Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review
Abstract
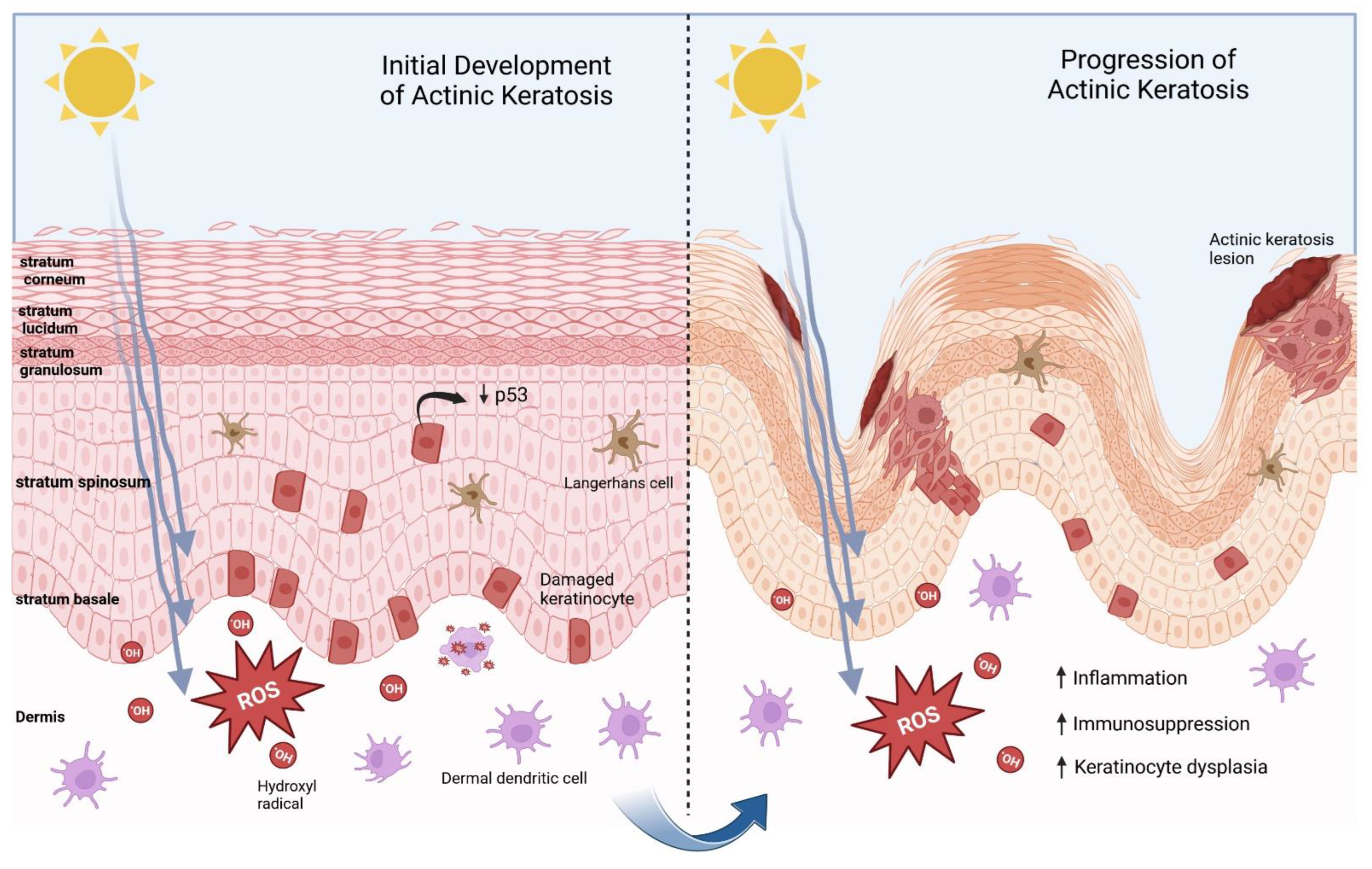
1. Introduction
2. Chemoprophylaxis against AK
2.1. Nicotinamide Chemoprophylaxis
2.2. Acitretin Chemoprophylaxis
2.3. Topical 5-Fluorouracil Chemoprophylaxis
3. Treatment Modalities for AKs
3.1. Topical 5-Flurouracil Treatment
3.1.1. 5-Fluorouracil and Calcipotriol
3.1.2. 5-Fluorouracil and Salicylic Acid
3.2. Imiquimod
3.3. Diclofenac as a Well-Tolerated but Only Mildly Effective Treatment Option
3.4. Tirbanibulin
3.5. Traditional Photodynamic Light Therapy
Daylight Photodynamic Therapy
3.6. Trial Comparisons of 5-FU, Imiquimod, Photodynamic Therapy and Diclofenac Treatment Options
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uhlenhake, E.E. Optimal treatment of actinic keratoses. Clin. Interv. Aging 2013, 8, 29–35. [Google Scholar] [CrossRef]
- Rosen, T.; Lebwohl, M.G. Prevalence and awareness of actinic keratosis: Barriers and opportunities. J. Am. Acad. Dermatol. 2013, 68 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef]
- Dréno, B.; Amici, J.M.; Basset-Seguin, N.; Cribier, B.; Claudel, J.P.; Richard, M.A. Management of actinic keratosis: A practical report and treatment algorithm from AKT eam TM expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1141–1149. [Google Scholar] [CrossRef]
- Chetty, P.; Choi, F.; Mitchell, T. Primary care review of actinic keratosis and its therapeutic options: A global perspective. Dermatol. Ther. (Heidelb) 2015, 5, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.C.V.; Motta Valéria, R.V.; Pantoja, P.C.; Ilha CS de, O.; Magalhães Renata, F.; Galadari, H.; Leonardi, G.R. Actinic keratosis—Review for clinical practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef]
- Darlington, S.; Williams, G.; Neale, R.; Frost, C.; Green, A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch. Dermatol. 2003, 139, 451–455. [Google Scholar] [CrossRef]
- Placzek, M.; Eberlein-König, B.; Przybilla, B. Association between actinic keratoses and potentially photosensitizing drugs. N. Engl. J. Med. 1999, 341, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, S.; Jansen, M.H.E.; Nelemans, P.J.; Kessels, J.; Arits, A.; de Rooij, M.J.M.; Essers, B.A.B.; Quaedvlieg, P.J.F.; Kelleners-Smeets, N.W.J.; Mosterd, K. Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 634–640. [Google Scholar] [CrossRef]
- Zalaudek, I.; Piana, S.; Moscarella, E.; Longo, C.; Zendri, E.; Castagnetti, F.; Pellacani, G.; Lallas, A.; Argenziano, G. Morphologic grading and treatment of facial actinic keratosis. Clin. Dermatol. 2014, 32, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, M.R.; Gniadecki, R.; Skovgaard, G.L. Putative cancer stem cells in cutaneous malignancies. Exp. Dermatol. 2007, 16, 297–301. [Google Scholar] [CrossRef]
- Preston, D.S.; Stern, R.S. Non-melanoma cancer of the skin. N. Engl. J. Med. 1992, 327, 1649–1662. [Google Scholar] [CrossRef]
- Tucci, M.G.; Offidani, A.; Lucarini, G.; Simonelli, L.; Amati, S.; Cellini, A.; Biagini, G.; Ricotti, G. Advances in the understanding of malignant transformation of keratinocytes: An immunohistochemical study. J. Eur. Acad. Dermatol. Venereol. 1998, 10, 118–124. [Google Scholar] [CrossRef]
- Berman, B.; Perez, O.A.; Zell, D. Immunological strategies to fight skin cancer. Skin Ther. Lett. 2006, 11, 1–7. [Google Scholar]
- Hashim, P.W.; Chen, T.; Rigel, D.; Bhatia, N.; Kircik, L.H. Actinic Keratosis: Current Therapies and Insights Into New Treatments. J. Drugs Dermatol. 2019, 18, S161–S166. [Google Scholar] [PubMed]
- Du Thanh, A.; Guillot, B. Actinic keratosis: When is a skin biopsy necessary? Eur. J. Dermatol. 2012, 18, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Cornejo, C.M.; Jambusaria-Pahlajani, A.; Willenbrink, T.J.; Schmults, C.D.; Arron, S.T.; Ruiz, E.S. Field cancerization: Treatment. J. Am. Acad. Dermatol. 2020, 83, 719. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Cerio, R.; Dirschka, T.; Figueras Nart, I.; Lear, J.T.; Peris, K.; Ruiz de Casas, A.; Kaleci, S.; Pellacani, G. A Novel Actinic Keratosis Field Assessment Scale for Grading Actinic Keratosis Disease Severity. Acta Derm.-Venereol. 2017, 97, 1108–1113. [Google Scholar] [CrossRef]
- Figueras Nart, I.; Cerio, R.; Dirschka, T.; Dréno, B.; Lear, J.T.; Pellacani, G.; Peris, K.; Ruiz de Casas, A. Defining the actinic keratosis field: A literature review and discussion. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 544. [Google Scholar] [CrossRef]
- Stockfleth, E.; Ferrandiz, C.; Grob, J.J.; Leigh, I.; Pehamberger, H.; Kerl, H. Development of a treatment algorithm for actinic keratoses: A European consensus. Eur. J. Dermatol. 2008, 18, 651–659. [Google Scholar]
- McIntyre, W.J.; Downs, M.R.; Bedwell, S.A. Treatment options for actinic keratoses. Am. Fam. Physician 2007, 76, 667–671. [Google Scholar]
- Thompson, S.C.; Jolley, D.; Marks, R. Reduction of solar keratoses by regular sunscreen use. N. Engl. J. Med. 1993, 329, 1147–1151. [Google Scholar] [CrossRef]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide Prevents Ultraviolet Radiation-induced Cellular Energy Loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, H.M. A review of nicotinamide: Treatment of skin diseases and potential side effects. J. Cosmet. Dermatol. 2014, 13, 324–328. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 13, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Vestergaard, M.; Radojkovic, B.; Damian, D. Randomized, double-blinded, placebo controlled study to assess the effect of topical 1% nicotinamide on actinic keratoses. Br. J. Dermatol. 2010, 162, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Surjana, D.; Halliday, G.M.; Martin, A.J.; Moloney, F.J.; Damian, D.L. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J. Investig. Dermatol. 2012, 132, 1497–1500. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Mainville, L.; Smilga, A.S.; Fortin, P.R. Effects of Nicotinamide in Skin Cancer and Actinic Keratosis Chemoprophylaxis, and Adverse Effects Related to Nicotinamide: A Systematic Review and Meta-Analysis. J. Cutan. Med. Surg. 2022, 26, 297–308. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Cogorno, L.; Calvi, C.; Marsano, L.A.; Parodi, A. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: A case-control study. Eur. J. Dermatol. 2017, 27, 382–385. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Dalziell, R.A.; McKenzie, C.A.; Lowe, P.M.; Eris, J.M.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Bielski, V.A.; et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br. J. Dermatol. 2015, 175, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Women’s College Hospital. Skin Cancer Prevention with Nicotinamide in Transplant Recipients-Pilot Trial (SPRINTR-Pilot). NCT03769285. Available online: https://clinicaltrials.gov/ct2/show/NCT03769285 (accessed on 26 December 2022).
