First-Trimester Screening for HELLP Syndrome—Prediction Model Based on MicroRNA Biomarkers and Maternal Clinical Characteristics
Abstract
1. Introduction
2. Results
2.1. Identification of Risk Factors for the Development of HELLP Syndrome
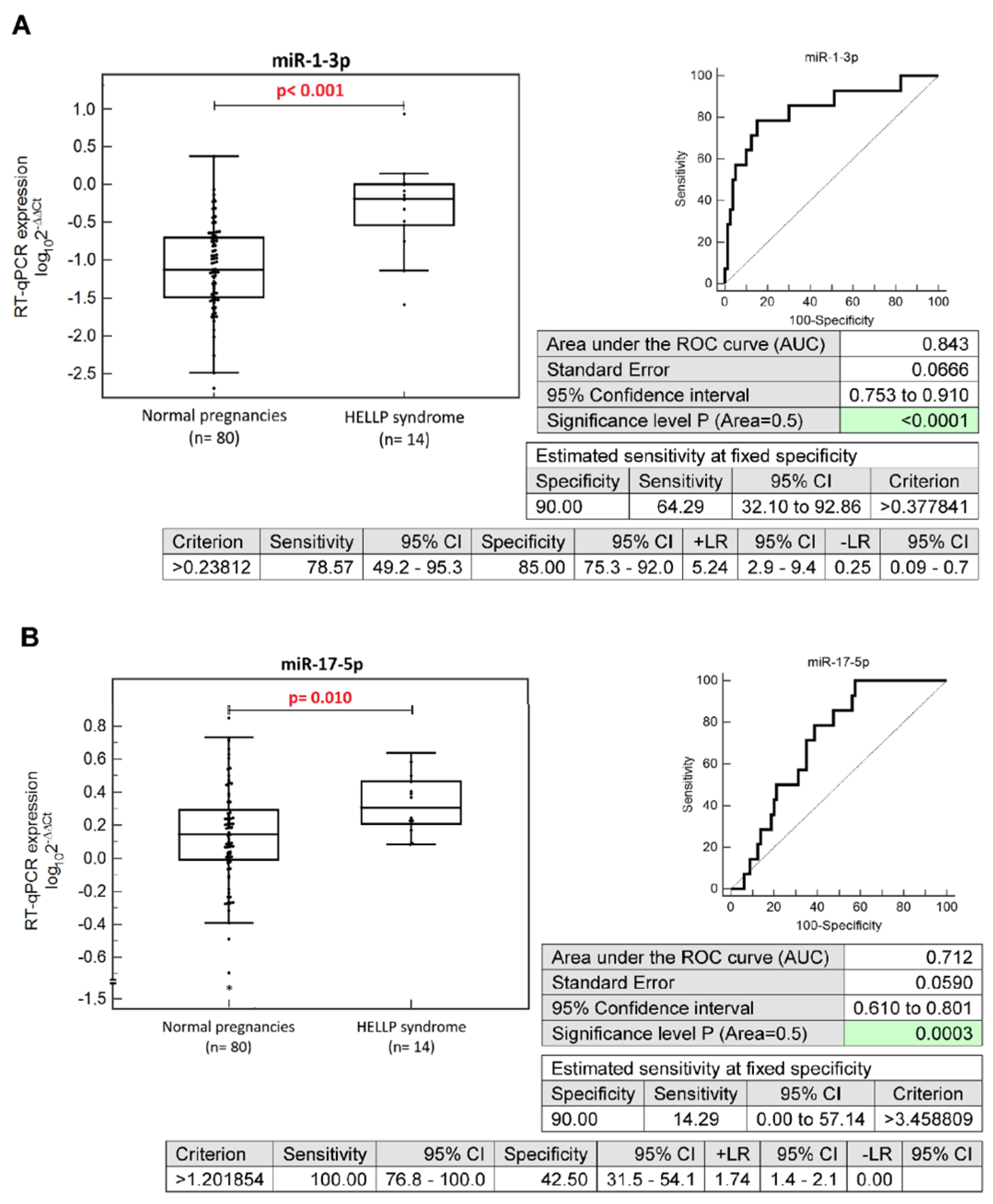
2.2. Altered Expression Profiles of MicroRNAs during the First Trimester of Gestation in Pregnancies Developing HELLP Syndrome
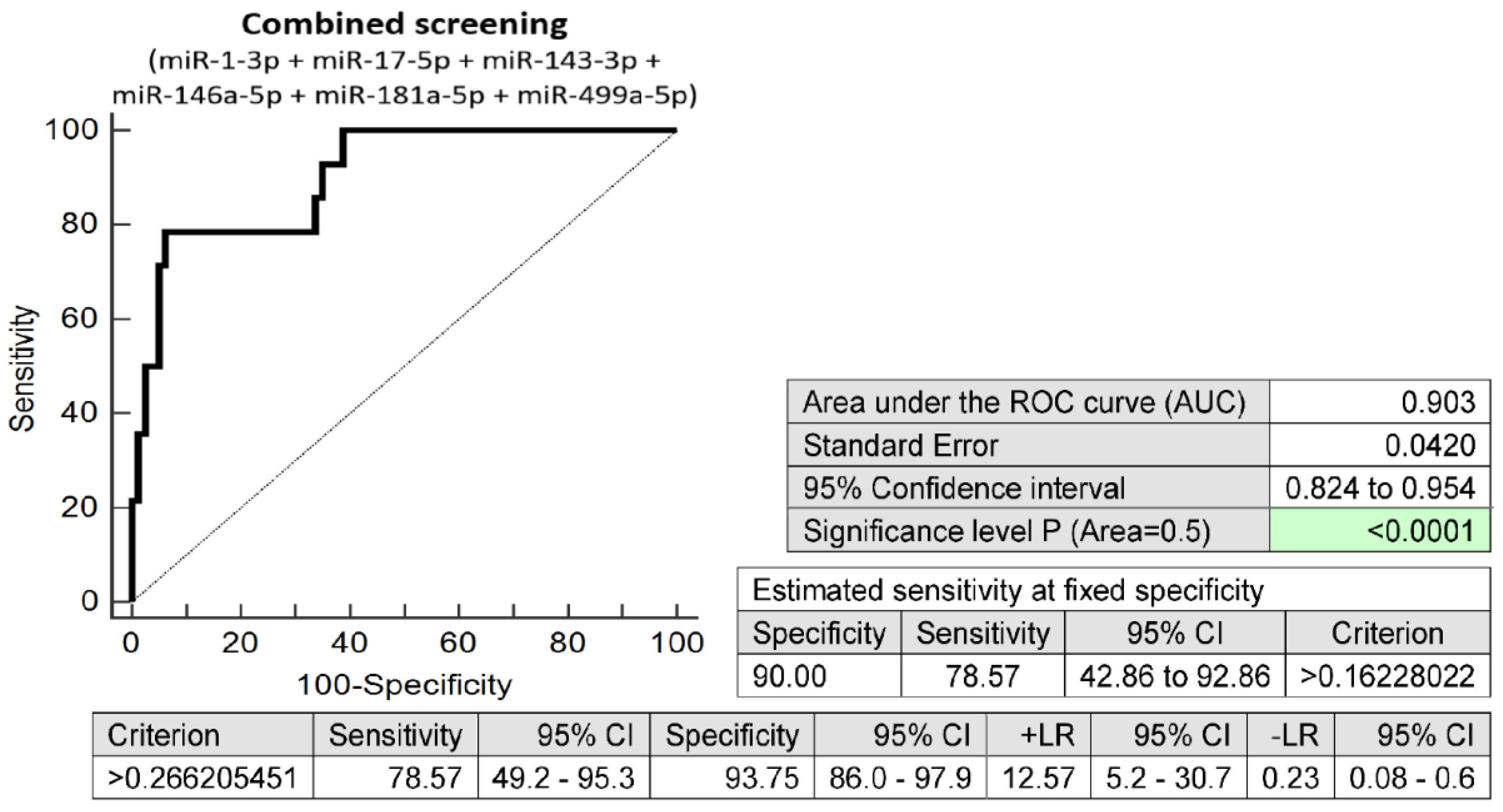
2.3. The Prediction Model for HELLP Syndrome—The Combination of Six MicroRNAs Only
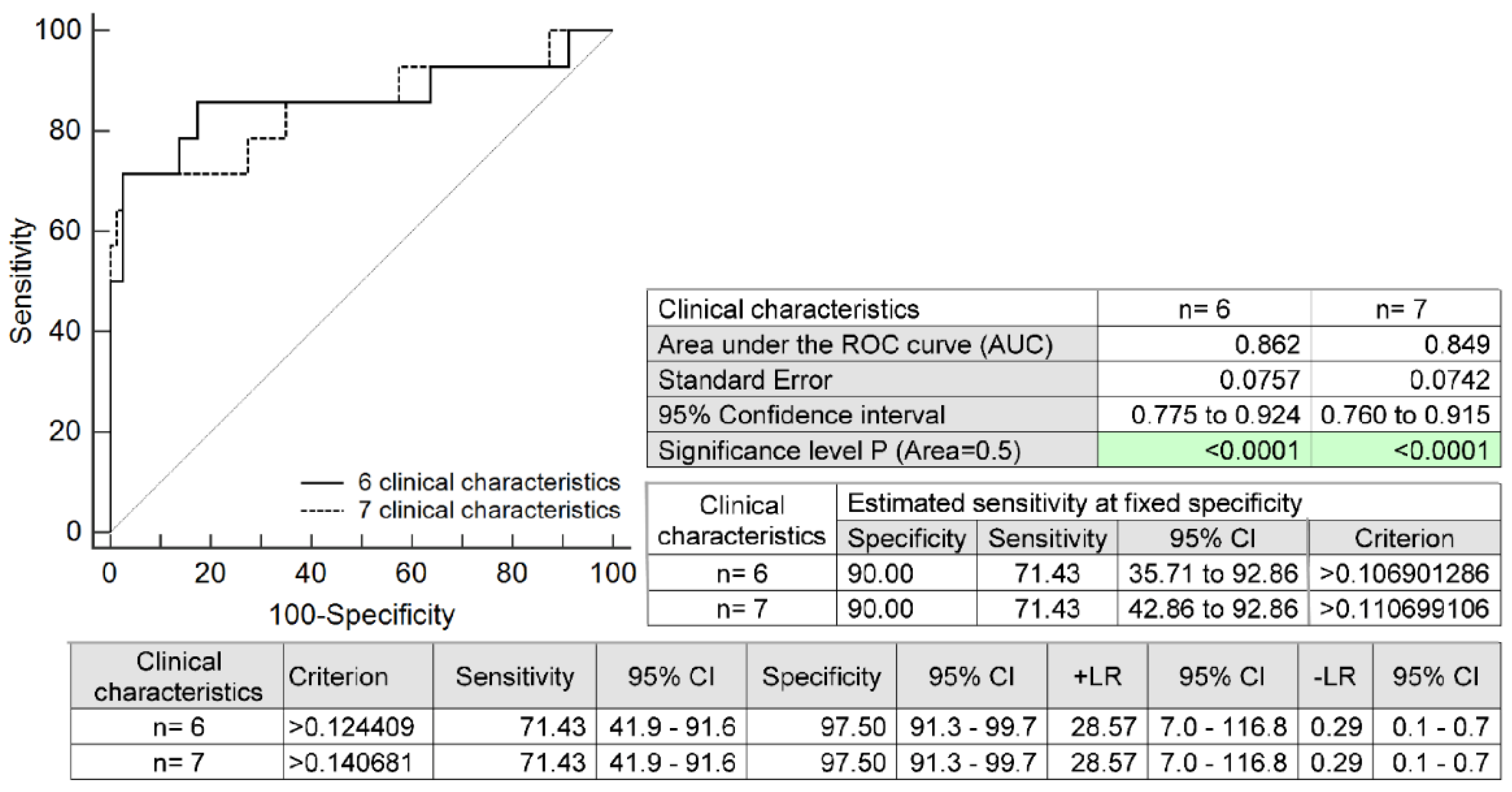
2.4. The Prediction Model for HELLP Syndrome Based on Selected Maternal Clinical Characteristics Only
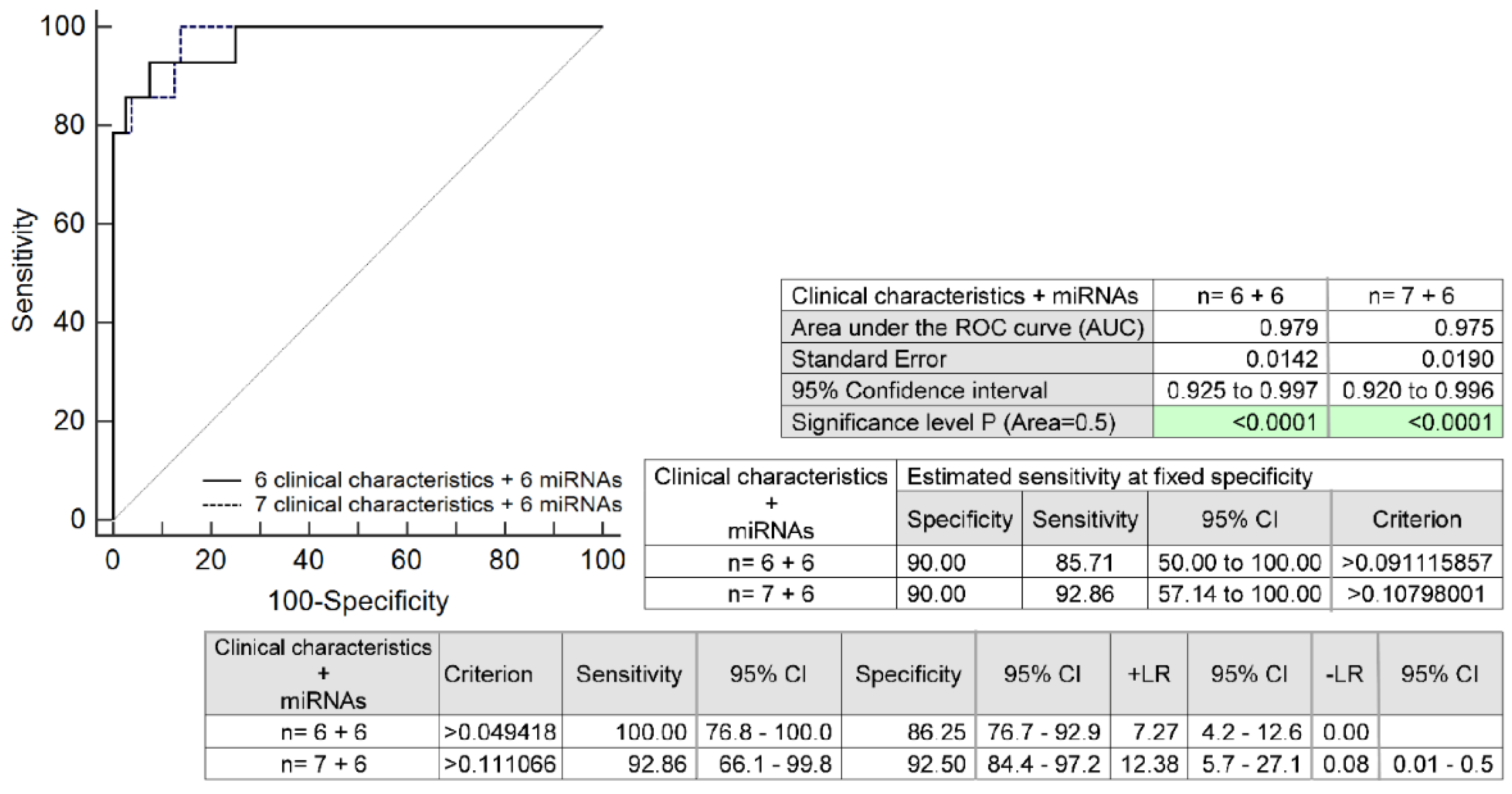
2.5. The Full Prediction Model for HELLP Syndrome Based on the Combination of Six MicroRNAs and Selected Maternal Clinical Characteristics
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Processing of Samples
4.3. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinstein, L. Syndrome of hemolysis, elevated liver enzymes and low platelet count; a severe consequence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1982, 142, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.A. The HELLP syndrome. Acta. Clin. Belg. 2010, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Svendsen, E.; Abildgaard, U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy Childbirth 2009, 26, 8. [Google Scholar] [CrossRef]
- Waterstone, M.; Bewley, S.; Wolfe, C. Incidence and predictors of severe obstetrics morbidity: Case-control study. BJM 2001, 322, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.N., Jr.; Rinehart, B.K.; May, W.L.; Magann, E.F.; Terrone, D.A.; Blake, P.G. The spectrum of severe preeclampsia: Comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am. J. Obstet. Gynecol. 1999, 180, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Gasem, T.; Al Jama, F.E.; Burshaid, S.; Rahman, J.; Al Suleiman, S.A.; Rahman, M.S. Maternal and fetal outcome of pregnancy complicated by HELLP syndrome. J. Matern. Fetal. Neonatal. Med. 2009, 22, 1140–1143. [Google Scholar] [CrossRef]
- Isler, C.M.; Rinehart, B.K.; Terrone, D.A.; May, W.L.; Magann, E.F.; Martin, J.N., Jr. The importance of parity to major maternal morbidity in the eclamptic mother with HELLP syndrome. Hypertens. Preganncy 2003, 22, 287–294. [Google Scholar] [CrossRef]
- Barton, J.R.; Sibai, B.M. Care of pregnancy complicated by HELLP syndrome. Obstet. Gynecol. Clin. N. Am. 1991, 18, 165–179. [Google Scholar] [CrossRef]
