The Role of Light-Regulated Auxin Signaling in Root Development
Abstract
1. Introduction
2. Light Signaling in Root Development
2.1. Light Perception
2.2. Photoreceptors Involved in Root Development
2.3. Key Components in Response to Light in Root Development
| Photoreceptors | Genes | Response to Light | Species | Function | References |
|---|---|---|---|---|---|
| phyA | - | far red (FR) | Arabidopsis thaliana | root hair formation | [24] |
| phyA and phyB | - | red light (RL) | A. thaliana | root hair formation | [24] |
| phyA and phyB | - | - | A. thaliana | root elongation and irregular root hair formation | [20] |
| phyA, phyB, and phyE | - | - | A. thaliana | lateral root formation | [28] |
| phyD | - | - | A. thaliana | lateral root formation | [25] |
| PhyA, PhyB1, and PhyB2 | NaPhyA, NaPhyB1, and NaPhyB2 | - | Nicotiana attenuata | shoot-root development | [26] |
| phyA and phyB | - | - | A. thaliana | lateral root formation | [25] |
| PhyB | - | - | Lotus japonicus | root nodule formation | [27] |
| PhyB | - | - | A. thaliana | root growth | [28] |
| PhyB | IAA14, ARF7 and ARF19 | A. thaliana | adventitious root formation | [29] | |
| CRY1 and CRY2 | - | blue light (BL) | A. thaliana | primary root elongation | [30] |
| CRY1 and CRY2 | - | white light | A. thaliana | primary root elongation | [34] |
| CRY1 and CRY2 | - | white light | Solanum lycopersicum L. | primary root elongation | [35] |
| CRY1 | - | BL | A. thaliana | lateral root formation | [36] |
| CRY1 | - | BL | Glycine max L. Merr. | root nodulation | [37] |
| PHOT1 | - | BL | A. thaliana | root phototropism | [40] |
| PHOT1 and PHOT2 | RPT2 and JAC1 | BL | A. thaliana | hypocotyl phototropism | [4] |
| UVR8 | - | low-fluence UV-B | A. thaliana | hypocotyl development | [44] |
| UVR8 | MYB73/MYB77 | UV-B-dependent manner | A. thaliana | lateral root development and hypocotyl elongation | [44] |
| PIF3 | - | white light | A. thaliana | primary root development | [46] |
| PIF4 | - | - | A. thaliana | primary root growth | [47] |
| PIF5 | - | BL | A. thaliana | hypocotyl elongation | [48] |
| PIF1 | HB1 | - | A. thaliana | hypocotyl elongation | [50] |
| PIF1, PIF2, PIF3, PIF4 and PIF5 | - | darkness | A. thaliana | adventitious root formation | [56] |
| COP1 | - | darkness | A. thaliana | primary root length | [55] |
| HY5 | HY5 and NRT2.1 | - | A. thaliana | root growth | [57] |
| HY5 | HY5 | white light | A. thaliana | root growth | [56] |
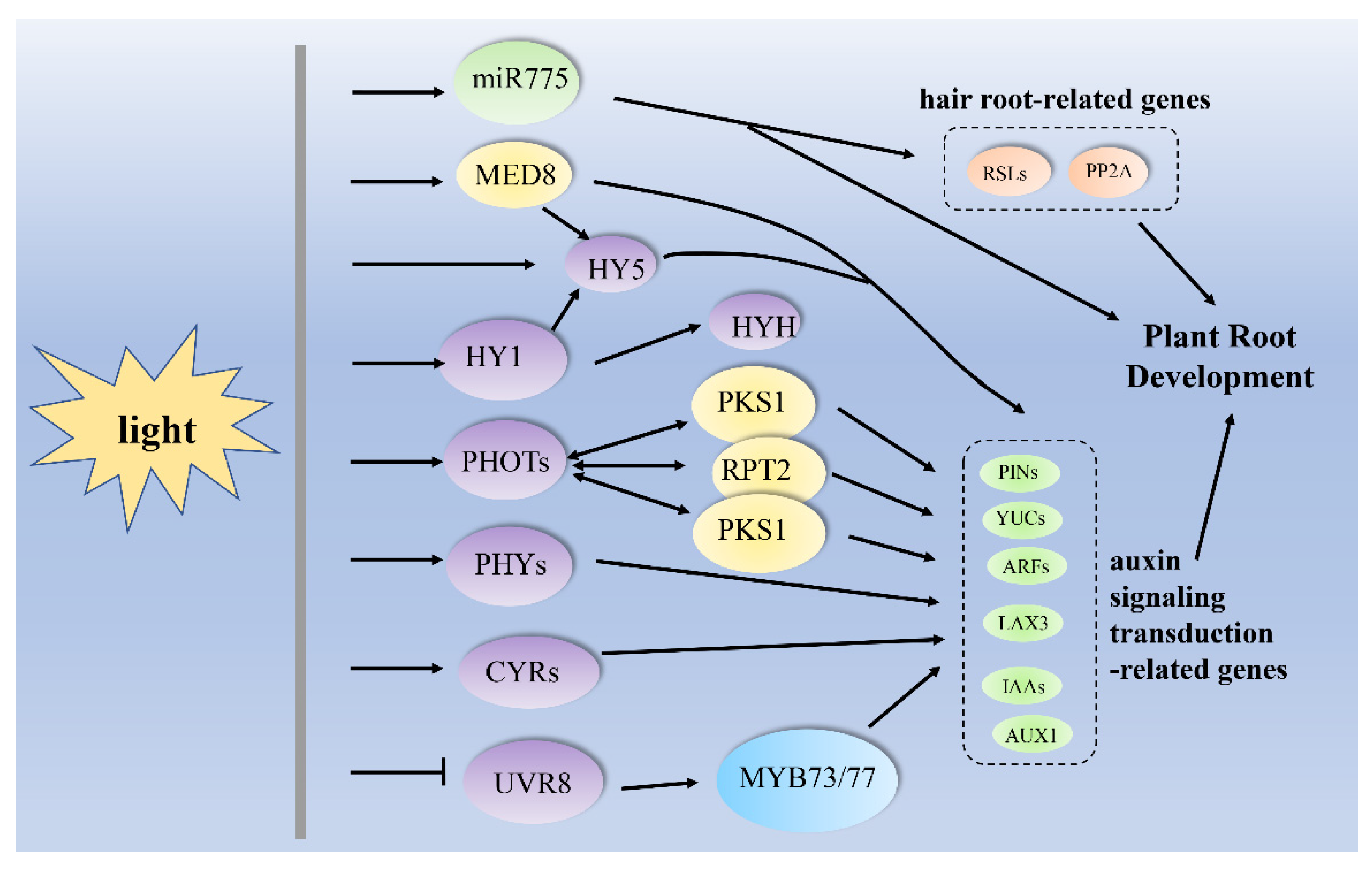
3. Light Regulates Root Growth and Development via the Auxin-Signaling Transduction Pathway
3.1. Primary Root, Root Hair and Growth and Development
3.2. Lateral Root and Adventitious Root Growth and Development
3.3. Rhizoid, Seminal and Crown Root Development
| Light Treatment | Genes/Proteins | Species | Function | References |
|---|---|---|---|---|
| darkness to light | miR775, PIN1, PIN2, AUXR1, YUC1 and YUC4, HY5 | Arabidopsis thaliana, (A. thaliana) | primary root growth | [58] |
| light | MED18 | A. thaliana | primary root elongation | [59] |
| direct light | PIN2 | A. thaliana | root hair formation | [60] |
| light | miR775, RSL2, RSL4, PP2A, HY5, PIN1, PIN2, AUXR1, YUC1 and YUC4 | A. thaliana | root hair formation | [58] |