- Tee, L.Y.; Sultana, R.; Tam, S.Y.; Oh, C.C. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: Systematic review and meta-analyses. J. Am. Acad. Dermatol. 2021, 84, 528–529. [Google Scholar] [CrossRef]
- Patton, T.F.L.; Wolverton, S.E. Comprehensive Dermatologic Therapy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–262.e244. [Google Scholar]
- Harwood, C.A.; Toland, A.E.; Proby, C.M.; Euvrard, S.; Hofbauer, G.F.L.; Tommasino, M.; Bouwes Bavinck, J.N.; KeraCon Consortium. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br. J. Dermatol. 2017, 177, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Mudigonda, T.; Levender, M.M.; O’Neill, J.L.; West, C.E.; Pearce, D.J.; Feldman, S.R. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: A review of single- and multicenter retrospective studies from 2006 to 2010. Dermatol. Surg. 2013, 39 Pt 1, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Overgaard, N.H.; Burke, M.T.; Isbel, N.; Frazer, I.H.; Simpson, F.; Wells, J.W. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? Int. J. Cancer 2016, 138, 281–292. [Google Scholar] [CrossRef]
- Bavinck, J.N.; Tieben, L.M.; Van Der Woude, F.J.; Tegzess, A.M.; Hermans, J.; Ter Schegget, J.; Vermeer, B.J. Prevention of skin cancers and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: A double-blinded, placebo controlled study. J. Clin. Oncol. 1995, 13, 1933–1938. [Google Scholar] [CrossRef]
- Zito, P.; Mazzoni, T. Acitretin. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- De Graaf, Y.; Euvrard, S.; Bouwes-Bavinck, J.N. Systemic and Topical Retinoids in the Management of Skin Cancer in Organ Transplant Recipients. Dermatol. Surg. 2004, 30, 656–661. [Google Scholar] [PubMed]
- Badri, O.; Schmults, C.D.; Karia, P.S.; Ruiz, E.S. Efficacy and Cost Analysis for Acitretin for Basal and Squamous Cell Carcinoma Prophylaxis in Renal Transplant Recipients. Dermatol. Surg. 2021, 47, 125–126. [Google Scholar] [CrossRef]
- Patton, T.; Ferris, L.K. Wolverton Comprehensive Dermatologic Drug Therapy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Solomon-Cohen, E.; Reiss-Huss, S.; Hodak, E.; Davidovici, B. Low-Dose Acitretin for Secondary Prevention of Keratinocyte Carcinomas in Solid-Organ Transplant Recipients. Dermatology 2022, 238, 161–166. [Google Scholar] [CrossRef]
- Allnutt, K.J.; Vogrin, S.; Li, J.; Goh, M.S.; Brennand, S.; Davenport, R.; Chong, A.H. A long-term cohort study of acitretin for prevention of keratinocyte carcinoma in solid organ transplant recipients. Australas. J. Dermatol. 2022, 63, 121–126. [Google Scholar] [CrossRef]
- Ceilley, R. Mechanisms of action of topical 5-fluorouracil: Review and implications for the treatment of dermatological disorders. J. Dermatol. Treat. 2012, 23, 83–89. [Google Scholar] [CrossRef]
- Pomerantz, H.; Hogan, D.; Eilers, D.; Swetter, S.M.; Chen, S.C.; Jacob, S.E.; Warshaw, E.M.; Stricklin, G.; Dellavalle, R.P.; Sidhu-Malik, N.; et al. Long-term Efficacy of Topical Fluorouracil Cream, 5%, for Treating Actinic Keratoses: A Randomized Clinical Trial. JAMA Dermatol. 2015, 151, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A.; Thwin, S.S.; Siegel, J.A.; Marcolivio, K.; Means, A.D.; Leader, N.F.; Shaw, F.M.; Hogan, D.; Eilers, D.; Swetter, S.M.; et al. Chemoprevention of Basal and Squamous Cell Carcinoma With a Single Course of Fluorouracil, 5%, Cream: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Phibbs, C.S.; Chow, A.; Weinstock, M.A.; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial Group. Impact of topical fluorouracil cream on costs of treating keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis. J. Am. Acad. Dermatol. 2018, 79, 501–507. [Google Scholar] [CrossRef]
- Love, W.E.; Bernhard, J.D.; Bordeaux, J.S. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: A systematic review. Arch. Dermatol. 2009, 145, 1431–1438. [Google Scholar] [CrossRef]
- Prince, G.T.; Cameron, M.C.; Fathi, R.; Alkousakis, T. Topical 5-fluorouracil in dermatologic disease. Int. J. Dermatol. 2018, 57, 1259–1264. [Google Scholar] [CrossRef]
- Stockfleth, E.; Bégeault, N.; Delarue, A. Intensity of Local Skin Reactions During 5-Fluorouracil Treatment Related to the Number of Actinic Keratosis Lesions: A Post Hoc, Exploratory Analysis. Dermatol. Ther. 2022, 12, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Jury, C.S.; Ramraka-Jones, V.S.; Gudi, V.; Herd, R.M. A randomized trial of topical 5% 5-fluorouracil (Efudix cream) in the treatment of actinic keratoses comparing daily with weekly treatment. Br. J. Dermatol. 2005, 153, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Ramchatesingh, B.; Martínez Villarreal, A.; Arcuri, D.; Lagacé, F.; Setah, S.A.; Touma, F.; Al-Badarin, F.; Litvinov, I.V. The Use of Retinoids for the Prevention and Treatment of Skin Cancers: An Updated Review. Int. J. Mol. Sci. 2022, 23, 12622. [Google Scholar] [CrossRef]
- Ingham, A.I.; Weightman, W. The efficacy and safety of topical 5% 5-fluorouracil in renal transplant recipients for the treatment of actinic keratoses. Australas. J. Dermatol. 2014, 55, 204–208. [Google Scholar] [CrossRef]
- Loven, K.; Stein, L.; Furst, K.; Levy, S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin. Ther. 2002, 24, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Werschler, W.P. Considerations for use of Fluorouracil cream 0.5% for the treatment of actinic keratosis in elderly patients. J. Clin. Aesthetic Dermatol. 2008, 1, 22–27. [Google Scholar]
- Dohil, M.A. Efficacy, safety, and tolerability of 4% 5-fluorouracil cream in a novel patented aqueous cream containing peanut oil once daily compared with 5% 5-fluorouracil cream twice daily: Meeting the challenge in the treatment of actinic keratosis. J. Drugs Dermatol. 2016, 15, 1218–1224. [Google Scholar] [PubMed]
- Jansen, M.H.; Kessels, J.P.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.; van Pelt, H.P.; Quaedvlieg, P.J.; Essers, B.A.; Steijlen, P.M.; Kelleners-Smeets, N.W.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Merks, I.; Nelemans, P.J.; Kelleners-Smeets, N.W.J.; Mosterd, K.; Essers, B.A.B. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherla. Br. J. Dermatol. 2020, 183, 738–744. [Google Scholar] [CrossRef]
- Kishi, P.; Price, C.J. Life-Threatening Reaction with Topical 5-Fluorouracil. Drug Saf.-Case Rep. 2018, 5, 4. [Google Scholar] [CrossRef]
- Cohen, P.R. Topical application of 5-fluorouracil 5 percent cream associated with severe neutropenia: Discussion of a case and review of systemic reactions after topical treatment with 5-fluorouracil. Dermatol. Online J. 2018, 24, 13030. [Google Scholar] [CrossRef]
- Diasio, R.B.; Offer, S.M. Testing for Dihydropyrimidine Dehydrogenase Deficiency to Individualize 5-Fluorouracil Therapy. Cancers 2022, 14, 3207. [Google Scholar] [CrossRef]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef]
- Kalil, C.L.P.V.; Reinehr, C.P.H.; Bakos, R.M. Short-Term Follow-Up of a Randomized Controlled Trial of 0.5% and 5% 5-Fluorouracil After Microneedling for Treatment of Facial Actinic Keratoses. Dermatol. Surg. 2022, 48, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Kromenacker, B.W.; Brucks, E.S.; Hendricks, A.; Shi, V.Y. Reducing unpleasant side effects of topical 5-Fluorouracil treatment for actinic keratosis: A randomized controlled trial. J. Dermatol. Treat. 2020, 31, 175–179. [Google Scholar] [CrossRef]
- Heuser, C.L.; Heuser, G.G.; Casagrande, J.; de Fátima Pavan Zanella, J.; Winkelmann, E.R. Peeling with 70% glicolic acid followed by 5% 5-fluorouracil as well as 5% 5-fluorouracil cream are effective methods for the treatment of actinic keratoses on upper limbs: A randomized clinical trial. Dermatol. Ther. 2020, 33, e13459. [Google Scholar] [CrossRef]
- West, C.E.; Kwatra, S.G.; Choi, J.; Von Hoff, D.; Booth, L.; Dent, P. A novel plant-derived compound is synergistic with 5-fluorouracil and has increased apoptotic activity through autophagy in the treatment of actinic keratoses. J. Dermatol. Treat. 2022, 33, 590–591. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J. Clin. Investig. 2017, 127, 106–116. [Google Scholar] [CrossRef]
- Dilek Seckin, M.D.; Cerman, A.A.; Ayfer Yildiz, M.D.; Ergun, T. Can topical calcipotriol be a treatment alternative in actinic keratoses? A preliminary report. J. Drugs Dermatol. 2009, 8, 451–454. [Google Scholar] [PubMed]
- Rosenberg, A.R.; Tabacchi, M.; Ngo, K.H.; Wallendorf, M.; Rosman, I.S.; Cornelius, L.A.; Demehri, S. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight 2019, 4, e125476. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.Y.; Nguyen, M.; Moore, S. Cyclic calcipotriene 0.005% foam and 1% 5-fluorouracil cream after cryotherapy in treatment of hyperkeratotic actinic keratosis: A retrospective study. J. Am. Acad. Dermatol. 2021, 84, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, J. Influence of keratolytics on cutaneous pharmacokinetics of glucocorticoids. J. Der Dtsch. Dermatol. Ges. 2021, 19, 554–561. [Google Scholar] [CrossRef]