- Sibai, B.M. Maternal morbidity and mortality in 442 pregnancy with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am. J. Obstet. Gynecol. 1993, 169, 1000–1006. [Google Scholar] [CrossRef]
- Khumsat, R.; Wongwananurak, T.; Boriboonhirunsarn, D. Incidence and risk factors of HELLP syndrome in Thai pregnant women with severe pre-eclampsia. Thai J. Obstet. Gynaecol. 2008, 16, 192–198. [Google Scholar]
- Abraham, K.A.; Connolly, G.; Farrell, J.; Walshe, J.J. The HELLP syndrome, a prospective study. Ren. Fail 2001, 23, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.L. Liver disease in pregnancy. Med. Clin. N. Am. 1996, 80, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, U.; Heimdal, K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Raval, D.S.; Co, S.; Reid, M.A.; Pildes, R. Maternal and neonatal outcome of pregnancies complicated with maternal HELLP syndrome. J. Perinatol. 1997, 17, 266–269. [Google Scholar] [PubMed]
- Visser, W.; Wallenburg, H.C.S. Temporising management of severe pre-eclampsia with and without the HELLP syndrome. Br. J. Obstet. Gynaecol 1995, 102, 111–117. [Google Scholar] [CrossRef]
- Harms, K.; Rath, W.; Herting, E.; Kuhn, W. Maternal hemolysis, elevated liver enzymes, low platelet count, and neonatal outcome. Am. J. Perinatol. 1995, 12, 1–7. [Google Scholar] [CrossRef]
- Audibert, F.; Friedman, S.A.; Frangieh, A.Y.; Sibai, B.M. Clinical utility of strict diagnostic criteria for the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am. J. Obstet. Gynecol. 1996, 175, 460–464. [Google Scholar] [CrossRef]
- Van Pampus, M.G.; Wolf, H.; Westenberg, S.M.; van der Post, J.A.M.; Bonsel, G.J.; Treffers, P.E. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 76, 31–36. [Google Scholar] [CrossRef]
- Aloizos, S.; Seretis, C.; Liakos, N.; Aravosita, P.; Mystakelli, C.; Kanna, E.; Gourgiotis, S. HELLP syndrome: Understanding and management of a pregnancy-specific disease. J. Obstet. Gynaecol. 2013, 33, 331–337. [Google Scholar] [CrossRef]
- Backes, C.H.; Markham, K.; Moorehead, P.; Cordero, L.; Nankervis, C.A.; Giannone, P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy 2011, 2011, 214365. [Google Scholar] [CrossRef]
- Ganzevoort, W.; Rep, A.; de Vries, J.I.; Bonsel, G.J.; Wolf, H.; PETRA-investigators. Prediction of maternal complications and adverse infant outcome at admission for temporizing management of early-onset severe hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2006, 195, 495–503. [Google Scholar] [CrossRef]
