With Refractory Solid Tumors or Neurofibromatosis Type I and Plexiform Neurofibromas. J. Clin. Oncol. 2006, 24, 507–516. [Google Scholar] [CrossRef]
- Widemann, B.C.; Dombi, E.; Gillespie, A.; Wolters, P.L.; Belasco, J.; Goldman, S.; Korf, B.R.; Solomon, J.; Martin, S.; Salzer, W.; et al. Phase 2 ran-domized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipi-farnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014, 16, 707–718. [Google Scholar] [CrossRef]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.; Cantor, A.; Perentesis, J.; Schorry, E.; Ullrich, N.; Gutmann, D.H.; et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: A Neurofibromatosis Clinical Trials Consortium phase II study. Neuro-Oncology 2014, 17, 596–603. [Google Scholar] [CrossRef]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.A.; Cantor, A.; Korf, B.; Perentesis, J.; Gutmann, D.H.; Schorry, E.; et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: An NF clinical trials consortium phase II study. Pediatr. Blood Cancer 2013, 61, 982–986. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Dombi, E.; Potter, D.M.; Goldman, S.; Allen, J.C.; Pollack, I.F.; Widemann, B.C. Phase I trial of pegylated interferon- -2b in young patients with plexiform neurofibromas. Neurology 2011, 76, 265–272. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Dombi, E.; Steinberg, S.M.; Goldman, S.; Kieran, M.W.; Ullrich, N.J.; Pollack, I.F.; Goodwin, A.; Manley, P.E.; Fangusaro, J.; et al. Phase II trial of pegylated interferon alfa-2b in young patients with neu-rofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017, 19, 289–297. [Google Scholar]
- Robertson, K.A.; Nalepa, G.; Yang, F.-C.; Bowers, D.C.; Ho, C.Y.; Hutchins, G.D.; Croop, J.M.; Vik, T.A.; Denne, S.C.; Parada, L.F.; et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: A phase 2 trial. Lancet Oncol. 2012, 13, 1218–1224. [Google Scholar] [CrossRef]
- Banerjee, A.; Jakacki, R.I.; Onar-Thomas, A.; Wu, S.; Nicolaides, T.; Poussaint, T.Y.; Fangusaro, J.; Phillips, J.; Perry, A.; Turner, D.; et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncology 2017, 19, 1135–1144. [Google Scholar] [CrossRef]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. [Google Scholar] [CrossRef]
- Gross, A.; Bishop, R.; Widemann, B.C. Selumetinib in Plexiform Neurofibromas. N. Engl. J. Med. 2017, 376, 1195. [Google Scholar]
- Plotkin, S.R.; Blakeley, J.O.; Dombi, E.; Fisher, M.J.; Hanemann, C.O.; Walsh, K.; Wolters, P.L.; Widemann, B.C. Achieving consensus for clinical trials: The REiNS International Collaboration. Neurology 2013, 81, S1–S5. [Google Scholar] [CrossRef]
- McCowage, G.B.; Mueller, S.; Pratilas, C.A.; Hargrave, D.R.; Moertel, C.L.; Whitlock, J.; Fox, E.; Hingorani, P.; Russo, M.W.; Dasgupta, K.; et al. Trametinib in pediatric patients with neurofibromatosis type 1 (NF-1)–associated plexiform neurofibroma: A phase I/IIa study. J. Clin. Oncol. 2018, 36, 10504. [Google Scholar] [CrossRef]
- Listernick, R.; Charrow, J.; Gutmann, D.H. Intracranial gliomas in neurofibromatosis type 1. Am. J. Med. Genet. 1999, 89, 38–44. [Google Scholar] [CrossRef]
- Lewis, R.A.; Gerson, L.P.; Axelson, K.A.; Riccardi, V.M.; Whitford, R.P. von Recklinghausen neurofibromatosis. II. Incidence of optic gliomata. Ophthalmology 1984, 91, 929–935. [Google Scholar] [CrossRef]
