Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Adults with Long COVID: Secondary Analysis of a Randomized Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Population and Concentrations of l-Arginine Metabolites at Baseline
2.2. l-Arginine Metabolism in Participants with Long COVID and Controls
2.3. Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Participants with Long COVID
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. l-Arginine Metabolism Assessment
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and endothelial function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef]
- Durante, W. Targeting Arginine in COVID-19-induced immunopathology and vasculopathy. Metabolites 2022, 12, 240. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Zhang, C.; Hein, T.W.; Wang, W.; Miller, M.W.; Fossum, T.W.; McDonald, M.M.; Humphrey, J.D.; Kuo, L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 2004, 44, 935–943. [Google Scholar] [CrossRef]
- Pernow, J.; Jung, C. The Emerging role of arginase in endothelial dysfunction in diabetes. Curr. Vasc. Pharmacol. 2016, 14, 155–162. [Google Scholar] [CrossRef]
- Rouzaut, A.; Subirá, M.L.; De Miguel, C.; Domingo-De-Miguel, E.; González, A.; Santiago, E.; López-Moratalla, N. Co-expression of inducible nitric oxide synthase and arginases in different human monocyte subsets. Apoptosis regulated by endogenous NO. Biochim. Biophys. Acta 1999, 1451, 319–333. [Google Scholar] [CrossRef]
- Blanc, R.S.; Richard, S. Arginine methylation: The coming of age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Freitag, D.F.; Laukkanen, J.A.; Chowdhury, S.; Gobin, R.; Mayr, M.; Di Angelantonio, E.; Chowdhury, R. Asymmetric dimethylarginine and cardiovascular risk: Systematic review and meta-analysis of 22 prospective studies. J. Am. Heart Assoc. 2015, 4, e001833. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, Q.; Li, X.; Chen, C.; Liu, J.; Ye, Y.; Ruan, Y.; Hei, Z. Asymmetric dimethylarginine and all-cause mortality: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44692. [Google Scholar] [CrossRef]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; Donkor, K.; Kansakar, U.; Trimarco, V.; Mone, P.; et al. l-arginine and COVID-19: An update. Nutrients 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of l-arginine plus vitamin c supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: A single-blind randomized controlled trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Rees, C.A.; Rostad, C.A.; Mantus, G.; Anderson, E.J.; Chahroudi, A.; Jaggi, P.; Wrammert, J.; Ochoa, J.B.; Ochoa, A.; Basu, R.K.; et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101708118. [Google Scholar] [CrossRef]
- Sacchi, A.; Grassi, G.; Notari, S.; Gili, S.; Bordoni, V.; Tartaglia, E.; Casetti, R.; Cimini, E.; Mariotti, D.; Garotto, G.; et al. Expansion of myeloid derived suppressor cells contributes to platelet activation by L-arginine deprivation during SARS-CoV-2 infection. Cells 2021, 10, 2111. [Google Scholar] [CrossRef]
- Reizine, F.; Lesouhaitier, M.; Gregoire, M.; Pinceaux, K.; Gacouin, A.; Maamar, A.; Painvin, B.; Camus, C.; Le Tulzo, Y.; Tattevin, P.; et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J. Clin. Immunol. 2021, 41, 515–525. [Google Scholar] [CrossRef]
- Karu, N.; Kindt, A.; van Gammeren, A.J.; Ermens, A.A.M.; Harms, A.C.; Portengen, L.; Vermeulen, R.C.H.; Dik, W.A.; Langerak, A.W.; van der Velden, V.H.J.; et al. Severe COVID-19 is characterised by perturbations in plasma amines correlated with immune response markers, and linked to inflammation and oxidative stress. Metabolites 2022, 12, 618. [Google Scholar] [CrossRef]
- Hannemann, J.; Balfanz, P.; Schwedhelm, E.; Hartmann, B.; Ule, J.; Müller-Wieland, D.; Dahl, E.; Dreher, M.; Marx, N.; Böger, R. Elevated serum SDMA and ADMA at hospital admission predict in-hospital mortality of COVID-19 patients. Sci. Rep. 2021, 11, 9895. [Google Scholar] [CrossRef]
- Tosato, M.; Ciciarello, F.; Zazzara, M.B.; Pais, C.; Savera, G.; Picca, A.; Galluzzo, V.; Coelho-Júnior, H.J.; Calvani, R.; Marzetti, E.; et al. Nutraceuticals and dietary supplements for older adults with long COVID-19. Clin. Geriatr. Med. 2022, 38, 565–591. [Google Scholar] [CrossRef]
- Fiorentino, G.; Coppola, A.; Izzo, R.; Annunziata, A.; Bernardo, M.; Lombardi, A.; Trimarco, V.; Santulli, G.; Trimarco, B. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: A randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 2021, 40, 101125. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and cardiovascular disease: An update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Đukić, I.; Kaličanin, N.; Sencanski, M.; Pajovic, S.B.; Milicevic, J.; Prljic, J.; Paessler, S.; Prodanović, R.; Glisic, S. Inhibition of SARS-CoV-2 Mpro with vitamin C, L-arginine and a aitamin C/L-arginine combination. Front. Biosci. Landmark Ed. 2023, 28, 8. [Google Scholar] [CrossRef]
- Izzo, R.; Trimarco, V.; Mone, P.; Aloè, T.; Capra Marzani, M.; Diana, A.; Fazio, G.; Mallardo, M.; Maniscalco, M.; Marazzi, G.; et al. Combining L-arginine with vitamin C improves long-COVID symptoms: The LINCOLN Survey. Pharmacol. Res. 2022, 183, 106360. [Google Scholar] [CrossRef]
- A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 27 February 2023).
