Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment
Abstract
1. Introduction
2. Results
2.1. Resveratrol Increases 5-FU Sensitivity and Acts Anti-Carcinogenic via ß1-Integrin Receptors in CRC Cells
2.1.1. Reduction of CRC Cell Vitality
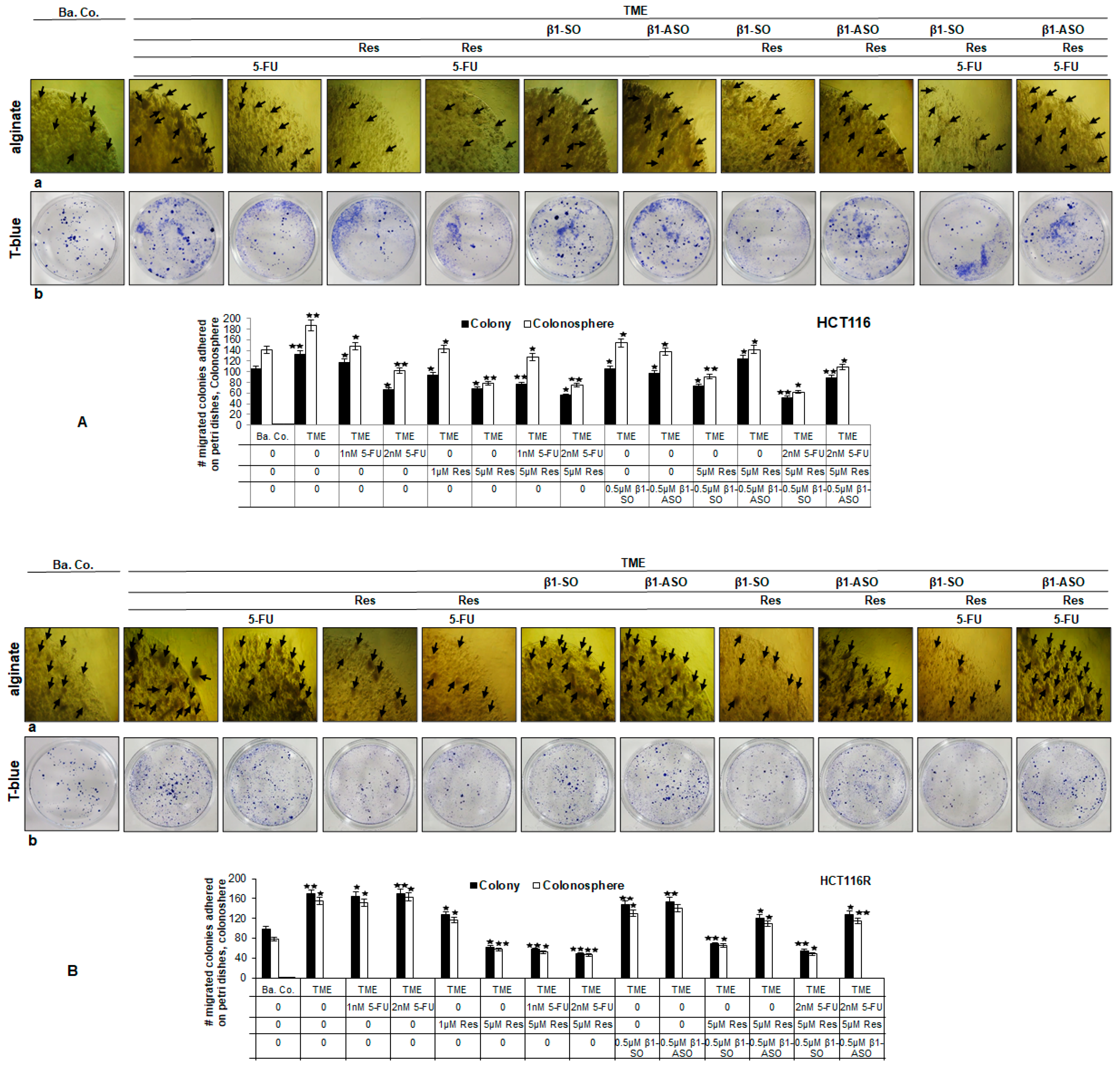
2.1.2. Reduction of CRC Cell Colony Formation