- Packer, R.J.; Lange, B.; Ater, J.; Nicholson, H.S.; Allen, J.; Walker, R.; Prados, M.; Jakacki, R.; Reaman, G.; Needles, M.N. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J. Clin. Oncol. 1993, 11, 850–856. [Google Scholar] [CrossRef]
- Listernick, R.; Ferner, R.E.; Liu, G.T.; Gutmann, D.H. Optic pathway gliomas in neurofibromatosis-1: Controversies and rec-ommendations. Ann. Neurol. 2007, 61, 189–198. [Google Scholar] [CrossRef]
- Kondyli, M.; Larouche, V.; Saint-Martin, C.; Ellezam, B.; Pouliot, L.; Sinnett, D.; Legault, G.; Crevier, L.; Weil, A.; Farmer, J.-P.; et al. Trametinib for progressive pediatric low-grade gliomas. J. Neuro-Oncology 2018, 140, 435–444. [Google Scholar] [CrossRef]
- Romo, C.; Slobogean, B.; Blair, L.; O Blakeley, J. RARE-54. MEK INHIBITION FOR AGGRESSIVE GLIOMAS IN ADULTS WITH NEUROFIBROMATOSIS TYPE 1. Neuro-Oncology 2019, 21, vi233. [Google Scholar] [CrossRef]
- Rauen, K.A.; Alsaegh, A.; Ben-Shachar, S.; Berman, Y.; Blakeley, J.; Cordeiro, I.; Elgersma, Y.; Evans, D.G.; Fisher, M.J.; Frayling, I.M.; et al. First International Conference on RASopathies and Neurofibromatoses in Asia: Identification and advances of new therapeutics. Am. J. Med. Genet. Part A 2019, 179, 1091–1097. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Moriya, M.; Watanabe, Y.; Miyagawa-Tomita, S.; Niihori, T.; Oba, D.; Ono, M.; Kure, S.; Ogura, T.; Matsubara, Y.; et al. New BRAF knockin mice provide a pathogenetic mechanism of developmental defects and a therapeutic approach in cardio-facio-cutaneous syndrome. Hum. Mol. Genet. 2014, 23, 6553–6566. [Google Scholar] [CrossRef]
- Andelfinger, G.; Marquis, C.; Raboisson, M.-J.; Théoret, Y.; Waldmüller, S.; Wiegand, G.; Gelb, B.D.; Zenker, M.; Delrue, M.-A.; Hofbeck, M. Hypertrophic Cardiomyopathy in Noonan Syndrome Treated by MEK-Inhibition. J. Am. Coll. Cardiol. 2019, 73, 2237–2239. [Google Scholar] [CrossRef]
- Waters, A.M.; Beales, P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011, 26, 1039–1056. [Google Scholar] [CrossRef]
- Oud, M.M.; Lamers, I.J.C.; Arts, H.H. Ciliopathies: Genetics in Pediatric Medicine. J. Pediatr. Genet. 2016, 6, 018–029. [Google Scholar] [CrossRef]
- Khayyeri, H.; Barreto, S.; Lacroix, D. Primary cilia mechanics affects cell mechanosensation: A computational study. J. Theor. Biol. 2015, 379, 38–46. [Google Scholar] [CrossRef]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.J.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary Ca2+ Signals Through Endothelial TRPV4 Channels Regulate Vascular Function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef]
- Feather, S.A.; Winyard, P.J.; Dodd, S.; Woolf, A. Oral-facial-digital syndrome type 1 is another dominant polycystic kidney disease: Clinical, radiological and histopathological features of a new kindred. Nephrol. Dial. Transplant. 1997, 12, 1354–1361. [Google Scholar] [CrossRef]
- Beales, P.L.; Elcioglu, N.; Woolf, A.S.; Parker, D.; Flinter, F.A. New criteria for improved diagnosis of Bardet-Biedl syn-drome: Results of a population survey. J. Med. Genet. 1999, 36, 437–446. [Google Scholar] [CrossRef]
