Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches
Abstract
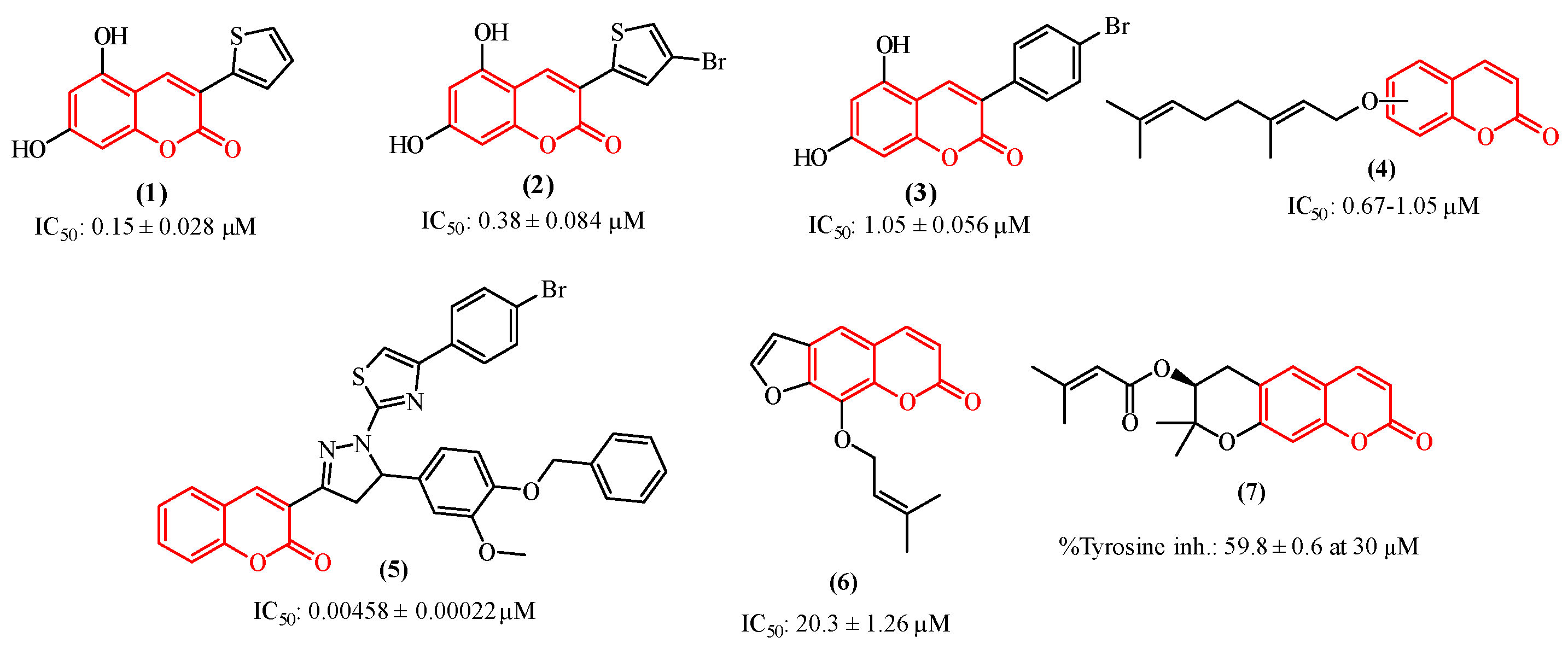
1. Introduction
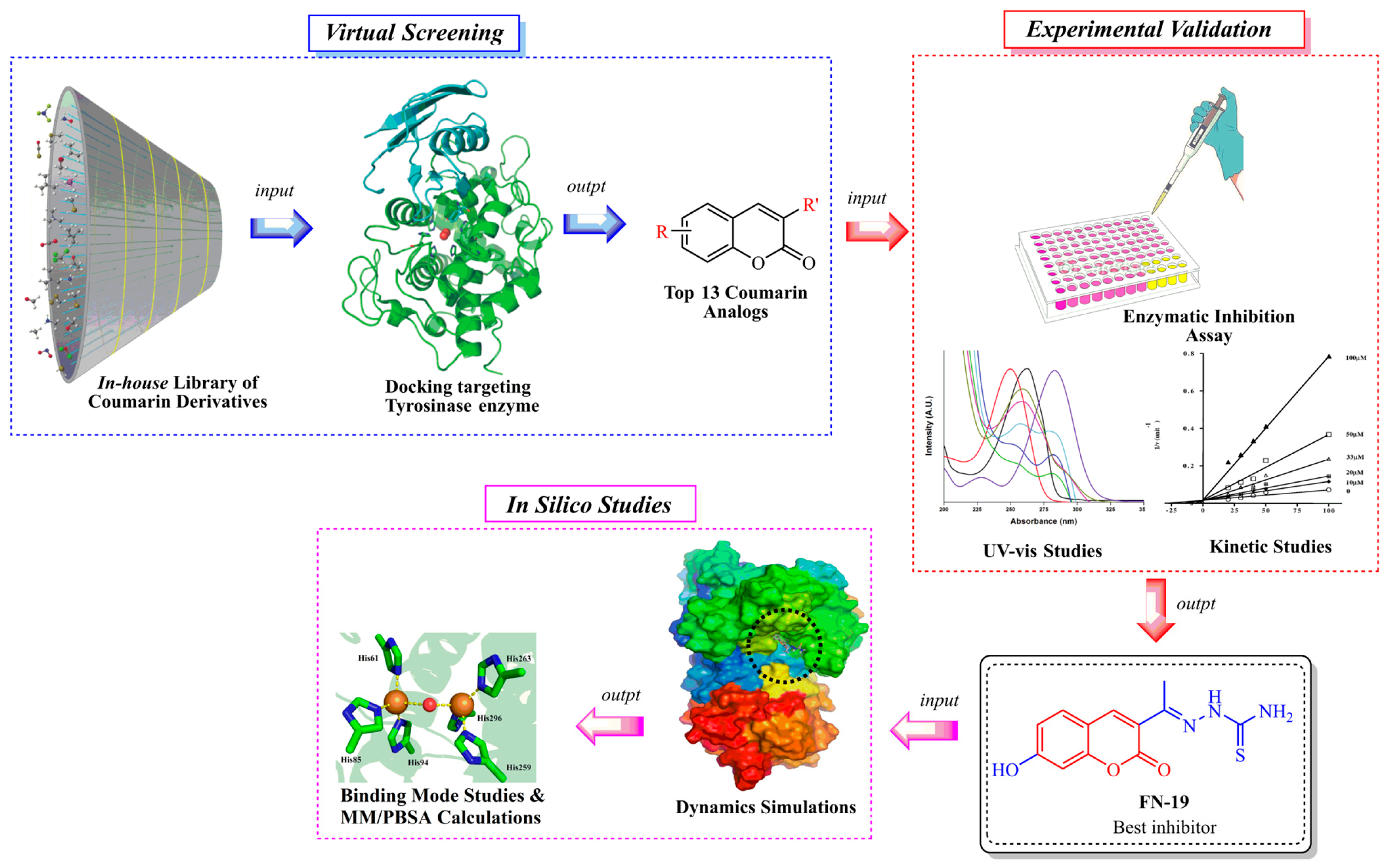
2. Results and Discussions
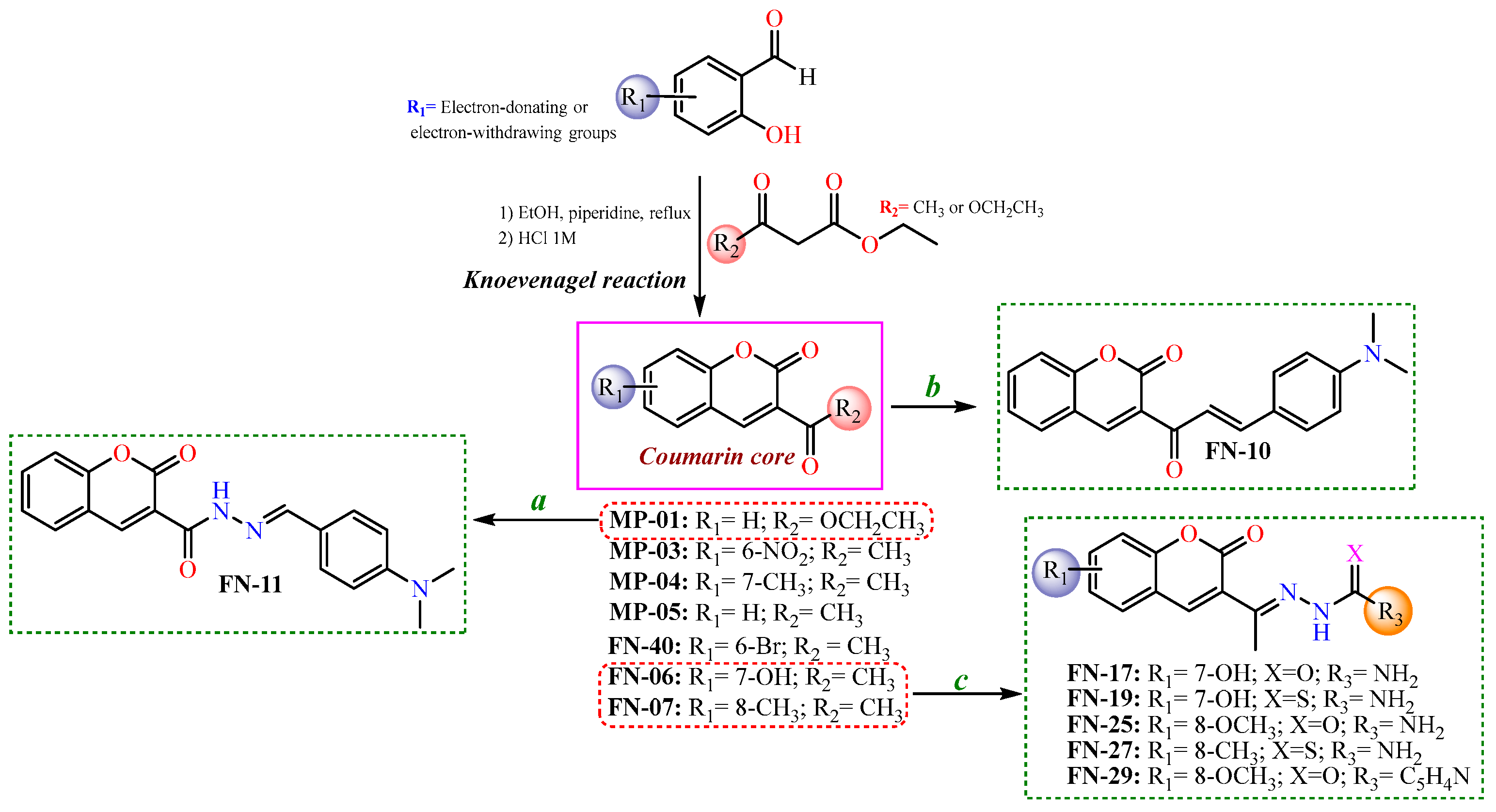
2.1. Chemistry
2.2. Mushroom Inhibition Assays
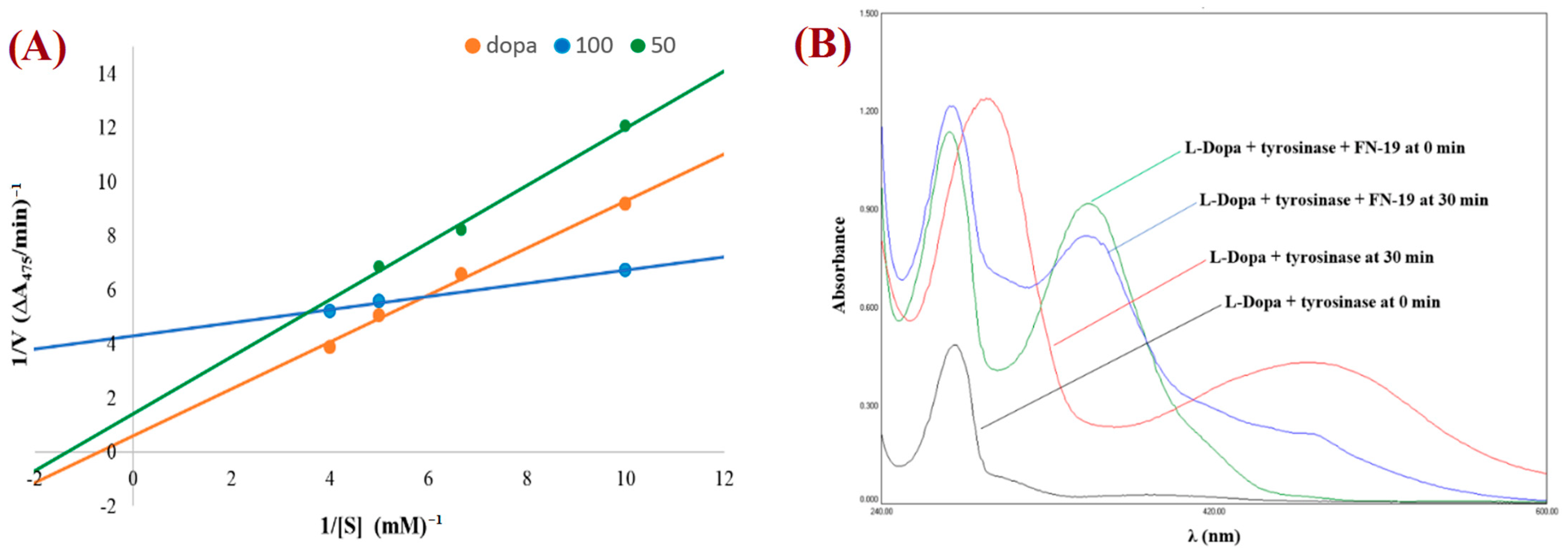
2.3. Kinetic Analysis of the Inhibition of Tyrosinase
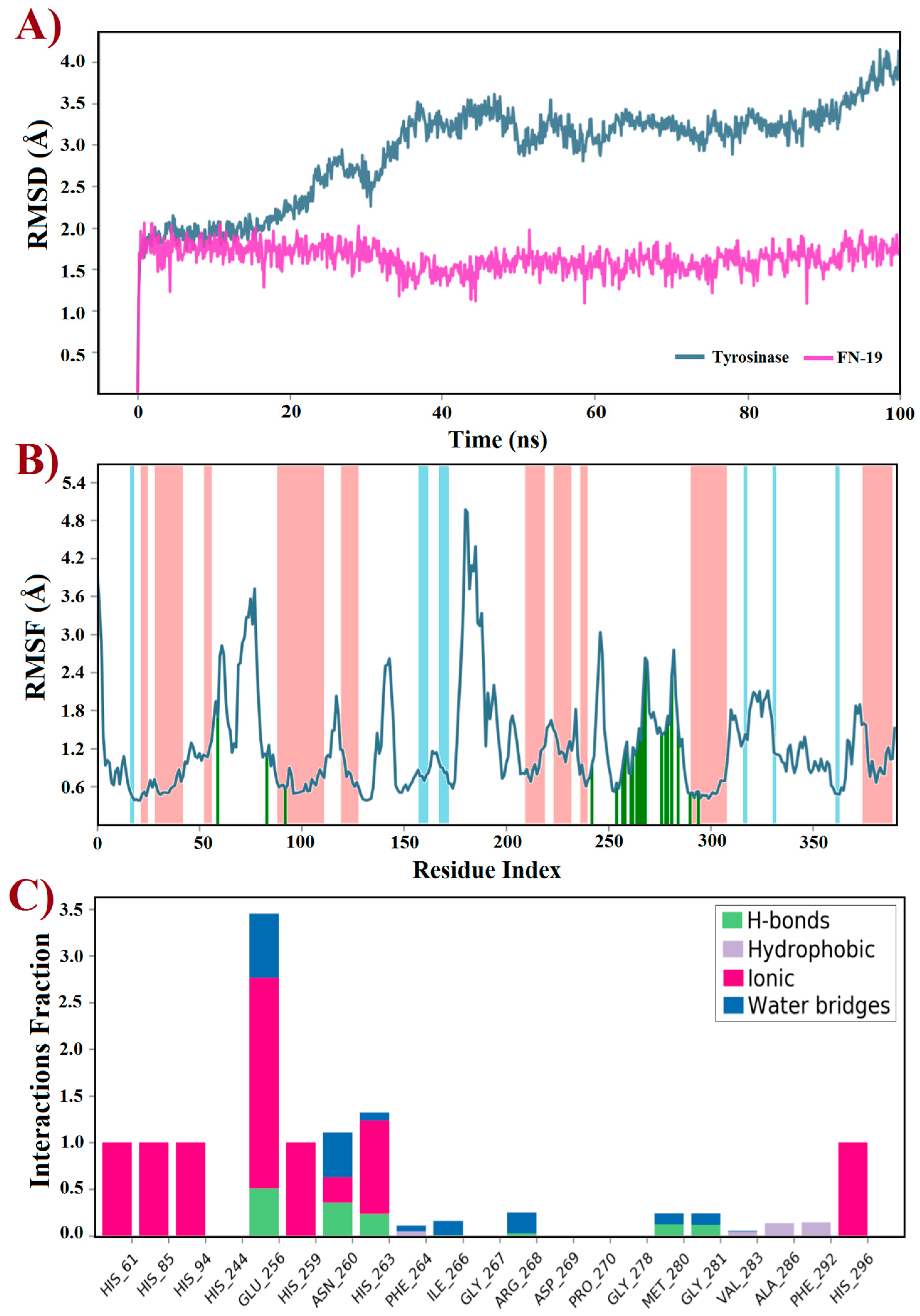
2.4. Molecular Dynamics (MD) Simulations and Docking
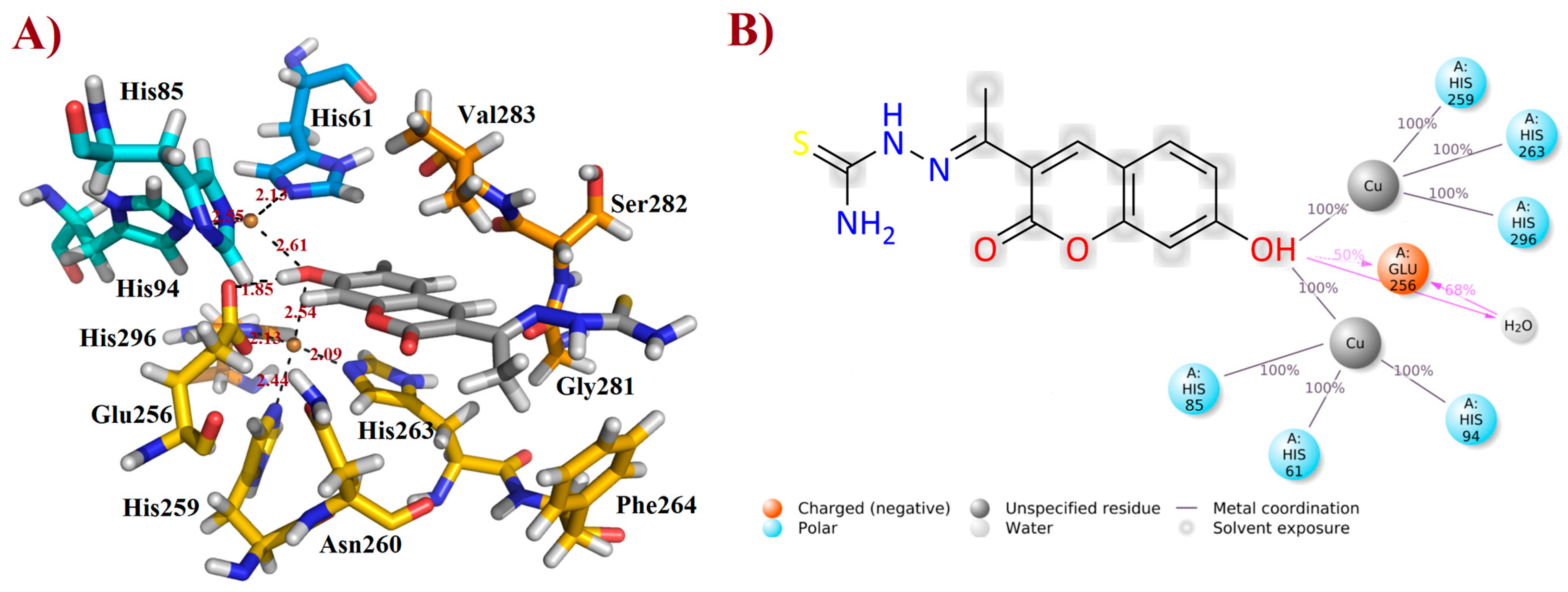
2.5. Binding Mode Analysis
2.6. Molecular Mechanics Poisson–Boltzmann Surface Area (MM/PBSA) Calculations
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis and Characterization of Coumarin-Based Analogs
3.1.2. General Procedure for the Synthesis of 3-Ketocoumarins and Ethyl-Coumarin-3-Carboxylate Intermediaries
- Description of 3. -Acetyl-7-hydroxy-2H-chromen-2-one (FN-06)
- Description of 3. -Acetyl-8-methoxy-2H-chromen-2-one (FN-07)
- Description of Ethyl 2-oxo-2H-chromene-3-carboxylate (MP-01)
- Description of 3. -Acetyl-6-nitro-2H-chromen-2-one (MP-03)