2.1.3. Reduction of CRC Cell Invasion
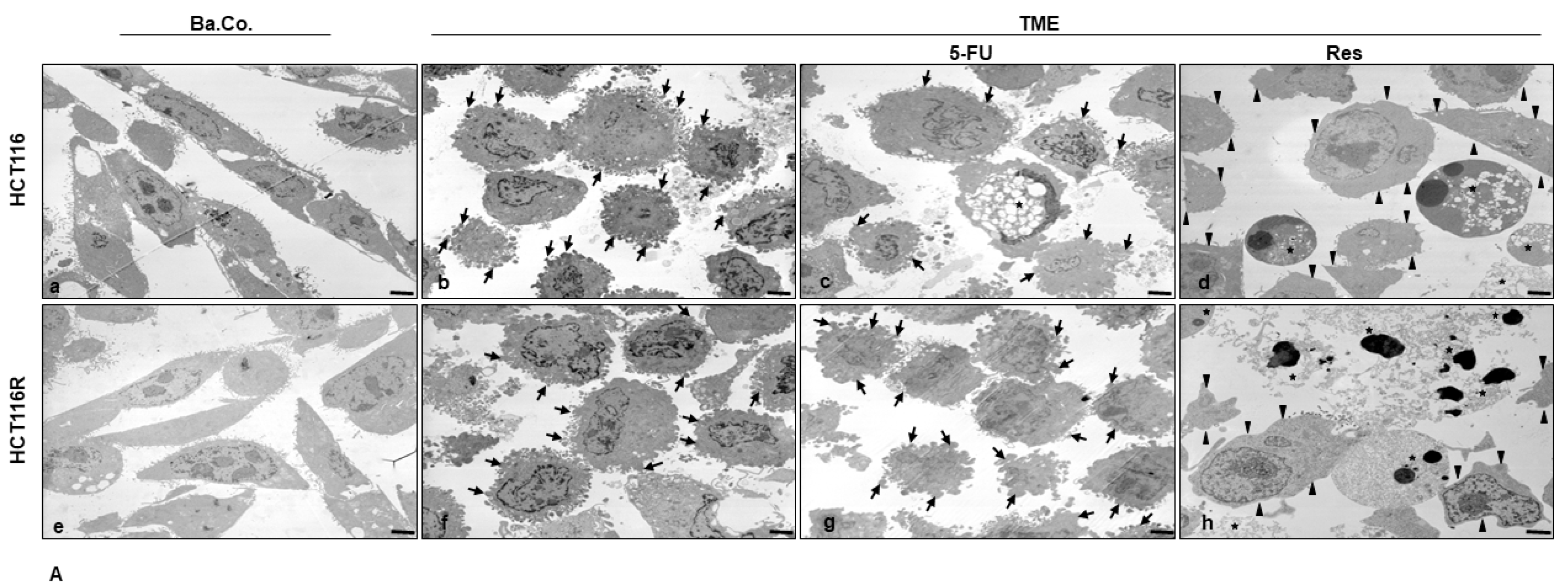
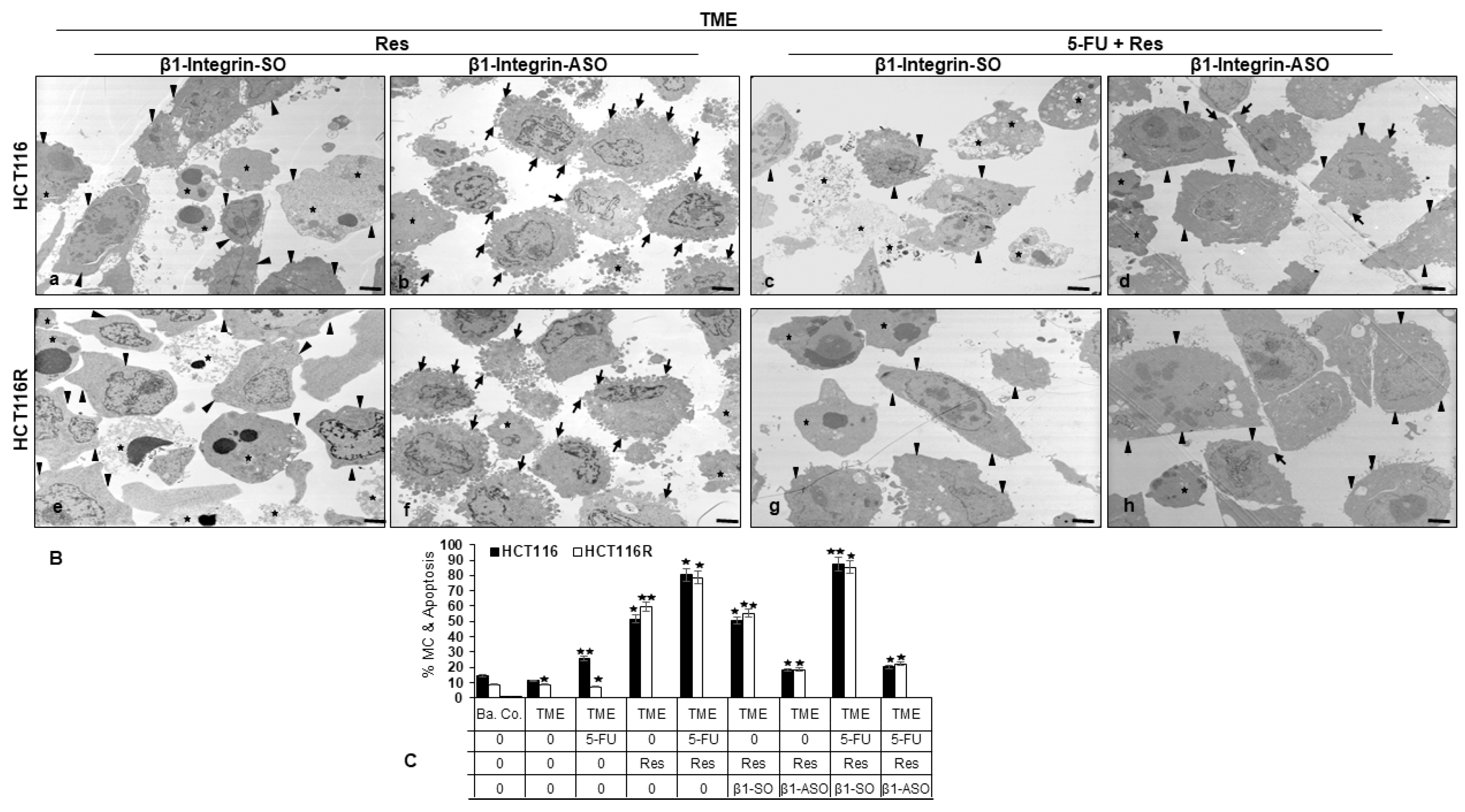
2.1.4. Reduction of CRC Cell’s Mesenchymal Phenotype
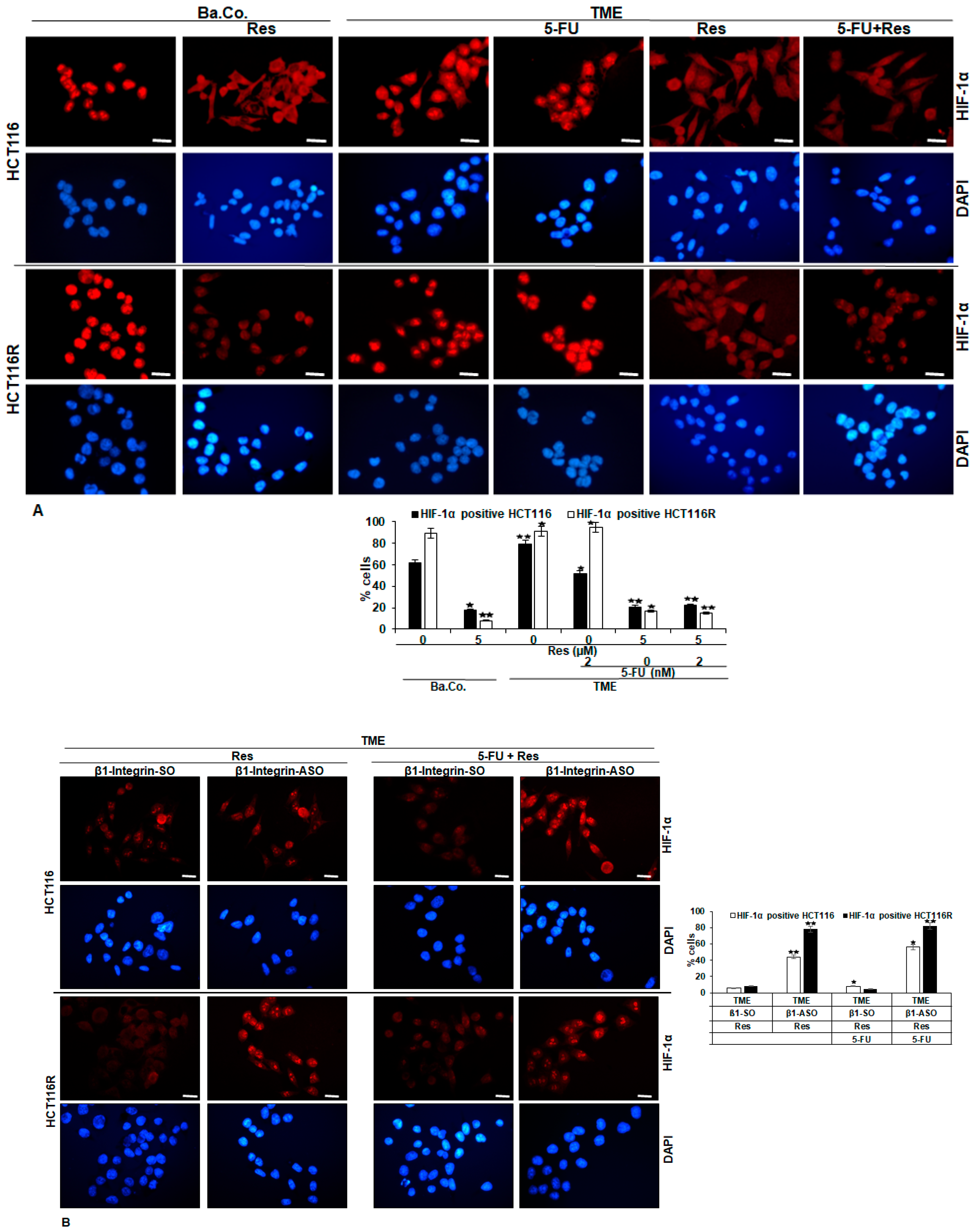
2.2. HIF-1α Is Involved in Resveratrol-Promoted Chemosensitising CRC Cells to 5-FU