- Tobin, J.L.; Di Franco, M.; Eichers, E.; May-Simera, H.; Garcia, M.; Yan, J.; Quinlan, R.; Justice, M.J.; Hennekam, R.C.; Briscoe, J.; et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet–Biedl syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 6714–6719. [Google Scholar] [CrossRef]
- Brancati, F.; Italy, U.D.N.; Camerota, L.; Colao, E.; Vega-Warner, V.; Zhao, X.; Zhang, R.; Bottillo, I.; Castori, M.; Caglioti, A.; et al. Biallelic variants in the ciliary gene TMEM67 cause RHYNS syndrome. Eur. J. Hum. Genet. 2018, 26, 1266–1271. [Google Scholar] [CrossRef]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase, E.; link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef]
- Cantagrel, V.; Silhavy, J.L.; Bielas, S.L.; Swistun, D.; Marsh, S.E.; Bertrand, J.Y.; Audollent, S.; Attié-Bitach, T.; Holden, K.R.; Dobyns, W.B.; et al. Mutations in the Cilia Gene ARL13B Lead to the Classical Form of Joubert Syndrome. Am. J. Hum. Genet. 2008, 83, 170–179. [Google Scholar] [CrossRef]
- Noor, A.; Windpassinger, C.; Patel, M.; Stachowiak, B.; Mikhailov, A.; Azam, M.; Irfan, M.; Siddiqui, Z.K.; Naeem, F.; Paterson, A.; et al. CC2D2A, Encoding A Coiled-Coil and C2 Domain Protein, Causes Autosomal-Recessive Mental Retardation with Retinitis Pigmentosa. Am. J. Hum. Genet. 2008, 82, 1011–1018. [Google Scholar] [CrossRef]
- Delous, M.; Baala, L.; Salomon, R.; Laclef, C.; Vierkotten, J.; Tory, K.; Golzio, C.; Lacoste, T.; Besse, L.; Ozilou, C.; et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat. Genet. 2007, 39, 875–881. [Google Scholar] [CrossRef]
- Baala, L.; Romano, S.; Khaddour, R.; Saunier, S.; Smith, U.M.; Audollent, S.; Ozilou, C.; Faivre, L.; Laurent, N.; Foliguet, B.; et al. The Meckel-Gruber Syndrome Gene, MKS3, Is Mutated in Joubert Syndrome. Am. J. Hum. Genet. 2007, 80, 186–194. [Google Scholar] [CrossRef]
- Parisi, M.A.; Bennett, C.; Eckert, M.L.; Dobyns, W.; Gleeson, J.G.; Shaw, D.W.; McDonald, R.; Eddy, A.; Chance, P.F.; Glass, I.A. The NPHP1 Gene Deletion Associated with Juvenile Nephronophthisis Is Present in a Subset of Individuals with Joubert Syndrome. Am. J. Hum. Genet. 2004, 75, 82–91. [Google Scholar] [CrossRef]
- Dixon-Salazar, T.; Silhavy, J.L.; Marsh, S.E.; Louie, C.M.; Scott, L.C.; Gururaj, A.; Al-Gazali, L.; Al-Tawari, A.A.; Kayserili, H.; Sztriha, L.; et al. Mutations in the AHI1 Gene, Encoding Jouberin, Cause Joubert Syndrome with Cortical Polymicrogyria. Am. J. Hum. Genet. 2004, 75, 979–987. [Google Scholar] [CrossRef]
- Sayer, J.; Otto, E.; O’Toole, J.F.; Nurnberg, G.; Kennedy, M.A.; Becker, C.; Hennies, H.C.; Helou, J.; Attanasio, M.; Fausett, B.V.; et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006, 38, 674–681. [Google Scholar] [CrossRef]
- Coene, K.; Roepman, R.; Doherty, D.; Afroze, B.; Kroes, H.Y.; Letteboer, S.J.; Ngu, L.H.; Budny, B.; van Wijk, E.; Gorden, N.T.; et al. OFD1 Is Mutated in X-Linked Joubert Syndrome and Interacts with LCA5-Encoded Lebercilin. Am. J. Hum. Genet. 2009, 85, 465–481. [Google Scholar] [CrossRef]
- Valente, E.M.; Silhavy, J.L.; Brancati, F.; Barrano, G.; Krishnaswami, S.R.; Castori, M.; Lancaster, M.A.; Boltshauser, E.; Boccone, L.; Al-Gazali, L.; et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 2006, 38, 623–625. [Google Scholar] [CrossRef]