- Van Lieshout, L.C.E.W.; Koek, G.H.; Spaanderman, M.A.; van Runnard Heimel, P.J. Placenta derived factors involved in the pathogenesis of the liver in the syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP): A review. Pregnancy Hypertens. 2019, 18, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ling, G.J.; Zhang, S.Q.; Zhai, W.Q.; Chen, Y.J. Effect of HELLP syndrome on acute kidney injury in pregnancy and pregnancy outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 657. [Google Scholar] [CrossRef] [PubMed]
- Pavlis, T.; Aloizos, S.; Aravosita, P.; Mystakelli, C.; Petrochilou, D.; Dimopoulos, N.; Gourgiotis, S. Diagnosis and surgical management of spontaneous hepatic rupture associated with HELLP syndrome. J. Surg. Educ. 2009, 66, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): Much ado about nothing? Am. J. Obstet. Gynecol. 1990, 162, 311–316. [Google Scholar] [CrossRef]
- Sibai, B.M. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet. Gynecol. 2004, 103, 981–991. [Google Scholar] [CrossRef]
- Sibai, B.M. Imitators of severe pre-eclampsia/eclampsia. Clin. Perinatol. 2004, 31, 835–852. [Google Scholar] [CrossRef]
- Martin, J.N., Jr.; Blake, P.G.; Perry, K.G., Jr.; McCaul, J.F.; Hess, L.W.; Martin, R.W. The natural history of HELLP syndrome: Patterns of disease progression and regression. Am. J. Obstet. Gynecol. 1991, 164, 1500–1509. [Google Scholar] [CrossRef]
- Martin, J.N., Jr.; Rose, C.H.; Briery, C.M. Understanding and managing HELLP syndrome: The integral role of aggressive glucocorticoids for mother and child. Am. J. Obstet. Gynecol. 2006, 195, 914–934. [Google Scholar] [CrossRef]
- Malmström, O.; Morken, N.H. HELLP syndrome, risk factors in first and second pregnancy: A population-based cohort study. Acta. Obstet. Gynecol. Scand. 2018, 97, 709–716. [Google Scholar] [CrossRef]
- Leeners, B.; Neumaier-Wagner, P.M.; Kuse, S.; Mütze, S.; Rudnik-Schöneborn, S.; Zerres, K.; Rath, W. Recurrence risks of hypertensive diseases in pregnancy after HELLP syndrome. J. Perinat. Med. 2011, 39, 673–678. [Google Scholar] [CrossRef]
- Habli, M.; Eftekhari, N.; Wiebracht, E.; Bombrys, A.; Khabbaz, M.; How, H.; Sibai, B. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am. J. Obstet. Gynecol. 2009, 201, e1–e5. [Google Scholar] [CrossRef]
- Hupuczi, P.; Rigó, B.; Sziller, I.; Szabó, G.; Szigeti, Z.; Papp, Z. Follow-up analysis of pregnancies complicated by HELLP syndrome. Fetal. Diagn. Ther. 2006, 21, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.E.; Hinshaw, K.; Kurinczuk, J.J.; Knight, M. Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver enzymes, low platelets syndrome. Obstet. Gynecol. 