| red light (RL) | PIN3 | A. thaliana | lateral root development | [62] |
| blue light (BL) | PIN1, PIN3 and PIN4 | A. thaliana | lateral root development | [62] |
| white light with FR light | - | A. thaliana | decreased lateral root density | [10] |
| far red (FR) light | HY5, ARF19, PIN3 and LAX3 | A. thaliana | lateral root development | [10] |
| UV-B | HAT2, SUAR23, MYB73/MYB77, UVR8, IAA29, SAUR28, SAUR68, SAUR-like and SAUR-like-3 | A. thaliana | lateral root growth | [16] |
| BL | PIN3, PHOT1 and PHOT2 | A. thaliana | adventitious root formation | [63] |
| - | CRYs | Physcomitrella patens | rhizoid development | [67] |
| white light | PHYA and PHYB | Oryza sativa L. | seminal root development | [68] |
| BL | CRY1, PHOT2, PIN3 | A. thaliana | root negative phototropism | [45] |
| BL | PHOT1, PIN1 and PIN2 | A. thaliana | root negative phototropism | [46] |
| BL | PIN2 | A. thaliana | root negative phototropism | [70] |
| white light | ZM2G141383 | Zea mays | root gravitropism | [71] |
| - | HY5, GLK2, IAA14, ARF7 and ARF19 | A. thaliana | root greening | [72] |
| - | HY1, HY5, HYH, AUX1, PIN1, PIN2, PIN3 and PIN7, | A. thaliana | lateral root branching | [73] |
| - | HY5, AXR2/IAA7 and SLR/IAA14 | A. thaliana | lateral root branching | [74] |
4. Light-Regulated Tropic Movement, Root Greening and Root Branching through Auxin Signaling
4.1. Root Negative Phototropism
4.2. Gravitropism/U-Turn Formation at Root Apex
4.3. Root Greening and Root Branching
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Sheng, Y. Phototropin2-mediated hypocotyl phototropism is negatively regulated by JAC1 and RPT2 in Arabidopsis. Plant Physiol. Biochem. 2021, 164, 289–298. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Arena, C.; Pesce, G. The role of light quality of photoperiodic lighting on photosynthesis, flowering and metabolic profiling in Ranunculus asiaticus L. Physiol. Plant 2020, 170, 187–201. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.; Wang, R.; Wang, L.; Zhang, C.; Xu, W.; Wang, S.; Jiu, S. The Role of Strigolactones in the Regulation of Root System Architecture in Grapevine (Vitis vinifera L.) in Response to Root-Restriction Cultivation. Int. J. Mol. Sci. 2021, 22, 8799. [Google Scholar] [CrossRef]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.; Fragoso, V.; Shin, K.; et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef] [PubMed]
- van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell. 2018, 30, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Chen, P.; Liang, T.; Li, X.; Liu, H. UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 2020, 39, e101928. [Google Scholar] [CrossRef] [PubMed]
- van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.; Xu, M.; Qi, W.; Shi, C.; Li, X.; Ma, J.; Tian, D.; Shou, J.; Wu, H.; et al. Transmembrane kinase 1-mediated auxin signal regulates membrane-associated clathrin in Arabidopsis roots. J. Integr. Plant Biol. 2023, 65, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Halliday, K.J.; Martínez-García, J.F.; Josse, E.M. Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H. Coordinated Shoot and Root Responses to Light Signaling in Arabidopsis. Plant Commun. 