- Kyttälä, M.; Tallila, J.; Salonen, R.; Kopra, O.; Kohlschmidt, N.; Paavola-Sakki, P.; Peltonen, L.; Kestilä, M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat. Genet. 2006, 38, 155–157. [Google Scholar] [CrossRef]
- Smith, U.M.; Consugar, M.; Tee, L.J.; McKee, B.M.; Maina, E.N.; Whelan, S.; Morgan, N.; Goranson, E.; Gissen, P.; Lilliquist, S.; et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat. Genet. 2006, 38, 191–196. [Google Scholar] [CrossRef]
- Tallila, J.; Jakkula, E.; Peltonen, L.; Salonen, R.; Kestilä, M. Identification of CC2D2A as a Meckel Syndrome Gene Adds an Important Piece to the Ciliopathy Puzzle. Am. J. Hum. Genet. 2008, 82, 1361–1367. [Google Scholar] [CrossRef]
- Caridi, G.; Murer, L.; Bellantuono, R.; Sorino, P.; Caringella, D.; Gusmano, R.; Ghiggeri, G.M. Renal-retinal syndromes: Asso-ciation of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am. J. Kidney Dis. 1998, 32, 1059–1062. [Google Scholar] [CrossRef]
- Omran, H.; Sasmaz, G.D.; Häffner, K.; Volz, A.; Olbrich, H.; Melkaoui, R.; Otto, E.; Wienker, T.F.; Korinthenberg, R.; Brandis, M.; et al. Identification of a Gene Locus for Senior-Løken Syndrome in the Region of the Nephronophthisis Type 3 Gene. J. Am. Soc. Nephrol. 2002, 13, 75–79. [Google Scholar] [CrossRef]
- Schuermann, M.J.; Otto, E.; Becker, A.; Saar, K.; Rüschendorf, F.; Polak, B.C.; Ala-Mello, S.; Hoefele, J.; Wiedensohler, A.; Haller, M.; et al. Mapping of gene loci for nephronophthisis type 4 and Sen-ior-Løken syndrome, to chromosome 1p36. Am. J. Hum. Genet. 2002, 70, 1240–1246. [Google Scholar] [CrossRef]
- Otto, E.; Hoefele, J.; Ruf, R.; Mueller, A.M.; Hiller, K.S.; Wolf, M.T.; Schuermann, M.J.; Becker, A.; Birkenhäger, R.; Sudbrak, R.; et al. A Gene Mutated in Nephronophthisis and Retinitis Pigmentosa Encodes a Novel Protein, Nephroretinin, Conserved in Evolution. Am. J. Hum. Genet. 2002, 71, 1161–1167. [Google Scholar] [CrossRef]
- Ferrante, M.I.; Feather, S.A.; Bulfone, A.; Wright, V.; Ghiani, M.; Selicorni, A.; Gammaro, L.; Scolari, F.; Woolf, A.S.; Sylvie, O.; et al. Identification of the Gene for Oral-Facial-Digital Type I Syndrome. Am. J. Hum. Genet. 2001, 68, 569–576. [Google Scholar] [CrossRef]
- Perrault, I.; Delphin, N.; Hanein, S.; Gerber, S.; Dufier, J.L.; Roche, O.; Defoort-Dhellemmes, S.; Dollfus, H.; Fazzi, E.; Mun-nich, A.; et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum. Mutat. 2007, 28, 416. [Google Scholar] [CrossRef]
- Marlhens, F.; Bareil, C.; Griffoin, J.-M.; Zrenner, E.; Amalric, P.; Eliaou, C.; Liu, S.-Y.; Harris, E.; Redmond, T.M.; Arnaud, B.; et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat. Genet. 1997, 17, 139–141. [Google Scholar] [CrossRef]
- Wang, H.; Hollander, A.I.D.; Moayedi, Y.; Abulimiti, A.; Li, Y.; Collin, R.W.; Hoyng, C.B.; Lopez, I.; Bray, M.; Lewis, R.A.; et al. Mutations in SPATA7 Cause Leber Congenital Amaurosis and Juvenile Retinitis Pigmentosa. Am. J. Hum. Genet. 2009, 84, 380–387. [Google Scholar] [CrossRef]
- Sohocki, M.M.; Sullivan, L.S.; Tirpak, D.L.; Daiger, S.P. Comparative analysis of aryl-hydrocarbon receptor interacting pro-tein-like 1 (Aipl1), a gene associated with inherited retinal disease in humans. Mamm. Genome 2001, 12, 566–568. [Google Scholar]