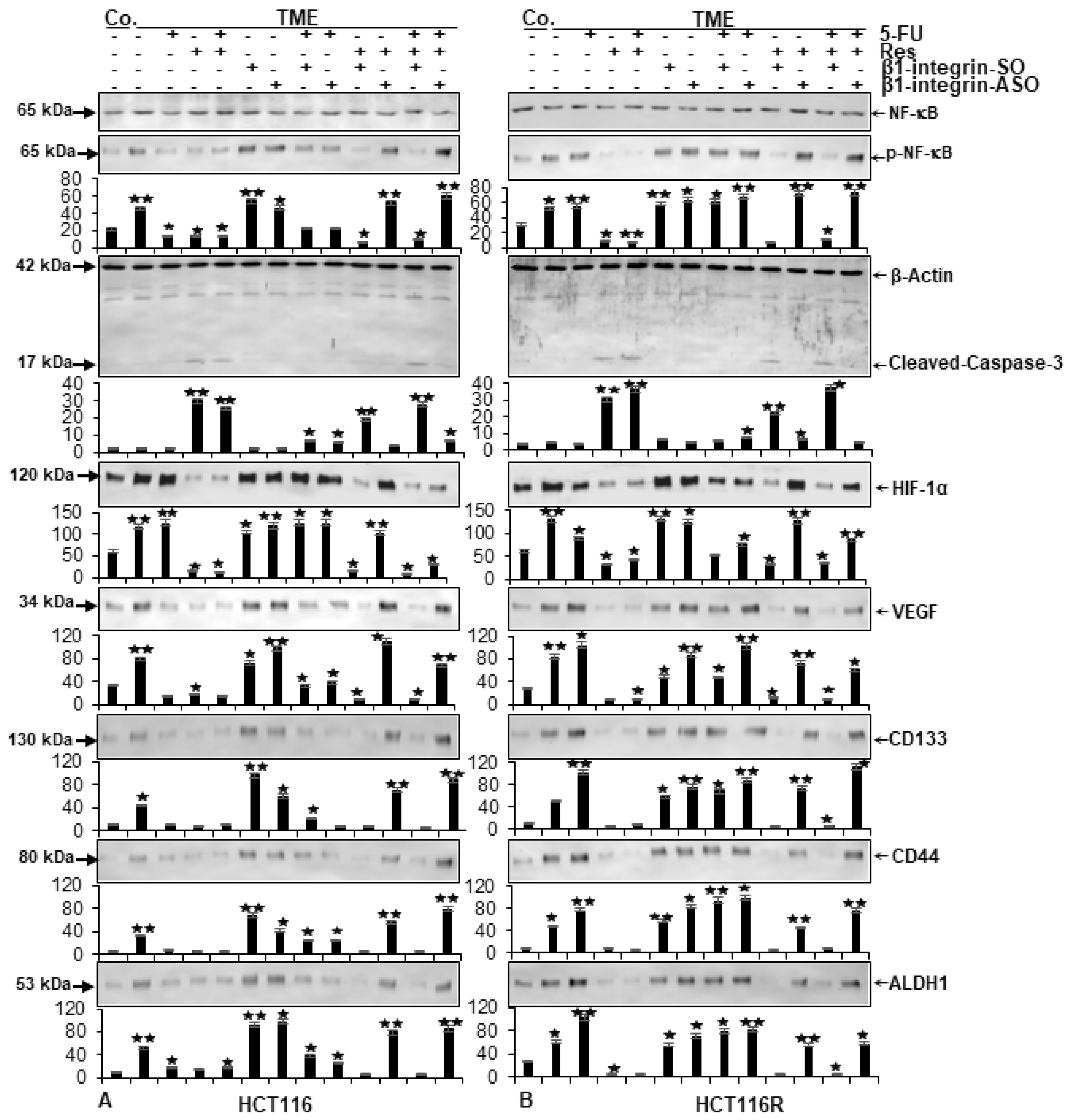
2.3. ß1-Integrin Participated in Resveratrol-Mediated Down-Regulation of NF-kB Activation and Related Gene End Products
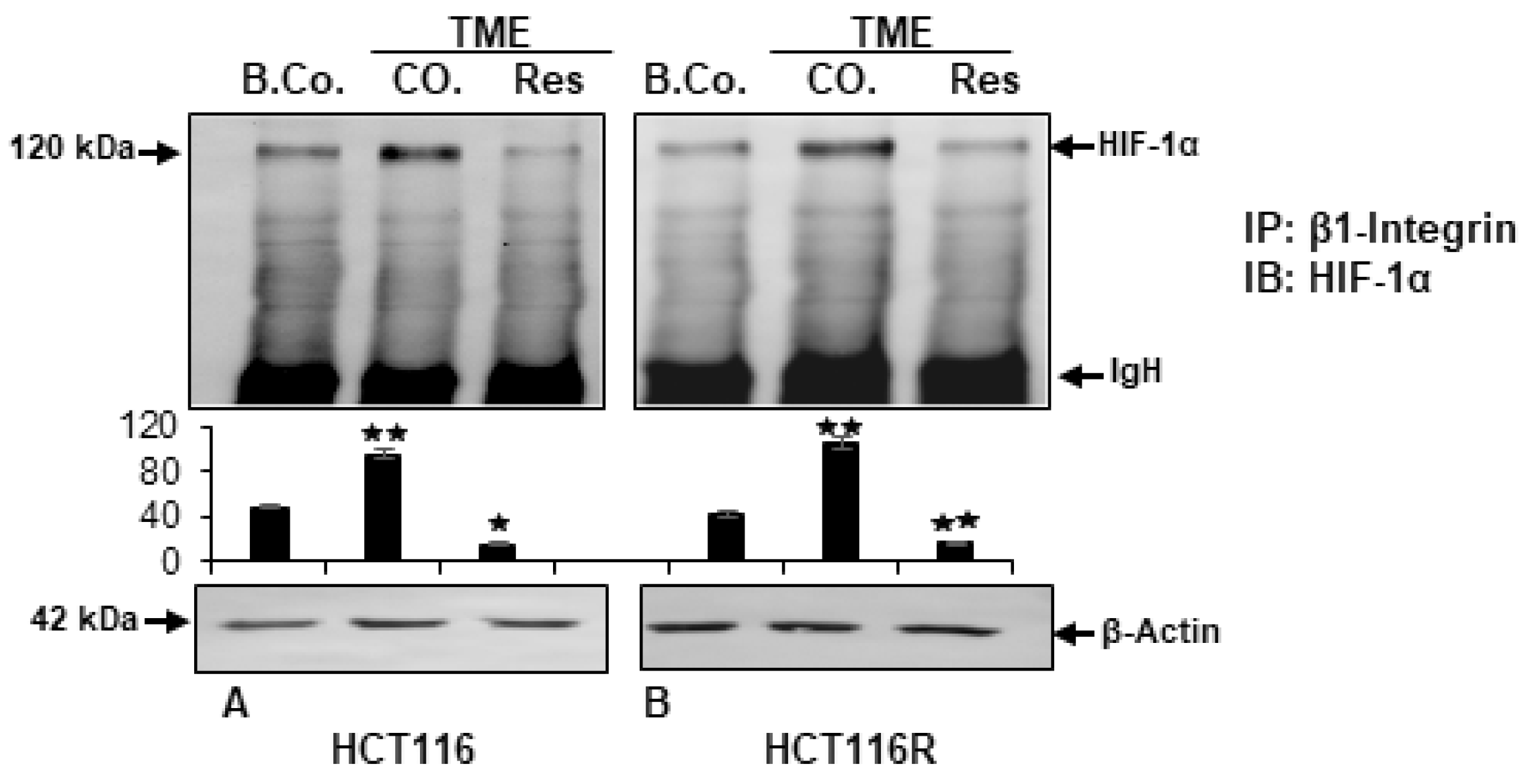
2.4. Resveratrol Inhibits β1-Integrin/HIF-1a Axis in CRC Cells
3. Discussion
4. Materials and Methods
4.1. Trial Substances
4.2. Cell Types and Conditioning
4.3. Knockdown of β1-Integrin
4.4. Alginate Drop Preparation
4.5. Carcinogenic Tumor Microenvironment
4.6. Vitality Assay
4.7. Proliferation Assay
4.8. Invasion Assay
4.9. Immunofluorescence Microscopy
4.10. Electron Microscopic Evaluation
4.11. Western Blot Analysis
4.12. Immunoprecipitation Assay
4.13. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Darmkrebs. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Darmkrebs/darmkrebs_node.html (accessed on 19 May 2022).
- AWMF. Leitlinien Kolorektales Karzinom. Available online: https://register.awmf.org/de/leitlinien/detail/021-007OL (accessed on 19 May 2022).