2020, 1, 100026. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Rai, N.; O’Hara, A.; Farkas, D.; Safronov, O.; Ratanasopa, K.; Wang, F.; Lindfors, A.V.; Jenkins, G.I.; Lehto, T.; Salojärvi, J.; et al. The photoreceptor UVR8 mediates the perception of both UV-B and UV-A wavelengths up to 350 nm of sunlight with responsivity moderated by cryptochromes. Plant Cell Environ. 2020, 43, 1513–1527. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Shin, D.H.; Cho, M.H.; Kim, T.L.; Yoo, J.; Kim, J.I.; Han, Y.J.; Song, P.S.; Jeon, J.S.; Bhoo, S.H.; Hahn, T.R. A small GTPase activator protein interacts with cytoplasmic phytochromes in regulating root development. J. Biol. Chem. 2010, 285, 32151–32159. [Google Scholar] [CrossRef]
- Schafer, E.; Bowle, C. Phytochrome-mediated photoperception and signal transduction in higher plants. EMBO Rep. 2002, 3, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.A. Phytochrome A: Functional diversity and polymorphism. Photochem. Photobiol. Sci. 2004, 3, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z.; Mullen, J.L.; Correll, M.J.; Hangarter, R.P. Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 2003, 131, 1411–1417. [Google Scholar] [CrossRef]
- Warnasooriya, S.N.; Montgomery, B.L. Spatial-specific regulation of root development by phytochromes in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef]
- Oh, Y.; Fragoso, V.; Guzzonato, F.; Kim, S.G.; Park, C.M.; Baldwin, I.T. Root-expressed phytochromes B1 and B2, but not PhyA and Cry2, regulate shoot growth in nature. Plant Cell Environ. 2018, 41, 2577–2588. [Google Scholar] [CrossRef]
- Shigeyama, T.; Tominaga, A.; Arima, S.; Sakai, T.; Inada, S.; Jikumaru, Y.; Kamiya, Y.; Uchiumi, T.; Abe, M.; Hashiguchi, M.; et al. Additional cause for reduced JA-Ile in the root of a Lotus japonicus phyB mutant. Plant Signal. Behav. 2012, 7, 746–748. [Google Scholar] [CrossRef]
- Gil, K.E.; Ha, J.H.; Park, C.M. Abscisic acid-mediated phytochrome B signaling promotes primary root growth in Arabidopsis. Plant Signal. Behav. 2018, 13, e1473684. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Wang, Y.L.; Zhong, Y.; Chao, Z.F.; Gao, Y.Q.; Han, M.L.; Xu, L.; Chao, D.Y. Phytochrome B inhibits darkness-induced hypocotyl adventitious root formation by stabilizing IAA14 and suppressing ARF7 and ARF19. Plant J. 2021, 105, 1689–1702. [Google Scholar] [CrossRef]
- Canamero, R.C.; Bakrim, N.; Bouly, J.P.; Garay, A.; Dudkin, E.E.; Habricot, Y.; Ahmad, M. Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 2006, 224, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida-Mayama, T.; Saka, T.; Hanada, A.; Uehara, Y.; Asami, T.; Yamaguchi, S. Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 2010, 62, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Sulli, M.; Zhang, L.; Aprea, G.; Jiménez-Gómez, J.M.; Bendahmane, A.; Perrotta, G.; Giuliano, G.; Facella, P. Pivotal Roles of Cryptochromes 1a and 2 in Tomato Development and Physiology. Plant Physiol. 2019, 179, 732–748. [Google Scholar] [CrossRef]