- Hollander, A.I.D.; Heckenlively, J.R.; Born, L.I.V.D.; de Kok, Y.J.; van der Velde-Visser, S.D.; Kellner, U.; Jurklies, B.; van Schooneveld, M.J.; Blankenagel, A.; Rohrschneider, K.; et al. Leber Congenital Amaurosis and Retinitis Pigmentosa with Coats-like Exudative Vasculopathy Are Associated with Mutations in the Crumbs Homologue 1 (CRB1) Gene. Am. J. Hum. Genet. 2001, 69, 198–203. [Google Scholar] [CrossRef]
- Gerber, S.; Perrault, I.; Hanein, S.; Barbet, F.; Ducroq, D.; Ghazi, I.; Martin-Coignard, D.; Leowski, C.; Homfray, T.; Dufier, J.-L.; et al. Complete exon-intron structure of the RPGR-interacting protein (RPGRIP1) gene allows the identification of mutations underlying Leber congenital amaurosis. Eur. J. Hum. Genet. 2001, 9, 561–571. [Google Scholar] [CrossRef]
- Freund, C.L.; Wang, Q.-L.; Chen, S.; Muskat, B.L.; Wiles, C.D.; Sheffield, V.C.; Jacobson, S.G.; Mclnnes, R.R.; Zack, D.J.; Stone, E.M. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat. Genet. 1998, 18, 311–312. [Google Scholar] [CrossRef]
- Hollander, A.I.D.; Johnson, K.; De Kok, Y.J.M.; Klebes, A.; Brunner, H.G.; Knust, E.; Cremers, F.P.M. CRB1 has a cytoplasmic domain that is functionally conserved between human and Drosophila. Hum. Mol. Genet. 2001, 10, 2767–2773. [Google Scholar] [CrossRef]
- Bowne, S.J.; Sullivan, L.S.; Mortimer, S.E.; Hedstrom, L.; Zhu, J.; Spellicy, C.J.; Gire, A.I.; Hughbanks-Wheaton, D.; Birch, D.G.; Lewis, R.A.; et al. Spectrum and Frequency of Mutations in IMPDH1 Associated with Autosomal Dominant Retinitis Pig-mentosa and Leber Congenital Amaurosis. Investig. Opthalmol. Vis. Sci. 2006, 47, 34–42. [Google Scholar] [CrossRef]
- Friedman, J.S.; Chang, B.; Kannabiran, C.; Chakarova, C.; Singh, H.P.; Jalali, S.; Hawes, N.L.; Branham, K.; Othman, M.; Filippova, E.; et al. Premature Truncation of a Novel Protein, RD3, Exhibiting Subnuclear Localization Is Associated with Retinal Degeneration. Am. J. Hum. Genet. 2006, 79, 1059–1070. [Google Scholar] [CrossRef]
- Janecke, A.; Thompson, D.; Utermann, G.; Becker, C.; Hübner, C.A.; Schmid, E.; McHenry, C.L.; Nair, A.R.; Rüschendorf, F.; Heckenlively, J.; et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat. Genet. 2004, 36, 850–854. [Google Scholar] [CrossRef]
- Mykytyn, K.; Nishimura, D.Y.; Searby, C.; Shastri, M.; Yen, H.-J.; Beck, J.S.; Braun, T.A.; Streb, L.M.; Cornier, A.S.; Cox, G.F.; et al. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat. Genet. 2002, 31, 435–438. [Google Scholar] [CrossRef]
- Nishimura, D.Y.; Searby, C.C.; Carmi, R.; Elbedour, K.; Van Maldergem, L.; Fulton, A.B.; Lam, B.L.; Powell, B.R.; Swiderski, R.E.; Bugge, K.E.; et al. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2). Hum. Mol. Genet. 2001, 10, 865–874. [Google Scholar] [CrossRef]
- Chiang, A.P.; Nishimura, D.; Searby, C.; Elbedour, K.; Carmi, R.; Ferguson, A.L.; Secrist, J.; Braun, T.; Casavant, T.; Stone, E.M.; et al. Comparative Genomic Analysis Identifies an ADP-Ribosylation Factor–like Gene as the Cause of Bardet-Biedl Syndrome (BBS3). Am. J. Hum. Genet. 2004, 75, 475–484. [Google Scholar] [CrossRef]
- Mykytyn, K.; Braun, T.; Carmi, R.; Haider, N.B.; Searby, C.C.; Shastri, M.; Beck, G.; Wright, A.F.; Iannaccone, A.; Elbedour, K.; et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat. Genet. 2001, 28, 188–191. [Google Scholar] [CrossRef]