- Description of 3. -Acetyl-7-methyl-2H-chromen-2-one (MP-04)
- Description of 3. -Acetyl-2H-chromen-2-one (MP-05)
3.1.3. General Procedure for preparation of coumarin–chalcone (FN-10)
- Description of 3. -((E)-3-(4-(Dimethylamino)phenyl)acryloyl)-2H-chromen-2-one (FN-10)
3.1.4. General Procedure for Preparation of Coumarin–N-acylhydrazone (FN-11)
- Description of (E)-N’-(4-(dimethylamino)benzylidene)-2-methylene-2H-chromene-3-carbohydrazide (FN-11)
3.1.5. General Procedure for Synthesis of Semicarbazone–, Thiosemicarbazone–, and Isonicotinoylhydrazone–Coumarin Derivatives
- Description of (1E)-1-(1-(7-Hydroxy-2-oxo-2H-chromen-3-yl)ethylidene)semicarbazide (FN-17)
- Description of (1E)-1-(1-(7-Hydroxy-2-oxo-2H-chromen-3-yl)ethylidene)thiosemicarbazide (FN-19)
- Description of (1E)-1-(1-(8-Methoxy-2-oxo-2H-chromen-3-yl)ethylidene)semicarbazide (FN-25)
- Description of (1E)-1-(1-(8-Methoxy-2-oxo-2H-chromen-3-yl)ethylidene)thiosemicarbazide (FN-27)
- Description of (13E)-N’-(1-(8-Methoxy-2-oxo-2H-chromen-3-yl)ethylidene)isonicotinohydrazide (FN-29)
3.2. Mushroom Inhibition Assays
3.3. Kinetic Analysis of the Inhibition of Tyrosinase
3.4. Binding Analysis and Molecular Dynamics (MD) Simulations
3.5. Molecular Mechanics Poisson–Boltzmann Surface Area (MM/PBSA) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 December 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural Products: An Upcoming Therapeutic Approach to Cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.; Silbermann, M.; Amash, A.; Gincel, D.; Abdel-Sattar, E.; Sarikahya, N.B. Medicinal Plants and Natural Active Compounds for Cancer Chemoprevention/Chemotherapy. Evid.-Based Complement. Altern. Med. 2017, 2017, 7952417. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.; Shah, V.; Shah, K.; Shah, R.; Shah, M. State-of-the-Art Machine Learning Techniques for Melanoma Skin Cancer Detection and Classification: A Comprehensive Review. Intell. Med. 2022. [Google Scholar] [CrossRef]
- CDC. What Is Skin Cancer? Available online: https://www.cdc.gov/cancer/skin/basic_info/what-is-skin-cancer.htm (accessed on 11 September 2022).