- ADMA—Overview: Asymmetric Dimethylarginine, Plasma. Available online: https://www.mayocliniclabs.com/test-catalog/Overview/607697#Clinical-and-Interpretive (accessed on 27 February 2023).
- ADMA/SDMA|Test Detail|Quest Diagnostics. Available online: https://testdirectory.questdiagnostics.com/test/test-detail/94153/admasdma?q=ADMA%2FSDMA&cc=MASTER (accessed on 27 February 2023).
- ADMA/SDMA. Available online: https://www.clevelandheartlab.com/wp-content/uploads/2018/11/CHL-D070-AUG2018-ADMA-SDMA-Practitioner-One-Pager.pdf (accessed on 27 February 2023).
- Dean, M.J.; Ochoa, J.B.; Sanchez-Pino, M.D.; Zabaleta, J.; Garai, J.; Del Valle, L.; Wyczechowska, D.; Baiamonte, L.B.; Philbrook, P.; Majumder, R.; et al. Severe COVID-19 is characterized by an impaired type I interferon response and elevated levels of arginase producing granulocytic myeloid derived suppressor cells. Front. Immunol. 2021, 12, 695972. [Google Scholar] [CrossRef]
- Wu, P.; Chen, D.; Ding, W.; Wu, P.; Hou, H.; Bai, Y.; Zhou, Y.; Li, K.; Xiang, S.; Liu, P.; et al. The trans-omics landscape of COVID-19. Nat. Commun. 2021, 12, 4543. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Batiha, G.E.-S. COVID-19 and L-arginine supplementations: Yet to find the missed key. Curr. Protein Pept. Sci. 2022, 23, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Sourij, H.; Meinitzer, A.; Pilz, S.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; März, W. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 2011, 218, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Surdacki, A.; Nowicki, M.; Sandmann, J.; Tsikas, D.; Boeger, R.H.; Bode-Boeger, S.M.; Kruszelnicka-Kwiatkowska, O.; Kokot, F.; Dubiel, J.S.; Froelich, J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol. 1999, 33, 652–658. [Google Scholar] [CrossRef]
