Abstract
Chlorophyll and heme are essential molecules for photosynthesis and respiration, which are competing branches of the porphyrin metabolism pathway. Chlorophyll and heme balance regulation is very important for the growth and development of plants. The chimeric leaves of Ananas comosus var. bracteatus were composed of central photosynthetic tissue (PT) and marginal albino tissue (AT), which were ideal materials for the study of porphyrin metabolism mechanisms. In this study, the regulatory function of ALA content on porphyrin metabolism (chlorophyll and heme balance) was revealed by comparing PT and AT, 5-Aminolevulinic Acid (ALA) exogenous supply, and interference of hemA expression. The AT remained similar in porphyrin metabolism flow level to the PT by keeping an equal ALA content in both tissues, which was very important for the normal growth of the chimeric leaves. As the chlorophyll biosynthesis in AT was significantly inhibited, the porphyrin metabolism flow was directed more toward the heme branch. Both tissues had similar Mg2+ contents; however, Fe2+ content was significantly increased in the AT. The chlorophyll biosynthesis inhibition in the white tissue was not due to a lack of Mg2+ and ALA. A 1.5-fold increase in ALA content inhibited chlorophyll biosynthesis while promoting heme biosynthesis and hemA expression. The doubling of ALA content boosted chlorophyll biosynthesis while decreasing hemA expression and heme content. HemA expression interference resulted in a higher ALA content and a lower chlorophyll content, while the heme content remained at a relatively low and stable level. Conclusively, a certain amount of ALA was important for the stability of porphyrin metabolism and the normal growth of plants. The ALA content appears to be able to regulate chlorophyll and heme content by bidirectionally regulating porphyrin metabolism branch direction.
1. Introduction
Ananas comosus var. bracteatus, also known as a variegated pineapple, is a perennial evergreen herb that belongs to the genus Bromeliaceae [1]. It is planted as an ornamental plant due to its beautiful photosynthetic-albino chimeric leaf and red fruit. Its chimeric leaf is made up of central photosynthetic tissue (PT, which contains chlorophyll and is found in the center of the leaves) and marginal albino tissue (AT, which lacks chlorophyll and is found on both edges of the leaves) [2]. Over the years, studies on Ananas comosus var. bracteatus have revealed biological mechanisms that control the leaf type, and they are ideal research materials for further revealing plant growth, porphyrin metabolism, and development regulation mechanisms [3,4,5]. Leaf albinos are common leaf color mutants that exhibit an inability to produce chloroplasts as well as defects in chlorophyll biosynthesis. However, the albino tissues of chimeric leaves are different from the very weak albino mutations; they can grow well since they cooperate with the normal photosynthetic leaf tissue in growth and development. The porphyrin regulation mechanism of the chimeric leaves must be different from the albino mutant, and it is interesting to reveal the porphyrin metabolism regulation mechanism of the chimeric tissue types. There are enzymatic steps involved in porphyrin metabolism and synthesis [6]. These reactions are closely regulated to prevent phytotoxic accumulation and ensure the continuous supply of enzymatic capacity to cognate cells [7]. In plants, the reactions are coordinated by chlorophyll synthesis [8], mediated by retrograde signals from chloroplasts [9] and cell cellular metabolism processes [10]. Studies have shown that the albino of the leaf is closely related to chloroplast development [11], chlorophyll synthesis [12], and heme metabolism [13]. Leaf color mutation, also known as chlorophyll mutation, is directly or indirectly related to chlorophyll biosynthesis dynamics [14].
The crucial co-factors and pigments for chlorophyll, heme, and phytochromobilin are produced by the porphyrin biosynthesis pathway [14]. All living cells produce porphyrin through a process called biosynthesis, and the first committed intermediary in this process is ALA [15]. In most cases, protoporphyrinogen oxidase is the last enzyme before the branch in the porphyrin biosynthetic pathway catalyzes the oxidation of protophorinogen IX to Protoporphyrin IX (Proto IX). Protophorinogen IX is then directed to the magnesium (Mg) and iron (Fe) branches for chlorophyll and heme biosynthesis, respectively [16,17].
Chlorophyll and heme are essential molecules for photosynthesis and respiration, generated through porphyrin metabolism [18,19]. The chlorophyll photo-pigments are necessary for photosynthesis, while heme is the precursor of the phytochrome component for the photosynthetic electron transport chain b6/f complex [20,21]. As the first and key precursor of porphyrin metabolism, ALA is generated through three-step enzymatic reactions catalyzed by glutamyl tRNA reductase (GluTR), which is coded by the hemA gene [22]. The GluTR catalyzation steps involve the synthesis of ALA from glutamyl tRNA, which is the first step of chlorophyll synthesis and a key rate-limiting step that determines how fast the whole chlorophyll pathway can be carried out [23]. Moreover, ALA is catalyzed to form the Protoporphyrin Ⅸ complex that binds to Mg2+ or Fe2+ and then enters the chlorophyll synthesis pathway and the heme synthesis pathway, respectively [24]. In higher plants, GluTR is always maintained in a stable homeostasis to avoid the unnecessary synthesis of tetrapyrrole compounds. In rice, for example, an uncontrolled accumulation of porphyrins and magnesium porphyrins resulted in a high formation of reactive oxygen species [25]. It has been revealed in tomatoes that heme content has a negative feedback control on GluTR and ALA content, and consequently the chlorophyll content [26]. Furthermore, heme acts on approximately 30 amino acid residues of the N-terminus of GluTR1 proteins, inhibiting GluTR activity in Arabidopsis thaliana [27]. The AtGluTR protein was encoded by AtHemA1, which regulates the balance of chlorophyll and heme metabolism in Arabidopsis thaliana [28]. Heme is not only involved in the synthesis of oxide-reductase co-groups but also in protein stabilization [29]. In Pakchoi, high expression of hemA inhibited the synthesis of chlorophyll, resulting in lower chlorophyll content and yellowing leaves [30]. In Arabidopsis thaliana, the AtHemA1 expression resulted in the decrease in ALA, chlorophyll, and heme contents and a significant decrease in GluTR enzyme activity [31,32]. The protein encoded by HemA influences the role of GluTR in controlling the balance of chlorophyll and heme biosynthesis [33]. This shows that the interaction mechanism between ALA content, hemA expression, and GluTR content is a key regulatory element of porphyrin metabolism.