- Torrefranca, A.B.; Santiago, A.P.D.; Lingao, M.D.; Racoma, M.J.C. Novel compound heterozygous pathogenic BBS5 variants in Filipino siblings with Bardet-Biedl syndrome (BBS). Ophthalmic Genet. 2020, 41, 621–624. [Google Scholar] [CrossRef]
- Katsanis, N.; Beales, P.L.; Woods, M.O.; Lewis, R.A.; Green, J.S.; Parfrey, P.S.; Ansley, S.J.; Davidson, W.S.; Lupski, J.R. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat. Genet. 2000, 26, 67–70. [Google Scholar] [CrossRef]
- Badano, J.; Ansley, S.J.; Leitch, C.C.; Lewis, R.A.; Lupski, J.R.; Katsanis, N. Identification of a Novel Bardet-Biedl Syndrome Protein, BBS7, That Shares Structural Features with BBS1 and BBS2. Am. J. Hum. Genet. 2003, 72, 650–658. [Google Scholar] [CrossRef]
- Tadenev, A.L.; Kulaga, H.M.; May-Simera, H.L.; Kelley, M.W.; Katsanis, N.; Reed, R.R. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc. Natl. Acad. Sci. USA 2011, 108, 10320–10325. [Google Scholar] [CrossRef]
- Nishimura, D.Y.; Swiderski, R.E.; Searby, C.; Berg, E.M.; Ferguson, A.L.; Hennekam, R.; Merin, S.; Weleber, R.G.; Biesecker, L.G.; Stone, E.M.; et al. Comparative Genomics and Gene Expression Analysis Identifies BBS9, a New Bardet-Biedl Syndrome Gene. Am. J. Hum. Genet. 2005, 77, 1021–1033. [Google Scholar] [CrossRef]
- Stoetzel, C.; Laurier, V.; Davis, E.E.; Muller, J.; Rix, S.; Badano, J.L.; Leitch, C.C.; Salem, N.; Chouery, E.; Corbani, S.; et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat. Genet. 2006, 38, 521–524. [Google Scholar] [CrossRef]
- Chiang, A.P.; Beck, J.S.; Yen, H.J.; Tayeh, M.K.; Scheetz, T.E.; Swiderski, R.E.; Nishimura, D.Y.; Braun, T.A.; Kim, K.Y.; Huang, J.; et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11). Proc. Natl. Acad. Sci. USA 2006, 103, 6287–6292. [Google Scholar] [CrossRef]
- Stoetzel, C.; Muller, J.; Laurier, V.; Davis, E.E.; Zaghloul, N.A.; Vicaire, S.; Jacquelin, C.; Plewniak, F.; Leitch, C.C.; Sarda, P.; et al. Identification of a Novel BBS Gene (BBS12) Highlights the Major Role of a Vertebrate-Specific Branch of Chaperonin-Related Proteins in Bardet-Biedl Syndrome. Am. J. Hum. Genet. 2007, 80, 1–11. [Google Scholar] [CrossRef]
- Leitch, C.C.; Zaghloul, N.A.; Davis, E.; Stoetzel, C.; Diaz-Font, A.; Rix, S.; Al-Fadhel, M.; Lewis, R.A.; Eyaid, W.; Banin, E.; et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 2008, 40, 443–448. [Google Scholar] [CrossRef]
- Kim, S.K.; Shindo, A.; Park, T.J.; Oh, E.C.; Ghosh, S.; Gray, R.S.; Lewis, R.A.; Johnson, C.A.; Attie-Bittach, T.; Katsanis, N.; et al. Planar Cell Polarity Acts Through Septins to Control Collective Cell Movement and Ciliogenesis. Science 2010, 329, 1337–1340. [Google Scholar] [CrossRef]
- Otto, E.A.; Hurd, T.W.; Airik, R.; Chaki, M.; Zhou, W.; Stoetzel, C.; Patil, S.B.; Levy, S.; Ghosh, A.K.; Murga-Zamalloa, C.A.; et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010, 42, 840–850. [Google Scholar] [CrossRef]
- Collin, G.B.; Marshall, J.D.; Ikeda, A.; So, W.V.; Russell-Eggitt, I.; Maffei, P.; Beck, S.; Boerkoel, C.F.; Sicolo, N.; Martin, M.; et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nat. Genet. 2002, 31, 74–78. [Google Scholar] [CrossRef]