- Schlaak, M.; Simon, J.C. Topical treatment of actinic keratoses with low-dose 5-fluorouracil in combination with salicylic acid—Pilot study. J. Der Dtsch. Dermatol. Ges. 2010, 8, 174–178. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Dirschka, T.; Prechtl, A.; Melzer, A. Efficacy of low-dose 5-fluorouracil/salicylic acid in actinic kerstosis in relation to treatment duration. J. Der Dtsch. Dermatol. Ges. 2015, 13, 430–438. [Google Scholar]
- Stockfleth, E.; von Kiedrowski, R.; Dominicus, R.; Ryan, J.; Ellery, A.; Falqués, M.; Ivanoff, N.; Azeredo, R.R. Efficacy and Safety of 5-Fluorouracil 0.5%/Salicylic Acid 10% in the Field-Directed Treatment of Actinic Keratosis: A Phase III, Randomized, Double-Blind, Vehicle-Controlled Trial. Dermatol. Ther. 2016, 7, 81–96. [Google Scholar] [CrossRef]
- Reinhold, U.; Hadshiew, I.; Melzer, A.; Prechtl, A. Low-dose 5-fluorouracil in combination with salicylic acid for the treatment of actinic keratoses on the hands and/or forearms—Results of a non-interventional study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 455–462. [Google Scholar] [CrossRef]
- Garofalo, V.; Geraci, F.; Di Prete, M.; Lanna, C.; Lozzi, F.; Cosio, T.; Lambiase, S.; Gaeta Shumak, R.; Di Raimondo, C.; Diluvio, L.; et al. Early clinical response to 5-fluorouracil 0.5% and salicylic acid 10% topical solution in the treatment of actinic keratoses of the head: An observational study. J. Dermatol. Treat. 2022, 33, 2264–2669. [Google Scholar] [CrossRef]
- Dirschka, T.; Pellacani, G.; Micali, G.; Malvehy, J.; Stratigos, A.J.; Casari, A.; Schmitz, L.; Gupta, G.; Athens AK Study Group; Panagiotopoulos, A.; et al. A proposed scoring A proposed scoring the head: Actinic keratosis area and severity index. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Meyer, T.; Benninghoff, B.; Christophers, E. Successful treatment of actinic keratosis with imiquimod cream 5%: A report of six cases. Br. J. Dermatol. 2001, 144, 1050–1053. [Google Scholar] [CrossRef]
- Imbertson, L.M.; Couture, A.M.; Gibson, S.J.; Smith, R.M.; Miller, R.L.; Reiter, M.J.; Wagner, T.L.; Tomai, M.A.; Beaurline, J.M. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J. Investig. Dermatol. 1998, 110, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int. J. Immunol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Gerritsen, M.J.P.; Gupta, G.; Ortonne, J.P.; Serresi, S.; Bichel, J.; Lee, J.H.; Fox, T.L.; Alomar, A. Imiquimod 5% cream for the treatment of actinic keratosis: Results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J. Am. Acad. Dermatol. 2004, 51, 547–555. [Google Scholar] [CrossRef]
- Ooi, T.; Barnetson, R.S.; Zhuang, L.; McKane, S.; Lee, J.H.; Slade, H.B.; Halliday, G.M. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: A randomized controlled trial. Br. J. Dermatol. 2006, 154, 72–78. [Google Scholar] [CrossRef]
- Oyama, S.; Funasaka, Y.; Tsuchiya, S.I.; Kawana, S.; Saeki, H. Increased number of mast cells in the dermis in actinic keratosis lesions effectively treated with imiquimod. J. Dermatol. 2017, 44, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Gaspari, A.A. Mechanism of action and other potential roles of an immune response modifier. Cutis 2007, 79 (Suppl. S4), s36–s45. [Google Scholar]
- Bubna, A.K. Imiquimod—Its role in the treatment of cutaneous malignancies. Indian J. Pharmacol. 2015, 47, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Schön, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Schön, M. TLR7 and TLR8 as targets in cancer therapy. Oncogene 2008, 27, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Persaud, A.N.; Shamuelova, E.; Sherer, D.; Lou, W.; Singer, G.; Cervera, C.; Lamba, S.; Lebwohl, M.G. Clinical effect of imiquimod 5% cream in the treatment of actinic keratosis. J. Am. Acad. Dermatol. 2002, 47, 553–556. [Google Scholar] [CrossRef]
- Lee, P.K.; Harwell, W.B.; Loven, K.H.; Phillips, T.J.; Whiting, D.A.; Andres, K.L.; Lee, J.H. Long-term clinical outcomes following treatment of actinic keratosis with imiquimod 5% cream. Dermatol. Surg. 2005, 31, 659–664. [Google Scholar] [CrossRef]
- Ulrich, C.; Busch, J.O.; Meyer, T.; Nindl, I.; Schmook, T.; Sterry, W.; Stockfleth, E. Successful treatment of multiple actinic keratoses in organ transplant patients with topical 5% imiquimod: A report of six cases. Br. J. Dermatol. 2006, 155, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Sterry, W.; Carey-Yard, M.; Bichel, J. Multicentre, open-label study using imiquimod 5% cream in one or two 4-week courses of treatment for multiple actinic keratoses on the head. Br. J. Dermatol. 2007, 157 (Suppl. S2), 41–46. [Google Scholar] [CrossRef]
- Alomar, A.; Bichel, J.; McRae, S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br. J. Dermatol. 2007, 157, 133–141. [Google Scholar] [CrossRef]
- Gebauer, K.; Shumack, S.; Cowen, P.S. Effect of dosing frequency on the safety and efficacy of imiquimod 5% cream for treatment of actinic keratosis on the forearms and hands: A phase II, randomized placebo-controlled trial. Br. J. Dermatol. 2009, 161, 897–903. [Google Scholar] [CrossRef]
- M’barek, L.B.; Mebazaa, A.; Euvrard, S.; Frances, C.; Thervet, E.; Morel, P.; Menasché, S.; Legendre, C.; Lebbe, C. 5% topical imiquimod tolerance in transplant recipients. Dermatology 2007, 215, 130–133. [Google Scholar] [CrossRef]
- Ulrich, C.; Bichel, J.; Euvrard, S.; Guidi, B.; Proby, C.M.; Van De Kerkhof, P.C.M.; Amerio, P.; Rønnevig, J.; Slade, H.B.; Stockfleth, E. Topical immunomodulation under systemic immunosuppression: Results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br. J. Dermatol. 2007, 157 (Suppl. S2), 25–31. [Google Scholar] [CrossRef]
- Stockfleth, E.; Gupta, G.; Peris, K.; Aractingi, S.; Dakovic, R.; Alomar, A. Reduction in lesions from Lmax: A new concept for assessing efficacy of field-directed therapy for actinic keratosis. Results with imiquimod 3.75%. Eur. J. Dermatol. 2014, 24, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kopera, D. Earliest stage treatment of actinic keratosis with imiquimod 3.75% cream: Two case reports-Perspective for non melanoma skin cancer prevention. Dermatol. Ther. 2020, 33, e13517. [Google Scholar] [CrossRef]
- Zavattaro, E.; Veronese, F.; Landucci, G.; Tarantino, V.; Savoia, P. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: Our case series. J. Dermatol. Treat. 2020, 31, 285–289. [Google Scholar] [CrossRef]
- Rivers, J.K.; Rosoph, L.; Provost, N.; Bissonnette, R. Open-label study to assess the safety and efficacy of imiquimod 5% cream applied once daily three times per week in cycles for treatment of actinic keratoses on the head. J. Cutan. Med. Surg. 2008, 12, 97–101. [Google Scholar] [CrossRef]
- Stockfleth, E. Lmax and imiquimod 3.75%: The new standard in AK management. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.I.; Skinner, S.L.; Marbury, T.C.; Owens, M.L.; Kurup, S.; McKane, S.; Greene, R.J. Pharmacokinetics and safety of imiquimod 5% cream in the treatment of actinic keratoses of the face, scalp, or hands and arms. Arch. Dermatol. Res. 2004, 296, 6–11. [Google Scholar] [CrossRef]
- Heikkinen, A.K.; Susitaival, P. Severe Systemic Reaction to Topical Imiquimod. Acta Derm. Venereol. 2011, 91, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Hanger, C.; Dalrymple, J.; Hepburn, D. Systemic side effects from topical imiquimod. N. Z. Med. J. 2005, 118, U1682. [Google Scholar]
- Bhatia, N. Local skin reactions and the onset of influenza-like signs and symptoms induced by imiquimod. JAAD Int. 2022, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.G. Diclofenac gel in the treatment of actinic keratoses. Ther. Clin. Risk Manag. 2011, 7, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Fecker, L.F.; Stockfleth, E.; Braun, F.K.; Rodust, P.M.; Schwarz, C.; Köhler, A.; Leverkus, M.; Eberle, J. Enhanced death ligand-induced apoptosis in cutaneous SCC cells by treatment with diclofenac/hyaluronic acid correlates with downregulation of c-FLIP. J. Investig. Dermatol. 2010, 130, 2098–2109. [Google Scholar] [CrossRef]
- Singer, K.; Dettmer, K.; Unger, P.; Schönhammer, G.; Renner, K.; Peter, K.; Siska, P.J.; Berneburg, M.; Herr, W.; Oefner, P.J.; et al. Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis. Front. Oncol. 2019, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.K.; McLean, D.I. An open study to assess the efficacy and safety of topical 3% diclofenac in a 2.5% hyaluronic acid gel for the treatment of actinic keratoses. Arch. Dermatol. 1997, 133, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.E., Jr.; Taylor, J.R.; Tschen, E.; Kang, S. Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int. J. Dermatol. 2001, 40, 709–713. [Google Scholar] [CrossRef]
