Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways
Abstract
1. Introduction
2. Results
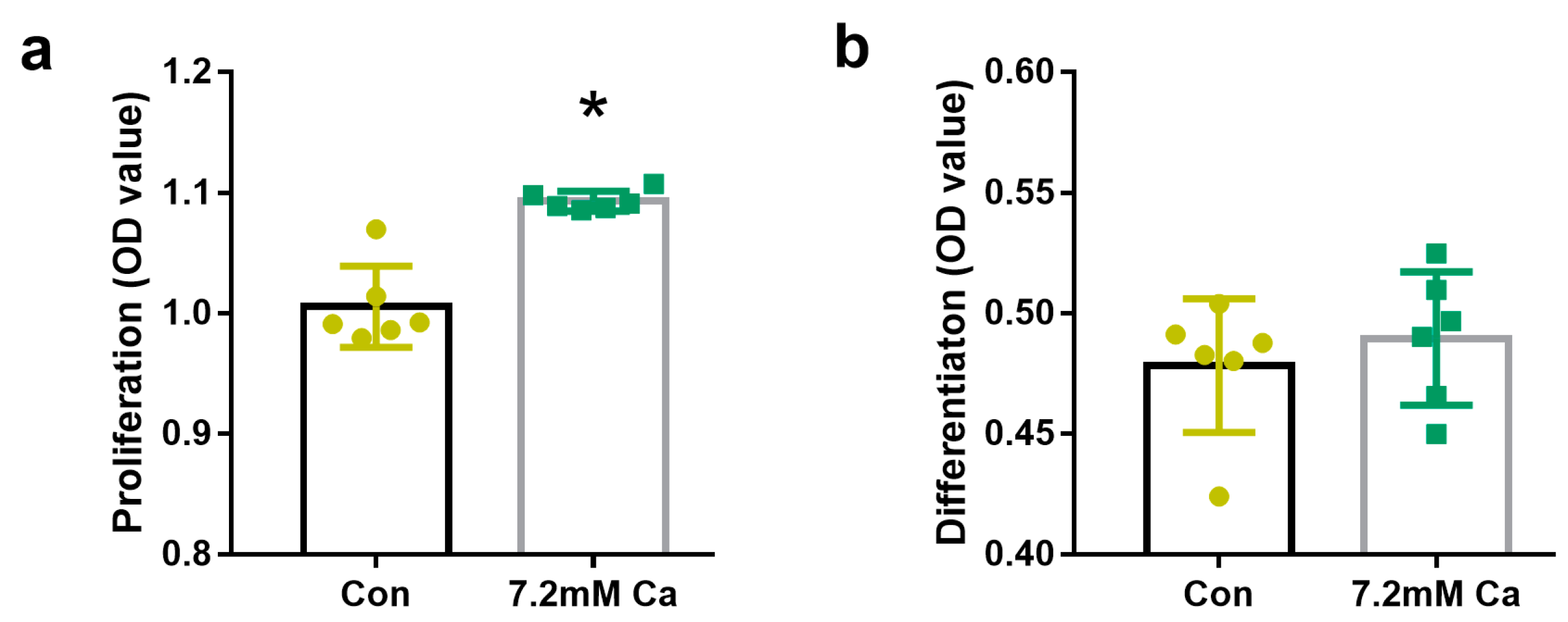
2.1. Extracellular Calcium Levels Affected Osteoblasts Proliferation but Not Osteoblasts Differentiation
2.2. Metabolomics Analysis
2.3. Effects of Extracellular Calcium on Different Pathways Associated with Energy Metabolism
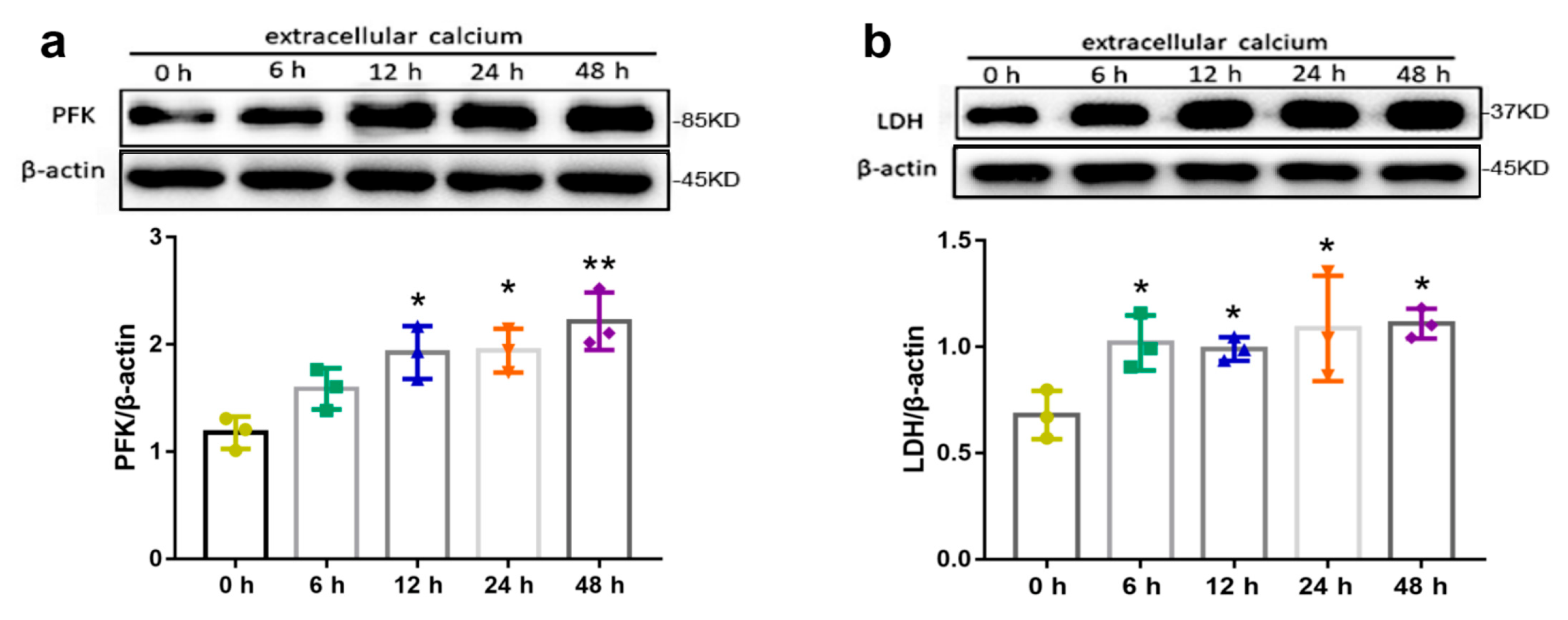
2.4. Effects of Extracellular Calcium on Metabolism Enzymes
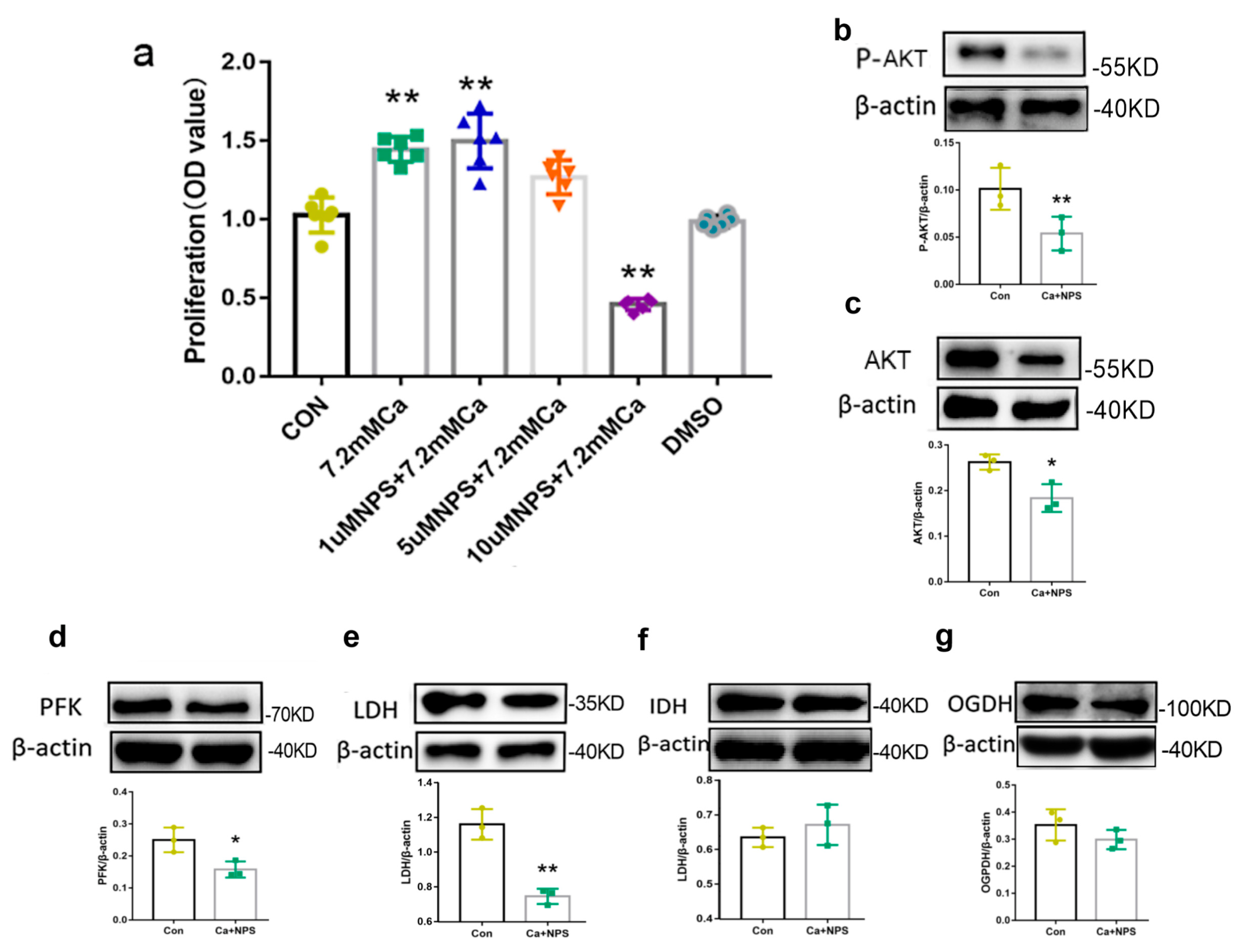
2.5. AKT Activation Regulated Metabolism via High Extracellular Calcium
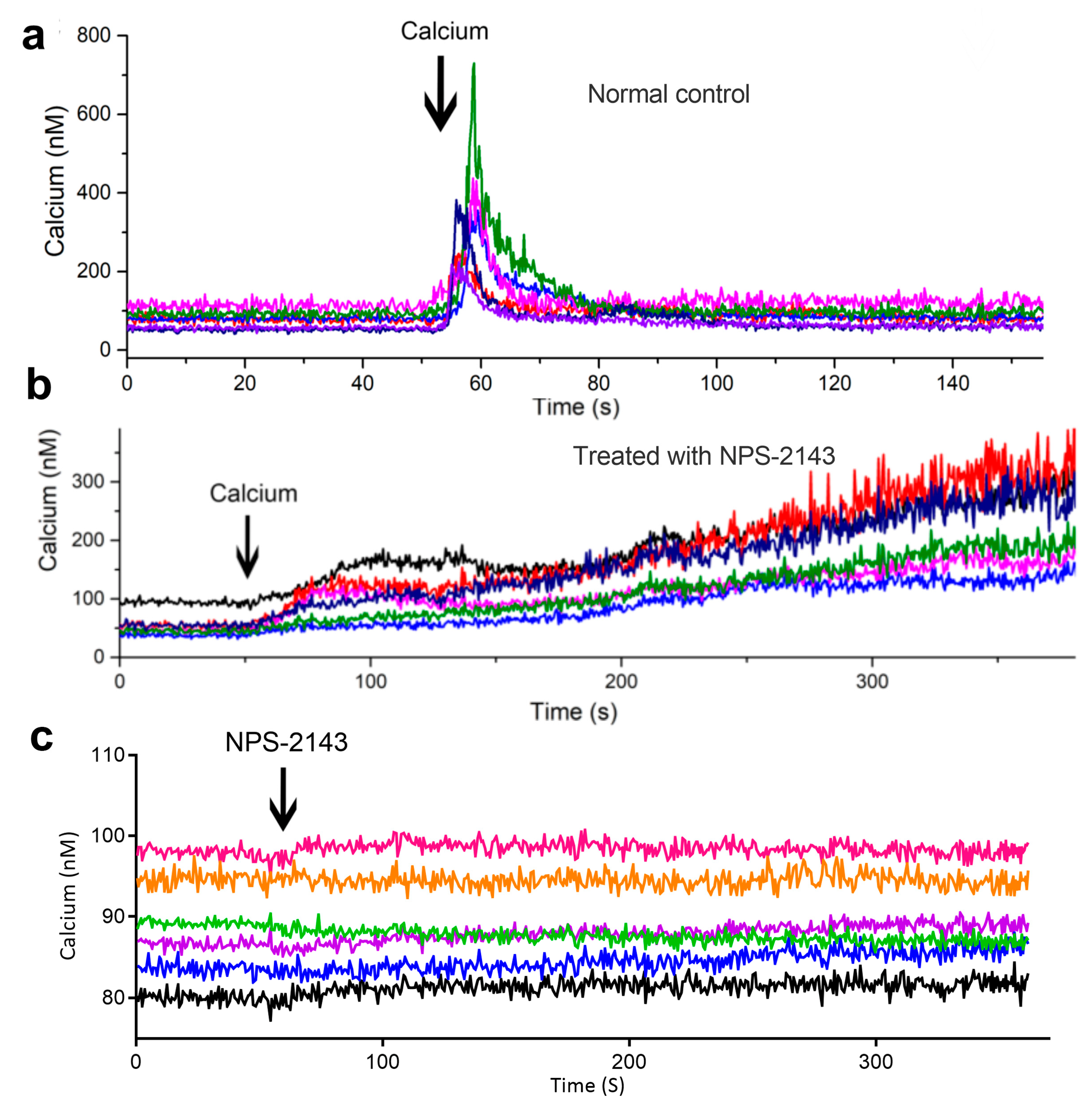
2.6. Extracellular Calcium Induces Intracellular Ca2+ Transients Which Rely on Calcium-Sensing Receptor (CaSR) in Osteoblasts
2.7. CaSR Promotes Osteoblast Proliferation by Regulating Oxidative Metabolism
3. Discussion
4. Materials and Methods
4.1. Cell Culture, G0 Cycle Treatment, and Inhibitory Experiment
4.2. Cell Proliferation Assay, Alkaline Phosphatase (ALP) Activity Assay, and Lactate Concentration Measurements
4.3. Calcium Imaging
4.4. Metabolomics Analysis
4.5. Western Blotting Analysis
4.6. Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boudot, C.; Saidak, Z.; Boulanouar, A.K.; Petit, L.; Gouilleux, F.; Massy, Z.; Brazier, M.; Mentaverri, R.; Kamel, S. Implication of the calcium sensing receptor and the Phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone 2010, 46, 1416–1423. [Google Scholar] [CrossRef]
- Berger, C.E.M.; Rathod, H.; Gillespie, J.I.; Horrocks, B.R.; Datta, H.K. Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: Evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J. Bone Miner. Res. 2001, 16, 2092–2102. [Google Scholar] [CrossRef]