- ACS. Key Statistics for Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 11 September 2022).
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol./Oncol. Clin. N. Am. 2021, 35, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many Ways to Resistance: How Melanoma Cells Evade Targeted Therapies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Liu, L.; Wang, F.; Ouyang, L.; Zhang, L.; Hu, X.; Wang, G. Recent Advances in the Design and Discovery of Synthetic Tyrosinase Inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U. Azole Inhibitors of Mushroom and Human Tyrosinases: Current Advances and Prospects of Drug Development for Melanogenic Dermatological Disorders. Eur. J. Med. Chem. 2022, 239, 114525. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus Bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to Identify Inhibitors of Melanin Biosynthesis via the Quality Control of Tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Kanteev, M.; Goldfeder, M.; Fishman, A. Structure-Function Correlations in Tyrosinases. Protein Sci. 2015, 24, 1360–1369. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-Based Specific Detection and Inhibition of Tyrosinase and Their Application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The Four Oxidation States of the Active Site and Their Relevance to Enzymatic Activation, Oxidation and Inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and Tyrosinase Inhibitory Properties of Some Novel Derivatives of Kojic Acid. Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar]
- Lim, J.T.E.; Frcpi; Fams. Treatment of Melasma Using Kojic Acid in a Gel Containing Hydroquinone and Glycolic Acid. Dermatol. Surg. 1999, 25, 282–284. [Google Scholar] [CrossRef]
- Brasil, E.M.; Canavieira, L.M.; Cardoso, É.T.C.; Silva, E.O.; Lameira, J.; Nascimento, J.L.M.; Eifler-Lima, V.L.; Macchi, B.M.; Sriram, D.; Bernhardt, P.V.; et al. Inhibition of Tyrosinase by 4 H-chromene Analogs: Synthesis, Kinetic Studies, and Computational Analysis. Chem. Biol. Drug Des. 2017, 90, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Silva, J.R.A.; Cardoso, D.T.C.; Silva, E.O.; Lameira, J.; Nascimento, J.L.M.D.; Brasil, D.D.S.B.; Alves, C.N. Combined Kinetic Studies and Computational Analysis on Kojic Acid Analogs as Tyrosinase Inhibitors. Molecules 2014, 19, 9591–9605. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Araújo, R.S.A.; Mendonça-Junior, F.J. Coumarins: Synthetic Approaches and Pharmacological Importance. In Natural Products and Drug Discovery: From Pharmacochemistry to Pharmacological Approaches; Diniz, M.d.F.F.M., Scotti, L., Scotti, M.T., Alves, M.F., Eds.; UFPB: João Pessoa, Brazil, 2018. [Google Scholar]
- Mahmoodi, N.O.; Jalalifard, Z.; Fathanbari, G.P. Green Synthesis of Bis-coumarin Derivatives Using Fe(SD) 3 as a Catalyst and Investigation of Their Biological Activities. J. Chin. Chem. Soc. 2020, 67, 172–182. [Google Scholar] [CrossRef]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Liu, S.; Sun, Y.; Zhang, S.; Wang, L. Design, Synthesis and Evaluation of 3-Substituted Coumarin Derivatives as Anti-Inflammatory Agents. Chem. Pharm. Bull. 2020, 68, 443–446. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Ibraheem, H.H.; Jassim, L.S.; Al-Amiery, A.A. Antioxidant Activity of Coumarine Compounds. Al-Nahrain J. Sci. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Liang, C.-G.; Sun, Y.-Q.; Teng, P.; Wang, J.-Q.; Zhang, W.-H. Design, Synthesis and Antifungal Activities of Novel Pyrrole- and Pyrazole-Substituted Coumarin Derivatives. Mol. Divers. 2019, 23, 915–925. [Google Scholar] [CrossRef]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological Properties of Coumarins. Mini-Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-Containing Hybrids and Their Anticancer Activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin Derivatives as Anticancer Agents for Lung Cancer Therapy: A Review. Anti-Cancer Agents Med. Chem. 2018, 18, 964–984. [Google Scholar] [CrossRef]
- de Araújo, R.S.A.; Carmo, J.D.O.D.S.; Silva, S.L.d.O.; da Silva, C.R.A.C.; Souza, T.P.M.; de Mélo, N.B.; Bourguignon, J.-J.; Schmitt, M.; de Aquino, T.M.; Rodarte, R.S.; et al. Coumarin Derivatives Exert Anti-Lung Cancer Activity by Inhibition of Epithelial–Mesenchymal Transition and Migration in A549 Cells. Pharmaceuticals 2022, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Dimić, D.S.; Kaluđerović, G.N.; Avdović, E.H.; Milenković, D.A.; Živanović, M.N.; Potočňák, I.; Samoľová, E.; Dimitrijević, M.S.; Saso, L.; Marković, Z.S.; et al. Synthesis, Crystallographic, Quantum Chemical, Antitumor, and Molecular Docking/Dynamic Studies of 4-Hydroxycoumarin-Neurotransmitter Derivatives. Int. J. Mol. Sci. 2022, 23, 1001. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, S.-Y.; Hyun, C.-G. 8-Methoxycoumarin Enhances Melanogenesis via the MAPKase Signaling Pathway. Pharmazie 2019, 74, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Cha, S.-B.; Park, M.-C.; Park, S.-A.; Kim, H.-S.; Woo, W.-H.; Mun, Y.-J. Scopoletin Stimulates Melanogenesis via CAMP/PKA Pathway and Partially P38 Activation. Biol. Pharm. Bull. 2017, 40, 2068–2074. [Google Scholar] [CrossRef]
