Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective
Abstract
1. Introduction
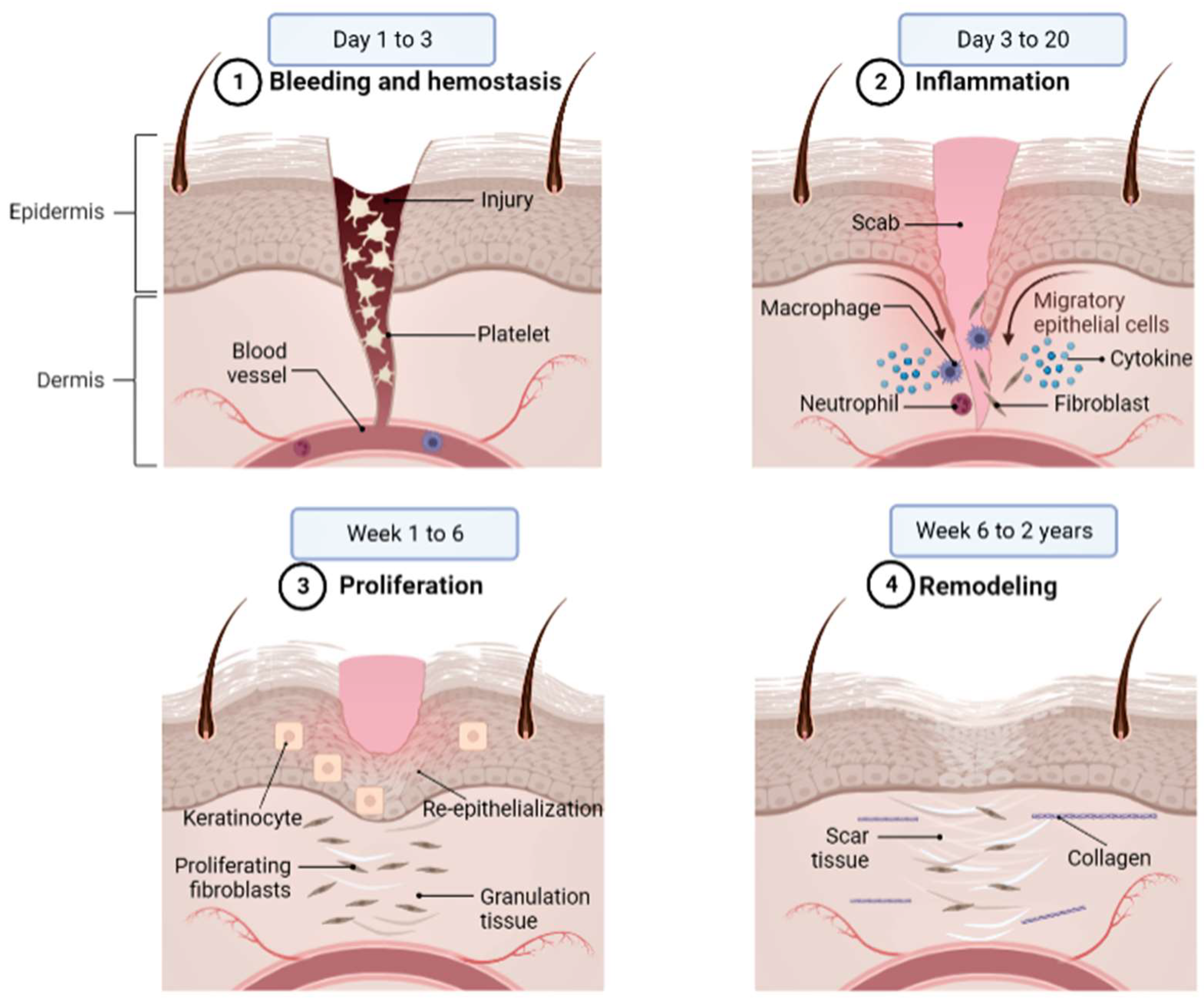
2. Wound Healing
3. Current Treatment for Treating Wound Healing
3.1. Wound Dressings
3.2. Antibiotics
3.3. Surgical Methods
4. Socioeconomic Effects of Morbidity and Mortality from Chronic Wounds
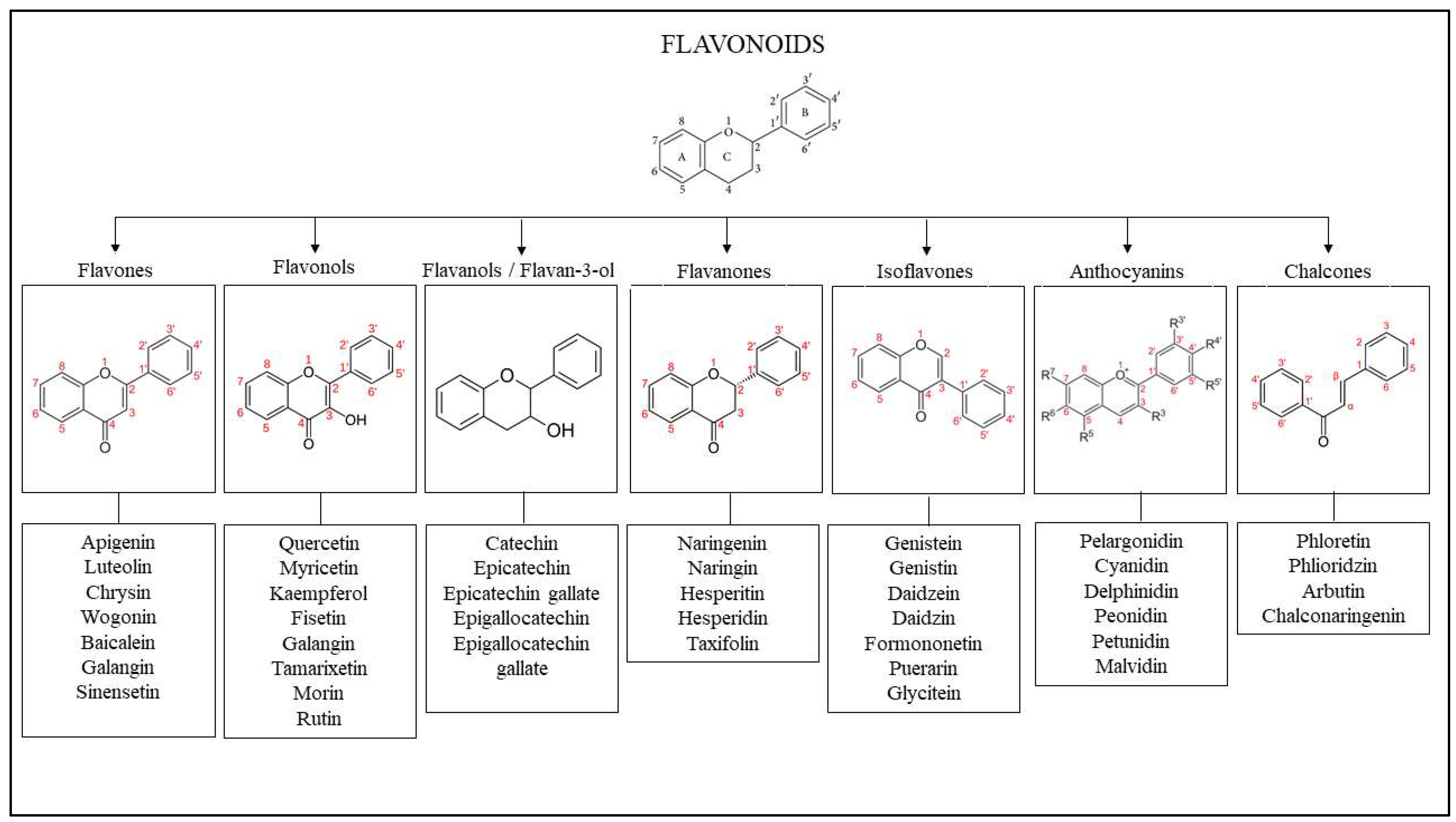
5. The Effect of Flavonoids on Wound Healing
5.1. Efficacy Evidence of Flavonoid as a Wound Healing Agent
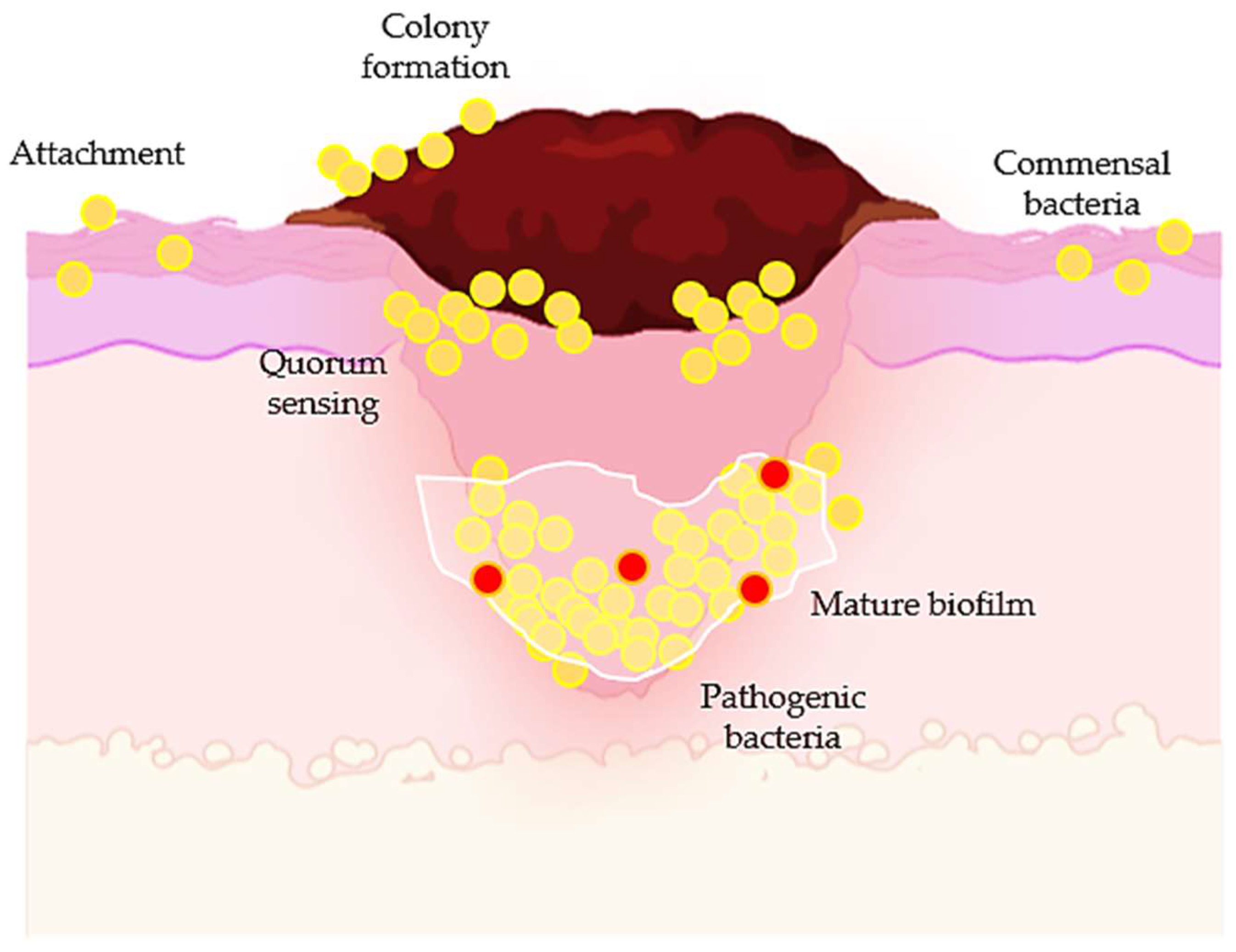
5.2. Antibacterial Properties of Flavonoids in Wound Healing
6. Pathways Involved in Wound Healing
6.1. Wnt/β-Catenin Pathway
6.2. Hippo Pathway
6.3. Transforming Growth Factor β (TGF-β) Pathway
6.4. Hedgehog Pathway
6.5. Jun N-Terminal Kinase (JNK) Pathway
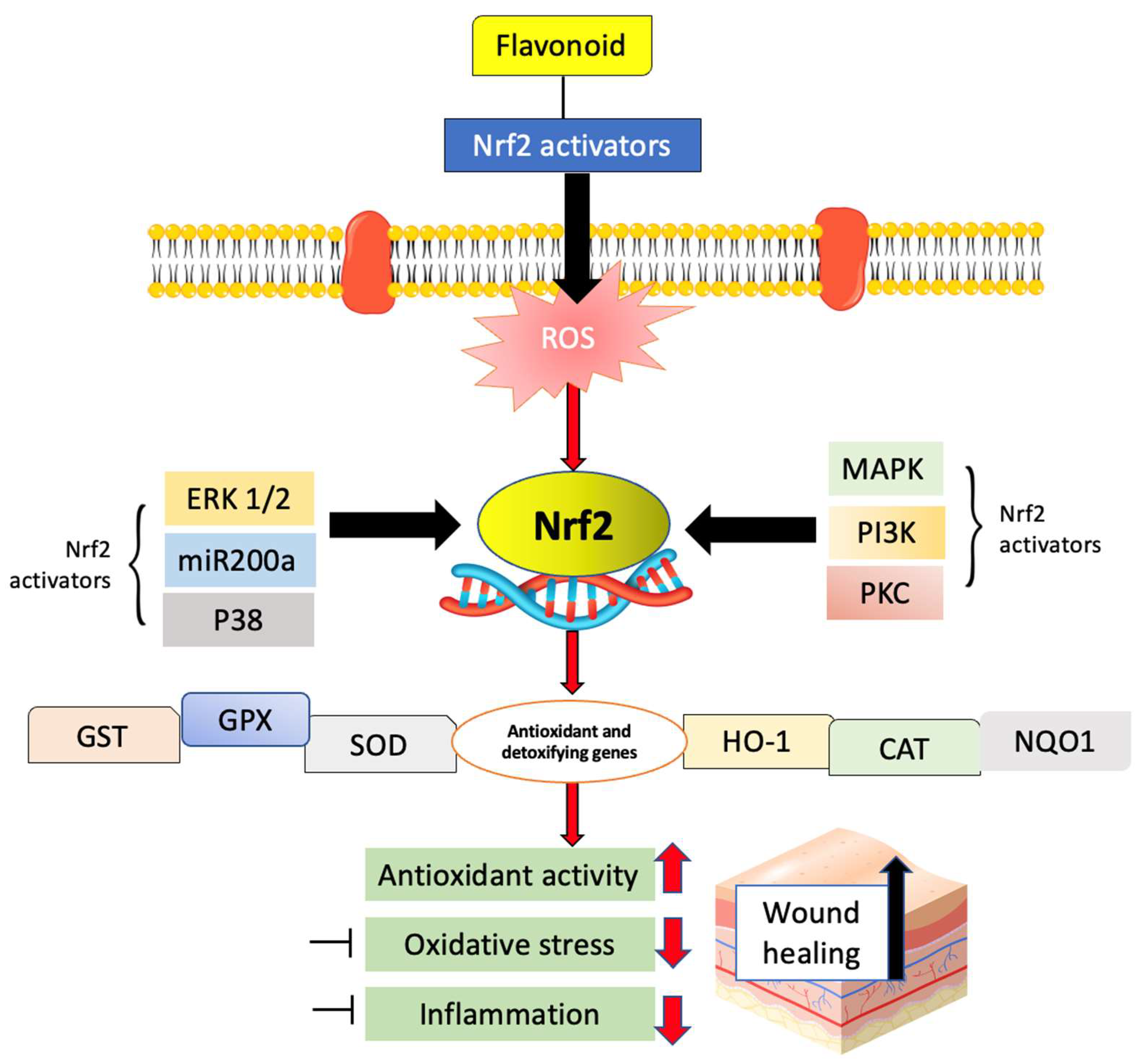
6.6. Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element (Nrf2/ARE) and Nuclear Factor-κB (NF-κB) Pathways
6.7. Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase (MAPK/ERK) and Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (P13/AKT) Pathway
6.8. Focal Adhesion Kinase (FAK)/Src and p38 Mitogen-Activated Kinase (MAPK)
6.9. Transforming Growth Factor/Suppressor of Mothers against Decapentaplegic (TGF-ß/Smads) and Angiopoietin-1/Tie-2 (Ang-1/Tie-2) Pathways