- Zancan, P.; Sola-Penna, M. Calcium influx: A possible role for insulin modulation of intracellular distribution and activity of 6-phosphofructo-1-kinase in human erythrocytes. Mol. Genet. Metab. 2005, 86, 392–400. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.G.; Denton, R.M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem. J. 1980, 190, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, H.; Kerkhofs, M.; La Rovere, R.M.; Bultynck, G. Endoplasmic Reticulum-Mitochondrial Ca2+ Fluxes Underlying Cancer Cell Survival. Front. Oncol. 2017, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of Mitochondrial Ca2+ in the Regulation of Cellular Energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1334–1341. [Google Scholar] [CrossRef]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, C423–C435. [Google Scholar] [CrossRef]
- Murphy, A.N.; Kelleher, J.K.; Fiskum, G. Submicromolar Ca-2+ Regulates Phosphorylating Respiration by Normal Rat-Liver and as-30d Hepatoma Mitochondria by Different Mechanisms. J. Biol. Chem. 1990, 265, 10527–10534. [Google Scholar] [CrossRef]
- Li, X.R.; Sung, X.D.; Carmeliet, P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Esen, E.; Chen, J.Q.; Karner, C.M.; Okunade, A.L.; Patterson, B.W.; Long, F.X. WNT-LRP5 Signaling Induces Warburg Effect through mTORC2 Activation during Osteoblast Differentiation. Cell Metab. 2013, 17, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Shares, B.H.; Busch, M.; White, N.; Shum, L.; Eliseev, R.A. Active mitochondria support osteogenic differentiation by stimulating beta-catenin acetylation. J. Biol. Chem. 2018, 293, 16019–16027. [Google Scholar] [CrossRef]