The chimeric leaves of Ananas comosus var. bracteatus have effective photosynthesis ability and grow normally, which provide typical normal photosynthetic tissues and albino tissues for studies on the porphyrin metabolism internal regulation mechanism in normally growing plants. Research results from chimeric leaves can make up for the shortcoming of studying porphyrin metabolism using albino mutants that do not grow normally. In this study, a comprehensive analysis of chlorophyll and heme biosynthesis in the photosynthetic central tissue and marginal albino tissue of Ananas comosus var. bracteatus chimeric leaves was conducted to reveal the relationship and balance between the two main branches of porphyrin metabolism. Exogenous ALA supply and hemA gene expression interference were used to reveal the regulatory function of ALA content on chlorophyll and heme biosynthesis balance.
2. Results
2.1. Porphyrin Metabolism Characters in Chimeric Leaves
Porphyrin metabolism provides the vital pigments for chlorophyll and heme [34]. Its biosynthesis begins with the formation of 5-aminolevulinic acid (ALA) as the first step [35]. The study examined the porphyrin metabolism characters in the PT and WT of Ananas comosus var. bracteatus chimeric leaves (Figure 1D). Unlike the albino mutation, chimeric leaves grow well, making them an ideal material for further research into the porphyrin metabolism mechanism.
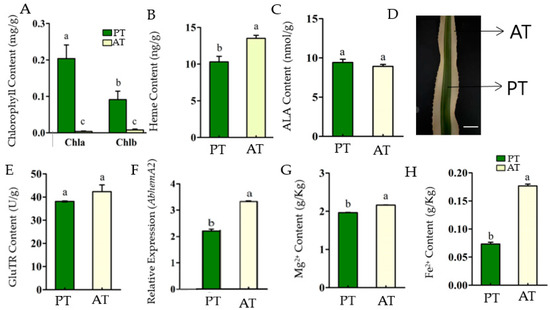
Figure 1.
The porphyrin metabolism characterizes in the central photosynthetic tissue (PT) and marginal albino tissue (AT) of the chimeric leaves of Ananas comosus var. bracteatus. Chlorophyll content (A), heme content (B), 5-aminolevulinic acid content (C), Chimeric leaf (Photosynthesis tissue (PT) and marginal Albino tissue (AT) at 100 µm scale bar (D), GluTR content (E), Relative expression of AbHemA2 (F), Mg2+ content (G), and Fe2+ content (H). The bars show how each bar differs from the others. The means denoted by the same letter did not differ significantly at p < 0.05.
The chlorophyll content of AT was significantly lower than that of PT (p < 0.01) (Figure 1A). However, the heme content in AT was significantly higher than that in PT (p < 0.05) (Figure 1B). Moreover, the heme to Chl ratio in AT was significantly higher than in PT. As the results suggest, the porphyrin metabolism flow in the AT is directed more to the heme branch than the chlorophyll branch, which resulted in the accumulation of heme and the lack of chlorophyll.
The rate-limiting step of porphyrin biosynthesis is ALA formation [36]. ALA is the first precursor of the chlorophyll synthesis pathway, synthesized by GluTR catalyzation. ALA plays a critical role in the chlorophyll biosynthesis pathway [37]. There was no significant difference in ALA content between PT and AT (Figure 1C). It was suggested that the inhibition of chlorophyll accumulation in AT did not result in the accumulation of ALA. The ratio of Chl/ALA in AT was lower than that of PT, while the Heme/ALA ratio was significantly higher than that of PT. Since the ALA content is basically the same between PT and AT, it was suggested that ALA in PT mainly flowed to the chlorophyll synthesis pathway, while AT mainly flowed to the heme synthesis pathway.
It was revealed that ALA is generated through three-step enzymatic reactions catalyzed by GluTR, coded by the hemA gene [22]. There was no significant difference in GluTR content between PT and AT (Figure 1E), while the expression level of AbHemA2 was significantly higher in AT than in PT (Figure 1F). It was indicated that both PT and AT maintained a similar specific level of porphyrin metabolism by maintaining a certain level of GluTR and ALA content. Additionally, the GluTR content was not only determined by hemA expression level. The translation and modification may affect the protein content as well.