- Gervasoni, J.; Bonelli, F.; Zuppi, C.; Zappacosta, B.; Mordente, A.; Calvani, R.; Persichilli, S. Determination of asymmetric dimethyl arginine in human serum by liquid chromatography-tandem mass spectrometry: Clinical application in hypertensive subjects. Clin. Chem. Lab. Med. 2011, 49, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, K.; Mittermayer, F.; Wolzt, M.; Schernthaner, G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care 2007, 30, 1834–1839. [Google Scholar] [CrossRef]
- Chirinos, J.A.; David, R.; Bralley, J.A.; Zea-Díaz, H.; Muñoz-Atahualpa, E.; Corrales-Medina, F.; Cuba-Bustinza, C.; Chirinos-Pacheco, J.; Medina-Lezama, J. Endogenous nitric oxide synthase inhibitors, arterial hemodynamics, and subclinical vascular disease: The PREVENCION Study. Hypertension 2008, 52, 1051–1059. [Google Scholar] [CrossRef]
- Maas, R.; Xanthakis, V.; Polak, J.F.; Schwedhelm, E.; Sullivan, L.M.; Benndorf, R.; Schulze, F.; Vasan, R.S.; Wolf, P.A.; Böger, R.H.; et al. Association of the endogenous nitric oxide synthase inhibitor ADMA with carotid artery intimal media thickness in the Framingham Heart Study offspring cohort. Stroke 2009, 40, 2715–2719. [Google Scholar] [CrossRef]
- Janes, F.; Cifù, A.; Pessa, M.E.; Domenis, R.; Gigli, G.L.; Sanvilli, N.; Nilo, A.; Garbo, R.; Curcio, F.; Giacomello, R.; et al. ADMA as a possible marker of endothelial damage. A study in young asymptomatic patients with cerebral small vessel disease. Sci. Rep. 2019, 9, 14207. [Google Scholar] [CrossRef]
- Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C.U. Arginine derivatives in cerebrovascular diseases: Mechanisms and clinical implications. Int. J. Mol. Sci. 2020, 21, 1798. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Yanamadala, S.; Eng, C.; Pinsky, D.J.; Marmur, J.D. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in men with acute coronary syndrome referred for coronary angiography. Coron. Artery Dis. 2009, 20, 112–117. [Google Scholar] [CrossRef]
- Valkonen, V.P.; Päivä, H.; Salonen, J.T.; Lakka, T.A.; Lehtimäki, T.; Laakso, J.; Laaksonen, R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 2001, 358, 2127–2128. [Google Scholar] [CrossRef] [PubMed]