- Pintus, F.; Matos, M.J.; Vilar, S.; Hripcsak, G.; Varela, C.; Uriarte, E.; Santana, L.; Borges, F.; Medda, R.; Di Petrillo, A.; et al. New Insights into Highly Potent Tyrosinase Inhibitors Based on 3-Heteroarylcoumarins: Anti-Melanogenesis and Antioxidant Activities, and Computational Molecular Modeling Studies. Bioorg. Med. Chem. 2017, 25, 1687–1695. [Google Scholar] [CrossRef]
- Pintus, F.; Floris, S.; Fais, A.; Era, B.; Kumar, A.; Gatto, G.; Uriarte, E.; Matos, M.J. Hydroxy-3-Phenylcoumarins as Multitarget Compounds for Skin Aging Diseases: Synthesis, Molecular Docking and Tyrosinase, Elastase, Collagenase and Hyaluronidase Inhibition, and Sun Protection Factor. Molecules 2022, 27, 6914. [Google Scholar] [CrossRef]
- Matos, M.J.; Varela, C.; Vilar, S.; Hripcsak, G.; Borges, F.; Santana, L.; Uriarte, E.; Fais, A.; Di Petrillo, A.; Pintus, F.; et al. Design and Discovery of Tyrosinase Inhibitors Based on a Coumarin Scaffold. RSC Adv. 2015, 5, 94227–94235. [Google Scholar] [CrossRef]
- Roh, E.-J. Inhibitory Effects of Coumarin Derivatives on Tyrosinase. Molecules 2021, 26, 2346. [Google Scholar] [CrossRef]
- Saeed, A.; Mahesar, P.A.; Channar, P.A.; Abbas, Q.; Larik, F.A.; Hassan, M.; Raza, H.; Seo, S.-Y. Synthesis, Molecular Docking Studies of Coumarinyl-Pyrazolinyl Substituted Thiazoles as Non-Competitive Inhibitors of Mushroom Tyrosinase. Bioorg. Chem. 2017, 74, 187–196. [Google Scholar] [CrossRef]
- Pynam, H.; Dharmesh, S.M. Antioxidant and Anti-Inflammatory Properties of Marmelosin from Bael (Aegle Marmelos L.); Inhibition of TNF-α Mediated Inflammatory/Tumor Markers. Biomed. Pharmacother. 2018, 106, 98–108. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.-H.; Youn, K.; Jun, M. Decursin Prevents Melanogenesis by Suppressing MITF Expression through the Regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β Cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Cardoso, S.H.; Barreto, M.B.; Lourenço, M.C.S.; Henriques, M.d.G.M.d.O.; Candéa, A.L.P.; Kaiser, C.R.; de Souza, M.V.N. Antitubercular Activity of New Coumarins. Chem. Biol. Drug Des. 2011, 77, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Hałdys, K.; Latajka, R. Thiosemicarbazones with Tyrosinase Inhibitory Activity. Medchemcomm 2019, 10, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Cabezudo, I.; Ayelen Ramallo, I.; Alonso, V.L.; Furlan, R.L.E. Effect Directed Synthesis of a New Tyrosinase Inhibitor with Anti-Browning Activity. Food Chem. 2021, 341, 128232. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.A.; Almeida, M.A.; Marins-Goulart, C.; Chaves, O.A.; Echevarria, A.; de Oliveira, M.C.C. Thiosemicarbazones as Inhibitors of Tyrosinase Enzyme. Bioorg. Med. Chem. Lett. 2017, 27, 3546–3550. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, G.; Zeng, Q.-H.; Li, Y.; Wu, Y.; Liu, H.; Wang, J.J.; Zhao, Y. Synthesis, Antioxidant and Anti-Tyrosinase Activity of 1,2,4-Triazole Hydrazones as Antibrowning Agents. Food Chem. 2021, 341, 128265. [Google Scholar] [CrossRef]
- Ghani, U. Carbazole and Hydrazone Derivatives as New Competitive Inhibitors of Tyrosinase: Experimental Clues to Binuclear Copper Active Site Binding. Bioorg. Chem. 2019, 83, 235–241. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Sakeh, N.M.; Zareen, S.; Gul, S.; Lo, K.M.; Ul-Haq, Z.; Shah, S.A.A.; Ahmad, S. Design and Synthesis of Chalcone Derivatives as Potent Tyrosinase Inhibitors and Their Structural Activity Relationship. J. Mol. Struct. 2015, 1085, 97–103. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Serra, S.; Corda, M.; Fadda, M.B.; Era, B.; Fais, A. Tyrosine-like Condensed Derivatives as Tyrosinase Inhibitors. J. Pharm. Pharmacol. 2012, 64, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Majidi, K.; Bassam, K.; Abdi, M.; Daneshvar, M.; Moayedi, S.S.; Pourhesabi, S.; Attarroshan, M.; Boumi, S.; Kabiri, M.; et al. Synthesis, Biological Evaluation, and Molecular Docking Analysis of Novel 1, 3, 4-Thiadiazole -Based Kojic Acid Derivatives as Tyrosinase Inhibitors. J. Mol. Struct. 2022, 1268, 133707. [Google Scholar] [CrossRef]
- Mahdavi, A.; Mohammadsadeghi, N.; Mohammadi, F.; Saadati, F.; Nikfard, S. Evaluation of Inhibitory Effects of Some Novel Phenolic Derivatives on the Mushroom Tyrosinase Activity: Insights from Spectroscopic Analyses, Molecular Docking and in Vitro Assays. Food Chem. 2022, 387, 132938. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fan, L.; Ding, Z. The Inhibition Mechanisms between Asparagus Polyphenols after Hydrothermal Treatment and Tyrosinase: A Circular Dichroism Spectrum, Fluorescence, and Molecular Docking Study. Food Biosci. 2022, 48, 101790. [Google Scholar] [CrossRef]