- Rivers, J.K.; Arlette, J.; Shear, N.; Guenther, L.; Carey, W.; Poulin, Y. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br. J. Dermatol. 2002, 146, 94–100. [Google Scholar] [CrossRef]
- Cayirli, M.; Köse, O.; Demiriz, M. Clinical, dermoscopic and immunohistochemical assessment of actinic keratoses and evaluation of the effectiveness of diclofenac therapy with immunohistochemical analysis. Arch. Dermatol. Res. 2013, 305, 389–395. [Google Scholar] [CrossRef]
- Pflugfelder, A.; Welter, A.K.; Leiter, U.; Weide, B.; Held, L.; Eigentler, T.K.; Dirschka, T.; Stockfleth, E.; Nashan, D.; Garbe, C. German Dermatologic Cooperative Oncology Group. Open label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: A trial of the German Dermatologic Cooperative Oncology Group. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 48–53. [Google Scholar] [CrossRef]
- Tampucci, S.; Carpi, S.; Digiacomo, M.; Polini, B.; Fogli, S.; Burgalassi, S.; Macchia, M.; Nieri, P.; Manera, C.; Monti, D. Diclofenac-Derived Hybrids for Treatment of Actinic Keratosis and Squamous Cell Carcinoma. Molecules 2019, 24, 1793. [Google Scholar] [CrossRef]
- Kempers, S.; DuBois, J.; Forman, S.; Poon, A.; Cutler, E. Tirbanibulin ointment 1% as a novel treatment for actinic keratosis: Phase 1 and 2 results. J. Drugs Dermatol. 2020, 19, 1093–1100. [Google Scholar] [CrossRef]
- Fallah-Tafti, A.; Foroumadi, A.; Tiwari, R.; Shirazi, A.N.; Hangauer, D.G.; Bu, Y.; Akbarzadeh, T.; Parang, K.; Shafiee, A. Thiazolyl N-benzyl-substituted acetamide derivatives: Synthesis, Src kinase inhibitory and anticancer activities. Eur. J. Med. Chem. 2011, 46, 4853–4858. [Google Scholar] [CrossRef]
- Anbalagan, M.; Ali, A.; Jones, R.K.; Marsden, C.G.; Sheng, M.; Carrier, L.; Bu, Y.; Hangauer, D.; Rowan, B.G. Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts. Mol. Cancer Ther. 2012, 11, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Kempers, S.; Lain, E.; Schlesinger, T.; Tyring, S.; Forman, S.; Ablon, G.; Martin, G.; Wang, H.; Cutler, D.L.; et al. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N. Engl. J. Med. 2021, 384, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Karrer, S.; Sauerwald, A.; Landthaler, M. Photodynamic therapy with topical application of 5-aminolevulinic acid in the treatment of actinic keratoses: An initial clinical study. Dermatology 1996, 192, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Szeimiesa, R.M.; Karrera, S.; Radakovic-Fijanb, S.; Tanewb, A.; Calzavara-Pintonc, P.G.; Zanec, C.; Sidoroffd, A.; Hempele, M.; Ulrichf, J.; Proebstleg, T.; et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: A prospective, randomized study. J. Am. Acad. Dermatol. 2002, 47, 258–262. [Google Scholar] [CrossRef]
- Nokes, B.; Apel, M.; Jones, C.; Brown, G.; Lang, J.E. Aminolevulinic acid (ALA): Photodynamic detection and potential therapeutic applications. J. Surg. Res. 2013, 181, 262–271. [Google Scholar] [CrossRef]
- Warren, C.B.; Lohser, S.; Wene, L.C.; Pogue, B.W.; Bailin, P.L.; Maytin, E.V. Noninvasive fluorescence monitoring of protoporphyrin IX production and clinical outcomes in actinic keratoses following short-contact application of 5-aminolevulinate. J. Biomed. Opt. 2010, 15, 051607. [Google Scholar] [CrossRef]
- Lui, H.; Anderson, R.R. Photodynamic therapy in dermatology—Shedding a different light on skin-disease. Arch. Dermatol. 1992, 128, 1631–1636. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. Photodynamic therapy: Update 2006 Part 2: Clinical results. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 439–451. [Google Scholar] [CrossRef]
- Rollakanti, K.R.; Anand, S.; Davis, S.C.; Pogue, B.W.; Maytin, E.V. Noninvasive Optical Imaging of UV-Induced Squamous Cell Carcinoma in Murine Skin: Studies of Early Tumor Development and Vitamin D Enhancement of Protoporphyrin IX Production. Photochem. Photobiol. 2015, 97, 1469–1478. [Google Scholar] [CrossRef]
- Piacquadio, D.J.; Chen, D.M.; Farber, H.F.; Fowler, J.F., Jr.; Glazer, S.D.; Goodman, J.J.; Hruza, L.L.; Jeffes, E.W.; Ling, M.R.; Phillips, T.J.; et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: Investigator-blinded, phase 3, multicenter trials. Arch. Dermatol. 2004, 140, 41–46. [Google Scholar] [CrossRef]
- Berman, B.; Bhatia, N.; Piacquadio, D.; Houlihan, A.; Davidson, D.; Siegel, D. Aminolevulinic Acid 20 % solution photodynamic therapy in the treatment of actinic keratoses on the upper extremities: A post hoc analysis of a phase 3, randomized, vehicle-controlled trial. Photodiagnosis Photodyn. Ther. 2020, 32, 102013. [Google Scholar] [CrossRef]
- Reinhold, U.; Dirschka, T.; Ostendorf, R.; Aschoff, R.; Berking, C.; Philipp-Dormston, W.G.; Hahn, S.; Lau, K.; Jäger, A.; Schmitz, B.; et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLE. Br. J. Dermatol. 2016, 175, 696–705. [Google Scholar] [CrossRef]
- Reinhold, U. A review of BF-200 ALA for the photodynamic treatment of mild-to-moderate actinic keratosis. Future Oncol. 2017, 13, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Reinhold, U.; Dominicus, R.; Aschoff, R.; Szeimies, R.M.; Dirschka, T. Red light photodynamic therapy with BF-200 ALA showed superior efficacy in the treatment of actinic keratosis on the extremities, trunk, and neck in a vehicle-controlled phase III study. J. Am. Acad. Dermatol. 2021, 85, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Bai-Habelski, J.C.; Ko, A.; Ortland, C.; Stocker, M.; Ebeling, A.; Reinhold, U. 5-ALA loaded self-adhesive patch-PDT is effective and safe in the treatment of actinic keratoses on hands and arms. Exp. Dermatol. 2022, 31, 1385–1391. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Z.; Wang, P.; Zhu, L.; Zhang, L.; Zho, Z.; Shi, L.; Wang, X. Comparison of efficacy, adverse effects and costs between 20 % ALA-PDT and 10 % ALA-PDT for the treatment of actinic keratosis in Chinese patients. Photodiagnosis Photodyn. Ther. 2020, 31, 101605. [Google Scholar] [CrossRef]
- Pariser, D.M.; Lowe, N.J.; Stewart, D.M.; Jarratt, M.T.; Lucky, A.W.; Pariser, R.J.; Yamauchi, P.S. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: Results of a prospective randomized multicenter trial. J. Am. Acad. Dermatol. 2003, 48, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Vinciullo, C.; Francis, D.; Spelman, L.; Nguyen, R.; Fergin, P.; Thai, K.E.; Murrell, D.; Weightman, W.; Anderson, C.; et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: A prospective, randomized study. J. Dermatol. Treat. 2003, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lonsdorf, A.S.; Keller, A.; Hartmann, J.; Enk, A.H.; Gholam, P. Ablative Fractional Laser-assisted Low-irradiance Photodynamic Therapy for Treatment of Actinic Keratoses in Organ Transplant Recipients: A Prospective, Randomized, Intraindividual Controlled Trial. Acta Derm.-Venereol. 2022, 102, adv00694. [Google Scholar] [CrossRef] [PubMed]
- Vallecorsa, P.; Di Venosa, G.; Ballatore, M.B.; Ferreyra, D.; Mamone, L.; Sáenz, D.; Calvo, G.; Durantini, E.; Casas, A. Novel meso-substituted porphyrin derivatives and its potential use in photodynamic therapy of cancer. BMC Cancer 2021, 21, 547. [Google Scholar] [CrossRef]
- Bullock, T.A.; Negrey, J.; Hu, B.; Warren, C.B.; Hasan, T.; Maytin, E.V. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: An interventional cohort-controlled trial. J. Am. Acad. Dermatol. 2022, 87, 80–86. [Google Scholar] [CrossRef]
- Steeb, T.; Niesert, A.C.; French, L.E.; Berking, C.; Heppt, M.V. Microneedling-assisted photodynamic therapy for the treatment of actinic keratosis: Results from a systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 515–519. [Google Scholar] [CrossRef]
- Jhanker, Y.; Mbano, M.N.; Ponto, T.; Espartero, L.J.L.; Yamada, M.; Prow, T.; Benson, H.A. Comparison of physical enhancement technologies in the skin permeation of methyl amino levulinic acid (mALA). Int. J. Pharm. 2021, 610, 121258. [Google Scholar] [CrossRef]
- Wulf, H.C.; Heerfordt, I.M. Counteracting Side-effects of Photodynamic Therapy for Actinic Keratoses. Anticancer Res. 2022, 42, 5017–5020. [Google Scholar] [CrossRef]
- Heerfordt, I.M.; Wulf, H.C. Pain and stinging associated with pretreatment in photodynamic therapy of actinic keratosis. Photodiagnosis Photodyn. Ther. 2019, 25, 225–226. [Google Scholar] [CrossRef]