- Krempl, T.K.; Maas, R.; Sydow, K.; Meinertz, T.; Böger, R.H.; Kähler, J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur. Heart J. 2005, 26, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Poludasu, S.; Yanamadala, S.; Frishman, W.H.; Eng, C.; Pinsky, D.J.; Marmur, J.D. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis 2010, 210, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Nabika, T.; Bokura, H.; Suyama, Y.; Kobayashi, S.; Yamaguchi, S.; Masuda, J. Evaluation of asymmetric dimethylarginine and homocysteine in microangiopathy-related cerebral damage. Am. J. Hypertens. 2009, 22, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Endres, H.G.; Schwedhelm, E.; Darius, H.; Atzler, D.; Lüneburg, N.; von Stritzky, B.; Maas, R.; Thiem, U.; Benndorf, R.A.; et al. Asymmetric dimethylarginine as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J. Intern. Med. 2010, 269, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Laurence, J. Long COVID endotheliopathy: Hypothesized mechanisms and potential therapeutic approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.Y.; Wang, S.I.; Wei, J.C.C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Strobel, J.; Müller, F.; Zolk, O.; Endreß, B.; König, J.; Fromm, M.F.; Maas, R. Transport of asymmetric dimethylarginine (ADMA) by cationic amino acid transporter 2 (CAT2), organic cation transporter 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1). Amino Acids 2013, 45, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J. Cardiovasc. Pharmacol. 1992, 20, S60–S62. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Zhao, J.F.; Lin, S.J.; Shyue, S.K.; Guo, B.C.; Lu, T.M.; Lee, T.S. Asymmetric dimethylarginine limits the efficacy of simvastatin activating endothelial nitric oxide synthase. J. Am. Heart Assoc. 2016, 5, e003327. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef]
- Böger, G.I.; Rudolph, T.K.; Maas, R.; Schwedhelm, E.; Dumbadze, E.; Bierend, A.; Benndorf, R.A.; Böger, R.H. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin: Effect of combination with oral L-arginine. J. Am. Coll. Cardiol. 2007, 49, 2274–2282. [Google Scholar] [CrossRef]