Generally in higher photosynthetic plants, the synthetic pathway of tetrapyrrole compounds is divided into two parts: ferrochelatase catalyzes the reaction insertion of Fe2+ into Proto IX to form Heme, and magnesium chelatase catalyzes the reaction insertion of Mg2+ into Proto IX to form Mg2+ protoporphyrin IX and then chlorophyll [24]. Moreover, Mg2+ is a component of chlorophyll involved in photosynthesis, and the synthesis of chlorophyll that requires the presence of Fe2+ [38]. Together, the Mg2+ and Fe2+ contents were closely related to the synthesis of chlorophyll and heme processes. In this study, the Mg2+ and Fe2+ contents in AT were significantly higher than those in PT (p < 0.05), especially the content of Fe2+, which was about 2.4 times to PT (Figure 1G,H). The presence of adequate levels of Mg2+ in AT suggested that Mg2+ content was not the limiting factor in AT chlorophyll biosynthesis. The Mg2+ content in AT may be promoted by the high concentrations of Fe2+ in it to maintain the specific level of ALA and the balance of porphyrin metabolism because Mg2+ and Fe2+ are co-factors that work in parallel [38,39]. The Mg2+ and Fe2+ contents were closely related to the synthesis of chlorophyll and heme processes. The Mg2+ and Fe2+ contents of AT were significantly higher than those of PT (p < 0.05), especially the content of Fe2+, which was about 2.4 times to PT (Figure 1F,G).
2.2. Effects of Exogenous ALA Supplementation on Porphyrin Metabolism
In order to study the effects of endogenous ALA content on porphyrin metabolism, exogenous ALA was supplied to increase the endogenous ALA content of the leaves. Different concentrations (0, 100, and 200 mg/L) of exogenous ALA were supplied to the photosynthetic leaves, and the endogenous ALA contents were detected after 2 days. After the treatment with exogenous ALA treatments of 100 mg/L and 200 mg/L, respectively, there was an increase in the endogenous ALA content in the leaves by approximately 1.4 and 2 times, respectively (Figure 2A). The results showed that exogenous ALA can effectively increase endogenous ALA levels, and that the higher the exogenous ALA concentration, the higher the endogenous ALA concentration.
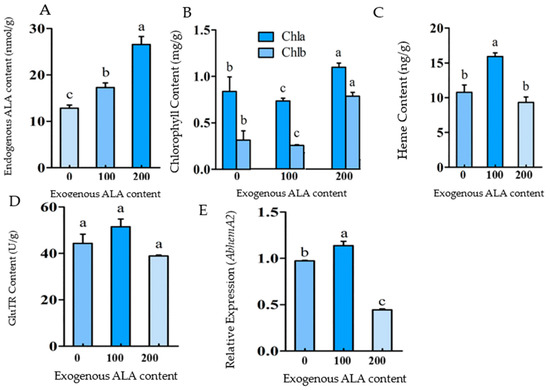
Figure 2.
The effects of exogenous ALA supplementation on porphyrin metabolism. The effect of exogenous ALA supply on endogenous ALA content (A), The effect of exogenous ALA supply on chlorophyll content (B), The effect of exogenous ALA supply on heme content (C), The effect of exogenous ALA supply on GluTR content (D), and the effect of exogenous ALA supply on AbHemA2 expression (E). The bars represent how each bar is different from the others. The means denoted by the same letter did not differ significantly at p < 0.05.
The chlorophyll and heme contents were determined 2 days after the exogenous ALA treatment. The heme content of the 100 mg/L ALA treatment group significantly increased, which was about 1.5 times that of the control group (0 mg/L); however, the chlorophyll content decreased significantly (Figure 2B). It was proposed that 100 mg/L exogenous ALA (endogenous ALA content increased to about 1.5 times) promoted heme synthesis while inhibiting chlorophyll synthesis. When the exogenous ALA was increased to 200 mg/L, the heme content decreased with no significant differences from the control, but the chlorophyll content increased significantly (Figure 2C). It was indicated that 200 mg/L exogenous ALA (the endogenous ALA content increased to about 2 times) promoted the biosynthesis of chlorophyll. In conclusion, these results confirmed that ALA content can regulate the branch direction (chlorophyll or heme) of the porphyrin metabolism. A certain improvement of ALA content (about 1.5 times) can promote the biosynthesis of heme and inhibit the biosynthesis of chlorophyll, but an excessive enhancement of ALA (about 2 times) will promote the biosynthesis of chlorophyll and maintain heme biosynthesis on a certain level.
In the balance regulation of chlorophyll and heme biosynthesis, ALA content plays an important bidirectional regulatory role. GluTR is the key enzyme in ALA biosynthesis and is encoded by the HemA gene. The increase in endogenous ALA content caused by exogenous ALA supply did not appear to affect GluTR content significantly (Figure 2D). It was discovered that the increase in ALA content caused by exogenous ALA supply did not result in GluTR feedback regulation. The relative expression of AbHemA2 increased significantly under 100 mg/L ALA treatment and decreased significantly under 200 mg/L (Figure 2E). The exogenous-supply-induced ALA content increase can regulate the expression of the AbHemA2 gene bidirectionally.
2.3. Effects of HemA Expression on Porphyllrin Metabolism
In order to reveal the effects of hemA gene expression on porphyrin metabolism, the conserved sequence fragment of the hemA gene between tobacco and Ananas comosus var. bracteatus was used to construct the interference vector (pTCK303-AbHemA2-RNAi) and transformed into tobacco. Compared to the wild-type plant, the interference plant was yellowish and some parts of the leaves even turned albino (Figure 3).