2014, 123, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Poon, L.C.; Nicolaides, K.H.; Baschat, A.A. First trimester prediction of HELLP syndrome. Prenat. Diagn. 2016, 36, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Pusl, T.; Beuers, U. Intrahepatic cholestasis of pregnancy. Orphanet. J. Rare Dis. 2007, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Mortensen, J.H.; Nagy, B. Genetic aspects of preeclampsia and the HELLP syndrome. J. Pregnancy 2014, 2014, 910751. [Google Scholar] [CrossRef]
- Stojanovska, V.; Zenclussen, A.C. Innate and Adaptive Immune Responses in HELLP Syndrome. Front. Immunol. 2020, 11, 667. [Google Scholar] [CrossRef]
- Muetze, S.; Leeners, B.; Ortlepp, J.R.; Kuse, S.; Tag, C.G.; Weiskirchen, R.; Gressner, A.M.; Rudnik-Schoeneborn, S.; Zerres, K.; Rath, W. Maternal factor V Leiden mutation is associated with HELLP syndrome in Caucasian women. Acta. Obstet. Gynecol. Scand 2008, 87, 635–642. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Ding, X.; Wang, Y.; Han, Y. Effects of serum from patients with early-onset pre-eclampsia, HELLP syndrome, and antiphospholipid syndrome on fatty acid oxidation in trophoblast cells. Arch. Gynecol. Obstet. 2015, 292, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kongwattanakul, K.; Saksiriwuttho, P.; Chaiyarach, S.; Thepsuthammarat, K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int. J. Womens. Health 2018, 10, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Adorno, M.; Maher-Griffiths, C.; Grush Abadie, H.R. HELLP Syndrome. Crit. Care. Nurs. Clin. N. Am. 2022, 34, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Harris, S.; Addison, A.; Bean, C. HELLP Syndrome: Pathophysiology and Current Therapies. Curr. Pharm. Biotechnol. 2018, 19, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Asadikalameh, Z.; Maddah, R.; Maleknia, M.; Nassaj, Z.S.; Ali, N.S.; Azizi, S.; Dastyar, F. Bioinformatics analysis of microarray data to identify hub genes, as diagnostic biomarker of HELLP syndrome: System biology approach. J. Obstet. Gynaecol. Res. 2022, 48, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.C.; Dierking, E. Intensive Care Unit issues in eclampsia and HELLP syndrome. Int. J. Crit. Illn. Inj. Sci. 2017, 7, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Feinberg, B.B.; Burwick, R.M. Thrombotic microangiopathies of pregnancy: Differential diagnosis. Pregnancy Hypertens 2018, 12, 29–34. [Google Scholar] [CrossRef]