- Walker, H.A.; McGing, E.; Fisher, I.; Böger, R.H.; Bode-Böger, S.M.; Jackson, G.; Ritter, J.M.; Chowienczyk, P.J. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: Lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J. Am. Coll. Cardiol. 2001, 38, 499–505. [Google Scholar] [CrossRef]
- Speakman, L.L.; Michienzi, S.M.; Badowski, M.E. Vitamins, supplements and COVID-19: A review of currently available evidence. Drugs Context 2021, 10, 2021-6-2. [Google Scholar] [CrossRef]
- Veljković, M.; Pavlović, D.R.; Stojanović, N.M.; Džopalić, T.; Popović Dragonjić, L. Behavioral and dietary habits that could influence both COVID-19 and non-communicable civilization disease prevention-what have we learned up to now? Medicina 2022, 58, 1686. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Galluzzo, V.; Ciciarello, F.; Tosato, M.; Zazzara, M.B.; Pais, C.; Savera, G.; Calvani, R.; Picca, A.; Marzetti, E.; Landi, F. Association between vitamin D status and physical performance in COVID-19 survivors: Results from the Gemelli against COVID-19 Post-Acute Care project. Mech. Ageing Dev. 2022, 205, 111684. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Polli, S.; Uberti, F. Cooperative effects of Q10, vitamin D3, and L-arginine on cardiac and endothelial cells. J. Vasc. Res. 2018, 55, 47–60. [Google Scholar] [CrossRef]
- Landi, F.; Gremese, E.; Bernabei, R.; Fantoni, M.; Gasbarrini, A.; Settanni, C.R.; Benvenuto, F.; Bramato, G.; Carfì, A.; Ciciarello, F.; et al. Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin. Exp. Res. 2020, 32, 1613–1620. [Google Scholar] [CrossRef]
- Indicazioni ad Interim sui Principi di Gestione del Long-COVID, Versione del 1° Luglio 2021. Available online: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19+15_2021.pdf/a97f5be0-983b-efaa-2638-3cafc8380296?t=1625124332301 (accessed on 24 February 2023).