- Pirnar, T.; Neuhauser, E.B.D. Asphyxiating thoracic dystrophy of the newborn. Am. J. Roentgenol Radium. Ther. Nucl. Med. 1966, 98, 358–364. [Google Scholar] [CrossRef]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef]
- Habas, R.; Dawid, I.B. Dishevelled and Wnt signaling: Is the nucleus the final frontier? J. Biol. 2005, 4, 2. [Google Scholar] [CrossRef]
- LeCarpentier, Y.; Schussler, O.; Hébert, J.-L.; Vallée, A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells 2020, 9, 2125. [Google Scholar] [CrossRef]
- Zhong, W.; Oguljahan, B.; Xiao, Y.; Nelson, J.; Hernandez, L.; Garcia-Barrio, M.; Francis, S.C. Serum and glucocorti-coid-regulated kinase 1 promotes vascular smooth muscle cell proliferation via regulation of β-catenin dy-namics. Cell Signal. 2014, 26, 2765–2772. [Google Scholar] [CrossRef]
- Cicenas, J.; Meskinyte-Kausiliene, E.; Jukna, V.; Rimkus, A.; Simkus, J.; Soderholm, D. SGK1 in Cancer: Biomarker and Drug Target. Cancers 2022, 14, 2385. [Google Scholar] [CrossRef]
- Colosimo, P.F.; Tolwinski, N.S. Wnt, Hedgehog and junctional Armadillo/beta-catenin establish planar polarity in the Dro-sophila embryo. PLoS ONE 2006, 1, e9. [Google Scholar] [CrossRef]
- Colosimo, P.F.; Liu, X.; Kaplan, N.A.; Tolwinski, N.S. GSK3beta affects apical-basal polarity and cell-cell adhesion by regulating aPKC levels. Dev. Dyn. 2010, 239, 115–125. [Google Scholar]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium 2005, 38, 439–446. [Google Scholar] [CrossRef]
- Sumida, T.; Lincoln, M.R.; Ukeje, C.M.; Rodriguez, D.M.; Akazawa, H.; Noda, T.; Naito, A.T.; Komuro, I.; Dominguez-Villar, M.; Hafler, D.A. Activated β-catenin in Foxp3+ regulatory T cells links inflammatory environments to autoimmunity. Nat. Immunol. 2018, 19, 1391–1402. [Google Scholar] [CrossRef]
- Abdelhamed, Z.A.; Abdelmottaleb, D.I.; El-Asrag, M.E.; Natarajan, S.; Wheway, G.; Inglehearn, C.F.; Toomes, C.; Johnson, C.A. The ciliary Frizzled-like receptor Tmem67 regulates canonical Wnt/β-catenin signalling in the developing cerebellum via Hoxb5. Sci. Rep. 2019, 9, 5446. [Google Scholar] [CrossRef]
- Abdelhamed, Z.A.; Wheway, G.; Szymanska, K.; Natarajan, S.; Toomes, C.; Inglehearn, C.; Johnson, C.A. Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syn-drome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects. Hum. Mol. Genet. 2013, 22, 1358–1372. [Google Scholar] [CrossRef]
- Preston, D.; Simpson, S.; Halm, D.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Blazer-Yost, B.L. Activation of TRPV4 stimulates transepithelial ion flux in a porcine choroid plexus cell line. Am. J. Physiol. Physiol. 2018, 315, C357–C366. [Google Scholar] [CrossRef]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An aqua-porin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef]
- Takayama, Y.; Shibasaki, K.; Suzuki, Y.; Yamanaka, A.; Tominaga, M. Modulation of water efflux through functional in-teraction between TRPV4 and TMEM16A/anoctamin 1. FASEB J. 2014, 28, 2238–2248. [Google Scholar] [CrossRef]
- Singh, S.K.; O’Hara, B.; Talukder, J.R.; Rajendran, V.M. Aldosterone induces active K+secretion by enhancing mucosal ex-pression of Kcnn4c and Kcnma1 channels in rat distal colon. Am. J. Physiol. Physiol. 2012, 302, C1353–C1360. [Google Scholar] [CrossRef]