- Alsantali, R.I.; Mughal, E.U.; Naeem, N.; Alsharif, M.A.; Sadiq, A.; Ali, A.; Jassas, R.S.; Javed, Q.; Javid, A.; Sumrra, S.H.; et al. Flavone-Based Hydrazones as New Tyrosinase Inhibitors: Synthetic Imines with Emerging Biological Potential, SAR, Molecular Docking and Drug-Likeness Studies. J. Mol. Struct. 2022, 1251, 131933. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Peng, X.; Li, S.; Liu, B.; Chu, C. Recent Discovery of Tyrosinase Inhibitors in Traditional Chinese Medicines and Screening Methods. J. Ethnopharmacol. 2023, 303, 115951. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular Docking. In Molecular Modeling of Proteins; Kukol, A., Ed.; Methods Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 365–382. [Google Scholar]
- Tiwari, A.; Singh, S. Computational Approaches in Drug Designing. In Bioinformatics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–217. [Google Scholar]
- Kumar, D.; Meena, M.K.; Kumari, K.; Kumar, R.V.; Bahadur, I.; Jain, P.; Singh, P. Exploring the Effect of Temperature on Inhibition of Non-Structural Protease 3 of Chikungunya Virus Using Molecular Dynamics Simulations and Thermodynamics Parameters. J. Mol. Liq. 2021, 335, 116164. [Google Scholar] [CrossRef]
- Kahn, V.; Andrawis, A. Inhibition of Mushroom Tyrosinase by Tropolone. Phytochemistry 1985, 24, 905–908. [Google Scholar] [CrossRef]
- Espín, J.C.; Wichers, H.J. Slow-Binding Inhibition of Mushroom (Agaricus bisporus) Tyrosinase Isoforms by Tropolone. J. Agric. Food Chem. 1999, 47, 2638–2644. [Google Scholar] [CrossRef]
- Kahn, V. Tropolone—A Compound That Can Aid in Differentiating between Tyrosinase and Peroxidase. Phytochemistry 1985, 24, 915–920. [Google Scholar] [CrossRef]
- Gusakov, E.A.; Topchu, I.A.; Mazitova, A.M.; Dorogan, I.V.; Bulatov, E.R.; Serebriiskii, I.G.; Abramova, Z.I.; Tupaeva, I.O.; Demidov, O.P.; Toan, D.N.; et al. Design, Synthesis and Biological Evaluation of 2-Quinolyl-1,3-Tropolone Derivatives as New Anti-Cancer Agents. RSC Adv. 2021, 11, 4555–4571. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson−Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Gilson, M.K.; Honig, B. Calculation of the Total Electrostatic Energy of a Macromolecular System: Solvation Energies, Binding Energies, and Conformational Analysis. Proteins Struct. Funct. Bioinform. 1988, 4, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.C.; Aynechi, T.; Case, D.A.; Kuntz, I.D. Estimation of Absolute Free Energies of Hydration Using Continuum Methods: Accuracy of Partial Charge Models and Optimization of Nonpolar Contributions. J. Chem. Theory Comput. 2006, 2, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate Calculation of Hydration Free Energies Using Macroscopic Solvent Models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Still, W.C.; Tempczyk, A.; Hawley, R.C.; Hendrickson, T. Semianalytical Treatment of Solvation for Molecular Mechanics and Dynamics. J. Am. Chem. Soc. 1990, 112, 6127–6129. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sharma, M.; Dalal, V.; Singh, V.; Tomar, S.; Kumar, P. Multifunctional Inhibitors of SARS-CoV-2 by MM/PBSA, Essential Dynamics, and Molecular Dynamic Investigations. J. Mol. Graph. Model. 2021, 107, 107969. [Google Scholar] [CrossRef]
- Sharma, M.; Mahto, J.K.; Dhaka, P.; Neetu, N.; Tomar, S.; Kumar, P. MD Simulation and MM/PBSA Identifies Phytochemicals as Bifunctional Inhibitors of SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 40, 12048–12061. [Google Scholar] [CrossRef] [PubMed]
- Vilela, G.G.; Silva, W.F.d.S.; Batista, V.d.M.; Silva, L.R.; Maus, H.; Hammerschmidt, S.J.; Costa, C.A.C.B.; Moura, O.F.d.S.; de Freitas, J.D.; Coelho, G.L.; et al. Fragment-Based Design of α-Cyanoacrylates and α-Cyanoacrylamides Targeting Dengue and Zika NS2B/NS3 Proteases. New J. Chem. 2022, 46, 20322–20346. [Google Scholar] [CrossRef]
- De Brito, W.A.; Dantas, M.G.; Nogueira, F.H.A.; Da Silva-Júnior, E.F.; De Araújo-Júnior, J.X.; De Aquino, T.M.; Ribeiro, A.N.; Solon, L.G.D.S.; Aragão, C.F.S.; Gomes, A.P.B. Development and Validation of HPLC-DAD and UHPLC-DAD Methods for the Simultaneous Determination of Guanylhydrazone Derivatives Employing a Factorial Design. Molecules 2017, 22, 1394. [Google Scholar] [CrossRef]
- Passos, G.F.S.; Gomes, M.G.M.; de Aquino, T.M.; de Araújo-Júnior, J.X.; de Souza, S.J.M.; Cavalcante, J.P.M.; dos Santos, E.C.; Bassi, Ê.J.; da Silva-Júnior, E.F. Computer-Aided Design, Synthesis, and Antiviral Evaluation of Novel Acrylamides as Potential Inhibitors of E3-E2-E1 Glycoproteins Complex from Chikungunya Virus. Pharmaceuticals 2020, 13, 141. [Google Scholar] [CrossRef]
- Yakoub, K.; Jung, S.; Sattler, C.; Damerow, H.; Weber, J.; Kretzschmann, A.; Cankaya, A.S.; Piel, M.; Rösch, F.; Haugaard, A.S.; et al. Structure–Function Evaluation of Imidazopyridine Derivatives Selective for δ-Subunit-Containing γ-Aminobutyric Acid Type A (GABAA) Receptors. J. Med. Chem. 2018, 61, 1951–1968. [Google Scholar] [CrossRef]