- Yang, C.S.; Kuhn, H.; Cohen, L.M.; Kroumpouzos, G. Aminolevulinic Acid Photodynamic Therapy in the Treatment of Erosive Pustular Dermatosis of the Scalp: A Case Series. JAMA Dermatol. 2016, 152, 694–697. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Landthaler, M.; Karrer, S. Non-oncologic indications for ALA-PDT. J. Dermatolog. Treat. 2002, 13 (Suppl. S1), s13–s18. [Google Scholar] [CrossRef] [PubMed]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patient treated with methyl aminolevulinate compare to aminolevulinic acid. J. Drugs Dermatol. 2006, 5, 353–356. [Google Scholar]
- Kaw, U.; Ilyas, M.; Bullock, T.; Rittwage, L.; Riha, M.; Vidimos, A.; Hu, B.; Warren, C.B.; Maytin, E.V. A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: Bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J. Am. Acad. Dermatol. 2020, 82, 862–868. [Google Scholar] [CrossRef]
- Salvio, A.G.; Stringasci, M.D.; Requena, M.B.; de Oliveira, E.R.; da Costa Medeiro, M.M.; Bagnato, V.S. Field cancerization treatment: Adjustments to an ALA red light photodynamic therapy protocol to improve pain tolerance. Photodiagnosis Photodyn. Ther. 2021, 35, 102415. [Google Scholar] [CrossRef]
- Brumana, M.B.; Milani, M.; Puviani, M. Efficacy of lidocaine 7 %, tetracaine 7 % self-occlusive cream in reducing MAL-cPDT-associated pain in subjects with actinic keratosis: A randomized, single-blind, vehicle-controlled trial (The “3P-Trial”). Photodiagnosis Photodyn. Ther. 2020, 30, 101758. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Szczepanik-Kułak, P.; Raczkiewicz, D.; Niewiedzioł, M.; Gerkowicz, A.; Kowalczuk, D.; Kwaśny, M.; Krasowska, D. Topical Photodynamic Therapy with Different Forms of 5-Aminolevulinic Acid in the Treatment of Actinic Keratosis. Pharmaceutics 2022, 14, 346. [Google Scholar] [CrossRef]
- Meierhofer, C.; Silic, K.; Urban, M.V.; Tanew, A.; Radakovic, S. The impact of occlusive vs non-occlusive application of 5-aminolevulinic acid (BF-200 ALA) on the efficacy and tolerability of photodynamic therapy for actinic keratosis on the scalp and face: A prospective within-patient comparison trial. Photodermatol. Photoimmunol. Photomed. 2021, 37, 56–62. [Google Scholar] [CrossRef]
- Vicentini, C.; Vignion-Dewalle, A.S.; Thecua, E.; Lecomte, F.; Maire, C.; Deleporte, P.; Behal, H.; Kerob, D.; Duhamel, A.; Mordon, S.; et al. Photodynamic therapy for actinic keratosis of the forehead and scalp: A randomized, controlled, phase II clinical study evaluating the noninferiority of a new protocol involving irradiation with a light-emitting, fabric-based device (the Flexitheralight protocol) compared with the conventional protocol involving irradiation with the Aktilite CL 128 lamp. Br. J. Dermatol. 2019, 765–773. [Google Scholar]
- Dubois, M.; Abi Rached, H.; Dezoteux, F.; Maire, C.; Vicentini, C.; Behal, H.; Thecua, E.; Lecomte, F.; Mordon, S.; Mortier, L. Real-life evaluation of the treatment of actinic keratoses by textile photodynamic therapy (FLUXMEDICARE® device). Photodiagnosis Photodyn. Ther. 2021, 34, 102213. [Google Scholar] [CrossRef] [PubMed]
- Braathen, L.R. Daylight photodynamic therapy in private practice in Switzerland: Gain without pain. Acta. Derm. Venereol. 2012, 92, 653–654. [Google Scholar] [CrossRef]
- Dirschka, T.; Ekanayake-Bohlig, S.; Dominicus, R.; Aschoff, R.; Herrera-Ceballos, E.; Botella-Estrada, R.; Hunfeld, A.; Kremser, M.; Schmitz, B.; Lübbert, H.; et al. A randomized, intraindividual, non-inferiority, Phase III study comparing daylight photodynamic therapy with BF-200 ALA gel and MAL cream for the treatment of actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Maire, C.; Vignion-Dewalle, A.S.; Cartier, H.; Mordon, S. Artificial white light photodynamic therapy for actinic keratosis: A study of 38 patients in private office practice. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e165–e167. [Google Scholar] [CrossRef]
- Creusot, M.; Mordon, S. Clinical evaluation of a short illumination duration (1 hour) when performing photodynamic therapy of actinic keratosis using the Dermaris light source. Photodiagnosis Photodyn. Ther. 2021, 36, 102618. [Google Scholar] [CrossRef] [PubMed]
- Bai-Habelski, J.C.; Medrano, K.; Palacio, A.; Reinhold, U. No room for pain: A prospective study showing effective and nearly pain-free treatment of actinic keratosis with simulated daylight photodynamic therapy (SDL-PDT) using the IndoorLux® System in combination with BF-200 ALA (Ameluz®). Photodiagnosis Photodyn. Ther. 2022, 37, 102692. [Google Scholar] [CrossRef]
- de Oliveira Bento, C.; Pantaleão, L.; de Souza, M.B.; Vilar, E.A.G.; Luiz, R.R.; Soares Filho, P.J.; Gismondi, R.A.O.C.; Issa, M.C.A. Comparison of clinical and histologic findings in daylight photodynamic therapy for skin field cancerization: A randomized controlled four-arm study on physical methods-assisted delivery of methyl aminolevulinate. Photodiagnosis Photodyn. Ther. 2021, 35, 102404. [Google Scholar] [CrossRef]
- Piaserico, S.; Piccioni, A.; Garcìa-Rodrigo, C.G.; Sacco, G.; Pellegrini, C.; Fargnoli, M.C. Sequential treatment with calcitriol and methyl aminolevulinate-daylight photodynamic therapy for patients with multiple actinic keratoses of the upper extremities. Photodiagnosis Photodyn. Ther. 2021, 34, 102325. [Google Scholar] [CrossRef] [PubMed]
- Bernad, I.; Aguado, L.; Núñez-Córdoba, J.M.; Redondo, P. Daylight photodynamic therapy for prevention of new actinic keratosis and keratinocyte carcinomas in organ transplants. A cryotherapy-controlled randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1464–1470. [Google Scholar] [CrossRef]
- Hasan, Z.U.; Ahmed, I.; Matin, R.N.; Homer, V.; Lear, J.T.; Ismail, F.; Whitmarsh, T.; Green, A.C.; Thomson, J.; Milligan, A.; et al. opical treatment of actinic keratoses in organ transplant recipients: A feasibility study for SPOT (Squamous cell carcinoma Prevention in Organ transplant recipients using Topical treatments). Br. J. Dermatol. 2022, 187, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, R.; Levandoski, K.A.; Zhu, Z.; Sokil, M.; Chren, M.M.; Friedman, G.D.; Asgari, M.M. A real-world, community-based cohort study comparing the effectiveness of topical fluorouracil versus topical imiquimod for the treatment of actinic keratosis. J. Am. Acad. Dermatol. 2018, 78, 710–716. [Google Scholar] [CrossRef]
- Cortelazzi, C.; Odorici, G.; Castagnetti, E.; Pellacani, G.; Di Nuzzo, S. Comparative study of imiquimod 3.75% vs. photodynamic therapy for actinic keratosis of the scalp. Photodermatol Photoimmunol. Photomed. 2021, 37, 404–409. [Google Scholar] [CrossRef]
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: A comparison of clinical and histological outcomes including 1-year follow-up. Br. J. Dermatol. 2007, 157, 34–40. [Google Scholar] [CrossRef]
- Segatto, M.M.; Dornelles, S.I.T.; Silveira, V.B.; Frantz, G.D.O. Comparative study of actinic keratosis treatment with 3% diclofenac sodium and 5% 5-fluorouracil. An. Bras. De Dermatol. 2013, 88, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.; Dirschka, T.; Ostendorf, R.; Kerl, H.; Kunstfeld, R. Long-term clinical outcomes of imiquimod 5% cream vs. diclofenac 3% gel for actinic keratosis on the face or scalp: A pooled analysis of two randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 82–89. [Google Scholar] [CrossRef]
- Sáenz-Guirado, S.; Cuenca-Barrales, C.; Vega-Castillo, J.; Linares-Gonzalez, L.; Ródenas-Herranz, T.; Molina-Leyva, A.; Ruiz-Villaverde, R. Combined versus conventional photodynamic therapy with 5-aminolaevulinic acid nanoemulsion (BF-200 ALA) for actinic keratosis: A randomized, single-blind, prospective study. Photodermatol. Photoimmunol. Photomed. 2022, 38, 334–342. [Google Scholar] [CrossRef] [PubMed]
| Study (n = x) [Citation] | Field, Spot or Systemic | Location | Strength | Dosage | Results | ADR |
|---|---|---|---|---|---|---|
| Nicotinamide (Immunocompetent) | ||||||
| Moloney et al., 2010 [26] | Field | Face, scalp, upper limbs | 1% gel | Twice Daily | Adult: 21.8% count reduction—3 months 24.6% count reduction—6 months Elderly: 22% count reduction—3 months 10% count reduction—6 months (non-significant) | Not reported |
| Surjana et al., 2012 n = 36 (Study 1) n = 41 (Study 2) [27] | Systemic | Face, scalp, upper limbs | 500 mg | Twice daily × 4 months (Study 1) Daily × 4 months (Study 2) | Study 1: 35% count reduction—2 months 35% count reduction—4 months Study 2: 15% count reduction—2 months 29% count reduction—4 months | Not reported |
| Chen et al., 2015 n = 386 [28] | Systemic | Face, scalp, forearms, hands | 500 mg | Twice daily × 12 months | 12 months: 23% less NMSC vs. placebo 13% count reduction vs. placebo | Not significant |
| Nicotinamide (Immunocompromised) | ||||||