- Munyon, W.H.; Merchant, D.J. The Relation Between Glucose Utilization, Lactic Acid Production and Utilization and the Growth Cycle of L Strain Fibroblasts. Exp. Cell Res. 1959, 17, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Liu, R.; Li, J.; Zhang, C.; Wang, Y.; Cai, Q.; Qian, X.; Xia, Y.; Zheng, Y.; Piao, Y.; et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 2017, 8, 949. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Calcium Signal. 2020, 1131, 1–6. [Google Scholar]
- Tonelli, F.M.P.; Santos, A.K.; Gomes, D.A.; da Silva, S.L.; Gomes, K.N.; Ladeira, L.O.; Resende, R.R. Stem Cells and Calcium Signaling. Calcium Signal. 2012, 740, 891–916. [Google Scholar]
- Tao, R.; Sun, H.Y.; Lau, C.P.; Tse, H.F.; Lee, H.C.; Li, G.R. Cyclic ADP ribose is a novel regulator of intracellular Ca2+ oscillations in human bone marrow mesenchymal stem cells. J. Cell Mol. Med. 2011, 15, 2684–2696. [Google Scholar] [CrossRef]
- Smedler, E.; Uhlen, P. Frequency decoding of calcium oscillations. Biochim. Biophys. Acta 2014, 1840, 964–969. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chattopadhyay, N.; Kifor, O.; Butters, R.R., Jr.; Sugimoto, T.; Brown, E.M. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J. Bone Miner. Res. 1998, 13, 1530–1538. [Google Scholar] [CrossRef]