7. Clinical Trials/Human Studies on Flavonoids as a Wound Healing Agent
8. Conclusions
9. Future Perspective and Future Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care New Rochelle 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Shenbagamurthi, S.; Brem, H. Lower extremity ulcers: Venous, arterial, or diabetic? Emerg. Med. 2009, 41, 18–24. [Google Scholar]
- Smith, F.; Sharp, A. Undertaking a person-centred assessment of patients with chronic wounds. Nurs. Stand. 2019, 34, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chronic Wound Care Market Size GIT. Chronic Wound Care Market Size, Growth. Industry Trends 2029: Market Research Report. 2022. Available online: https://www.fortunebusinessinsights.com/industry-reports/chronic-wound-care-market-100222 (accessed on 30 November 2022).
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Enoch, S.; Grey, J.E.; Harding, K.G. ABC of wound healing. Non-surgical and drug treatments. BMJ Clin. Res. Ed. 2006, 332, 900–903. [Google Scholar] [CrossRef]
- Bobo, J.K. Nicotine dependence and alcoholism epidemiology and treatment. J. Psychoact. Drugs 1989, 21, 323–329. [Google Scholar] [CrossRef]
- Spillane, J.F. Cocaine: From Medical Marvel to Modern Menace in the United States, 1884–1920; The Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Tearns, J. Account of the pulvis parturiens, a remedy for quickening childbirth. Med. Repos. N. Y. 1808, 5, 308–309. [Google Scholar]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; Serna-Saldívar, S.O. Anti-inflammatory glycosylated flavonoids as therapeutic agents for treatment of diabetes-impaired wounds. Curr. Top. Med. Chem. 2015, 15, 2456–2463. [Google Scholar] [CrossRef]
- Aslam, M.S.; Ahmad, M.S.; Riaz, H.; Raza, S.A.; Hussain, S.; Qureshi, O.S.; Maria, P.; Hamzah, Z.; Javed, O. Role of flavonoids as wound healing agent. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; IntechOpen: London, UK, 2018; pp. 95–102. [Google Scholar]
- Carvalho, M.T.; Araujo-Filho, H.G.; Barreto, A.S.; Quintans-Junior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Campos, A.C.; Growth, A.K.; Branco, A.B. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Gupta, B.; Ghosh, S.K. Mobile metadata assisted community database of chronic wound images. Wound Med. 2014, 6, 34–42. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Maynard, J. 5 Lifestyle Factors That Affect Wound Healing California; Shield HealthCare Inc.: Valencia, CA, USA, 2016; Available online: http://www.shieldhealthcare.com/community/skin-preservation/2016/03/17/5-lifestyle-factors-affect-wound-healing/ (accessed on 10 November 2022).