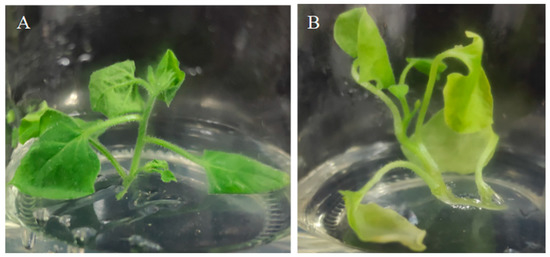
Figure 3.
The wild type (A) and pTCK303-AbHemA2-RNAi transgenetic plants (B).
The expression of hemA in the three transgenic tobacco lines (Ri1, Ri2, and Ri3) was significantly lower than the wild type (p < 0.05) (Figure 4A). It was confirmed that the expression of hemA was inhibited effectively. The GluTR content of the transgenic plants decreased significantly as hemA gene expression decreased (Figure 4B), indicating that hemA expression regulation affected GluTR content. It was worth noting that the ALA content of the transgenic plants increased significantly (about 1.5 times to that of the wild type) (Figure 4C), which was not in accordance with the decreasing hemA expression and GluTR content. However, the chlorophyll and heme content of the transgenic plants were significantly lower than that of the wild type (Figure 4D,E). The increased accumulation of ALA (<1.5 times) in the transgenic plants resulted in a greater decrease in chlorophyll biosynthesis, while the heme content remained at a relatively stable lower level.
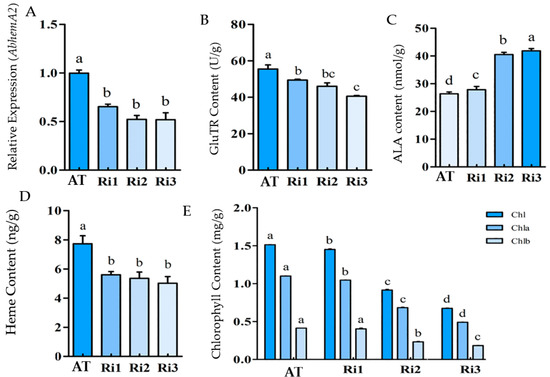
Figure 4.
Effects of HemA expression interference on porphyrin metabolism. Relative HemA expression using Tubulin as the reference gene (A), GluTR content (B), ALA content (C), heme content (D) and chlorophyll content (E). The means denoted by the same letter did not differ significantly at p < 0.05.
3. Discussion
Chlorophyll and heme are the two main branches of the porphyrin metabolism pathway. The metabolism pathway begins with the first precursor, ALA, which is synthesized by the catalyzation of GluTR, and branches to heme or chlorophyll by the binding of protoporphyrin IX to Fe2+ or Mg2+ separately. Heme is an iron-containing cyclic tetrapyrrole compound and an intermediator in phytochrome and phycobilin synthesis [31]. Chlorophyll is synthesized by the magnesium branch of the porphyrin metabolism pathway and is very important for photosynthesis [40]. The synthesis of ALA is the key limitation step of chlorophyll and heme biosynthesis [41], and the content of heme has a negative feedback regulation on the content of ALA and GluTR content [13].
The central photosynthetic and marginal albino tissues of the chimeric leaves of Ananas comosus var. bracteatus cooperated to keep normal growth and development of the plants. Comparing the porphyrin metabolism characters between the photosynthetic and albino tissues of the chimeric leaves can better illustrate the internal cooperation mechanism of chlorophyll and heme biosynthesis in plants than using albino mutants as study material. The similar ALA and GluTR content of the photosynthetic and albino tissues of the chimeric leaves suggested that the two tissues kept a similar porphyrin metabolism level. Chlorophyll and heme are essential molecules for photosynthesis and respiration [18,19]. Maintaining a certain level of porphyrin metabolism in both photosynthetic and albino tissues is important for the normal growth of the chimeric leaves and plants. The albino tissues of the chimeric leaves stabilized porphyrin metabolism by increasing heme biosynthesis and compensating for the damage caused by chlorophyll biosynthesis inhibition. The accumulation of heme in the Arabidopsis mutation inhibited chlorophyll biosynthesis and resulted in leaf albinism [42]. The increased heme content in the albino tissue of Ananas comosus var. bracteatus chimeric leaves may give destructive feedback, regulating chlorophyll biosynthesis, resulting in the albino color of the leaves.
The biosynthesis of chlorophyll and heme is branched by the ion binding of protoporphyrin IX. Ferrous chelatase catalyzes the insertion of Fe2+ into Proto-IX to form the ferrum branches of Heme and phytochromes, while magnesium chelatase catalyzes the insertion of Mg2+ into Proto-IX to form the magnesium branch of Mg-protoporphyrin IX. [43]. The availability of Fe2+ and Mg2+ will affect the biosynthesis of heme and chlorophyll. As a component of chlorophyll, Mg2+ is an essential element for plant growth and development. It can participate in photosynthesis, maintain the stability of the cell membrane, and regulate the enzymes activity in cells [44,45]. The albino tissues of the chimeric leaves contained significantly more Fe2+ and Mg2+ than the photosynthetic tissues, indicating that the inhibition of chlorophyll biosynthesis in the albino tissues of the chimeric leaves was not due to a lack of Mg2+. It was reported that the expression level and protein abundance decrease in chlorophyll-biosynthesis-related genes and chloroplast development inhibition played important roles in the albino of Ananas comosus var. bracteatus chimeric leaves [46].