- Li, Z.Q.; Liu, T.J.; Gilmore, A.; Gomez, N.M.; Mitchell, C.H.; Li, Y.P.; Oursler, M.J.; Yang, S.Y. Regulator of G protein signaling protein 12 is required for osteoblast differentiation through controlling calcium channel/G alpha i-calcium oscillation-ERK signaling. J. Bone Miner. Res. 2018, 33, 93. [Google Scholar]
- Keinan, D.; Yang, S.Y.; Cohen, R.E.; Yuan, X.; Liu, T.J.; Li, Y.P. Role of regulator of G protein signaling proteins in bone. Front. Biosci. 2014, 19, 634–648. [Google Scholar] [CrossRef]
- Aguirre, A.; Gonzalez, A.; Planell, J.A.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1(+) CD34(+) progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Gorvin, C.M. Molecular and clinical insights from studies of calcium-sensing receptor mutations. J. Mol. Endocrinol. 2019, 63, R1–R16. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, H.; Vervliet, T.; Missiaen, L.; Parys, J.B.; De Smedt, H.; Bultynck, G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta 2014, 1843, 2164–2183. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Tran, T.T.; Kondo, T.; Luo, J.; Ueki, K.; Cantley, L.C.; Kahn, C.R. Phosphoinositide 3-kinase regulatory subunit p85 alpha suppresses insulin action via positive regulation of PTEN. Proc. Natl. Acad. Sci. USA 2006, 103, 12093–12097. [Google Scholar] [CrossRef] [PubMed]
- Boynton, A.L.; Whitfiel, J.F.; Isaacs, R.J.; Morton, H.J. Control of 3t3 Cell-Proliferation by Calcium. In Vitro 1974, 10, 12–17. [Google Scholar]
- Paul, D.; Ristow, H.J. Cell-Cycle Control by Ca++-Ions in Mouse 3t3 Cells and in Transformed 3t3 Cells. J. Cell. Physiol. 1979, 98, 31–39. [Google Scholar] [CrossRef]