- Moreira, M.W.L.; Rodrigues, J.J.P.C.; Al-Muhtadi, J.; Korotaev, V.V.; de Albuquerque, V.H.C. Neuro-fuzzy model for HELLP syndrome prediction in mobile cloud computing environments. Concurr. Comput. Pract. Exp. 2021, 22, e4651. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. Cardiovascular Disease-Associated MicroRNA Dysregulation during the First Trimester of Gestation in Women with Chronic Hypertension and Normotensive Women Subsequently Developing Gestational Hypertension or Preeclampsia with or without Fetal Growth Restriction. Biomedicines 2022, 10, 256. [Google Scholar]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines 2022, 10, 718. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First Trimester Prediction of Preterm Delivery in the Absence of Other Pregnancy-Related Complications Using Cardiovascular-Disease Associated MicroRNA Biomarkers. Int. J. Mol. Sci 2022, 23, 3951. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. Cardiovascular Disease-Associated MicroRNAs as Novel Biomarkers of First-Trimester Screening for Gestational Diabetes Mellitus in the Absence of Other Pregnancy-Related Complications. Int. J. Mol. Sci. 2022, 23, 10635. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Werler, M.M.; Mitchell, A.A. Gestational hypertension in pregnancies supported by infertility treatments: Role of infertility, treatments, and multiple gestations. Fertil. Steril. 2007, 88, 438–445. [Google Scholar] [CrossRef]
- Almasi-Hashiani, A.; Omani-Samani, R.; Mohammadi, M.; Amini, P.; Navid, B.; Alizadeh, A.; Khedmati Morasae, E.; Maroufizadeh, S. Assisted reproductive technology and the risk of preeclampsia: An updated systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 149. [Google Scholar] [CrossRef]
- Monseur, B.C.; Morris, J.R.; Hipp, H.S.; Berghella, V. Hypertensive disorders of pregnancy and infertility treatment: A population-based survey among United States women. J. Assist. Reprod. Genet. 2019, 36, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Obel, C.; Hammer Bech, B.; Olsen, J.; Basso, O. Infertility, infertility treatment, and fetal growth restriction. Obstet. Gynecol. 2007, 110, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil. Steril. 2016, 105, 73.e1–85.e6. [Google Scholar] [CrossRef]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: Comparison with NI-CE guidelines and ACOG recommendations. Ultrasound Obs. Gynecol. 2017, 49, 756–760. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obs. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef] [PubMed]
- The Fetal Medicine Foundation. Stratification of Pregnancy Management 11–13 Weeks’ Gestation. Available online: https://courses.fetalmedicine.com/fmf/show/861?locale=en (accessed on 4 October 2021).