- Filho, H.G.D.A.; Dias, J.D.S.; Quintans-Júnior, L.J.; Santos, M.R.V.; White, P.A.S.; Barreto, R.S.S.; Barreto, A.S.; Estevam, C.S.; Araujo, S.; Almeida, J.R.G.S.; et al. Phytochemical screening and analgesic profile of the lyophilized aqueous extract obtained from Chrysobalanus icaco leaves in experimental protocols. Pharm. Biol. 2016, 54, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef]
- Yan, T.; Cheng, F.; Wei, X.; Huang, Y.; He, J. Biodegradable collagen sponge reinforced with chitosan/calcium pyrophosphate nanoflowers for rapid hemostasis. Carbohydr. Polym. 2017, 170, 271–280. [Google Scholar] [CrossRef]
- Menezes, P.d.P.; Frank, L.A.; Lima, B.d.S.; de Carvalho, Y.M.B.G.; Serafini, M.R.; Quintans-Júnior, L.J.; Pohlmann, A.R.; Gutteres, S.S.; Araujo, A.A.S. Hesperetin-loaded lipid-core nanocapsules in polyamide: A new textile formulation for topical drug delivery. Int. J. Nanomed. 2017, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Intake of individual flavonoids and risk of carcinogenesis: Overview of epidemiological evidence. Nutr. Cancer 2017, 69, 1119–1150. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, B.; Ding, Y.; Xu, D.; Zhao, J.; Zhang, K. Rational engineering the DNA tetrahedrons of dual wavelength ratiometric electrochemiluminescence biosensor for high efficient detection of SARS-CoV-2 RdRp gene by using entropy-driven and bipedal DNA walker amplification strategy. Chem. Eng. J. 2022, 427, 131686. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the chronic-wound microbiome: Fungal communities are prevalent, dynamic, and associated with delayed healing. MBio 2016, 7, e01058-16. [Google Scholar] [CrossRef]
- Bertesteanu, S.; Triaridis, S.; Stankovic, M.; Lazar, V.; Chifiriuc, M.C.; Vlad, M.; Grigore, R. Polymicrobial wound infections: Pathophysiology and current therapeutic approaches. Int. J. Pharm. 2014, 463, 119–126. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Ni, P.-W.; Huang, Y.; Xie, T. Therapeutic strategies for chronic wound infection. Chin. J. Traumatol. 2022, 25, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.Ü.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial contribution in chronicity of wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Khan, M.N.; Davies, C.G. Advances in the management of leg ulcers–the potential role of growth factors. Int. Wound J. 2006, 3, 113–122. [Google Scholar] [CrossRef]
- Komarcević, A. The modern approach to wound treatment. Med. Pregl. 2000, 53, 363–368. [Google Scholar]
- Park, S.-N.; Kim, J.K.; Suh, H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials 2004, 25, 3689–3698. [Google Scholar] [CrossRef]
- Patrick, B.N.; Rivey, M.P.; Allington, D.R. Acute renal failure associated with vancomycin-and tobramycin-laden cement in total hip arthroplasty. Ann. Pharmacother. 2006, 40, 2037–2042. [Google Scholar] [CrossRef]
- Misra, A.; Nanchahal, J. Use of gauze soaked in povidone iodine for dressing acute open wounds. Plast. Reconstr. Surg. 2003, 111, 2105–2106. [Google Scholar]
- Hoekstra, M.; Hermans, M.; Richters, C.; Dutrieux, R. A histological comparison of acute inflammatory responses with a hydrofibre or tulle gauze dressing. J. Wound Care 2002, 11, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; de Castro, M.C.R. New dressings, including tissue-engineered living skin. Clin. Dermatol. 2002, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Voinchet, V.; Vasseur, P.; Kern, J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am. J. Clin. Dermatol. 2006, 7, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Liu, C.; Ponsero, A.J.; Armstrong, D.G.; Lipsky, B.A.; Hurwitz, B.L. The dynamic wound microbiome. BMC Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme mimicry for combating bacteria and biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Versey, Z.; Nizer, W.S.D.C.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-innate immune interface: Contribution to chronic wound formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- Attinger, C.; Wolcott, R. Clinically addressing biofilm in chronic wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burn. Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for treatment of infected diabetic foot ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Muktar, M.; Ismail, W.; Razak, S.; Razali, M.; Amin, K. Accelerated wound healing of physically cross linked gellan gum-virgin coconut oil hydrogel containing manuka honey. ASM Sci. J. 2018, 11, 166–182. [Google Scholar]
- Murandu, M.; Webber, M.; Simms, M. Use of granulated sugar therapy in the management of sloughy or necrotic wounds: A pilot study. J. Wound Care 2011, 20, 206–216. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Murthy, K.N.C. Challenges in healing wound: Role of complementary and alternative medicine. Front. Nutr. 2021, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Chen, B.-H.; Inbaraj, B.S. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Bogoev, S. Chronic Wounds as a Socioeconomic Burden. KNOWLEDGE-Int. J. 2021, 49, 885–888. [Google Scholar]
- Upton, D.; Upton, P. Quality of life and well-being. In Psychology of Wounds and Wound care in Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2015; pp. 85–111. [Google Scholar]
- Gupta, S.; Sagar, S.; Maheshwari, G.; Kisaka, T.; Tripathi, S. Chronic wounds: Magnitude, socioeconomic burden and consequences. Wounds Asia 2021, 4, 8–14. [Google Scholar]
- Kapp, S.; Santamaria, N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int. Wound J. 2017, 14, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Pal, S.; Saha, C. A review on structure–affinity relationship of dietary flavonoids with serum albumins. J. Biomol. Struct. Dyn. 2014, 32, 1132–1147. [Google Scholar] [CrossRef]
- Verma, M.L.; Sharma, S.; Saini, R.; Rani, V.; Kushwaha, R. Chapter 3— Bioflavonoids: Synthesis, functions and biotechnological applications. In Biotechnological Production of Bioactive Compounds; Verma, M.L., Chandel, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–105. [Google Scholar]
- Chaniad, P.; Tewtrakul, S.; Sudsai, T.; Langyanai, S.; Kaewdana, K. Anti-inflammatory, wound healing and antioxidant potential of compounds from Dioscorea bulbifera L. bulbils. PLoS ONE 2020, 15, e0243632. [Google Scholar] [CrossRef]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef]