- Ma, R.; Gan, L.; Ren, H.; Harrison, A.; Qian, J. PDK2-enhanced glycolysis promotes fibroblast proliferation in thyroid-associated ophthalmopathy. J. Mol. Endocrinol. 2020, 65, 163–174. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Signaling in Control of Cell Growth and Metabolism. Cold Spring Harb. Perspect. Biol. 2012, 4, a006783. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.F.; Kuo, H.C.; Li, J.H.; Wang, Y.S.; Chang, C.C.; Chen, K.C.; Chen, W.C.; Chiu, C.C.; Yang, S.Y.; Chang, W.C. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim. Biophys. Acta-Gen. Subj. 2011, 1810, 1278–1284. [Google Scholar] [CrossRef]
- Deliot, N.; Constantin, B. Plasma membrane calcium channels in cancer: Alterations and consequences for cell proliferation and migration. Biochim. Biophys. Acta 2015, 1848, 2512–2522. [Google Scholar] [CrossRef]
- Wiernan, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar]
- Gottlob, K.; Majewski, N.; Kennedy, S.; Kandel, E.; Robey, R.B.; Hay, N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001, 15, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, G.J.; Dey, S.; Gallagher-Colombo, S.M.; Daurio, N.A.; Tuttle, S.; Busch, T.M.; Lin, A.; Sun, R.; Esipova, T.V.; Vinogradov, S.A.; et al. The PI3K/Akt Pathway Regulates Oxygen Metabolism via Pyruvate Dehydrogenase (PDH)-E1 alpha Phosphorylation. Mol. Cancer Ther. 2015, 14, 1928–1938. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- Novellasdemunt, L.; Tato, I.; Navarro-Sabate, A.; Ruiz-Meana, M.; Mendez-Lucas, A.; Perales, J.C.; Garcia-Dorado, D.; Ventura, F.; Bartrons, R.; Rosa, J.L. Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids. J. Biol. Chem. 2013, 288, 10640–10651. [Google Scholar] [CrossRef]