- Satake, H.; Kagawa, Y.; Shinozaki, E.; Tanizawa, Y.; Jin, L.; Cai, Z.; Makiyama, A. Real-World Data Analysis of Second-Line Antiangiogenic Targeted Treatments Following Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies and First-Line FOLFOX for Patients with Metastatic Colorectal Cancer. Adv. Ther. 2022, 39, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Kaplan, M.A.; Uncu, D.; İnanç, M.; Kaya, S.; Dane, F.; Küçüköner, M.; Demirci, A.; Bilici, M.; Durnalı, A.G.; et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: A multicenter study. Int. J. Color. Dis. 2021, 36, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.P.; Miao, Z.F.; Huang, C.W.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Chang, T.K.; Chang, S.F.; Wang, J.Y. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866964. [Google Scholar] [CrossRef]
- Escalante, P.I.; Quiñones, L.A.; Contreras, H.R. Epithelial-Mesenchymal Transition and MicroRNAs in Colorectal Cancer Chemoresistance to FOLFOX. Pharmaceutics 2021, 13, 75. [Google Scholar] [CrossRef]
- Geevimaan, K.; Guo, J.Y.; Shen, C.N.; Jiang, J.K.; Fann, C.S.J.; Hwang, M.J.; Shui, J.W.; Lin, H.T.; Wang, M.J.; Shih, H.C.; et al. Patient-Derived Organoid Serves as a Platform for Personalized Chemotherapy in Advanced Colorectal Cancer Patients. Front. Oncol. 2022, 12, 883437. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. β1-Integrin plays a major role in resveratrol-mediated anti-invasion effects in the CRC microenvironment. Front. Pharmacol. 2022, 13, 978625. [Google Scholar] [CrossRef]
- Defeudis, A.; Cefaloni, L.; Giannetto, G.; Cappello, G.; Rizzetto, F.; Panic, J.; Barra, D.; Nicoletti, G.; Mazzetti, S.; Vanzulli, A.; et al. Comparison of radiomics approaches to predict resistance to 1st line chemotherapy in liver metastatic colorectal cancer. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Guadalajara, Mexico, 26–30 July 2021; pp. 3305–3308. [Google Scholar] [CrossRef]
- He, X.S.; Zou, S.Y.; Yao, J.L.; Yu, W.; Deng, Z.Y.; Wang, J.R.; Gan, W.J.; Wan, S.; Yang, X.Q.; Wu, H. Transcriptomic Analysis Identifies Complement Component 3 as a Potential Predictive Biomarker for Chemotherapy Resistance in Colorectal Cancer. Front. Mol. Biosci. 2021, 8, 763652. [Google Scholar] [CrossRef] [PubMed]
- Heslin, M.J.; Yan, J.; Weiss, H.; Shao, L.; Owens, J.; Lucas, V.S.; Diasio, R.B. Dihydropyrimidine dehydrogenase (DPD) rapidly regenerates after inactivation by eniluracil (GW776C85) in primary and metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2003, 52, 399–404. [Google Scholar] [CrossRef]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1322–1327. [Google Scholar]
- Amatori, F.; Di Paolo, A.; Del Tacca, M.; Fontanini, G.; Vannozzi, F.; Boldrini, L.; Bocci, G.; Lastella, M.; Danesi, R. Thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer and normal mucosa in patients. Pharm. Genom. 2006, 16, 809–816. [Google Scholar] [CrossRef]
- Bai, W.; Wu, Y.; Zhang, P.; Xi, Y. Correlations between expression levels of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase, and efficacy of 5-fluorouracil-based chemotherapy for advanced colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 12333–12345. [Google Scholar] [PubMed]
- Gustavsson, B.; Kaiser, C.; Carlsson, G.; Wettergren, Y.; Odin, E.; Lindskog, E.B.; Niyikiza, C.; Ma, D. Molecular determinants of efficacy for 5-FU-based treatments in advanced colorectal cancer: mRNA expression for 18 chemotherapy-related genes. Int. J. Cancer 2009, 124, 1220–1226. [Google Scholar] [CrossRef]
- Shigeta, K.; Ishii, Y.; Hasegawa, H.; Okabayashi, K.; Kitagawa, Y. Evaluation of 5-fluorouracil metabolic enzymes as predictors of response to adjuvant chemotherapy outcomes in patients with stage II/III colorectal cancer: A decision-curve analysis. World J. Surg. 2014, 38, 3248–3256. [Google Scholar] [CrossRef]
- Soong, R.; Shah, N.; Salto-Tellez, M.; Tai, B.C.; Soo, R.A.; Han, H.C.; Ng, S.S.; Tan, W.L.; Zeps, N.; Joseph, D.; et al. Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncology 2008, 19, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015, 98, 51–68. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Mahmoud-Ghoneim, D.; Adeghate, E.; Al-Akhras, M.A.; Al-Saadi, M.; Al-Rahmoun, S.; Hameed, R. Pancreas-protective effects of chlorella in STZ-induced diabetic animal model: Insights into the mechanism. J. Diabetes Mellit. 2011, 1, 36. [Google Scholar] [CrossRef]