- Hou, Y.; Xin, M.; Li, Q.; Wu, X. Glycyrrhizin micelle as a genistein nanocarrier: Synergistically promoting corneal epithelial wound healing through blockage of the HMGB1 signaling pathway in diabetic mice. Exp. Eye Res. 2021, 204, 108454. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Ali-Seyed, M.; Ayesha, S. Calotropis-A multi-potential plant to humankind: Special focus on its wound healing efficacy. Biocatal. Agric. Biotechnol. 2020, 28, 101725. [Google Scholar] [CrossRef]
- Saini, P.; Al-Shibani, N.; Sun, J.; Zhang, W.; Song, F.; Gregson, K.S.; Windsor, L.J. Effects of Calendula officinalis on human gingival fibroblasts. Homeopathy 2012, 101, 92–98. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Leo, A.; Gallelli, L.; Citraro, R.; Cione, E.; Caroleo, M.C.; Biagi, M.; Aiello, F.; Manetti, F. Quercetin-3-Oleate Contributes to Skin Wound Healing Targeting FFA1/GPR40. ChemistrySelect 2019, 4, 8429–8433. [Google Scholar] [CrossRef]
- Kumar, S.; Lakshmi, P.K.; Sahi, C.; Pawar, R.S. Sida cordifolia accelerates wound healing process delayed by dexamethasone in rats: Effect on ROS and probable mechanism of action. J. Ethnopharmacol. 2019, 235, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018, 17, 399. [Google Scholar]
- Yeh, C.-J.; Chen, C.-C.; Leu, Y.-L.; Lin, M.-W.; Chiu, M.-M.; Wang, S.-H. The effects of artocarpin on wound healing: In vitro and in vivo studies. Sci. Rep. 2017, 7, 15599. [Google Scholar] [CrossRef] [PubMed]
- Heinke, J.; Patterson, C.; Moser, M. Life is a pattern: Vascular assembly within the embryo. Front. Biosci. Elite Ed. 2012, 4, 2269. [Google Scholar] [CrossRef]
- Soni, H.; Singhai, A.K. A recent update of botanicals for wound healing activity. Int. Res. J. Pharm. 2012, 3, 1–7. [Google Scholar]
- Mishra, S.; Mishra, S.R.; Soni, H. Efficacy of hydrogel containing rutin in wound healing. EAS J. Pharm. Pharmacol. 2021, 3, 161–167. [Google Scholar]
- Wan, J.; Ma, T.; Jin, Y.; Qiu, S. The effects of morin on bone regeneration to accelerate healing in bone defects in mice. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420962909. [Google Scholar] [CrossRef] [PubMed]
- Américo, Á.V.L.D.S.; Nunes, K.M.; Assis, F.F.V.D.; Dias, S.R.; Passos, C.T.S.; Morini, A.C.; Araújo, J.A.D.; Castro, K.C.F.; Silva, S.K.R.D.; Barata, L.E.S.; et al. Efficacy of Phytopharmaceuticals from the Amazonian Plant Libidibia ferrea for Wound Healing in Dogs. Front. Vet. Sci. 2020, 7, 244. [Google Scholar] [CrossRef]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Fialová, S.B. Prenylated flavonoids in topical infections and wound healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of quercetin and curcumin combination on antibacterial, antioxidant, in vitro wound healing and migration of human dermal fibroblast cells. Int. J. Mol. Sci. 2022, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Besrour, N.; Oludemi, T.; Mandim, F.; Pereira, C.; Dias, M.I.; Soković, M.; Barros, L. Valorization of Juglans regia Leaves as Cosmeceutical Ingredients: Bioactivity Evaluation and Final Formulation Development. Antioxidants 2022, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.M.U.; Hanif, M.; Mahmood, K.; Aziz, M.; Abbas, G.; Latif, H. Wound-healing and antibacterial activity of the quercetin–4-formyl phenyl boronic acid complex against bacterial pathogens of diabetic foot ulcer. ACS Omega 2022, 7, 24415–24422. [Google Scholar] [CrossRef]
- Kuma, D.N.; Boye, A.; Kwakye-Nuako, G.; Boakye, Y.D.; Addo, J.K.; Asiamah, E.A.; Aboagye, E.A.; Martey, O.; Essuman, M.A.; Barku, V.Y.A. Wound Healing Properties and Antimicrobial Effects of Parkia clappertoniana Keay Fruit Husk Extract in a Rat Excisional Wound Model. BioMed Res. Int. 2022, 2022, 9709365. [Google Scholar] [CrossRef]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.; Eldahshan, O.A.; Singab, A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.O.M.; Vilegas, W.; Tangerina, M.M.P.; Arunachalam, K.; Balogun, S.O.; Orlandi-Mattos, P.E.; Colodel, E.M.; Martins, D.T.D.O. Lafoensia pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J. Ethnopharmacol. 2018, 219, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yoon, M.; Choi, K.-Y. Approaches for regenerative healing of cutaneous wound with an emphasis on strategies activating the Wnt/β-catenin pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, X.; Shi, X.; Zhao, J.; Chen, Y.; Yao, Q.; Sun, C.; Yang, J. Regulatory mechanisms of the Wnt/β-catenin pathway in diabetic cutaneous ulcers. Front. Pharmacol. 2018, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef]
- Sferrazza, G.; Corti, M.; Brusotti, G.; Pierimarchi, P.; Temporini, C.; Serafino, A.; Calleri, E. Nature-derived compounds modulating Wnt/β-catenin pathway: A preventive and therapeutic opportunity in neoplastic diseases. Acta Pharm. Sin. B 2020, 10, 1814–1834. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.-L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Lee, M.-J.; Byun, M.R.; Furutani-Seiki, M.; Hong, J.-H.; Jung, H.-S. YAP and TAZ regulate skin wound healing. J. Investig. Dermatol. 2014, 134, 518–525. [Google Scholar] [CrossRef]
- Huang, F.; Chen, Y.-G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18. [Google Scholar]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Kleinerman, R.; Lerman, O.Z.; Brown, D.; Galiano, R.; Gurtner, G.C.; Warren, S.M.; Levine, J.P.; Saadeh, P.B. Hedgehog signaling is essential for normal wound healing. Wound Repair Regen. 2008, 16, 768–773. [Google Scholar] [CrossRef]
- Gururajan, M.; Chui, R.; Karuppannan, A.K.; Ke, J.; Jennings, C.D.; Bondada, S. c-Jun N-terminal kinase (JNK) is required for survival and proliferation of B-lymphoma cells. Blood 2005, 106, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Fujiwara, T.; Matsuzaki, S.; Shingaki, K.; Taniguchi, M.; Miyata, S.; Tohyama, M.; Sakai, Y.; Yano, K.; Hosokawa, K.; et al. bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PloS ONE 2010, 5, e12228. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Serras, F.; Martín-Blanco, E.; Baguñà, J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 2005, 280, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-L.; Yen, G.-C. Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J. Agric. Food Chem. 2009, 57, 2576–2582. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wu, W.-B.; Fang, J.-Y.; Chiang, H.-S.; Chen, S.-K.; Chen, B.-H.; Chen, Y.-T.; Hung, C.-F. (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules 2007, 12, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Jeong, Y.-J.; Lee, Y.-J.; Kwon, H.-M.; Kang, Y.-H. (−) Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J. Nutr. 2005, 135, 707–713. [Google Scholar] [CrossRef]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2017, 161, 1–13. [Google Scholar] [CrossRef]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Pharmacological activation of Nrf2 promotes wound healing. Eur. J. Pharmacol. 2020, 886, 173395. [Google Scholar] [CrossRef]