- Sakaguchi, J.; Matsushita, T.; Watanabe, Y. DWARF4 accumulation in root tips is enhanced via blue light perception by cryptochromes. Plant Cell Environ. 2019, 42, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, Q.; Lin, J.; Deng, K.; Zhao, X.; Tang, D.; Liu, X. Arabidopsis cryptochrome-1 restrains lateral roots growth by inhibiting auxin transport. J. Plant Physiol. 2010, 167, 670–673. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, R.; Lyu, X.; Chen, J.; Zhang, X.; Wang, Z.; Deng, Z.; Wang, Y.; Wang, H.; Li, R.; et al. Differential light-dependent regulation of soybean nodulation by papilionoid-specific HY5 homologs. Curr. Biol. 2022, 32, 783–795.e5. [Google Scholar] [CrossRef]
- Fankhauser, C.; Christie, J.M. Plant phototropic growth. Curr. Biol. 2015, 25, R384–R389. [Google Scholar] [CrossRef]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 2001, 98, 6969–6974. [Google Scholar] [CrossRef]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant flavoprotein photoreceptors. Plant Cell Physiol. 2015, 56, 401–413. [Google Scholar] [CrossRef]
- Lariguet, P.; Fankhauser, C. Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J. 2004, 40, 826–834. [Google Scholar] [CrossRef]
- Liang, T.; Yang, Y.; Liu, H. Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol. 2019, 221, 1247–1252. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Z. UV-B Response: When UVR8 Meets MYBs. Trends Plant Sci. 2020, 25, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Fasano, R.; Gonzalez, N.; Tosco, A.; Dal Piaz, F.; Docimo, T.; Serrano, R.; Grillo, S.; Leone, A.; Inzé, D. Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol. Plant 2014, 7, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mayba, O.; Pfeiffer, A.; Shi, H.; Tepperman, J.M.; Speed, T.P.; Quail, P.H. A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 2013, 9, e1003244. [Google Scholar] [CrossRef]
- Bai, S.; Yao, T.; Li, M.; Guo, X.; Zhang, Y.; Zhu, S.; He, Y. PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol. Plant 2014, 7, 616–625. [Google Scholar] [CrossRef]
- Liu, G.; Gao, S.; Tian, H.; Wu, W.; Robert, H.S.; Ding, Z. Local Transcriptional Control of YUCCA Regulates Auxin Promoted Root-Growth Inhibition in Response to Aluminium Stress in Arabidopsis. PLoS Genet. 2016, 12, e1006360. [Google Scholar] [CrossRef]
- Soy, J.; Leivar, P.; Monte, E. PIF1 promotes phytochrome-regulated growth under photoperiodic conditions in Arabidopsis together with PIF3, PIF4, and PIF5. J. Exp. Bot. 2014, 65, 2925–2936. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, A.; Yamashino, T.; Mizuno, T. PHYTOCHROME-INTERACTING FACTORS PIF4 and PIF5 are implicated in the regulation of hypocotyl elongation in response to blue light in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2010, 74, 2538–2541. [Google Scholar] [CrossRef] [PubMed]
- Capella, M.; Ribone, P.A.; Arce, A.L.; Chan, R.L. Arabidopsis thaliana HomeoBox 1 (AtHB1), a Homedomain-Leucine Zipper I (HD-Zip I) transcription factor, is regulated by PHYTOCHROME-INTERACTING FACTOR 1 to promote hypocotyl elongation. New Phytol. 2015, 207, 669–682. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhang, Z.; Zhang, C.X.; Wang, Y.L.; Liu, C.B.; Wu, J.C.; Han, M.L.; Wang, Q.X.; Chao, D.Y. Phytochrome-interacting factors orchestrate hypocotyl adventitious root initiation in Arabidopsis. Development 2022, 149, dev200362. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Liu, R.; Hao, H.; Wang, Z.; Bi, Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 2011, 168, 1771–1779. [Google Scholar] [CrossRef]