- Rachel, R.A.; Yamamoto, E.A.; Dewanjee, M.K.; May-Simera, H.L.; Sergeev, Y.V.; Hackett, A.N.; Pohida, K.; Munasinghe, J.; Gotoh, N.; Wickstead, B.; et al. CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies. Hum. Mol. Genet. 2015, 24, 3775–3791. [Google Scholar] [CrossRef]
- Coppieters, F.; Lefever, S.; Leroy, B.P.; De Baere, E. CEP290, a gene with many faces: Mutation overview and presentation of CEP290base. Hum. Mutat. 2010, 31, 1097–1108. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.A.; Desai, N.J.; Hildebrandt, F.; Khanna, H. Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol. Vis. 2010, 16, 1373–1381. [Google Scholar]
- Bagher, P.; Beleznai, T.; Kansui, Y.; Mitchell, R.; Garland, C.J.; Dora, K.A. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca 2+ events, and IK Ca channels, reducing arteriolar tone. Proc. Natl. Acad. Sci. USA 2012, 109, 18174–18179. [Google Scholar] [CrossRef]
- Hochstetler, A.E.; Smith, H.M.; Preston, D.C.; Reed, M.M.; Territo, P.R.; Shim, J.W.; Fulkerson, D.; Blazer-Yost, B.L. TRPV4 antagonists ameliorate ventriculomegaly in a rat model of hydrocephalus. JCI Insight 2020, 5, 137646. [Google Scholar] [CrossRef]
- Ortuso, F.; Amato, R.; Artese, A.; D’Antona, L.; Costa, G.; Talarico, C.; Gigliotti, F.; Bianco, C.; Trapasso, F.; Schenone, S.; et al. In Silico Identification and Biological Evaluation of Novel Selective Serum/Glucocorticoid-Inducible Kinase 1 Inhibitors Based on the Pyrazolo-Pyrimidine Scaffold. J. Chem. Inf. Model. 2014, 54, 1828–1832. [Google Scholar] [CrossRef]
- D’Antona, L.; Dattilo, V.; Catalogna, G.; Scumaci, D.; Fiumara, C.V.; Musumeci, F.; Perrotti, G.; Schenone, S.; Tallerico, R.; Spoleti, C.B.; et al. In Preclinical Model of Ovarian Cancer, the SGK1 Inhibitor SI113 Counteracts the Development of Paclitaxel Resistance and Restores Drug Sensitivity. Transl. Oncol. 2019, 12, 1045–1055. [Google Scholar] [CrossRef]
- Hochstetler, A.E.; Smith, H.; Reed, M.; Hulme, L.; Perrotti, N.; D’Antona, L.; Schenone, S.; Musumeci, F.; Blazer-Yost, B. Inhibition of SGK1 ameliorates ventriculomegaly in a genetic rat model via regulation of TRPV4 in the choroid plexus. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Antona, L.; Amato, R.; Brescia, C.; Rocca, V.; Colao, E.; Iuliano, R.; Blazer-Yost, B.L.; Perrotti, N. Kinase Inhibitors in Genetic Diseases. Int. J. Mol. Sci. 2023, 24, 5276. https://doi.org/10.3390/ijms24065276
D’Antona L, Amato R, Brescia C, Rocca V, Colao E, Iuliano R, Blazer-Yost BL, Perrotti N. Kinase Inhibitors in Genetic Diseases. International Journal of Molecular Sciences. 2023; 24(6):5276. https://doi.org/10.3390/ijms24065276
Chicago/Turabian StyleD’Antona, Lucia, Rosario Amato, Carolina Brescia, Valentina Rocca, Emma Colao, Rodolfo Iuliano, Bonnie L. Blazer-Yost, and Nicola Perrotti. 2023. "Kinase Inhibitors in Genetic Diseases" International Journal of Molecular Sciences 24, no. 6: 5276. https://doi.org/10.3390/ijms24065276
APA StyleD’Antona, L., Amato, R., Brescia, C., Rocca, V., Colao, E., Iuliano, R., Blazer-Yost, B. L., & Perrotti, N. (2023). Kinase Inhibitors in Genetic Diseases. International Journal of Molecular Sciences, 24(6), 5276. https://doi.org/10.3390/ijms24065276