Although the biosynthesis of chlorophyll was significantly inhibited, the porphyrin metabolism flow in the albino tissue was maintained at a similar level to that in the photosynthetic tissues. To avoid ALA accumulation, the metabolism flow branched more toward the ferrum branch to form heme. It was reported that, as a non-protein amino acid, ALA acts like a growth regulator and plant hormone [15]. Keeping a relatively stable GluTR and ALA content may be one of the reasons why the albino tissue of the chimeric leaves can grow normally. In order to further analyze the regulation function of ALA content on porphyrin metabolism, exogenous ALA was supplied to regulate the endogenous ALA content of the plants. It is reported that exogenous supply of ALA can increase chlorophyll biosynthesis and photosynthesis of plants [47]. In our study, with the increased supplies of exogenous ALA, the endogenous ALA content of the leaves increased accordingly. When the endogenous ALA content increased to about 1.5 times, the biosynthesis of chlorophyll was inhibited and heme biosynthesis was promoted, apparently. When the endogenous ALA content increased to about 2 times, the biosynthesis of chlorophyll was promoted and the biosynthesis of heme decreased to be similar to that of the control. These results suggested that the plants need to keep ALA content at a specific level to maintain a certain level of porphyrin metabolism and a balance of chlorophyll and heme biosynthesis for the normal growth and development of the plant. These results showed that the plants stabilized porphyrin metabolism by redirecting metabolism flow to heme biosynthesis when ALA content increased appropriately (about 1.5 times), while chlorophyll biosynthesis would be increased, apparently, and heme biosynthesis would be kept at a lower level if ALA content increased more (about 2 times). The results further indicated that plants could not accumulate excessive heme, and maintaining the metabolic balance of chlorophyll and heme was very important for the normal growth of plants. Endogenous ALA content can regulate the biosynthesis of chlorophyll and heme bidirectionally, which functions effectively in the regulation of chlorophyll and heme balance and porphyrin metabolism stability.
The biosynthesis of ALA is catalyzed by the GluTR content, which is encoded by the hemA gene. The exogenous supply of ALA for Ananas comosus var. bracteatus leaves increased endogenous ALA content, but it did not affect GluTR content, apparently. It was suggested that ALA content did not have a feedback regulation function on GluTR content. When AtHemA1 gene expression was silenced, the contents of ALA, chlorophyll, and heme in leaves decreased to varying degrees, and the GluTR content also decreased significantly, which confirmed for the first time the role of AtHemA1 in chlorophyll and heme synthesis in higher plants [48]. The interference with hemA gene expression resulted in a decrease in GluTR, heme, and chlorophyll content. It was suggested that the porphyrin metabolism level was down-regulated by hemA interference. The decrease in chlorophyll content was more apparent than that of heme content, which indicated that heme-lacking was more damage for the plants than chlorophyll-lacking. The ALA content of the interference plants increased significantly, reaching approximately 1.5 times that of the wild type. The accumulation of ALA under the interference of hemA gene expression may be caused by the simultaneous inhibition of chlorophyll and heme biosynthesis, and the ALA accumulation may further inhibit chlorophyll biosynthesis while maintaining heme content at a relatively low level. This result was similar to the result of exogenous ALA supply experiment in banana plants [36].
4. Materials and Methods
4.1. Plant Material
Ananas comosus var. bracteatus chimeric plants with photosynthetic-albino striped leaves were used to detect pigment, ALA, heme, Mg2+, Fe2+ and GluTR contents, and hemA expression. Three biological replicates were set up for each sample.
4.2. Determination of Photosynthetic Pigments
The central photosynthetic and marginal albino tissues of the fresh and mature functional chimeric leaves were cut separately for the detection of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid (Car) contents [49]. About 0.1 g of each tissue was cut into 2 × 2 mm squares and soaked in 5 mL of 95% ethanol in the dark. The photosynthetic pigment isolation and measuring process were executed accordingly [4].
4.3. Determination of ALA Content
The ALA content was measured with some modifications [50]. The leaf tissues (0.5 g) were homogenized in 5 mL of trichloroacetic acid (w/v = 4%) and centrifuged at 10,000× g for 10 min. The assay mixture consisted of 10 mL of two combined supernatant, 5.7 mL of sodium acetate, and 0.15 mL of acetylacetone (pH 4.6). The assay medium was mixed and heated in a boiling water bath for 10 min. The extract was cooled to room temperature, and an equal volume of modified Ehrlich’s reagent was added (1:1), vortexed for 2 min, and incubated in the dark for 15 min. The extraction absorbance was measured at a wavelength of 553 nm, and the ALA standard curve was determined as described.
4.4. Determination of Heme Content
Fresh leaf samples (0.1 g each) were homogenized in motor with pistil in 0.9 mL of Phosphate Buffer Saline (PBS), pH 7.4, transferred to a 2 mL centrifuge tube, and centrifuged at 3000 rpm at 4 °C for 20 min. The supernatant was used for the Heme concentration assay. Heme content was determined using a Plant (Heme) ELISA Kit [Shanghai, China, mlbio.cn] and the content was measured with a microplate reader Model 680 (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions (Supplementary Materials) as described [51].
4.5. Determination of Mg2+ and Fe2+ Content
The central photosynthetic and marginal albino tissues of the fresh chimeric leaves were cut separately and oven dried. To 100 mL of a graduated digestion tube containing 15 mL of nitric acid and perchloric acid, mixed at a 4:1 ratio, 0.3 g of the tissue samples were added and allowed to soak overnight. At low and high temperatures, the samples were digested at 160 °C and 300 °C for about 1–2 h, respectively, and then cooled to room temperature. After sampling, according to the content type, the iron (Fe2+) and magnesium ion (Mg2+) standard curves were determined accordingly [40]. The absorbance was measured using an atomic absorption spectrophotometer at wavelengths less than 450 nm.