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Santucci, L.; Lomuscio, S.; Canu, F.; Primiano, A.; Persichilli, S.; Urbani, A.; Gervasoni, J. A rapid method for determination of underivatized arginine-related metabolites in human plasma using LC−MS/MS. In Proceedings of the 54° National Conference of Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica (SIBioC), Genoa, Italy, 5–7 October 2022; Volume 46. Available online: https://bc.sibioc.it/bc/numero/bcnum/206 (accessed on 18 November 2022).
- Ståhle, L.; Wold, S. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
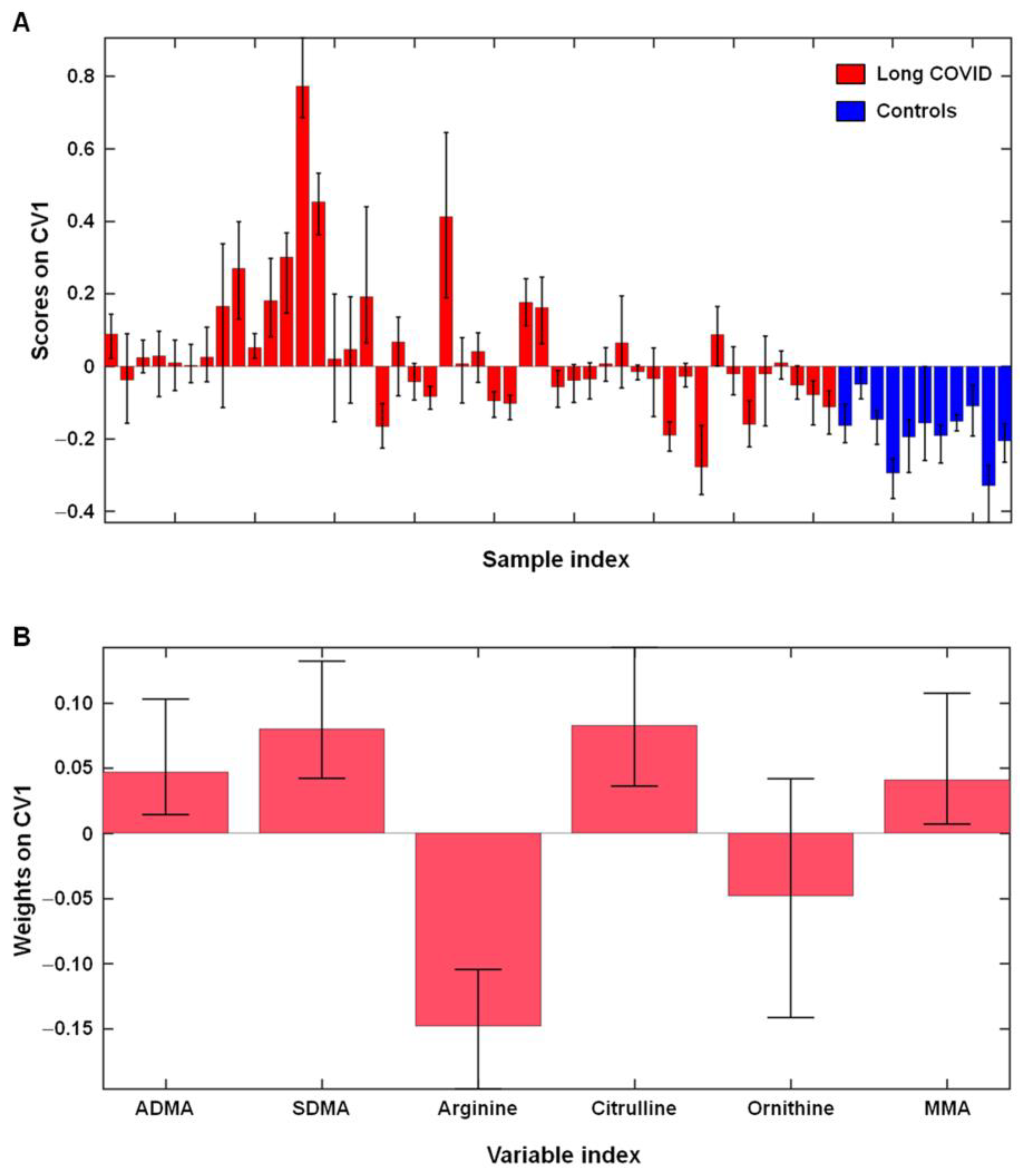
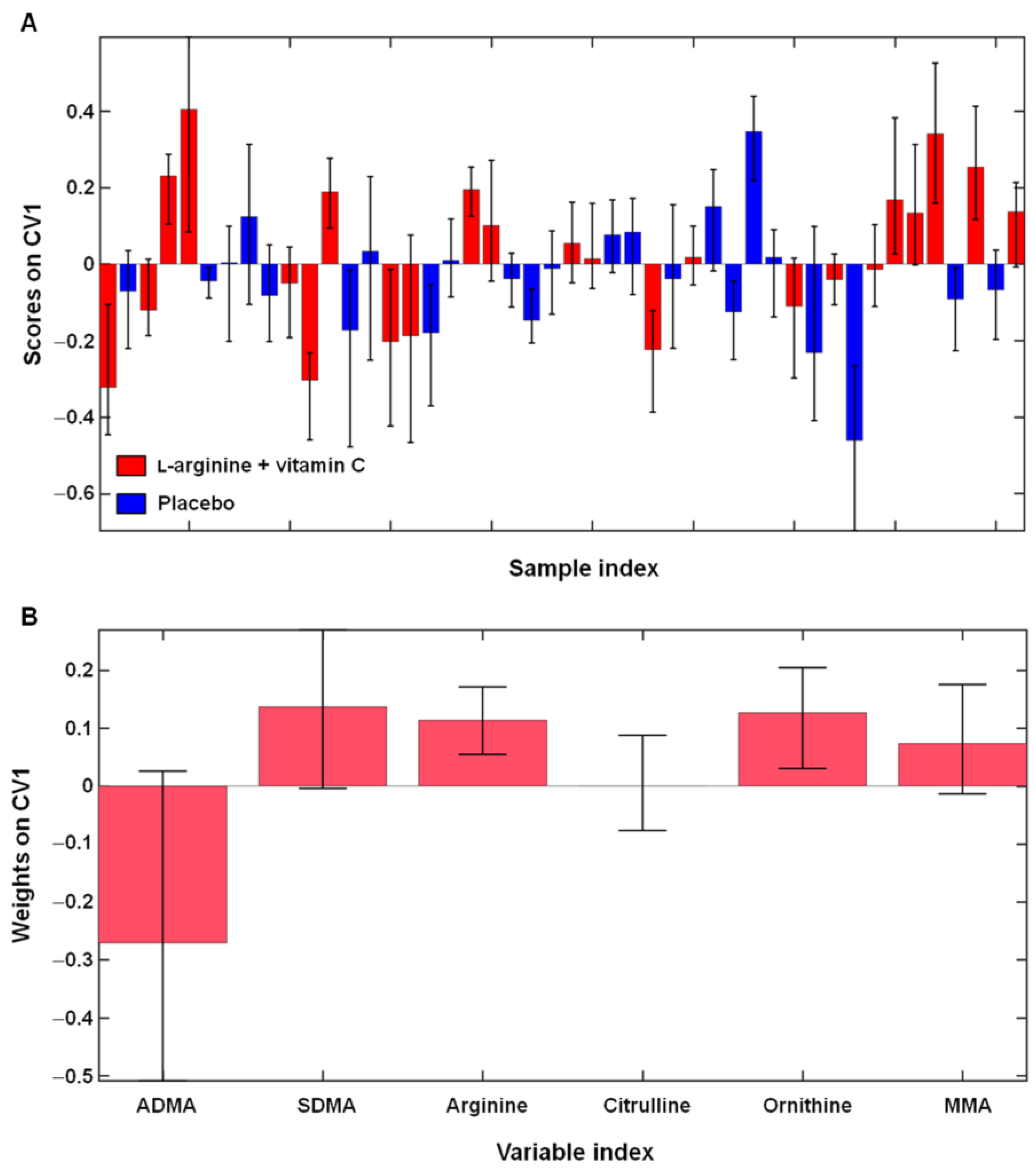

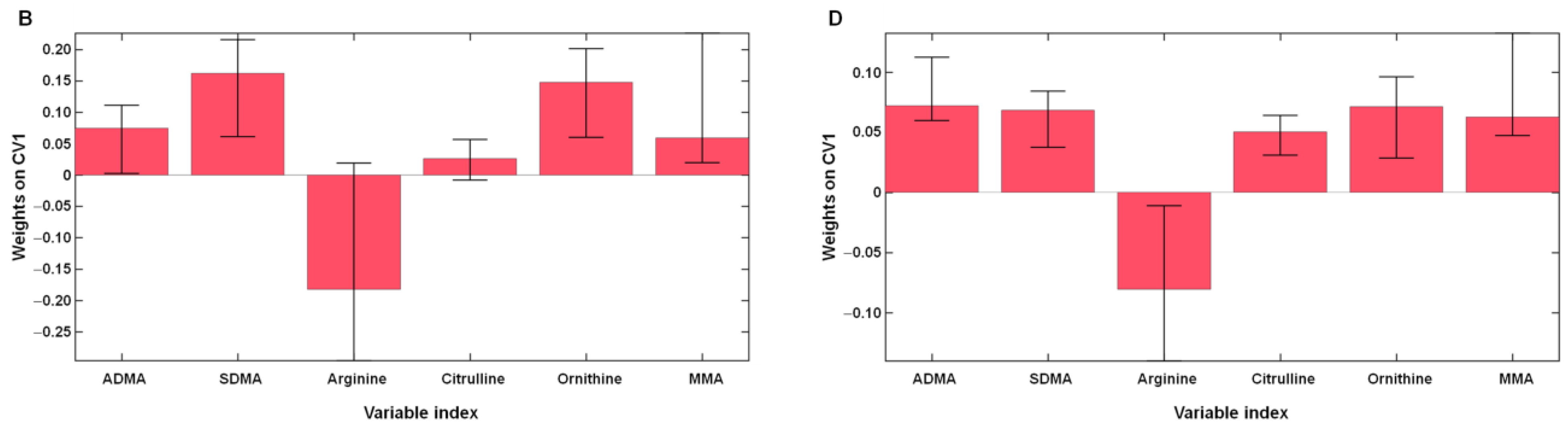

| Characteristic | Long COVID | Healthy Controls (n = 11) | |
|---|---|---|---|
| l-Arginine + Vitamin C (n = 23) | Placebo (n = 23) | ||
| Age, years | 47.3 ± 10.7 | 48.4 ± 8.0 | 48.8 ± 11.1 |
| Women, n (%) | 15 (65.2) | 15 (65.2) | 6 (55.5) |
| BMI, kg/m2 | 25.6 ± 5.6 | 25.6 ± 4.0 | 25.8 ± 4.7 |
| Glucose, mmol/L | 4.8 ± 0.6 | 4.8 ± 0.6 | 5.0 ± 0.3 |
| Total Cholesterol, mmol/L | 5.4 ± 1.1 | 5.3 ± 1.1 | 4.6 ± 0.5 |
| Albumin, mmol/L | 0.66 ± 0.05 | 0.65 ± 0.04 | 0.67 ± 0.05 |
| Creatinine, µmol/L | 69.7 ± 16.4 | 68.2 ± 14.9 | 64.0 ± 11.7 |
| CRP, nmol/L, median (IQR) | 33.3 (80.9) | 33.3 (22.9) | 33.1 (18.4) |
| Hemoglobin, g/L | 14.3 ± 1.5 | 14.2 ± 1.4 | 14.3 ± 1.2 |
| White Blood Cells, 109 L | 5.6 ± 2.0 | 6.1 ± 1.8 | 5.8 ± 2.0 |
| l-arginine, µM | 192.7 ± 74.1 | 196.6 ± 80.6 | 221.6 ± 31.3 |
| Citrulline, µM | 41.4 ± 13.2 | 41.6 ± 11.9 | 30.1 ± 7.5 |
| Ornithine, µM | 122.5 ± 43.6 | 124.9 ± 56.6 | 82.9 ± 12.5 |
| ADMA, µM | 0.60 ± 0.14 | 0.64 ± 0.19 | 0.48 ± 0.02 |
| MMA, µM | 0.13 ± 0.05 | 0.14 ± 0.06 | 0.10 ± 0.02 |
| SDMA, µM | 0.71 ± 0.15 | 0.77 ± 0.25 | 0.53 ± 0.11 |
| Arginine/ADMA | 320.9 ± 97.0 | 316.5 ± 103.2 | 462.8 ± 31.3 |
| Arginine/ornithine | 1.8 ± 1.0 | 1.8 ± 0.9 | 2.7 ± 0.4 |
| GABR | 1.3 ± 0.6 | 1.3 ± 0.6 | 2.0 ± 0.3 |
| Characteristic | l-Arginine + Vitamin C (n = 23) | Placebo (n = 23) | p |
|---|---|---|---|
| l-arginine, µM | 67.8 ± 90.6 | 5.3 ± 81.7 | 0.02 |
| Citrulline, µM | 4.0 ± 10.2 | 2.7 ± 9.2 | 0.65 |
| Ornithine, µM | 30.9 ± 53.0 | 8.8 ± 44.8 | 0.13 |