| Drago et al., 2017 n= 38 [30] | Systemic | Entire body | 500 mg | Daily × 6 months | 6 mo: Decreased AK size in 88% of patients 42% with complete clinical regression 0% of patients developed new AKs | Diarrhea × 1 case |
| Chen et al., 2016 n = 22 [31] | Systemic | Face, scalp, forearms, hands | 500 mg | Twice daily × 6 months | 6 months: 16% reduction in AK count (non-significant) | Not significant |
| Acitretin (Immunocompromised) | ||||||
| Solomon-Cohen et al., 2022 n = 34 [43] | Systemic | Unspecified | 10 mg | Daily × 2 years | ≥2 years: 53% reduction in pre-treatment KC | Not significant |
| Allnutt et al., 2022 n = 101 [44] | Systemic | Unspecified | 8.10–22.5 mg Mode: 10 mg | Daily × 2–9 years | Reduced KC development by at least 50% during first 5 years of treatment (IRR < 0.5) | Dose-dependant mucocutaneous xerosis, peripheral sensory neuropathy, visual hallucinations, diarrhea |
| 5-Fluorouracil (Immunocompetent) | ||||||
| Pomerantz et al., 2015 n = 932 [46] | Field | Face and ears | 5% cream | Twice daily × 4 weeks | 6 months follow ups: Fewer AKs compared to placebo (3.0 vs. 8.1) at 6 months and duration of the study Higher AK clearance vs. placebo (38% vs. 17%) Fewer hypertrophic AK lesions (0.23 vs. 0.41) | Erythema, pruritus, burning, soreness, tenderness, crusting, erosions, scaling, flaking, and swelling |
| Weinstock et al., 2018 n = 932 [47] | Field | Face and ears | 5% cream | Twice daily × 4 weeks | 12 months: 75% risk reduction in SCC (1% vs. 4% development) | See [44]. Notably, 87% of patients would repeat treatment course if effective in reducing future risk of skin cancer |
| Study (n = x) [Citation] | Field, Spot or Systemic | Location | Strength | Dosage | Results | ADR |
|---|---|---|---|---|---|---|
| 5-Flurouracil (Immunocompetent) | ||||||
| Jansen et al., 2019 n = 624 [58] | Field | Head | 5% cream | Twice daily × 4 weeks | 12 months: No treatment failure (>75% reduction) in 74.7% of 5-FU patients compared to 53.9% imiquimod, 37.7% MAL-PDT and 28.9% ingenol mebutate | Erythema, swelling, erosion, crusts, vesicles, scaling, pruritis, pain, burning |
| Kishi et al., 2018 n = 1 [60] | Field | Bilateral forearms | 0.5% cream | Daily × 30 days | Discontinuation of medication with ongoing effect requiring hospitalization. DPDD suspected but not confirmed | Lethargy, fever, fatigue, fever, mouth erosions, painful mucositis, weight loss |
| Cohen 2018 n = 1 [61] | Field | Face, lower lip | 5% cream | Daily × 1 week, then twice daily × 2 weeks | Discontinuation of medications while treating neutropenia. Recommencement of medications save 5-FU did not cause neutropenia | Severe neutropenia |
| Khalil et al., 2022 n = 44 [64] | Field | Face | 5% cream 0.5% anyhydrous serum | Intervention: 1.0 mm microneedling + 5% twice daily × 3 days + placebo × 12 days Or 0.5% twice daily × 3 days + placebo × 12 days Control: 5% cream twice daily × 15 days or 0.5% cream twice daily × 15 days | No statistical differences noted between microneedling + 3 days of treatment vs. 15 days of treatment alone at 3 mo (AK count 0.55 vs. 0.30 for drug alone) 5% 5-FU alone superior to 0.5% 5-FU alone. Microneedling + 5% FU superior to microneedling + 0.5% 5-FU in reducing AK lesions | Increased rate of erythema, crusting, exfoliation, scaling in 5-FU alone vs. microneedling + 5-FU. Slight but non-different ADR noted for 0.5% 5-FU and microneedling + 0.5% 5-FU |
| Maarouf et al., 2020 n = 30 [65] | Field | Face | 5% cream | 5-FU: Twice daily × 2 weeks Intervention: Applied to half of face twice daily × 2 weeks | 98.1% resolution of AK count by week 4 Clobetasol propionate 0.5% best at decreasing transepithelial water loss (TEWL) (p = 0.034), petrolatum jelly best at improving hydration (p = 0.019) and erythema (p = 0.014), CRSBE improved TEWL (p = 0.17) and hydration (p = 0.19) but no effect on erythema (p = 0.257) | Erythema, burning and scabbing with 5-FU. No suspected ADR for other interventions |
| Heuser et al., 2020 n = 17 [66] | Field | Upper limbs | 5-FU: 5% cream Glycolic acid: 70% | 5-FU: Twice daily × 2 months Glycolic acid: Every 15 days followed by 5-FU% solution on skin for 12H × 2 months | Significant reduction of 75% and 85.71% in the mean number of AK lesions and of 74.5% and 85.71% in the size of lesions on the upper limbs of patients treated with glycolic acid = 5% 5-FU solution and 5% 5-FU cream (p-value ≤ 0.001) No statistical difference between either treatment | Some erythema, pruritis and pain. No statistical difference between either treatment |
| 5-Flurouracil (Immunocomprimised) | ||||||
| Ingham et al., 2014 n = 8 [54] | Spot | Face | 5% cream | Twice daily × 3 week | 63 and 0% complete clearance rates at 8 weeks and 12 months, respectively. 100% patients had partial clearance (>75%) at weed 8 and 71% at 12 months, respectively. Average patients had 15 AK at week 0, 1 at week 8 and 3 at 12 months. Mean AK clearance rate was 98% at week 8 and 79% at 12 months | Mostly mild erythema, pruritis and flaking or scaling |
| 5-Fluorouracil + Calcipotriol (Immunocompetent) | ||||||
| Cunningham et al., 2017 n = 131 [68] | Field | Face, scalp, upper extremities | 5-FU: 5% cream Calcipotriol: 0.005% | Twice daily × 4 days | Calcipotriol plus 5-FU vs. Vaseline plus 5-FU × 4 days led to an 87.8% vs. 26.3% mean reduction in the number of actinic keratoses in participants (p < 0.0001) | 5-FU + calcipotriol led to more skin redness, burning sensation and delated erythema resolution compared to 5-FU + vaseline. No difference in redness onset, pruritis and scaling |
| Rosenberg et al., 2019 n = 86 [70] | Field | Face, scalp | 5-FU: 5% cream Calcipotriol: 0.005% | Twice daily × 4 days | 5-FU + calcipotriol–induced tissue-resident memory T (Trm) cell formation on face and scalp is associated with more erythema (p < 0.01). More epidermal Trm cells persisted in the 5-FU + calcipotriol–treated face and scalp skin (p = 0.0028) More participants remained SCC-free more than 1500-days after 5-FU + calcipotriol treatment (p = 0.0765), and significantly fewer developed SCC on the treated face and scalp within 3 years | Notable focus on erythema |
| Moore et al., 2021 n = 175 [71] | Spot (cryotherapy pre-treatment) + Field | Face | 5-FU: 5% cream Calcipotriol: 0.005% | Three week cycles of 5 nights on the face, 7 nights elsewhere then 2 weeks off before repeating | 5-FU + calcipotriol showed AKreduction at 101 to 200 days (9.55; p = 0.002) and 201 to 300 days (14.70; p = 0.001) post follow up. Small difference in AK clearance between 5-FU + calcipotriol (14.70; p ¼ 0.001) and cyclic vitamin D (14.18; p ¼ 0.004) at 201 to 300 days (Figure 1). 5-FU + calcipotriol demonstrates greater and earlier AK reduction compared to cryotherapy alone (p = 0.008) | Redness, dryness and pruritis |
| 5-Fluorouracil + salicylic acid (Immunocompetent) | ||||||
| Schlaak et al., 2010 n = 15 [73] | Spot | Face, scalp | 5-FU: 0.5% cream SA: 10% | 3 times per week × 4 weeks | 12 weeks: Complete response in 77%, partial response in 21% and non-response of 1 (2%) of surveyed AK lesions was achieved | Burning named as most notable. Redness, irritation, dryness and peeling also present |
| Szeimies et al., 2015 n = 1051 [74] | Spot | Face, head, arms, hands, legs, trunk | 5-FU: 0.5% cream SA: 10% | Once daily on up to 10 lesions | Mean Ak count decreased by approximately 70% during the observation period. Mean size of AK decreased by approximately 80%. About 50% of surveyed patients were treated less than 6 weeks | Pain, erythema, burning, irritation, discoloration, scabbing and erosion |
| Stockfletch et al., 2017 n = 166 [75] | Field | Face, scalp | 5-FU: 0.5% cream SA: 10% | Once daily × 12 weeks | 8 weeks following treatment: Complete clearance was found to be 49.5% vs. 18.2% with vehicle alone (p = 0.0006) Partial clearance was found to be 69.5% vs. 34.6% with vehicle alone (p = 0.0001). 99.1% of assessed patients experienced adverse events with treatment | Erythema, inflammation, and scabbing |
| Reinhold et al., 2017 n = 649 [76] | Spot | Hands, forearms | 5-FU: 0.5% cream SA: 10% | Once daily to a maximum of 10 lesions | 8 weeks after end of treatment: AK count reduction by 92% (0.3 lesions per patient (p < 0.0001)) Decrease in the size of the lesions by87% (p < 0.0001) | Erosion, irritation, pain, discharge, erythema, bleeding, macula, pruritis, rash, scar, ulcer in only 2% of patients |
| Garofalo et al., 2022 n = 40 [77] | Field | Face, scalp | 5-FU: 0.5% cream SA: 10% | Once daily for 12 weeks | AKASI score decreased from an initial score of 3.3 to a final score of 0.9. 12 week: 84% of assessed lesions showed complete clearance, partial clearance observed in 8% | Erythema, pruritis, erosion, bleeding |
| Imiquimod (Immunocompetent) | ||||||