4.6. Determination of GluTR Content
The GluTR content was determined using a Plant GluTR ELISA Kit, (Shanghai, China, mlbio.cn). The reference GluTR protein was diluted gradiently, and a standard curve was first established using the double-antibody sandwich enzyme-linked immunosorbent assay [52]. Fresh leaf samples (0.1 g) were homogenized in 0.9 mL PBS (pH 7.4) at 4 °C. The samples were centrifuged at 3000 rpm for 20 min, and the supernatant was used for the assay. The GluTR content (U/g) was calculated by a formula according to the standard curve [23]. Each sample was triplicated according to the manufacturer’s instructions (Supplementary Materials).
4.7. Supply of Exogenous ALA
Plants regenerated from Ananas comosus var. bracteatus stem by tissue culture [4] were used for the ALA exogenous supply experiments. The tissue culture-regenerated plants were sprayed with exogenous ALA solutions at 0 mg/L, 100 mg/L, and 200 mg/L overnight and kept in the dark. Each treatment was repeated three times and arranged randomly. After 2 days, fresh leaves were collected, cleaned, quickly frozen in liquid nitrogen, and stored at −80 °C for subsequent index determination.
4.8. Expression Interference of hemA Gene
The conserved sequence of hemA gene family of AbHemA2 was used for the construction of pTCK303-AbHemA2-RNAi. The vector was transformed into tobacco mediated by Agrobacterium tumefaciens. The expression analysis of AbHemA2 in the wild-type and transformed plants was performed by qRT-PCR using Tubulin as the reference gene.
4.9. Data Analysis
The analysis of variance (ANOVA) in SPSS 20.0 Version was used to analyze all of the experiment data, with p ≤ 0.05 probability significant values. The pigment content determination data were calculated and plotted using SPSS, and Graph Pad Prism 5.
5. Conclusions
The regulation functions of ALA content in Ananas comosus var. bracteatus porphyrin metabolism were demonstrated in this study by comparing the photosynthetic and albino parts of the chimeric leaves, exogenous ALA supply, and hemA expression interference. The albino tissue stabilized porphyrin metabolism levels by maintaining a certain ALA content and directing the metabolism flow more to the heme branch to avoid excessive accumulation of ALA. The increase in heme content in the albino tissue may negatively regulate chlorophyll biosynthesis, resulting in albino chimeric leaves. The inhibition of chlorophyll biosynthesis in albino tissue was not caused by a lack of Mg2+ and ALA. A stable level of ALA content is very important for the normal growth of the chimeric leaves. Endogenous ALA content increased as a result of exogenous ALA supply and interference of hemA expression. The ALA content did not have a feedback regulation function on the GluTR content, but it apparently regulated chlorophyll and heme biosynthesis bidirectionally. Appropriate increases in ALA content inhibit chlorophyll biosynthesis and redirect metabolism flow to heme biosynthesis, whereas excessive increases in ALA content promoted metabolism flow to chlorophyll biosynthesis and maintained heme content at a normal level.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24065274/s1.
Author Contributions
J.M. and J.L.; formal analysis, methodology, investigation, M.O.A.; writing—original draft preparation, S.L., X.L., J.D. and A.L. writing—review and editing, and visualization, J.M.; project administration, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, funding number 31971704, and Natural Science Foundation of Sichuan Province, funding number 2022NSFSC0090.
Institutional Review Board Statement
Not available.
Informed Consent Statement
Not available.
Data Availability Statement
Not available.
Acknowledgments
This work is grateful to Sichuan Agricultural University, China, for supporting this work.
Conflicts of Interest
Authors, declare in this work to have no conflict of interest.