- Süntar, I.; Çetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory role of Nrf2 signaling pathway in wound healing process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef]
- Emiroglu, G.; Coskun, Z.O.; Kalkan, Y.; Erdivanli, O.C.; Tumkaya, L.; Terzi, S.; Özgür, A.; Demirci, M.; Dursun, E. The effects of curcumin on wound healing in a rat model of nasal mucosal trauma. Evid. -Based Complement. Altern. Med. 2017, 2017, 9452392. [Google Scholar] [CrossRef]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.N.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic potential of luteolin on impaired wound healing in streptozotocin-induced rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.S.; Jung, S.-J.; Kim, D.; Park, H.J.; Cho, D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.S.; Li, M.L.; Chen, J.S.; Zhou, L.; Zhou, W. Application of Mono- and Disaccharides in Drug Targeting and Efficacy. ChemMedChem 2018, 13, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Hou, B.; Cai, W.; Chen, T.; Zhang, Z.; Gong, H.; Yang, W.; Qiu, L. Vaccarin hastens wound healing by promoting angiogenesis via activation of MAPK/ERK and PI3K/AKT signaling pathways in vivo. Acta Cir. Bras. 2020, 34, e201901202. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt pathway: Emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Wu, X.; Hui, B.; Han, S.; Gao, J.; Li, Y.; Shi, J.; Zhu, H.; Zhao, B.; et al. Simultaneous deactivation of FAK and Src improves the pathology of hypertrophic scar. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Li, W.; Nadelman, C.; Henry, G.; Fan, J.; Muellenhoff, M.; Medina, E.; Gratch, N.S.; Chen, M.; Han, J.; Woodley, D. The p38-MAPK/SAPK pathway is required for human keratinocyte migration on dermal collagen. J. Investig. Dermatol. 2001, 117, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.S.; Medicherla, S.; Wadsworth, S.; Cullen, B.; Silcock, D.; Ma, J.Y.; Mangadu, R.; Kerr, I.; Chakravarty, S.; Luedtke, G.L.; et al. p38 MAPK inhibition reduces diabetes-induced impairment of wound healing. Diabetes Metab. Syndr. Obes. Targets Ther. 2009, 2, 91. [Google Scholar] [CrossRef]
- Loughlin, D.T.; Artlett, C.M. Modification of collagen by 3-deoxyglucosone alters wound healing through differential regulation of p38 MAP kinase. PloS ONE 2011, 6, e18676. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv. Wound Care 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Taguchi, N.; Yuriguchi, M.; Ando, T.; Kitai, R.; Aoki, H.; Kunisada, T. Flavonoids with two OH groups in the B-ring promote pigmented hair regeneration. Biol. Pharm. Bull. 2019, 42, 1446–1449. [Google Scholar] [CrossRef]
- Xie, F.; Cai, W.; Liu, Y.; Li, Y.; DU, B.; Feng, L.; Qiu, L. Vaccarin attenuates the human EA. hy926 endothelial cell oxidative stress injury through inhibition of Notch signaling. Int. J. Mol. Med. 2015, 35, 135–142. [Google Scholar] [CrossRef]
- Teng, Y.-Y.; Zou, M.-L.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Yuan, Z.-D.; Wu, J.-J.; Ye, J.-X.; Yu, S.; Li, X.; et al. Dual-Action Icariin-Containing Thermosensitive Hydrogel for Wound Macrophage Polarization and Hair-Follicle Neogenesis. Front. Bioeng. Biotechnol. 2022, 10, 902894. [Google Scholar] [CrossRef]
- McKay, T.B.; Kivanany, P.B.; Nicholas, S.E.; Nag, O.K.; Elliott, M.H.; Petroll, W.M.; Karamichos, D. Quercetin Decreases Corneal Haze In Vivo and Influences Gene Expression of TGF-Beta Mediators In Vitro. Metabolites 2022, 12, 626. [Google Scholar] [CrossRef]
- Wibowo, I.; Utami, N.; Anggraeni, T.; Barlian, A.; Putra, R.E.; Indriani, A.D.; Masadah, R.; Ekawardhani, S. Propolis can improve caudal fin regeneration in zebrafish (Danio rerio) induced by the combined administration of Alloxan and glucose. Zebrafish 2021, 18, 274–281. [Google Scholar] [CrossRef]
- He, J.; Peng, H.; Wang, M.; Liu, Y.; Guo, X.; Wang, B.; Dai, L.; Cheng, X.; Meng, Z.; Yuan, L.; et al. Isoliquiritigenin inhibits TGF-β1-induced fibrogenesis through activating autophagy via PI3K/AKT/mTOR pathway in MRC-5 cells. Acta Biochim. Biophys. Sin. 2020, 52, 810–820. [Google Scholar] [CrossRef]
- Bernabe-Garcia, A.; Armero-Barranco, D.; Liarte, S.; Ruzafa-Martinez, M.; Ramos-Morcillo, A.J.; Nicolás, F.J. Oleanolic acid induces migration in Mv1Lu and MDA-MB-231 epithelial cells involving EGF receptor and MAP kinases activation. PLoS ONE 2017, 12, e0172574. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhou, C.; Qiu, W.; Wu, H.; Li, J.; Peng, J.; Qiu, M.; Liang, C.; Gao, J.; Luo, S. Total flavonoids from Semen Cuscutae target MMP9 and promote invasion of EVT cells via Notch/AKT/MAPK signaling pathways. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Han, X.; Chen, J.; Zhai, Y.; Lin, Y.; Ma, H.; Feng, F.; He, X.; Li, P. Huiyang Shengji decoction promotes wound healing in diabetic mice by activating the EGFR/PI3K/ATK pathway. Chin. Med. 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Ruttanapattanakul, J.; Wikan, N.; Potikanond, S.; Nimlamool, W. Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes. Pharmaceuticals 2022, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Liu, J.; Liu, G.; Zhou, W.; Liu, Y.; Hu, L.; Xiong, L.; Ye, S.; Wu, Y. Icariin promotes wound healing by enhancing the migration and proliferation of keratinocytes via the AKT and ERK signaling pathway. Int. J. Mol. Med. 2018, 42, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Sharma, M.; Kumar, V.; Joshi, V.G. Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol. Immunotoxicol. 2021, 43, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Coleridge-Smith, P.D. Leg ulcer treatment. J. Vasc. Surg. 2009, 49, 804–808. [Google Scholar] [CrossRef]
- Coleridge-Smith, P.; Lok, C.; Ramelet, A.-A. Venous leg ulcer: A meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 198–208. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Perry, C.M. Micronised purified flavonoid fraction. Drugs 2003, 63, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.; Ibrahem, M. Management of aphthous ulceration with topical quercetin: A randomized clinical trial. J. Contemp. Dent. Pract. 2010, 11, E9–E16. [Google Scholar] [CrossRef]