- Dakhale, G.N.; Chaudhari, H.V.; Shrivastava, M. Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharmacol. Sci. 2011, 2011, 195271. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Murali, C.; Al Madhoon, A.; Amin, A. Salvadora persica (Miswak): Antioxidant and promising antiangiogenic insights. Am. J. Plant Sci. 2018, 9, 1228. [Google Scholar] [CrossRef]
- Brockmueller, A.; Samuel, S.M.; Mazurakova, A.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Curcumin, calebin A and chemosensitization: How are they linked to colorectal cancer? Life Sci. 2023, 318, 121504. [Google Scholar] [CrossRef] [PubMed]
- Ehala, S.; Vaher, M.; Kaljurand, M. Characterization of phenolic profiles of Northern European berries by capillary electrophoresis and determination of their antioxidant activity. J. Agric. Food Chem. 2005, 53, 6484–6490. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef]
- Dariya, B.; Behera, S.K.; Srivani, G.; Farran, B.; Alam, A.; Nagaraju, G.P. Computational analysis of nuclear factor-κB and resveratrol in colorectal cancer. J. Biomol. Struct. Dyn. 2021, 39, 2914–2922. [Google Scholar] [CrossRef]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Ho Szeto, C.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef]
- Brockmueller, A.; Shayan, P.; Shakibaei, M. Evidence That β1-Integrin Is Required for the Anti-Viability and Anti-Proliferative Effect of Resveratrol in CRC Cells. Int. J. Mol. Sci. 2022, 23, 4714. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.T.; Lin, Y.T.; Tang, S.P.; Luo, C.K.; Tsai, C.T.; Shun, C.T.; Chen, C.C. Metabolic targeting of HIF-1α potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene 2020, 39, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Shi, M.; Danenberg, K.D.; Gardner, H.; Barrett, C.; Jacques, C.J.; Sherod, A.; Iqbal, S.; El-Khoueiry, A.; Yang, D.; et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics 2007, 8, 1705–1713. [Google Scholar] [CrossRef]
- Fan, L.F.; Dong, W.G.; Jiang, C.Q.; Qian, Q.; Yu, Q.F. Role of Hypoxia-inducible factor-1 alpha and Survivin in colorectal carcinoma progression. Int. J. Color. Dis. 2008, 23, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.H.; Lee, K.H. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef]
- Wu, H.; Liang, X.; Fang, Y.; Qin, X.; Zhang, Y.; Liu, J. Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomed. Pharmacother. Biomed. Pharmacother. 2008, 62, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Hsu, Y.M.; Chen, C.C.; Chen, L.L.; Lee, C.C.; Huang, T.S. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J. Biol. Chem. 2010, 285, 25458–25466. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol Regulates Colorectal Cancer Cell Invasion by Modulation of Focal Adhesion Molecules. Nutrients 2017, 9, 73. [Google Scholar] [CrossRef]
- Ochiai, T.; Ohta, K.; Iida, M.; Kumagai, Y.; Mitsunori, Y.; Aihara, A.; Noguchi, N.; Tanaka, S.; Arii, S.; Yamazaki, S. High resectability of colorectal liver metastases with aggressive chemotherapy in the era of molecular target-based agents. Hepato-Gastroenterol. 2013, 60, 955–960. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, S.; Zhou, J.; Li, X. Effect of resveratrol on drug resistance in colon cancer chemotherapy. RSC Adv. 2019, 9, 2572–2580. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Singh, M.; Ashraf, B.; Masoodi, T.; Prasad, C.P.; Sharma, A.; Maacha, S.; Karedath, T.; Hashem, S.; et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. 2022, 42, 689–715. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sameri, S.; Liskova, A.; Zhai, K.; Varghese, E.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers 2021, 13, 188. [Google Scholar] [CrossRef]
- Kang, Y.; Lv, R.; Feng, Z.; Zhu, J. Tumor-associated macrophages improve hypoxia-induced endoplasmic reticulum stress response in colorectal cancer cells by regulating TGF-β1/SOX4. Cell. Signal. 2022, 99, 110430. [Google Scholar] [CrossRef]
- Vatandoust, S.; Price, T.J.; Karapetis, C.S. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol. 2015, 21, 11767–11776. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hamada, J.; Takeichi, N.; Fujikawa, S.; Kobayashi, H. Ultrastructural differences in junctional intercellular communication between highly and weakly metastatic clones derived from rat mammary carcinoma. Cancer Res. 1990, 50, 358–362. [Google Scholar]