References
- Vincent, L.; Anushma, P.; Vasugi, C.; Rekha, A.; Shiva, B. Genetic Resources of Tropical Fruits. In Conservation and Utilization of Horticultural Genetic Resources; Springer: Berlin/Heidelberg, Germany, 2019; pp. 79–116. [Google Scholar]
- Ogata, T.; Yamanaka, S.; Shoda, M.; Urasaki, N.; Yamamoto, T. Current status of tropical fruit breeding and genetics for three tropical fruit species cultivated in Japan: Pineapple, mango, and papaya. Breed. Sci. 2016, 66, 69–81. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Y.; Mao, M.; He, Y.; Adjei, M.O.; Yang, W.; Hu, H.; Liu, J.; Feng, L.; Zhang, H. Metabolome and transcriptome profiling reveals anthocyanin contents and anthocyanin-related genes of chimeric leaves in Ananas comosus var. bracteatus. BMC Genom. 2021, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kanakala, S.; He, Y.; Zhong, X.; Yu, S.; Li, R.; Sun, L.; Ma, J. Physiological characterization and comparative transcriptome analysis of white and green leaves of Ananas comosus var. bracteatus. PLoS ONE 2017, 12, e0169838. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Xue, Y.; He, Y.; Zhou, X.; Rafique, F.; Hu, H.; Liu, J.; Feng, L.; Yang, W.; Li, X. Systematic identification and comparative analysis of lysine succinylation between the green and white parts of chimeric leaves of Ananas comosus var. bracteatus . BMC Genom. 2020, 21, 383. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, H.-i.; Park, J.-H.; Kim, J.-G.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, Q.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef]
- Ma, W.; Yi, F.; Xiao, Y.; Yang, G.; Chen, F.; Wang, J. Isolation of leaf mesophyll protoplasts optimized by orthogonal design for transient gene expression in Catalpa bungei . Sci. Hortic. 2020, 274, 109684. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Chan, K.X.; Marchant, D.B.; Franks, P.J.; Randall, D.; Tee, E.E.; Chen, G.; Ramesh, S.; Phua, S.Y. Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proc. Natl. Acad. Sci. USA 2019, 116, 5015–5020. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Shi, Z.; Xie, Q.; Xing, Y.; Liu, C.; Chen, Q.; Zhu, H.; Wang, J.; Zhang, J. Albino Leaf1 that encodes the sole octotricopeptide repeat protein is responsible for chloroplast development. Plant Physiol. 2016, 171, 1182–1191. [Google Scholar]
- Feng, J.; Shi, Q.; Wang, X.; Wei, M.; Yang, F.; Xu, H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. 2010, 123, 521–530. [Google Scholar] [CrossRef]
- Zhao, M.-H.; Li, X.; Zhang, X.-X.; Zhang, H.; Zhao, X.-Y. Mutation mechanism of leaf color in plants: A review. Forests 2020, 11, 851. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Pattanayak, G.K. Chlorophyll biosynthesis in higher plants. Photosynth. Plast. Biol. Energy Convers. Carbon Assim. 2012, 34, 63–94. [Google Scholar]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Pham, N.-T.; Kim, J.-G.; Jung, S. Differential antioxidant responses and perturbed porphyrin biosynthesis after exposure to oxyfluorfen and methyl viologen in Oryza sativa . Int. J. Mol. Sci. 2015, 16, 16529–16544. [Google Scholar] [CrossRef]
- Willows, R.D. Biosynthesis of chlorophylls from protoporphyrin IX. Nat. Prod. Rep. 2003, 20, 327–341. [Google Scholar] [CrossRef]
- Velini, E.D.; Trindade, M.L.B.; Alves, E.; Catâneo, A.C.; Marino, C.L.; Maia, I.D.G.; Mori, E.S.; Furtado, E.L.; Guerrini, I.A.; Wilcken, C.F. Eucalyptus ESTs corresponding to the protoporphyrinogen IX oxidase enzyme related to the synthesis of heme, chlorophyll, and to the action of herbicides. Genet. Mol. Biol. 2005, 28, 548–554. [Google Scholar] [CrossRef]
- Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of chlorophyll and carotene pigments content in mountain melick (Melica nutans L.) in relation to edaphic variables. Forests 2020, 11, 1197. [Google Scholar] [CrossRef]
- De Montellano, P.R.O.; Wilks, A. Heme oxygenase structure and mechanism. Adv. Inorg. Chem. 2000, 51, 359–407. [Google Scholar]
- Sanchini, A.; Grosjean, M. Quantification of chlorophyll a, chlorophyll b and pheopigments a in lake sediments through deconvolution of bulk UV–VIS absorption spectra. J. Paleolimnol. 2020, 64, 243–256. [Google Scholar] [CrossRef]
- Jahn, D.; Moser, J.; Schubert, W.-D.; Heinz, D.W. Transfer RNA-dependent aminolevulinic acid formation: Structure and function of glutamyl-tRNA synthetase, reductase and glutamate-1-semialdehyde-2, 1-aminomutase. In Chlorophylls and Bacteriochlorophylls; Springer: Berlin/Heidelberg, Germany, 2006; pp. 159–171. [Google Scholar]
- Zeng, Z.-Q.; Lin, T.-Z.; Zhao, J.-Y.; Zheng, T.-H.; Xu, L.-F.; Wang, Y.-H.; Liu, L.-L.; Jiang, L.; Chen, S.-H.; Wan, J.-M. OsHemA gene, encoding glutamyl-tRNA reductase (GluTR) is essential for chlorophyll biosynthesis in rice (Oryza sativa). J. Integr. Agric. 2020, 19, 612–623. [Google Scholar] [CrossRef]
- Papenbrock, J.; Pfündel, E.; Mock, H.P.; Grimm, B. Decreased and increased expression of the subunit CHL I diminishes Mg chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J. 2000, 22, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, H.-J.; Lee, Y.; Kang, K.; Kim, Y.S.; Grimm, B.; Back, K. Toxic tetrapyrrole accumulation in protoporphyrinogen IX oxidase-overexpressing transgenic rice plants. Plant Mol. Biol. 2008, 67, 535–546. [Google Scholar] [CrossRef]