- Limsitthichaikoon, S.; Khampaenjiraroch, B.; Damrongrungruang, T.; Limphirat, W.; Thapphasaraphong, S.; Priprem, A. Topical oral wound healing potential of anthocyanin complex: Animal and clinical studies. Ther. Deliv. 2018, 9, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Damrongrungruang, T.; Paphangkorakit, J.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Davies, M.J.; Sungthong, B.; Priprem, A. Anthocyanin complex niosome gel accelerates oral wound healing: In vitro and clinical studies. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 02423. [Google Scholar] [CrossRef]
- Mallery, S.R.; Stoner, G.D.; Larsen, P.E.; Fields, H.W.; Rodrigo, K.A.; Schwartz, S.J.; Tian, Q.; Dai, J.; Mumper, R.J. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: Implications for oral cancer chemoprevention. Pharm. Res. 2007, 24, 728–737. [Google Scholar] [CrossRef]
- Shumway, B.S.; Kresty, L.A.; Larsen, P.E.; Zwick, J.C.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D.; Mallery, S.R. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 2008, 14, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Kubat, M.; Karabulut, Z.; Şengül, S. Effect of propolis on wound healing in sacrococcygeal pilonidal disease: A randomized controlled clinical trial. Pak. J. Pharm. Sci. 2021, 34, 1063–1067. [Google Scholar]
- Miguel, M.G.; Antunes, M.D. Is propolis safe as an alternative medicine? J. Pharm. Bioallied Sci. 2011, 3, 479. [Google Scholar] [CrossRef]
- Salazar-Gómez, A.; Alonso-Castro, A.J. Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review. Pharmaceuticals 2022, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Benedek, B.; Kopp, B.; Melzig, M.F. Achillea millefolium L. sl—Is the anti-inflammatory activity mediated by protease inhibition? J. Ethnopharmacol. 2007, 113, 312–317. [Google Scholar] [CrossRef]
- Hajhashemi, M.; Ghanbari, Z.; Movahedi, M.; Rafieian, M.; Keivani, A.; Haghollahi, F. The effect of Achillea millefolium and Hypericum perforatum ointments on episiotomy wound healing in primiparous women. J. Matern.-Fetal Neonatal Med. 2018, 31, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M.; et al. Development of a chitosan hydrogel containing flavonoids extracted from Passiflora edulis leaves and the evaluation of its antioxidant and wound healing properties for the treatment of skin lesions in diabetic mice. J. Biomed. Mater. Res. Part A 2020, 108, 654–662. [Google Scholar] [CrossRef]
- Fras-Zemljič, L.; Kokol, V.; Čakara, D. Antimicrobial and antioxidant properties of chitosan-based viscose fibres enzymatically functionalized with flavonoids. Text. Res. J. 2011, 81, 1532–1540. [Google Scholar] [CrossRef]
- İlk, S.; Ramanauskaitė, A.; Bilican, B.K.; Mulerčikas, P.; Çam, D.; Onses, M.S.; Torun, I.; Kazlauskaitė, S.; Baublys, V.; Aydın, Ö.; et al. Usage of natural chitosan membrane obtained from insect corneal lenses as a drug carrier and its potential for point of care tests. Mater. Sci. Eng. C 2020, 112, 110897. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, S.; Ganesh, N.; Srivastava, M.M.; Srivastava, S. Biofabrication and characterization of flavonoid-loaded Ag, Au, Au–Ag bimetallic nanoparticles using seed extract of the plant Madhuca longifolia for the enhancement in wound healing bio-efficacy. Prog. Biomater. 2019, 8, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Tseng, Y.-T.; Gupta, A.; Lin, Y.-F.; Sangili, A.; Huang, Y.-F.; Huang, C.-C.; Chang, H.-T. Anti-microbial/oxidative/inflammatory nanogels accelerate chronic wound healing. Smart Mater. Med. 2022, 3, 148–158. [Google Scholar] [CrossRef]

| Type of Dressings | Available Treatments | Functions | Limitations | Refs. |
|---|---|---|---|---|
| Medicated | Growth factor | Promotes tissue regeneration by angiogenesis and cellular proliferation. | The suitability for appropriate dressings is challenging for effective release at the wound site. | [40,41,42] |
| Antimicrobials | Prevents infections. | Cell and organ toxicity due to a high dose of prescription antibiotic. | [43] | |
| Traditional | Topical applications, e.g., povidone iodine, saline | Cleanse wound and irrigates dry wound. | Applicable only for small wounds and suppuration. | [44] |
| Modern | Hydrocolloids | Prevents mild exuding wounds from drying out and offers moist insulation for wound healing to take place. | Fibers are deposited upon dressing and need to be replaced during dressing change. | [45] |
| Foam | Provides prolonged moisture and effective absorbent. | Not applicable to dry scars and epithelial wounds. | [46] | |
| Bioactive, e.g., collagen, hydrogel | Promotes matrix formation and is biodegradable. | Required additional testing to compare with standard wound dressing. | [47,48] |
| Years | Flavonoid Types | Study Design | Efficacy of Flavonoids Treatment | Refs. |
|---|---|---|---|---|
| 2019 | Quercetin-3-oleate | In vitro study on HaCaT cell. | Quercetin stimulated the HaCaT cell by 51% compared to the untreated cell. The result suggested an increment in TGF-β production and MMP-9 release. | [77] |
| 2018 | Hesperidin | In vivo study on Sprague–Dawley rats. | Hesperidin enhanced healing in the chronic diabetic wound by up-regulating VEGF-c, Ang-1/Tie-2, TGF-β, and Smad-2/3 mRNA expression. | [79] |
| 2021 | Rutin | In vivo study on mice model. | Reduction of wound area was observed over a period between the 4th and 16th days. H2 hydrogel formulation was chosen as the best concentration to accommodate wound-healing activity. | [83] |
| 2020 | Morin (flavonoid extracted from Maclura pomifera plant) | In vivo study on 44-week-old mice. | Morin stimulated the Wnt pathway during osteoblasts development and promoted angiogenesis, thus, portraying a promising future in bone defects regeneration treatment. | [84] |
| 2018 | Quercetin | In vivo study on albino rats. | The quercetin loaded liposomal hydrogel accelerated fibroblast cell growth in 4 days of treatment on subjects with shaved back wound. | [72] |
| 2019 | Kaempferol | In vivo study on diabetic and non-diabetic rats. | Kaempferol was suggested to be an effective wound-healing agent because it was able to induce a 92.12% healing rate. | [87] |
| Samples | Models | Parameters | Results | Ref. |
|---|---|---|---|---|