- Casillas, A.L.; Toth, R.K.; Sainz, A.G.; Singh, N.; Desai, A.A.; Kraft, A.S.; Warfel, N.A. Hypoxia-Inducible PIM Kinase Expression Promotes Resistance to Antiangiogenic Agents. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 169–180. [Google Scholar] [CrossRef]
- Crociani, O.; Zanieri, F.; Pillozzi, S.; Lastraioli, E.; Stefanini, M.; Fiore, A.; Fortunato, A.; D’Amico, M.; Masselli, M.; De Lorenzo, E.; et al. hERG1 channels modulate integrin signaling to trigger angiogenesis and tumor progression in colorectal cancer. Sci. Rep. 2013, 3, 3308. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, H.; Zhang, Y.; Xiong, L.; Zeng, Z.; He, X.; Wang, F.; Wu, X.; Lan, P. Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin α(V) β(5). Mol. Oncol. 2021, 15, 3447–3467. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, B.; Nagaraju, G.P.; Shaib, W.L.; Alese, O.B.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol. Carcinog. 2017, 56, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Srivani, G.; Behera, S.K.; Dariya, B.; Aliya, S.; Alam, A.; Nagaraju, G.P. Resveratrol binds and inhibits transcription factor HIF-1α in pancreatic cancer. Exp. Cell Res. 2020, 394, 112126. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Zhang, X. Resveratrol Induces Apoptosis in Murine Prostate Cancer Cells via Hypoxia-Inducible Factor 1-alpha (HIF-1α)/Reactive Oxygen Species (ROS)/P53 Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8970–8976. [Google Scholar] [CrossRef]
- Nelson, D.R.; Hrout, A.A.; Alzahmi, A.S.; Chaiboonchoe, A.; Amin, A.; Salehi-Ashtiani, K. Molecular Mechanisms behind Safranal’s Toxicity to HepG2 Cells from Dual Omics. Antioxidants 2022, 11, 1125. [Google Scholar] [CrossRef]
- Abdu, S.; Juaid, N.; Amin, A.; Moulay, M.; Miled, N. Effects of Sorafenib and Quercetin Alone or in Combination in Treating Hepatocellular Carcinoma: In Vitro and In Vivo Approaches. Molecules 2022, 27, 8082. [Google Scholar] [CrossRef] [PubMed]
- Al-Akhras, M.A.; Aljarrah, K.; Al-Khateeb, H.; Jaradat, A.; Al-Omari, A.; Al-Nasser, A.; Masadeh, M.M.; Amin, A.; Hamza, A.; Mohammed, K.; et al. Introducing Cichorium Pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats. Electromagn. Biol. Med. 2012, 31, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Lashin, F.M.; Gamel, M.; Hassanin, S.O.; Abdalla, Y.; Amin, A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates In Vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar] [CrossRef]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Saud, S.M.; Li, W.; Morris, N.L.; Matter, M.S.; Colburn, N.H.; Kim, Y.S.; Young, M.R. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 2014, 35, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kunnumakkara, A.B.; Kumar, A.; Samec, M.; Kubatka, P.; Aggarwal, B.B.; Shakibaei, M. Multitargeting Effects of Calebin A on Malignancy of CRC Cells in Multicellular Tumor Microenvironment. Front. Oncol. 2021, 11, 650603. [Google Scholar] [CrossRef]
- Gao, H.; Tian, Q.; Zhu, L.; Feng, J.; Zhou, Y.; Yang, J. 3D Extracellular Matrix Regulates the Activity of T Cells and Cancer Associated Fibroblasts in Breast Cancer. Front. Oncol. 2021, 11, 764204. [Google Scholar] [CrossRef]
- Mueller, A.L.; Brockmueller, A.; Kunnumakkara, A.B.; Shakibaei, M. Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling. Int. J. Mol. Sci. 2022, 23, 1695. [Google Scholar] [CrossRef]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; De Souza, P. Differentiation of mesenchymal limb bud cells to chondrocytes in alginate beads. Cell Biol. Int. 1997, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockmueller, A.; Girisa, S.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4988. https://doi.org/10.3390/ijms24054988
Brockmueller A, Girisa S, Kunnumakkara AB, Shakibaei M. Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment. International Journal of Molecular Sciences. 2023; 24(5):4988. https://doi.org/10.3390/ijms24054988
Chicago/Turabian StyleBrockmueller, Aranka, Sosmitha Girisa, Ajaikumar B. Kunnumakkara, and Mehdi Shakibaei. 2023. "Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment" International Journal of Molecular Sciences 24, no. 5: 4988. https://doi.org/10.3390/ijms24054988
APA StyleBrockmueller, A., Girisa, S., Kunnumakkara, A. B., & Shakibaei, M. (2023). Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment. International Journal of Molecular Sciences, 24(5), 4988. https://doi.org/10.3390/ijms24054988