- Ryberg, M.; Terry, M.J. Analysis of protochlorophyllide reaccumulation in the phytochrome chromophore-deficient aurea and yg-2 mutants of tomato by in vivo fluorescence spectroscopy. Photosynth. Res. 2002, 74, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.S.; Banse, C.; Grimm, B. The GluTR-binding protein is the heme-binding factor for feedback control of glutamyl-tRNA reductase. Elife 2019, 8, e46300. [Google Scholar] [CrossRef]
- Kumar, A.M.; Söll, D. Antisense HEMA1 RNA expression inhibits heme and chlorophyll biosynthesis in Arabidopsis . Plant Physiol. 2000, 122, 49–56. [Google Scholar] [CrossRef]
- Stolárik, T.; Hedtke, B.; Šantrůček, J.; Ilík, P.; Grimm, B.; Pavlovič, A. Transcriptional and post-translational control of chlorophyll biosynthesis by dark-operative protochlorophyllide oxidoreductase in Norway spruce. Photosynth. Res. 2017, 132, 165–179. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yuan, L.; Zhou, H.; Hou, X.; Liu, T. Cold acclimation can specifically inhibit chlorophyll biosynthesis in young leaves of Pakchoi. BMC Plant Biol. 2021, 21, 172. [Google Scholar] [CrossRef]
- Nagai, S.; Koide, M.; Takahashi, S.; Kikuta, A.; Aono, M.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.-i.; Masuda, T. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis . Plant Physiol. 2007, 144, 1039–1051. [Google Scholar] [CrossRef]
- Hedtke, B.; Alawady, A.; Chen, S.; Börnke, F.; Grimm, B. HEMA RNAi silencing reveals a control mechanism of ALA biosynthesis on Mg chelatase and Fe chelatase. Plant Mol. Biol. 2007, 64, 733–742. [Google Scholar] [CrossRef]
- Vasileuskaya, Z.; Oster, U.; Beck, C.F. Mg-protoporphyrin IX and heme control HEMA, the gene encoding the first specific step of tetrapyrrole biosynthesis, in Chlamydomonas reinhardtii . Eukaryot. Cell 2005, 4, 1620–1628. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, W.; Wang, L.; Han, S.; Zhang, Y.; Liu, Q.; Liu, B.; Zhao, X. A Maize Necrotic Leaf Mutant Caused by Defect of Coproporphyrinogen III Oxidase in the Porphyrin Pathway. Genes 2022, 13, 272. [Google Scholar] [CrossRef]
- Zhao, C.; Li, S.; Du, C.; Gao, H.; Yang, D.; Fu, G.; Cui, H. Establishment of a Protoplasts-Based Transient Expression System in Banana (Musa spp.). Agronomy 2022, 12, 2648. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; de Los Santos-Villalobos, S.; Wróbel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M. 5-Aminolevulinic acid and 24-epibrassinolide improve the drought stress resilience and productivity of banana plants. Plants 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Oda-Yamamizo, C.; Kishimoto, S. Overexpression of CONSTANS-like 16 enhances chlorophyll accumulation in petunia corollas. Plant Sci. 2019, 280, 90–96. [Google Scholar] [CrossRef]
- Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ahmed, Z. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants 2022, 11, 651. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, D.; Qi, N.; Zhao, Y.; Hu, X.; Ren, N. Deeply mechanism analysis of hydrogen production enhancement of Ethanoligenens harbinense by Fe2+ and Mg2+: Monitoring at growth and transcription levels. Int. J. Hydrog. Energy 2017, 42, 19695–19700. [Google Scholar] [CrossRef]
- Korim, T. Effect of Mg2+-and Fe3+-ions on formation mechanism of aluminium titanate. Ceram. Int. 2009, 35, 1671–1675. [Google Scholar] [CrossRef]
- Richter, A.; Peter, E.; Pörs, Y.; Lorenzen, S.; Grimm, B.; Czarnecki, O. Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol. 2010, 51, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Woodson, J.D.; Perez-Ruiz, J.M.; Chory, J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 2011, 21, 897–903. [Google Scholar] [CrossRef]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole metabolism in Arabidopsis thaliana . Arab. Book/Am. Soc. Plant Biol. 2011, 9, e0145. [Google Scholar]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Wang, J.; Zhang, B.; Wang, W.; Lin, H.; Luan, S.; Gao, J.; Lan, W. The rice high-affinity K+ transporter OsHKT2; 4 mediates Mg2+ homeostasis under high-Mg2+ conditions in transgenic Arabidopsis . Front. Plant Sci. 2017, 8, 1823. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, J.; He, Y.; Yu, S.; Lin, Z.; Xiong, Y.; Rafique, F.; Jiang, F.; Sun, L.; Ma, M. Comparative transcriptomic and proteomic analyses of the green and white parts of chimeric leaves in Ananas comosus var. bracteatus . PeerJ 2019, 7, e7261. [Google Scholar] [CrossRef]
- Tanaka, T.; Hotta, Y.; Takaoka, H. New physiological effects of 5-aminolevulinic acid in plants: Increase of photosynthesis, chlorophyll content and plant growth. Phytoma España 2007, 193, 59–63. [Google Scholar]
- McCormac, A.C.; Fischer, A.; Kumar, A.M.; Söll, D.; Terry, M.J. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana . Plant J. 2001, 25, 549–561. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Hu, Y.; Song, D.; Gao, L.; Ajayo, B.S.; Wang, Y.; Huang, H.; Zhang, J.; Liu, H.; Liu, Y.; Yu, G. Optimization of isolation and transfection conditions of maize endosperm protoplasts. Plant Methods 2020, 16, 96. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, Y.; Jia, X.; Wang, Y.; Wu, T.; Xu, X.; Han, Z.; Zhang, Z.; Zhang, X. MicroRNA156 (miR156) negatively impacts mg-Protoporphyrin IX (mg-proto IX) biosynthesis and its plastid-nucleus retrograde signaling in apple. Plants 2020, 9, 653. [Google Scholar] [CrossRef]
- Gujral, N.; Suresh, M.R.; Sunwoo, H.H. Quantitative double antibody sandwich ELISA for the determination of gliadin. J. Immunoass. Immunochem. 2012, 33, 339–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