| Stockfletch et al., 2014 n = 319 [98] | Field | Face, scalp | 3.75% cream | Daily × 2 weeks on, off, on | 8 week after treatment: Median of 18 AK lesions werecleared corresponding to a median percentage reduction of 92.2% of all the patients’ AK lesions compared to 39.3% for placebo | Not reported |
| Kopera 2020 n = 2 [99] | Field | Face | 3.75% cream | Twice daily × 2 weeks | Complete healing within 2–4 weeks of AK lesion without sequelae | Burning, fatigue, mild erythema |
| Imiquimod (Immunocompromised) | ||||||
| Zavattaro et al., 2020 n = 13 [100] | Field | Scalp | 3.75% cream | Daily × 2 week on, off, on | 8 weeks follow up: Complete clearance in 46% of patients 38% of patients had a 50% reduction in AK count 15% of patients had an 80% reduction in AK count | Erythema, crust, rarely edema, asthenia and fatigue |
| Bhatia et al., 2022 n = 22 [106] | Field | Face, scalp, trunk, upper extremities | 3.75% cream | Daily × 2 week on, off, on | Systemic symptos occurred rarely but usually followed local skin reactions within a 7–11 day period | Local skin reactions assayed included symptoms erythema and pruritis. Some assayed systemic symptoms included fever, headache and fatigue |
| Diclofenac (Immunocompetent) | ||||||
| Singer et al., 2019 n = 28 [109] | Spot | Unspecified | Diclofenac: 3% Hyalorinic Acid: 2.5% gel | Twice daily × 12 weeks | Gene expression of glucose transporter-1 (GLUT-1) was increased in AK lesions compared to normal skin Decrease in epidermal CD1a+ cells but increased dermal CD8+ T cells in AK Diclofenac treatment reduced AK lactate and amino acid levels while inducing infiltration of dermal CD8+ T cells and high IFN-γ mRNA expression | Not reported |
| Çayirli et al., 2013 n = 44 [113] | Spot | Face, scalp | Diclofenac: 3% Hyalorinic Acid: 2.5% gel | Twice daily × 12 weeks | Immunohistochemical and histopathologic examinations revealed that 12-weeks might not be enough to treat AK Ki-67 (p = 0.042) and p63 (p = 0.030) expression decreased denoting an anti-proliferative effect Complete clearance seen in 19 lesions (32.8%). Significant improvement seen in 25 lesions (43.1%) and mild-moderate improvement in 9 lesions (15.5%). No improvement in 5 lesions (8.6%), Complete remission was observed at a significantly higher rate in Grade 3 lesions (p = 0.017) | Xerosis, erythema, crusting |
| Pflugfelder et al., 2012 n = 418 [114] | Unclear | Face, head | Diclofenac: 3% Hyalorinic Acid: 2.5% gel | Group A: Twice daily × 3 months Group B: Twice daily × 6 months | Complete clearance in 40% (Group A) and in 45% (Group B) of AK lesions (p = 0.38). Histopathological clearance in 30% (group A) and 40% (group B) of AK lesions (p = 0.16). Decreased size in 38% (group A) and 39% of (group B) of surveyed AK lesions | Erythema, scaling, edema, erosion, induration |
| Tirbanibulin (Immunocompetent) | ||||||
| Kempers et al., 2020 n = 30 (phase I) n = 168 (Phase II) [116] | Field | Phase I: Forearms Phase II: Face, scalp | 1% ointment | Daily × 3 or 5 days | Phase I: By day 45, 25% (50 mg over 25 cm2 × 3 days), 0% (200 mg over 100 cm2 × 3 days), 50% (50 mg over 25 cm2 × 5 days), and 12.5% (200 mg × 5 days over 100 cm2) of participants demonstrated complete AK clearance Phase II: More participants had complete clearance at day 57 in the 5-day vs. the 3-day cohort (at 50 mg over 25 cm2) (43% vs. 32%) Partial clearance rates were higher in the 5-day vs. the 3-day cohort (at 50 mg over 25 cm2) (56% vs. 52%) An overall average decrease in AK count occurred by day 15 in the 5-day (−2.5 [2.48]) vs. 3-day (−2.5 [2.22]) regimens which continued up to day 57 (−3.9 [2.00] and −3.4 [1.75], respectively) | Erythema, scaling, crusting |
| Blauvet et al., 2021 n = 702 [119] | Field | Face and scalp | 1% ointment | Daily × 5 days | Day 57: Complete AK clearance in 174 of 353 patients (49%) using tirbanibulin vs. vehicle (9%) after pooling data from both trials (44% clearance in Trial 1, 54% clearance in Trial 2). 12 mo: 47% AK recurrence in patients who initially had a complete response | Erythema, flaking, scaling, pain, pruritis |
| Traditional Photodynamic Therapy (Immunocompetent) | ||||||
| Berman et al., 2020 n = 269 [128] | Spot | Face, scalp, upper extremities | 20% ALA BLU-U illuminator | ALA applied twice prior to illumination; repeated × 1 if lesions noted after 8 weeks | 12 weeks follow up post-baseline: Clearance was 80.6% (vs. 45.5% placebo; p < 0.0001) and the mean decrease in cumulative disease area was 82.4% (vs. 42.6% placebo; p <0.0001) | Edema, erythema, hyperpigmentation, hypopigmentation, scaling, dryness, stinging, burning, oozing, vesiculation, crusting |
| Reinhold et al., 2016 n = 94 [129] | Field | Face and scalp | BF-200 ALA BF-Rhodo-LED Lamp | 1 session repeated × 1 if lesions still noted after 12 weeks | 12 weeks following treatment: ALA complete clearance at 91% (vs. 22% placebo, p < 0·0001) and complete clearance rate at 94·3% (vs. 32·9% placebo, p < 0·0001) after a maximum of two PDTs | Pain at application site, erythema, pruritus, scab, exfoliation, oedema and vesicles |
| Ulrich et al., 2021 n = 50 [131] | Field | Neck, trunk, extremities | BF-200 ALA BF-RhodoLED lamp (Biofrontera | 1 session Maximum of 2 session permitted | Complete clearance rates were 86.0% (vs. 32.9% for placebo; p < 0.0001) and patient complete clearance per patient’s side was 67.3% (vs. 12.2% for placebo, p < 0.0001). One-year overall lesion recurrence rate was 14.1% (vs. 27.4% placebo p = 0.0068) Patients were more satisfied with cosmetic outcome of ALA/PDT than vehicle/PDT | Pain, erythema, pruritis, edema, scab, exfoliation, vesicles |
| Bai-Habelski et al., 2022 n = 20 [132] | Field | Hands, arms | PD P 506 A patch Aktilite CL 128 or BF-RhodoLED illuminator | 3–8 AK lesions covered by one patch and illuminated × 1 followed by 2nd session 2 weeks later | Complete clearance at 78.0%(95% CI: [64.6%, 87.3%]), and the by-participant clearance calculation was at 78.7% (95% CI of [67.0%, 90.3%]) | Erythema, irritation, pain, burning, discomfort, pruritus, exfoliation, desquamation, scab, excision, vesicles, edema, inflammation, headache |
| Bullock et al., 2022 n = 58 [139] | Field | Face, scalp | ALA 20% Vit D 10,000 IU | 1 session | 3 to 6 months: Mean clearance rates were lower in patients with vitamin D deficiency(40.9% +/− 42%) than in patients with normal vitamin D levels (62.6% +/− 14.2%). Vitamin D supplementation significantly improved the overall AK lesion response (72.5% 6 13.6%) | Pain, erythema, warmth, exfoliation, tightness, scabbing, edema, blistering, erosions, hemorrhage, discharge, pigmentary changes |
| Urvashi et al., 2020 n = 23 [146] | Field | Face, scalp | ALA 20% Blu-U Illuminator | 1 session | Less pain during simultaneously illumination compared to conventional PDT 3 months follow up showed nearly identical clearance with bothsimultaneous and conventional treatment asdetermined by statistical testing of noninferiority +/− 15% margin | Burning, itching, redness, stinging, swelling, crusting, peeling |
| Salvio et al., 2021 n = 30 [147] | Field | Forearms, hands | ALA 20% 630 nm LED Prototype | 1 session | Pain comparison showed best results when illuminating 1.5 h with 2 min breaks compared to conventional PDT 30 days: No statistical significant difference in clearance when illuminating 1.5 h with 2 min pauses compared to conventional PDT | Pain predominantly assessed |
| Brumana et al., 2020 n = 50 [148] | Field | Face, scalp | MAL 16% 7% lidocaine/7% tetracaine cream Akilite Lamp | 1 session | Median values of pain VAS score with anesthetic application was reduced by 60% vs. placebo (3.0 vs. 7.5) (p = 0.0009) | Pain predominantly assessed |
| Bartosińska et al., 2022 n = 22 [149] | Field | Face, scalp | ALA-HCl: 12.7% MAL-HCL: 12.5% ALA-P: 17.5% Red Beam Pro+ | 1 session | Pain intensity during PDT was significantly lower with ALA-P (5.8 on average) in comparison to the areas treated with ALA-HCl or MAL-HCl (7.0 on average on 0–10 scale) 94% of patients rated obtained cosmetic effect as excellent. No significant difference in efficacy | Erythema, edema, desquamation, crusting, and pustules |
| Meierhofer et al., 2020 n = 45 [150] | Field | Face, scalp | BF-200 ALA BF-RhodoLED | 1 session | 3 months: Clearance rate of the target AK and total AK after PDT was 88.4% and 90.6% with occlusion and 58.1% (p = 0.001) and 70.4% (p = 0.04) with non-occlusion. 6 months: Clearance of target and total AK was 69.7% and 72.1% with occlusion and 30.2% (p < 0.001) and 35.6% (p = 0.001) with non-occlusion. Pain score and skin phototoxicity were significantly higherafter occlusive ALA application | Photoxicity assessed as the sum of erythema, edema, blistering |