- Izumiya, M.; Haniu, M.; Ueda, K.; Ishida, H.; Ma, C.; Ideta, H.; Sobajima, A.; Ueshiba, K.; Uemura, T.; Saito, N.; et al. Evaluation of MC3T3-E1 Cell Osteogenesis in Different Cell Culture Media. Int. J. Mol. Sci. 2021, 22, 7752. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Malgaroli, A.; Milani, D.; Meldolesi, J.; Pozzan, T. Fura-2 Measurement of Cytosolic Free Ca-2+ in Monolayers and Suspensions of Various Types of Animal-Cells. J. Cell Biol. 1987, 105, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Di, X.; Li, J.; Kang, Y.; Xie, W.; Sun, L.; Zhang, J. Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways. Int. J. Mol. Sci. 2023, 24, 4991. https://doi.org/10.3390/ijms24054991
Gao X, Di X, Li J, Kang Y, Xie W, Sun L, Zhang J. Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways. International Journal of Molecular Sciences. 2023; 24(5):4991. https://doi.org/10.3390/ijms24054991
Chicago/Turabian StyleGao, Xiaohang, Xiaohui Di, Jingjing Li, Yiting Kang, Wenjun Xie, Lijun Sun, and Jianbao Zhang. 2023. "Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways" International Journal of Molecular Sciences 24, no. 5: 4991. https://doi.org/10.3390/ijms24054991
APA StyleGao, X., Di, X., Li, J., Kang, Y., Xie, W., Sun, L., & Zhang, J. (2023). Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways. International Journal of Molecular Sciences, 24(5), 4991. https://doi.org/10.3390/ijms24054991