- Mazer Zumaeta, A.; Wright, A.; Syngelaki, A.; Maritsa, V.A.; Da Silva, A.B.; Nicolaides, K.H. Screening for pre-eclampsia at 11-13 weeks’ gestation: Use of pregnancy-associated plasma protein-A, placental growth factor or both. Ultrasound Obstet. Gynecol. 2020, 56, 400–407. [Google Scholar] [CrossRef]
- Stubert, J.; Koczan, D.; Richter, D.U.; Dieterich, M.; Ziems, B.; Thiesen, H.J.; Gerber, B.; Reimer, T. miRNA expression profiles determined in maternal sera of patients with HELLP syndrome. Hypertens. Pregnancy 2014, 33, 215–235. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of re-al-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Hympanova, L.; Krofta, L. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb. Res. 2016, 137, 126–140. [Google Scholar] [CrossRef]


| miR-1-3p | miR-16-5p | miR-17-5p | miR-20a-5p | miR-20b-5p | miR-21-5p | miR-23a-3p | miR-24-3p | miR-26a-5p | miR-29a-3p | miR-92a-3p | miR-100-5p | miR-103a-3p | miR-125b-5p | miR-126-3p | miR-130b-3p | miR-133a-3p | miR-143-3p | miR-145-5p | miR-146a-5p | miR-155-5p | miR-181a-5p | miR-195-5p | miR-199a-5p | miR-210-3p | miR-221-3p | miR-342-3p | miR-499a-5p | miR-574-3p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homeostasis of the cardiovascular system | + | ||||||||||||||||||||||||||||
| Angiogenesis | + | ||||||||||||||||||||||||||||
| Cardiac development | + | ||||||||||||||||||||||||||||
| Cardiac regeneration | + | ||||||||||||||||||||||||||||
| Adipogenic differentiation | + | ||||||||||||||||||||||||||||
| Obesity | + | + | + | + | + | + | |||||||||||||||||||||||
| Insulin resistance | + | + | + | ||||||||||||||||||||||||||
| Gestational diabetes mellitus | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||
| Diabetes mellitus (T1DM, T2DM) and its complications | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Hypercholesterolemia | + | + | + | ||||||||||||||||||||||||||
| Metabolic syndrome | + | ||||||||||||||||||||||||||||
| Hypertension | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Atherosclerosis | + | + | + | + | + | + | |||||||||||||||||||||||
| Myocardial infarction | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||
| Cerebral ischemic events | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| Coronary heart disease | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Pulmonary hypertension | + | + | + | + | + | + | + | ||||||||||||||||||||||
| Heart failure | + | + | + | + | + | + | + | + | + | + | + | + |
| Normal-Term Pregnancies (n = 80) | HELLP Overall (n = 14) | HELLP without PE (n = 7) | HELLP with PE (n = 7) | p-Value 1 Odds Ratio (OR) (95%CI) | p-Value 2 Odds Ratio (OR) (95%CI) | p-Value 3 Odds Ratio (OR) (95%CI) | |
|---|---|---|---|---|---|---|---|
| Maternal characteristics | |||||||
| Chronic hypertension | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.396 OR: 5.552 (0.106–291.135) | 0.244 OR: 10.733 (0.198–580.643) | 0.244 OR: 10.733 (0.198–580.643) |
| Autoimmune diseases (APS/SLE/SS/RA) | 0 (0%) | 3 (21.43%) 2 SLE and APS 1 SS | 2 (28.57%) 1 SLE and APS 1 SS | 1 (14.29%) 1 SLE and APS | 0.012 OR: 49.000 (2.375–1011.10) | 0.008 OR: 73.182 (3.114–1719.605) | 0.032 OR: 37.154 (1.372–1006.271) |
| Other autoimmune diseases | 0 (0%) | 2 (14.29%) 1 AIT 2 CD | 2 (28.57%) 1 AIT 2 CD | 0 (0%) | 0.028 OR: 32.200 (1.459–710.734) | 0.008 OR: 73.182 (3.114–1719.605) | 0.243 OR: 10.733 (0.198–580.643) |
| T1DM | 0 (0%) | 1 (7.14%) | 1 (14.28%) | 0 (0%) | 0.082 OR: 17.889 (0.692–462.359) | 0.032 OR: 37.154 (1.372–1006.271) | 0.243 OR: 10.733 (0.198–580.643) |
| T2DM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.396 OR: 5.552 (0.106–291.135) | 0.244 OR: 10.733 (0.198–580.643) | 0.244 OR: 10.733 (0.198–580.643) |
| Any kind of autoimmune disease (APS/SLE/SS/RA/T1DM/other) | 0 (0%) | 4 (28.57%) 1 SLE, APS, AIT 1 SLE, APS 1 SS, CD 1 T1DM, CD | 3 (42.86%) 1 SLE, APS, AIT 1 SS, CD 1 T1DM, CD | 1 (14.29%) 1 SLE, APS | 0.006 OR: 69.000 (3.464–1374.494) | 0.002 OR: 125.222 (5.576–2812.179) | 0.032 OR: 37.154 (1.372–1006.271) |