| Vicentini et al., 2019 n = 25 [151] | Field | Forehead, Scalp | MAL 16% FLUXIMEDICARE | 1 session, then 1 session × 3 months later if AK still present | 3 months: Clearance was non-inferior to that obtained with the conventional PDT (660% vs. 591%,respectively; absolute difference, 69%; 95% confidence interval–06% to 145%). Pain was significantly lower with the Flexitheralight protocol vs. conventional PDT (p < 00001) | Pain, erythema, edema |
| Dubois et al., 2021 n = 39 [152] | Field | Forehaead, scalp | MAL 16% FLUXIMEDICARE | 1 session | 3 months: Clearance was 72.6% (95% CI 67.9–77.0) 6 months: Clearance was 67.5% (95% CI 61.2–73.3) | Pain, erythema |
| Traditional Photodynamic Therapy (Immunosuppressed) | ||||||
| Lonsdorf et al., 2022 n = 18 [136] | Field | Face, scalp | 16% MAL BF-RhodoLED® | 1 session | 3 months: Low-irradiance photodynamic therapy combined with Er:YAG pre-treatment lesion re- sponse rate of 77.3 ± 23.6%) compared to MAL-PDT(61.8 ± 21.4%; p = 0.025) without worsening pain (p = 0.777) or cosmetic outcome (p = 0.157) | Pain |
| Daylight Photodynamic Therapy (Immunocompetent) | ||||||
| Dirschka et al., 2019 n = 52 [154] | Field | Face, scalp | BF-200 ALA MAL 16% | 1 session | 12 weeks: Complete clearance for 79.8% of AK lesions treated with BF-200 ALA gel and 76.5% of the lesions treated with MAL (p < 0.0001). 12 months: Recurrence for 19.9% of lesions treated with BF-200 ALA and 31.6% for lesions treated with MAL | Erythema, pain, pruritus, scab |
| Maire et al., 2020 n = 38 [155] | Field | Scalp | MAL 16% Dermaris | 1 session, repeated at 3 mo if more than 5 AK lesions present | 3 months: Complete clearance for 58% of patients after the initial treatment. 32% required another round of PDT11% of patients showed 1–4 AK lesions remaining, all of which weregrade I–II and subsequently cured with topical ingenol mebutate. 87% of patients experienced no pain Discomfort, pruritus rated as mild or less (97%) | Pain, pruritus,, discomfort, crusting evaluated |
| Creusot et al., 2021 n = 30 [156] | Field | Scalp | MAL 16% Dermaris | 1 session, repeated at 3 mo and 6 mo if lesions were still present | 6 months: 93% clearance reported Twenty-six patients (87%) experienced no pain during the first PDT | Mild pain, erythema, crusting, discomfort |
| Bai-Habelski et al., 2021 n = 12 [157] | Field | Face, scalp | BF-200 ALA IndoorLux | 2 session with no pre-defined interval between both treatments | Median clearance rate after second treatment was 83.75%33.3% of patients demonstrated complete clearance. Median size of the remaining lesions decreased by 42.9%. The first treatment was pain-free for 58.3%of patients | Pain predominantly assessed |
| Bento et al., 2021 n = 40 [158] | Field | Face | MAL 16% | 2 sessions 4 weeks apart | dPDT + physical interventions had better clinical and histologic results. AK-clearancewas higher after both 1 and 3 months with pretreatment-CO2 laser | Pain, erythema, edema |
| Piaserico et al., 2021 n = 36 [159] | Field | Dorsum of hands, forearms | MAL 16% Calcitriol 3 mg/g | 2 sessions 1 week apart Calcitriol: Daily before bedtime × 14 days | After 3 months, the overall lesionresponse rate and patient ≥ 75% clearance rate of CAL-DL-PDT were higher, albeit not significantly, than P-DL-PDT. According to grade, response rate of grouped AK II/III was significantly higher for CAL-DL-PDT than for P-DL-PDT while similar results were observed for grade I AK | Calcitriol: Erythema, itch dPDT: Erythema, edema, crusting, pustulation |
| Daylight Photodynamic Therapy (Immunodeficient) | ||||||
| Bernard et al., 2020 n = 24 [160] | Field | Face, scalp | MAL 160 mg/g | 2 sessions 15 days apart, followed by double sessions at 3 and 9 months | Daylight PDT showed significantly lower mean of new AK lesions compared to control side 3 months (4.2 [3.4] vs. 6.8 [4.8]; p < 0.001), 9 months (3.0 [3.3] vs. 4.3 [3.4]; p = 0.04) and 15 months (3.0 [4.6] vs. 4.8 [5.0]; p = 0.02) after treatment. Mean number was non-significant at 21 months (3.7 [3.5] vs. 5.0 [4.5]; p = 0.06). Most participants favored DPDT | Erythema, inflammation, blisters, crusting, pruritus, desquamation, burning, stinging |
| Comparative Trials (Immunocompetent) | ||||||
| Neugebauer et al., 2018 n = 5700 [162] | Field | Unspecified | 5-FU Imiquimod | Unspecifed | 5-FU reduced the short-term incidence (cumulative risk difference -4.54%), but not long-term incidence (cumulative risk difference -1.43%) of AKS compared to imiquimod treatment | Unspecified |
| Cortelazzi et al., 2020 n = 9 [163] | Field | Scalp | Imiquimod 3.75% 16% MAL Aktilite Lamp | Imiquimod: Daily for 14 days on, off on 14 days after treatment with MAL-PDT MAL-PDT: 1 session | Imiquimod has higher overall clearance rate than MAL-PDT (68.1% vs. 56.5%) Higher clearance rates for I and III degree AKs with imiquimod (68.8%, 64.5% and 75%) vs. 48%, 69.8%, and 66.7% with MAL-PDT) A higher total recurrence rate was noted for imiquimod compared with MAL-PDT (9.9% vs. 8.6%) after 12 months | Both treatments: burning, erythema, edema, erosions, and crusts, flu-like symptoms (fever, asthenia, headache, joint pain) |
| Segatto et al., 2013 n = 28 [165] | Unclear | Face, scalp, hands | 5-FU 5% Diclofenac 3% hyaluronic acid 2.5% | 5-FU: Twice daily × 4 weeks Diclofenac/Hyaluronic acid: twice daily × 12 weeks | Significant reduction in the number of AK lesions with 5-FU vs. diclofenac (p < 0.001). High degree of satisfaction for both 5-FU vs. diclofenac (73% and 77%, respectively; p = 0.827) | Erythema, edema, crusts and itching were significantly higher with 5-FU |
| Gollnick et al., 2020 n = 479 [166] | Field | Face, scalp | 5% Imiquimod 3% Diclofenac | Imiquimod: 3 nights per week × 4 week followed by 4 week treatment pause; additional 4 week treatment if lesions noted Diclofenac: Twice daily × 12 weeks | Grade III AK or invasive SCC transformation was observed until 3 yrs in 5.4% of patients treated with imiquimod vs. 11.0% of patients treated with diclofenac (absolute risk difference –5.6% [95% CI: 10.7%, –0.7%]) Time to histological change was longer with imiquimod vs. diclofenac (p = 0.0266) | Imiquimod: Pruritus, pain, irritation, inflammation, alopecia, anaemia, psoriasis Imiquimod: Pruritus, pain, dermatitis, irritation, inflammation, rash, alopecia, anemia |
| Sáenz-Guirado et al., 2022 n = 51 [167] | Field | Face, scalp, forehead | BF-200 ALA | Conventional PDT: 1 session ComboPDT: Daylight PDT followed by conventional PDT | Grade I and II AK reduction rate was similar between combo PDT and conventional PDT, with no statistically significant differences between either groups (Grade I: 76.67% vs. 86.63% [p = 0.094]) and (Grade II: 80.48% vs. 83.08% [p = 0.679]). Pain was significantly lower in the combo PDT group (2.56 vs. 5, p < 0.01), including local skin reactions | Combo PDT: Erythema, edema, crusting Conventional PDT: Erythema, Edema, Flaking, Crusting |
| Comparative Trials (Immunosuppressed) | ||||||
| Hasan et al., 2022 n = 40 [161] | Field | Head, Neck, Upper Limb | 5-FU Imiquimod 5% | As used in routine clinical practice, with repeat treatment permittable after 4 weeks | 5-FU and imiquimod were superior to sunscreen for AK clearance and prevention. 5-FU in particular was also more effective than imiquimod in AK clearance and prevention | 5-FU: Pruritus, fatigue, flu-like symptoms, headache, myalgia, photosensitivity, malaise, arthalgia, nausea, Imiquimod: Pruritus, fatigue, hypopigmentation, flu-like symptoms, headache, myalgia, dizziness, malise, arthalgia, nausea, vomiting, diarrhea, bruising |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcuri, D.; Ramchatesingh, B.; Lagacé, F.; Iannattone, L.; Netchiporouk, E.; Lefrançois, P.; Litvinov, I.V. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. Int. J. Mol. Sci. 2023, 24, 4989. https://doi.org/10.3390/ijms24054989
Arcuri D, Ramchatesingh B, Lagacé F, Iannattone L, Netchiporouk E, Lefrançois P, Litvinov IV. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. International Journal of Molecular Sciences. 2023; 24(5):4989. https://doi.org/10.3390/ijms24054989
Chicago/Turabian StyleArcuri, Domenico, Brandon Ramchatesingh, François Lagacé, Lisa Iannattone, Elena Netchiporouk, Philippe Lefrançois, and Ivan V. Litvinov. 2023. "Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review" International Journal of Molecular Sciences 24, no. 5: 4989. https://doi.org/10.3390/ijms24054989
APA StyleArcuri, D., Ramchatesingh, B., Lagacé, F., Iannattone, L., Netchiporouk, E., Lefrançois, P., & Litvinov, I. V. (2023). Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. International Journal of Molecular Sciences, 24(5), 4989. https://doi.org/10.3390/ijms24054989