| ADMA, µM | 0.07 ± 0.10 | 0.04 ± 0.14 | 0.56 |
| MMA, µM | 0.09 ± 0.17 | 0.03 ± 0.15 | 0.19 |
| SDMA, µM | 0.02 ± 0.04 | 0.00 ± 0.05 | 0.34 |
| Arginine/ADMA | 0.07 ± 0.14 | −0.01 ± 0.14 | 0.05 |
| Arginine/ornithine | 0.03 ± 0.17 | −0.02 ± 0.19 | 0.32 |
| GABR | 0.01 ± 0.19 | −0.03 ± 0.21 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvani, R.; Gervasoni, J.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Mario, C.; Gremese, E.; Lomuscio, S.; Paglionico, A.M.; et al. Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Adults with Long COVID: Secondary Analysis of a Randomized Clinical Trial. Int. J. Mol. Sci. 2023, 24, 5078. https://doi.org/10.3390/ijms24065078
Calvani R, Gervasoni J, Picca A, Ciciarello F, Galluzzo V, Coelho-Júnior HJ, Di Mario C, Gremese E, Lomuscio S, Paglionico AM, et al. Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Adults with Long COVID: Secondary Analysis of a Randomized Clinical Trial. International Journal of Molecular Sciences. 2023; 24(6):5078. https://doi.org/10.3390/ijms24065078
Chicago/Turabian StyleCalvani, Riccardo, Jacopo Gervasoni, Anna Picca, Francesca Ciciarello, Vincenzo Galluzzo, Hélio José Coelho-Júnior, Clara Di Mario, Elisa Gremese, Sara Lomuscio, Anna Maria Paglionico, and et al. 2023. "Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Adults with Long COVID: Secondary Analysis of a Randomized Clinical Trial" International Journal of Molecular Sciences 24, no. 6: 5078. https://doi.org/10.3390/ijms24065078
APA StyleCalvani, R., Gervasoni, J., Picca, A., Ciciarello, F., Galluzzo, V., Coelho-Júnior, H. J., Di Mario, C., Gremese, E., Lomuscio, S., Paglionico, A. M., Santucci, L., Tolusso, B., Urbani, A., Marini, F., Marzetti, E., Landi, F., & Tosato, M., on behalf of the Gemelli against COVID-19 Post-Acute Care Team. (2023). Effects of l-Arginine Plus Vitamin C Supplementation on l-Arginine Metabolism in Adults with Long COVID: Secondary Analysis of a Randomized Clinical Trial. International Journal of Molecular Sciences, 24(6), 5078. https://doi.org/10.3390/ijms24065078