| Trombophilic gene mutations | 0 (0%) | 2 (14.29%) | 1 (14.29%) | 1 (14.29%) | 0.028 OR: 32.200 (1.459–710.734) | 0.032 OR: 37.154 (1.372–1006.271) | 0.032 OR: 37.154 (1.372–1006.271) |
| Parity | |||||||
| Nulliparous | 40 (50.0%) | 10 (71.43%) | 5 (71.43%) | 5 (71.43%) | 0.147 OR: 2.500 (0.724–8.636) | 0.290 OR: 2.500 (0.458–13.649) | 0.290 OR: 2.500 (0.458–13.649) |
| Parous—History of GH | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.286 OR: 9.000 (0.159–511.016) | 0.185 OR: 16.200 (0.262–1000.106) | 0.185 OR: 16.200 (0.262–1000.106) |
| Parous—History of HELLP and/or PE | 0 (0%) | 2 (50.0%) | 1 (50.0%) | 1 (50.0%) | 0.009 OR: 81.000 (3.005–2183.284) | 0.016 OR: 81.000 (2.232–2939.904) | 0.016 OR: 81.000 (2.232–2939.904) |
| Parous—History of SGA/FGR | 1 (1.25%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.529 OR: 2.926 (0.103–83.058) | 0.345 OR: 5.267 (0.168–165.318) | 0.345 OR: 5.267 (0.168–165.318) |
| History of miscarriage (spontaneous pregnancy loss before 22 gestational weeks) | 16 (20.0%) | 1 (7.14%) | 0 (0%) | 1 (14.29%) | 0.273 OR: 0.308 (0.037–2.529) | 0.366 OR: 0.261 (0.014–4.800) | 0.716 OR: 0.667 (0.075–5.938) |
| History of perinatal death (death of a fetus/newborn between 22 gestational weeks (or weighing over 500 g) and 7 days after the birth) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.396 OR: 5.552 (0.106–291.135) | 0.244 OR: 10.733 (0.198–580.643) | 0.244 OR: 10.733 (0.198–580.643) |
| ART (IVF/ICSI/other) | 2 (2.5%) | 4 (28.57%) | 1 (14.29%) | 3 (42.86%) | 0.003 OR: 15.600 (2.526–96.339) | 0.149 OR: 6.500 (0.513–82.423) | 0.001 OR: 29.250 (3.758–227.682) |
| Smoking during pregnancy | 2 (2.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.960 OR: 1.083 (0.049–23.740) | 0.643 OR: 2.093 (0.092–47.761) | 0.643 OR: 2.093 (0.092–47.761) |
| Pregnancy details (first trimester of gestation) | |||||||
| Maternal age (years) | 32 (25–42) | 31 (24–49) | 28 (24–35) | 35 (26–49) | 0.686 | 0.381 | 1.0 |
| Advanced maternal age (≥35 years old at early stages of gestation) | 18 (22.50%) | 6 (42.86%) | 2 (28.57%) | 4 (57.14%) | 0.115 OR: 2.583 (0.793–8.419) | 0.715 OR: 1.378 (0.246–7.708) | 0.060 OR: 4.593 (0.940–22.437) |
| BMI (kg/m2) | 21.28 (17.16–29.76) | 23.62 (15.67–31.53) | 23.05 (15.67–31.53) | 24.24 (18.97–27.43) | 0.169 | 1.0 | 0.186 |
| BMI ≥ 30 kg/m2 | 0 (0%) | 1 (7.14%) | 1 (14.29%) | 0 (0%) | 0.082 OR: 17.889 (0.692–462.359) | 0.032 OR: 37.154 (1.372–1006.271) | 0.244 OR: 10.733 (0.198–580.643) |
| Gestational age at sampling (weeks) | 10.29 (9.57–13.71) | 10.64 (9.14–11.71) | 10.57 (9.71–11.57) | 10.86 (9.14–11.71) | 0.086 | 0.505 | 0.748 |
| MAP (mmHg) | 88.75 (67.67–103.83) | 92.83 (78.33–108.25) | 94.04 (78.33–108.25) | 92.83 (83.67–107.17) | 0.348 | 1.0 | 1.0 |
| MAP (MoM) | 1.05 (0.84–1.25) | 1.09 (0.93–1.30) | 1.13 (0.93–1.30) | 1.09 (0.97–1.22) | 0.440 | 1.0 | 1.0 |
| Mean UtA-PI | 1.39 (0.56–2.43) | 1.32 (0.84–2.21) | 1.32 (0.91–2.21) | 1.56 (0.84–2.13) | 0.952 | 1.0 | 1.0 |
| Mean UtA-PI (MoM) | 0.90 (0.37–1.55) | 0.85 (0.56–1.38) | 0.82 (0.56–1.38) | 1.03 (0.57–1.31) | 0.945 | 1.0 | 1.0 |
| PIGF serum levels (pg/mL) | 27.1 (8.1–137.0) | 24.2 (14.6–32.6) | 20.6 (14.6–32.6) | 24.85 (18.2–29.2) | 0.095 | 0.527 | 0.823 |
| PIGF serum levels (MoM) | 1.04 (0.38–2.61) | 0.94 (0.65–1.18) | 0.94 (0.69–1.18) | 0.89 (0.65–1.15) | 0.058 | 0.654 | 0.373 |
| PAPP-A serum levels (IU/L) | 1.49 (0.48–15.69) | 1.50 (0.28–5.93) | 1.16 (0.45–2.42) | 1.68 (0.28–5.93) | 0.668 | 0.375 | 1.0 |
| PAPP-A serum levels (MoM) | 1.17 (0.37–3.18) | 0.93 (0.32–3.93) | 0.69 (0.56–1.54) | 0.95 (0.32–3.93) | 0.258 | 0.168 | 1.0 |
| Free b-hCG serum levels (μg/L) | 60.21 (9.9–200.6) | 54.34 (13.74–162.5) | 45.35 (21.91–162.5) | 55.56 (13.74–161.6) | 0.364 | 0.649 | 1.0 |
| Free b-hCG serum levels (MoM) | 1.02 (0.31–3.57) | 1.01 (0.28–2.55) | 0.67 (0.47–2.55) | 1.26 (0.28–2.47) | 0.438 | 0.400 | 1.0 |
| Positive screening for PE and/or FGR by FMF algorithm | 0 (0%) | 4 (28.57%) | 2 (28.57%) | 2 (28.57%) | 0.006 OR: 69.000 (3.464–1374.494) | 0.008 OR: 73.182 (3.114–1719.605) | 0.008 OR: 73.182 (3.114–1719.605) |