- Jacobsen, N.E. (Ed.) NMR Data Interpretation Explained-Understanding 1D and 2D NMR Spectra of Organic Compounds and Natural Products, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; ISBN 9781118370223. [Google Scholar]
- Silva-Júnior, E.F.; Silva, E.P.S.; França, P.H.B.; Silva, J.P.N.; Barreto, E.O.; Silva, E.B.; Ferreira, R.S.; Gatto, C.C.; Moreira, D.R.M.; Siqueira-Neto, J.L.; et al. Design, Synthesis, Molecular Docking and Biological Evaluation of Thiophen-2-Iminothiazolidine Derivatives for Use against Trypanosoma Cruzi. Bioorg. Med. Chem. 2016, 24, 4228–4240. [Google Scholar] [CrossRef]
- Silva, L.R.; Guimarães, A.S.; do Nascimento, J.; do Santos Nascimento, I.J.; da Silva, E.B.; McKerrow, J.H.; Cardoso, S.H.; da Silva-Júnior, E.F. Computer-Aided Design of 1,4-Naphthoquinone-Based Inhibitors Targeting Cruzain and Rhodesain Cysteine Proteases. Bioorg. Med. Chem. 2021, 41, 116213. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds—Tables of Spectral Data, 4th ed.; Pretsch, E., Bühlmann, P., Badertscher, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]
- Santos-Junior, P.F.d.S.; Nascimento, I.J.d.S.; da Silva, E.C.D.; Monteiro, K.L.C.; de Freitas, J.D.; de Lima Lins, S.; Maciel, T.M.S.; Cavalcanti, B.C.; Neto, J.d.B.V.; de Abreu, F.C.; et al. Synthesis of Hybrids Thiazole–Quinoline, Thiazole–Indole and Their Analogs: In Vitro Anti-Proliferative Effects on Cancer Cell Lines, DNA Binding Properties and Molecular Modeling. New J. Chem. 2021, 45, 13847–13859. [Google Scholar] [CrossRef]
- He, X.; Shang, Y.; Zhou, Y.; Yu, Z.; Han, G.; Jin, W.; Chen, J. Synthesis of Coumarin-3-Carboxylic Esters via FeCl3-Catalyzed Multicomponent Reaction of Salicylaldehydes, Meldrum’s Acid and Alcohols. Tetrahedron 2015, 71, 863–868. [Google Scholar] [CrossRef]
- Jagtap, A.R.; Satam, V.S.; Rajule, R.N.; Kanetkar, V.R. The Synthesis and Characterization of Novel Coumarin Dyes Derived from 1,4-Diethyl-1,2,3,4-Tetrahydro-7-Hydroxyquinoxalin-6-Carboxaldehyde. Dye. Pigment. 2009, 82, 84–89. [Google Scholar] [CrossRef]
- Helmy, M.M.; Moustafa, M.H.; Eldeab, H.A. Microwave-Assisted Synthesis of New Series Some Acetyl Coumarin Derivatives and Studying of Some Their Pharmacological Activities. J. Pharm. Sci. Res. 2015, 7, 83–88. [Google Scholar]
- Rosada, B.; Bekier, A.; Cytarska, J.; Płaziński, W.; Zavyalova, O.; Sikora, A.; Dzitko, K.; Łączkowski, K.Z. Benzo[b]Thiophene-Thiazoles as Potent Anti-Toxoplasma Gondii Agents: Design, Synthesis, Tyrosinase/Tyrosine Hydroxylase Inhibitors, Molecular Docking Study, and Antioxidant Activity. Eur. J. Med. Chem. 2019, 184, 111765. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-H.; Ryu, S.Y.; Choi, E.J.; Kang, S.-H.; Chang, I.-M.; Min, K.R.; Kim, Y. Oxyresveratrol as the Potent Inhibitor on Dopa Oxidase Activity of Mushroom Tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-Łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-Derived Alkaloids as Potent Anticancer Agents: Synthesis, Tyrosinase Inhibition, Mechanism of Action, DFT Calculation, and Molecular Docking Studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A. Molecular Docking Using ArgusLab: An Efficient Shape-Based Search Algorithm and an Enhanced XScore Scoring Function. In Proceedings of the The 228th ACS National Meeting, Philadelphia, PA, USA, 22–26 August 2004. [Google Scholar]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL 0.99rc6 2006; DeLano Scientific LLC: Palo Alto, CA, USA, 2006. [Google Scholar]
- Lozano Untiveros, K.; da Silva, E.G.; de Abreu, F.C.; da Silva-Júnior, E.F.; de Araújo-Junior, J.X.; Mendoça de Aquino, T.; Armas, S.M.; de Moura, R.O.; Mendonça-Junior, F.J.B.; Serafim, V.L.; et al. An Electrochemical Biosensor Based on Hairpin-DNA Modified Gold Electrode for Detection of DNA Damage by a Hybrid Cancer Drug Intercalation. Biosens. Bioelectron. 2019, 133, 160–168. [Google Scholar] [CrossRef]
- Santana, C.C.; Silva-Júnior, E.F.; Santos, J.C.N.; Rodrigues, É.E.d.S.; da Silva, I.M.; Araújo-Júnior, J.X.; do Nascimento, T.G.; Oliveira Barbosa, L.A.; Dornelas, C.B.; Figueiredo, I.M.; et al. Evaluation of Guanylhydrazone Derivatives as Inhibitors of Candida Rugosa Digestive Lipase: Biological, Biophysical, Theoretical Studies and Biotechnological Application. Bioorg. Chem. 2019, 87, 169–180. [Google Scholar] [CrossRef]
- Roque Marques, K.M.; do Desterro, M.R.; de Arruda, S.M.; de Araújo Neto, L.N.; do Carmo Alves de Lima, M.; de Almeida, S.M.V.; da Silva, E.C.D.; de Aquino, T.M.; da Silva-Júnior, E.F.; de Araújo-Júnior, J.X.; et al. 5-Nitro-Thiophene-Thiosemicarbazone Derivatives Present Antitumor Activity Mediated by Apoptosis and DNA Intercalation. Curr. Top. Med. Chem. 2019, 19, 1075–1091. [Google Scholar] [CrossRef]