- Bours, R.; Kohlen, W.; Bouwmeester, H.J.; van der Krol, A. Thermoperiodic control of hypocotyl elongation depends on auxin-induced ethylene signaling that controls downstream PHYTOCHROME INTERACTING FACTOR3 activity. Plant Physiol. 2015, 167, 517–530. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Deng, X.W. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 2014, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Xu, H.; Shi, X.; Zhen, W.; Hu, Z.; Huang, J.; Zheng, Y.; Huang, P.; Zhang, K.X.; et al. HY5 Contributes to Light-Regulated Root System Architecture under a Root-Covered Culture System. Front. Plant Sci. 2019, 10, 1490. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.R.; Bhatia, C.; Sharma, A.; Badola, P.K.; Saxena, G.; Trivedi, P.K. miR775 integrates light, sucrose and auxin associated pathways to regulate root growth in Arabidopsis thaliana. Plant Sci. 2021, 313, 111073. [Google Scholar] [CrossRef]
- Raya-González, J.; Oropeza-Aburto, A.; López-Bucio, J.S.; Guevara-García, Á.A.; de Veylder, L.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR18 influences Arabidopsis root architecture, represses auxin signaling and is a critical factor for cell viability in root meristems. Plant J. 2018, 96, 895–909. [Google Scholar] [CrossRef]
- García-González, J.; Lacek, J.; Retzer, K. Dissecting Hierarchies between Light, Sugar and Auxin Action Underpinning Root and Root Hair Growth. Plants 2021, 10, 111. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. The plant hormone auxin beats the time for oscillating light-regulated lateral root induction. Development 2018, 145, dev169839. [Google Scholar] [CrossRef]
- Meng, L.; Wenjing, S.; Shangjun, L.; Jianxin, D.; Yali, Z.; Chengdong, W.; Xu, Y.; Wang, S. Light Quality Regulates Lateral Root Development in Tobacco Seedlings by Shifting Auxin Distributions. J. Plant Growth Regul. 2015, 34, 574–583. [Google Scholar] [CrossRef]
- Zhai, S.; Cai, W.; Xiang, Z.X.; Chen, C.Y.; Lu, Y.T.; Yuan, T.T. PIN3-mediated auxin transport contributes to blue light-induced adventitious root formation in Arabidopsis. Plant Sci. 2021, 312, 111044. [Google Scholar] [CrossRef] [PubMed]
- Fett-Neto, A.G.; Fett, J.P.; Veira Goulart, L.W.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464. [Google Scholar] [CrossRef]
- Jang, G.; Yi, K.; Pires, N.D.; Menand, B.; Dolan, L. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 2011, 138, 2273–2281. [Google Scholar] [CrossRef]
- Imaizumi, T.; Kadota, A.; Hasebe, M.; Wada, M. Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell. 2002, 14, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Nishiyama, T.; Sumikawa, N.; Kofuji, R.; Murata, T.; Hasebe, M. Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 2003, 130, 4835–4846. [Google Scholar] [CrossRef]
- Shimizu, H.; Tanabata, T.; Xie, X.; Inagaki, N.; Takano, M.; Shinomura, T.; Yamamoto, K.T. Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol. Plant 2009, 137, 289–297. [Google Scholar] [CrossRef]