| Aspirin intake during pregnancy | 0 (0%) | 5 (35.71%) | 3 (42.86%) | 2 (28.57%) | 0.003 OR: 93.210 (4.772–1820.792) | 0.002 OR: 125.222 (5.576–2812.179) | 0.008 OR: 73.182 (3.114–1719.605) |
| Pregnancy details (at delivery) | |||||||
| SBP (mmHg) | 122 (100–155) | - | 138 (102–157) | 150 (134–210) | - | 0.059 | <0.001 |
| DBP (mmHg) | 76 (60–90) | - | 80 (75–108) | 98 (86–140) | - | 0.189 | <0.001 |
| Proteinuria | 0 (0%) | - | 0 (0%) | 7 (100%) | - | 0.244 OR: 10.733 (0.198–580.643) | <0.001 OR: 2415.0 (44.642–130,644.585) |
| Gestational age at delivery (weeks) | 40.07 (37.57–42.0) | 35.79 (29.14–37.86) | 36.86 (36.0–37.86) | 32.71 (29.14–35.57) | <0.001 | <0.001 | <0.001 |
| Delivery at gestational age < 37 weeks | 0 (0%) | 11 (78.57%) | 4 (57.14%) | 7 (100%) | <0.001 OR: 529.000 (25.636–10,915.759) | 0.001 OR: 207.000 (9.217–4648.705) | <0.001 OR: 2415.000 (44.642–130,644.585) |
| RBC (×1012/L) | 4.16 (3.49–4.85) | 4.36 (3.43–4.81) | 4.51 (3.93–4.81) | 4.05 (3.43–4.38) | 0.629 | 0.213 | 0.683 |
| HGB (g/L) | 119 (89–149) | 121 (89–136) | 122 (101–136) | 120 (89–133) | 0.565 | 0.800 | 1.0 |
| TBIL (μmol/L) | 0.251 (0.146–0.585) | 0.672 (0.230–3.630) | 0.637 (0.386–3.630) | 0.950 (0.230–1.520) | <0.001 | 0.014 | 0.018 |
| ALT (IU/L) | 11.4 (5.40–18.61) | 71.12 (12.05–531.81) | 60.24 (12.05–435.77) | 93.64 (21.01–531.81) | <0.001 | 0.004 | <0.001 |
| AST (IU/L) | 19.21 (12.60–30.61) | 128.75 (28.81–1128.45) | 100.24 (28.81–1128.45) | 151.86 (53.61–348.14) | <0.001 | <0.001 | <0.001 |
| PLT (×109/L) | 237 (158–407) | 100 (47–149) | 104 (47–149) | 100 (48–120) | <0.001 | <0.001 | <0.001 |
| PT (s) | 13.5 (11.1–15.8) | 12.7 (11.1–14.1) | 12.8 (11.1–14.1) | 12.3 (11.1–13.5) | 0.016 | 1.0 | 0.017 |
| APTT (s) | 34.3 (27–45.9) | 33.8 (24.2–47.9) | 36.9 (27.6–47.9) | 30.8 (24.2–40.6) | 0.377 | 1.0 | 0.371 |
| FIB (mg/dL) | 5.03 (3.99–7.38) | 4.53 (1.48–6.80) | 4.53 (2.75–6.80) | 4.75 (1.48–5.91) | 0.245 | 1.0 | 0.792 |
| Fetal birth weight (grams) | 3470 (2920–4240) | 2410 (1230–3850) | 2800 (2150–3850) | 1845 (1230–2410) | <0.001 | 0.024 | <0.001 |
| Fetal sex | |||||||
| Boy | 40 (50.0%) | 7 (50.0%) | 4 (47.14%) | 3 (42.86%) | 1.000 OR: 1.000 (0.321–3.113) | 0.718 OR: 1.333 (0.280–6.344) | 0.718 OR: 0.750 (0.158–3.568) |
| Girl | 40 (50.0%) | 7 (50.0%) | 3 (42.86%) | 4 (57.14%) | |||
| Mode of delivery | |||||||
| Vaginal | 69 (86.25%) | 0 (0%) | 0 (0%) | 0 (0%) | <0.001 OR: 175.261 (9.765–3145.545) | 0.003 OR: 90.652 (4.841–1697.654) | 0.003 OR: 90.652 (4.841–1697.654) |
| CS | 11 (13.75%) | 14 (100%) | 7 (100%) | 7 (100%) | |||
| Apgar score < 7, 5 min | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.396 OR: 5.552 (0.106–291.135) | 0.244 OR: 10.733 (0.198–580.643) | 0.244 OR: 10.733 (0.198–580.643) |
| Apgar score < 7, 10 min | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.396 OR: 5.552 (0.106–291.135) | 0.244 OR: 10.733 (0.198–580.643) | 0.244 OR: 10.733 (0.198–580.643) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for HELLP Syndrome—Prediction Model Based on MicroRNA Biomarkers and Maternal Clinical Characteristics. Int. J. Mol. Sci. 2023, 24, 5177. https://doi.org/10.3390/ijms24065177
Hromadnikova I, Kotlabova K, Krofta L. First-Trimester Screening for HELLP Syndrome—Prediction Model Based on MicroRNA Biomarkers and Maternal Clinical Characteristics. International Journal of Molecular Sciences. 2023; 24(6):5177. https://doi.org/10.3390/ijms24065177
Chicago/Turabian StyleHromadnikova, Ilona, Katerina Kotlabova, and Ladislav Krofta. 2023. "First-Trimester Screening for HELLP Syndrome—Prediction Model Based on MicroRNA Biomarkers and Maternal Clinical Characteristics" International Journal of Molecular Sciences 24, no. 6: 5177. https://doi.org/10.3390/ijms24065177
APA StyleHromadnikova, I., Kotlabova, K., & Krofta, L. (2023). First-Trimester Screening for HELLP Syndrome—Prediction Model Based on MicroRNA Biomarkers and Maternal Clinical Characteristics. International Journal of Molecular Sciences, 24(6), 5177. https://doi.org/10.3390/ijms24065177