- Marques, R.A.; Gomes, A.O.C.V.; de Brito, M.V.; dos Santos, A.L.P.; da Silva, G.S.; de Lima, L.B.; Nunes, F.M.; de Mattos, M.C.; de Oliveira, F.C.E.; do Ó Pessoa, C.; et al. Annonalide and Derivatives: Semisynthesis, Cytotoxic Activities and Studies on Interaction of Annonalide with DNA. J. Photochem. Photobiol. B Biol. 2018, 179, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.d.m.; Macedo, T.S.; Teixeira, H.M.P.; Moreira, D.R.M.; Soares, M.B.P.; Pereira, A.L.d.C.; Serafim, V.d.L.; Mendonça-Júnior, F.J.B.; de Lima, M.d.C.A.; de Moura, R.O.; et al. Correlation between DNA/HSA-Interactions and Antimalarial Activity of Acridine Derivatives: Proposing a Possible Mechanism of Action. J. Photochem. Photobiol. B Biol. 2018, 189, 165–175. [Google Scholar] [CrossRef]
- da Silva-Junior, E.F.; Barcellos Franca, P.H.; Ribeiro, F.F.; Mendonca-Junior, F.J.B.; Scotti, L.; Scotti, M.T.; de Aquino, T.M.; de Araujo-Junior, J.X. Molecular Docking Studies Applied to a Dataset of Cruzain Inhibitors. Curr. Comput.-Aided. Drug Des. 2018, 14, 68–78. [Google Scholar] [CrossRef]
- da Silva-Junior, E.F.; Barcellos Franca, P.H.; Quintans-Junior, L.J.; Mendonca-Junior, F.J.B.; Scotti, L.; Scotti, M.T.; de Aquino, T.M.; de Araujo-Junior, J.X. Dynamic Simulation, Docking and DFT Studies Applied to a Set of Anti-Acetylcholinesterase Inhibitors in the Enzyme β-Secretase (BACE-1): An Important Therapeutic Target in Alzheimer’s Disease. Curr. Comput.-Aided. Drug Des. 2017, 13, 266–274. [Google Scholar] [CrossRef]
- Braga, T.C.; Silva, T.F.; Maciel, T.M.S.; da Silva, E.C.D.; da Silva-Júnior, E.F.; Modolo, L.V.; Figueiredo, I.M.; Santos, J.C.C.; de Aquino, T.M.; de Fátima, Â. Ionic Liquid-Assisted Synthesis of Dihydropyrimidin(Thi)One Biginelli Adducts and Investigation of Their Mechanism of Urease Inhibition. New J. Chem. 2019, 43, 15187–15200. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of Multiple Amber Force Fields and Development of Improved Protein Backbone Parameters. Proteins Struct. Funct. Bioinform. 2006, 65, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber Ff99SB Protein Force Field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
| Compound | IC50 [µM] | ±SD |
|---|---|---|
FN-06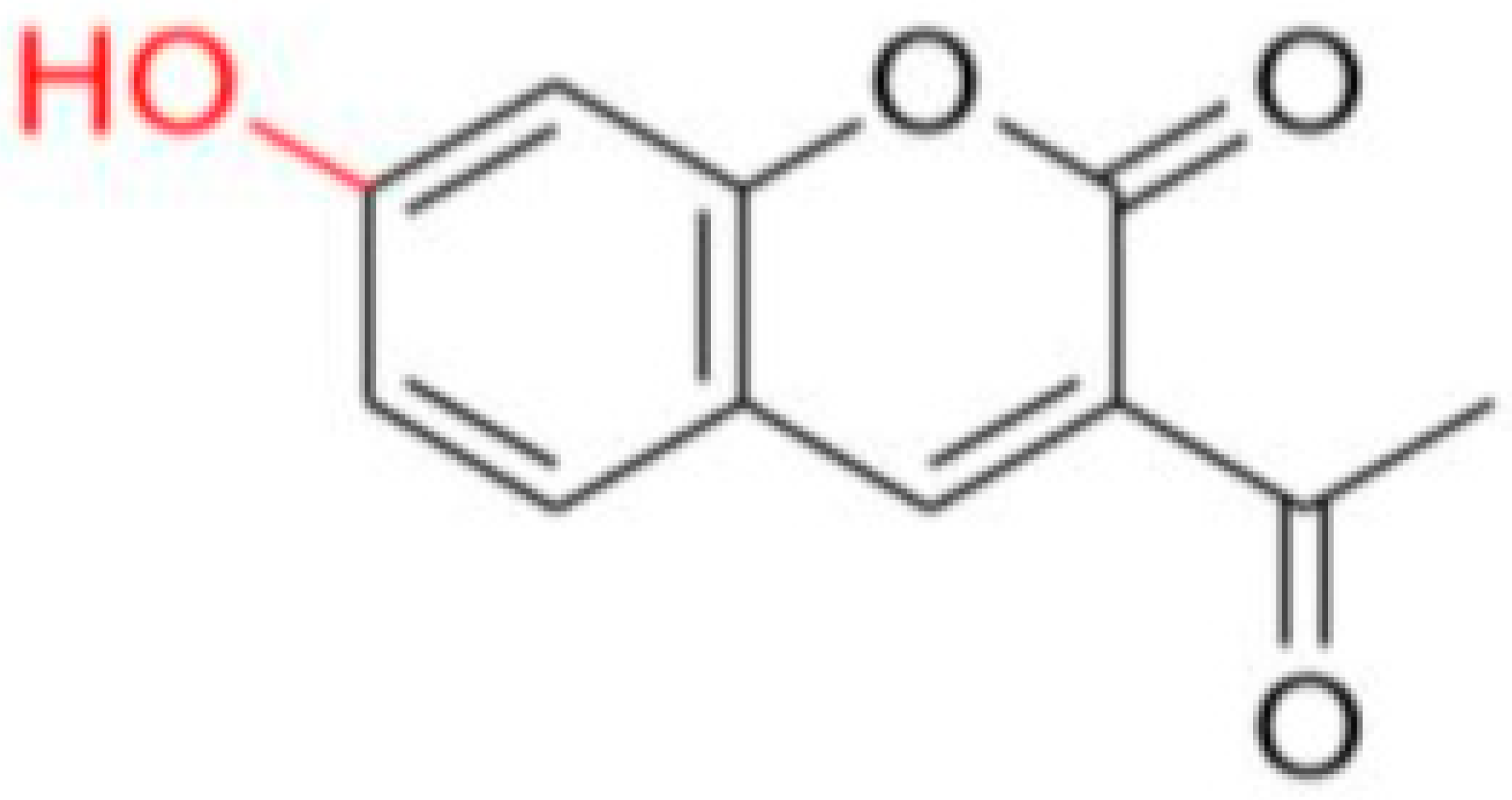 | 86.72 | 7.29 |
FN-07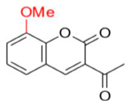 | 289.12 | 17.75 |
FN-10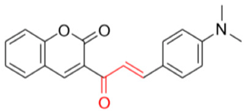 | 72.40 | 6.53 |
FN-11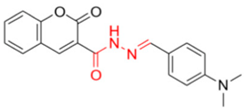 | 158.65 | 14.63 |
FN-17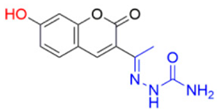 | 112.79 | 4.64 |
FN-19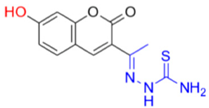 | 42.16 | 5.16 |
FN-25 | 192.35 | 11.08 |
FN-27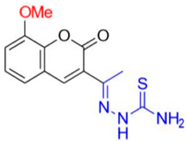 | 89.75 | 4.14 |
FN-29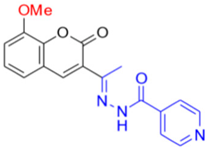 | precipitates | |
FN-40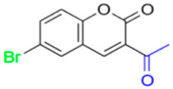 | 317.82 | 31.11 |
MP-03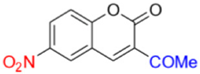 | 266.71 | 12.28 |
MP-04 | 222.03 | 30.80 |
MP-05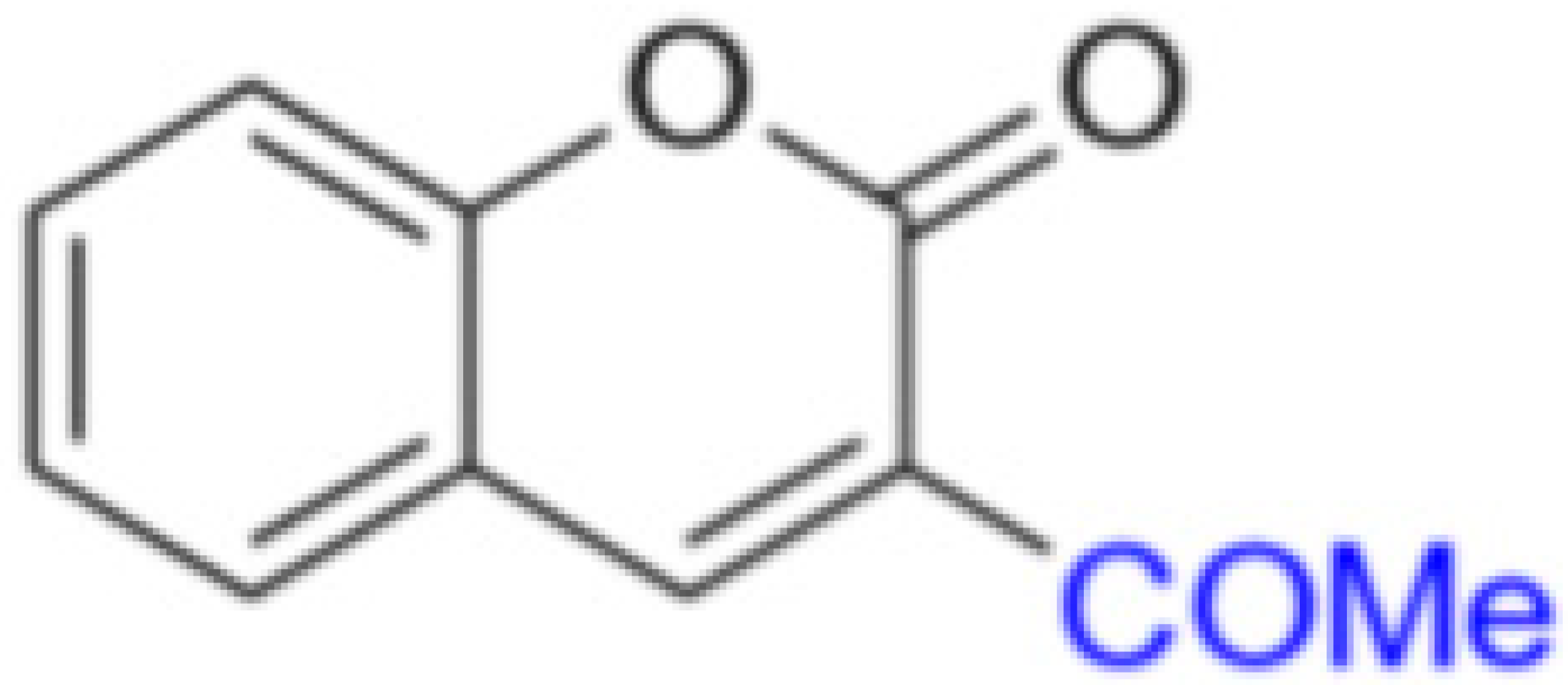 | 155.68 | 17.43 |
| Ascorbic acid | 386.50 | 11.95 |
| Kojic acid | 72.27 | 3.14 |
| Compound at 0.1 mM | Inhibitory Mechanism | Vmax | Km |
|---|---|---|---|
| FN-06 | Mixed | 0.97 | 4.775 |
| FN-07 | Mixed | 28.25 | 14.98 |
| FN-10 (0.05 mM) | Mixed | 2.02 | 0.73 |
| FN-11 | Mixed | 0.44 | 0.29 |
| FN-17 | Mixed | 1.33 | 1.73 |
| FN-19 | Mixed | 0.23 | 0.05 |
| FN-25 | Mixed | 0.96 | 0.74 |
| FN-27 | Mixed | 0.42 | 1.15 |
| FN-29 | Mixed | 0.41 | 0.03 |
| FN-40 | Competitive | 17.68 | 14.84 |
| MP-03 | Uncompetitive | 1.00 | 0.52 |
| MP-04 | Noncompetitive | 3.16 | 1.52 |
| MP-05 | Mixed | 0.50 | 0.87 |
| Energy Term | Tropolone (kcal/mol) ± SD | FN-19 (kcal/mol) ± SD |
|---|---|---|
| −75.8 ± 6.38 | −126.6 ± 10.00 | |
| −2.2 ± 0.79 | 49.7 ± 13.10 | |
| −78.0 ± 2.64 | −76.7 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, J.A.; Araújo, R.S.A.d.; Silva, F.N.d.; Cytarska, J.; Łączkowski, K.Z.; Cardoso, S.H.; Mendonça-Júnior, F.J.B.; Silva-Júnior, E.F.d. Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches. Int. J. Mol. Sci. 2023, 24, 5216. https://doi.org/10.3390/ijms24065216
Nunes JA, Araújo RSAd, Silva FNd, Cytarska J, Łączkowski KZ, Cardoso SH, Mendonça-Júnior FJB, Silva-Júnior EFd. Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches. International Journal of Molecular Sciences. 2023; 24(6):5216. https://doi.org/10.3390/ijms24065216
Chicago/Turabian StyleNunes, Jéssica Alves, Rodrigo Santos Aquino de Araújo, Fabrícia Nunes da Silva, Joanna Cytarska, Krzysztof Z. Łączkowski, Sílvia Helena Cardoso, Francisco Jaime Bezerra Mendonça-Júnior, and Edeildo Ferreira da Silva-Júnior. 2023. "Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches" International Journal of Molecular Sciences 24, no. 6: 5216. https://doi.org/10.3390/ijms24065216
APA StyleNunes, J. A., Araújo, R. S. A. d., Silva, F. N. d., Cytarska, J., Łączkowski, K. Z., Cardoso, S. H., Mendonça-Júnior, F. J. B., & Silva-Júnior, E. F. d. (2023). Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches. International Journal of Molecular Sciences, 24(6), 5216. https://doi.org/10.3390/ijms24065216