- Wang, S.J.; Ho, C.H.; Chen, H.W. Rice develop wavy seminal roots in response to light stimulus. Plant Cell Rep. 2011, 30, 1747–1758. [Google Scholar] [CrossRef]
- Wan, Y.; Jasik, J.; Wang, L.; Hao, H.; Volkmann, D.; Menzel, D.; Mancuso, S.; Baluška, F.; Lin, J. The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell. 2012, 24, 551–565. [Google Scholar] [CrossRef]
- Suzuki, H.; Yokawa, K.; Nakano, S.; Yoshida, Y.; Fabrissin, I.; Okamoto, T.; Baluška, F.; Koshiba, T. Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex. J. Exp. Bot. 2016, 67, 4581–4591. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M.; Aro, E.M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell. 2012, 24, 1081–1095. [Google Scholar] [CrossRef]
- Duan, X.; Xu, S.; Xie, Y.; Li, L.; Qi, W.; Parizot, B.; Zhang, Y.; Chen, T.; Han, Y.; Van Breusegem, F.; et al. Periodic root branching is influenced by light through an HY1-HY5-auxin pathway. Curr. Biol. 2021, 31, 3834–3847.e5. [Google Scholar] [CrossRef]
- Cluis, C.P.; Mouchel, C.F.; Hardtke, C.S. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 2004, 38, 332–347. [Google Scholar] [CrossRef]
- Zhang, K.X.; Xu, H.H.; Yuan, T.T.; Zhang, L.; Lu, Y.T. Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 2013, 76, 308–321. [Google Scholar]
- Inada, S.; Ohgishi, M.; Mayama, T.; Okada, K.; Sakai, T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell. 2004, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Lariguet, P.; Schepens, I.; Hodgson, D.; Pedmale, U.V.; Trevisan, M.; Kami, C.; de Carbonnel, M.; Alonso, J.M.; Ecker, J.R.; Liscum, E.; et al. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 2006, 103, 10134–10139. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; De Simone, S.N.; Bergmann-Honsberger, A.; Schepens, I.; Fankhauser, C.; Casal, J.J. PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol. 2008, 146, 108–115. [Google Scholar] [CrossRef]
- Blakeslee, J.J.; Bandyopadhyay, A.; Peer, W.A.; Makam, S.N.; Murphy, A.S. Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiol. 2004, 134, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Xu, H.H.; Gong, W.; Jin, Y.; Shi, Y.Y.; Yuan, T.T.; Li, J.; Lu, Y.T. Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PLoS ONE 2014, 9, e85720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The Role of Light-Regulated Auxin Signaling in Root Development. Int. J. Mol. Sci. 2023, 24, 5253. https://doi.org/10.3390/ijms24065253
Yun F, Liu H, Deng Y, Hou X, Liao W. The Role of Light-Regulated Auxin Signaling in Root Development. International Journal of Molecular Sciences. 2023; 24(6):5253. https://doi.org/10.3390/ijms24065253
Chicago/Turabian StyleYun, Fahong, Huwei Liu, Yuzheng Deng, Xuemei Hou, and Weibiao Liao. 2023. "The Role of Light-Regulated Auxin Signaling in Root Development" International Journal of Molecular Sciences 24, no. 6: 5253. https://doi.org/10.3390/ijms24065253
APA StyleYun, F., Liu, H., Deng, Y., Hou, X., & Liao, W. (2023). The Role of Light-Regulated Auxin Signaling in Root Development. International Journal of Molecular Sciences, 24(6), 5253. https://doi.org/10.3390/ijms24065253