| Juglans regia leaves | Staphylococcus lugdunensis, Proteus vulgaris, and Staphylococcus epidermidis. HaCaT cells. | Minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC). Wound scratch healing. Cell invasion. | HPLC profiles revealed 10 flavonoids and its derivatives. MIC of 2–4 mg/mL against S. lugdunensis and S. aureus. Good inhibitory effects against Gram-negative strains (P. vulgaris and S. lugdunensis) as compared to Gram-positive strains (S. epidermidis and S. aureus). About 28% of wound closure was recorded 40% inhibition against P. vulgaris-infected HaCaT cells. | [93] |
| Quercetin and 4-formyl phenyl boronic acid (4FPBA-Q complex) | Multidrug resistance of Salmonella typhi, S. aureus, Pseudomonas aeruginosa. Male Wistar albino rats. | MIC and MBC. Primary dermal irritation index. In vivo wound healing. | The 4FPBA-Q complex was more effective against P. aeruginosa and S. typhi. No sign of erythema upon 4FPBA-Q complex. Treatment with 4FPBA-Q complex demonstrated ample healing in 10 days. | [94] |
| Quercetin and Curcumin | S. aureus and P. aeruginosa. Human dermal fibroblasts (HDFB). | Disc diffusion assay. In vitro scratch assay. Migration assay. | A 1:1 ratio of quercetin/curcuminoid exhibited the strongest inhibition zone against both strains. Single compound did not show any inhibition. The percentage of wound closure is higher upon treatment with quercetin alone or in combination with curcuminoid. A 3:1 ratio of quercetin/curcuminoid significantly induced HDFB migration toward matrix crossing. | [90] |
| Parkia clappertoniana fruit husk extract (PCFHE) | Male Sprague–Dawley rats. S. aureus, Bacillus subtilis, Escherichia coli, P. aeruginosa, Klebsiella pneumoniae. | Excision wound model. H&E and collagen staining. Skin irritation test. MIC and MBC. | PCFHE-treated group showed potential wound-healing properties during epithelization and increased collagen content. PCFHE showed concentration dependent inhibition on all microbial growth except S. aureus. | [95] |
| Coccinia grandis leaves | Bacillus cereus. Male Wistar albino rats. | Daily observation of wound closure. Histopathological examination. | LC-ESI-MS/MS displayed that the extract was rich in flavonoids. Good healing ability and almost similar to the Fucidin-treated group. H&E staining portrayed complete re-epithelization of epidermis. Deposition of collagen fiber was as good as Fucidin-treated group. | [96] |
| Lafoensia capari leaves (HELp) | S. aureus, S. epidermidis, Streptococcus pyogenes, Enterococcus faecalis, S. typi, P. aeruginosa, Shigella flexneri, K. pneumoniae, E. coli. RAW 264.7 murine macrophages, Chinese hamster ovary epithelial cells, and L929 fibroblasts. Albino mice (Mus musculus), Swiss-Webster strain, and rats (Rattus norvegicus), Wistar strain. | Excision wound-healing model. Scratch wound-healing assay. Western blot analysis of p-ERK1/2 in vitro. | The contraction rates increase with a topical application of HELp on Day 2. At 10 and 30 mg/gel, HELp exhibited moderate re-epithelization and neovascularization through H&E staining. The rate of fibroblast migration increases by 25.1% and 35.3% at 0.1 and 0.03 ug/mL, respectively. HELp upregulated the expression of p-ERK1/2, which promoted cell proliferation. ESI-MS revealed that HELp was rich in phytochemicals, i.e., flavonoids that contributed to the wound-healing properties for in vivo and in vitro findings. | [97] |
| Pathways | Flavonoids | Experiments | Outcomes | Gene/Protein Detection | Refs. |
|---|---|---|---|---|---|
| Wnt/β-catenin | Quercetin | In vitro: CCK-8 and scratch assay (skin cells) In vivo: Histological staining (C57BL/6 mice), Western blot, RT-qPCR analysis, and molecular docking | Wound healing rates increased in dose-dependent manner compared to the control group; levels of inflammatory factors, including tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6 were significantly reduced after quercetin administration; improved level of GSH; molecular docking analysis validated the formed hydrogen bonds between quercetin and Ala195, Gln308, Asn369, and Lys372 residues of TERT. | Telomerase reverse transcriptase (TERT) | [98] |
| Flavonoids with two OH groups in the B-ring, such as sterubin, luteolin, and hydroxygekwanin | In vivo: Dorsal skin samples (2.25 cm) were excised from 7-week-old C57BL/6 mice; flavonoids (0.1% w/v) were dissolved in 50% ethanol; and 200 µL of the flavonoid solution was applied daily to the wounded area for 1 week. | Flavonoids with two OH groups in the B-ring, such as sterubin, luteolin, and hydroxygenkwanin, showed promising effects in regenerating black pigmented hairs, whereas those with one OH group in the B-ring showed no significant change. | N/A | [99] | |
| TGF-β | Hesperidin | In vivo: Diabetes was induced experimentally by streptozotocin (STZ, 55 mg/kg, i.p.) in Sprague–Dawley rats (180–220 g), and hesperidin (25, 50 and 100 mg/kg, p.o.) was administered for 21 days after wound stabilization. Various biochemical, molecular, and histopathological parameters were evaluated in wound tissue. | Hesperidin treatment showed a significant increase (p < 0.05) in percent wound closure and serum insulin level. Intraperitoneal administration of STZ caused significant down-regulation in VEGF-c, Ang-1, Tie-2, TGF-beta, and Smad 2/3 mRNA expression in wound tissues, whereas hesperidin (50 and 100 mg/kg) treatment showed significant up-regulation in these mRNA expressions. | VEGF-c, Ang-1/Tie-2, TGF-β and Smad-2/3 mRNA | [79] |
| Notch | Vaccarin | In vitro: In this study, the EAhy926 cells were exposed to 250, 500, and 1000 M H2O2 for 2 and 4 h in the absence or presence of vaccarin, and the cell injury induced by H2O2 was examined via sulforhodamine B (SRB) assay. Cell migratory ability, lactate dehydrogenase (LDH) leakage, malondialdehyde (MDA) levels, and decreasing superoxide dismutase (SOD) activity were evaluated by the wound-healing assay and corresponding assay kits. | Western blot detected the protein expressions of Notch1, Hes1, and caspase-3. Preincubation with vaccarin was found to protect EA.hy926 cells from H2O2-induced cell oxidative stress injury, which promoted cell viability and cell migratory ability and inhibited the level of LDH and MDA but enhanced the activity of SOD. In addition to the downregulation of Notch signaling, vaccarin treatments also downregulated caspase-3, a cell-apoptotic-pathway-related protein. | Notch1, Hes1 and Caspase-3 | [100] |
| Bone morphogenetic protein (BMP) pathway | Icariin | In vitro: CCK-8 assay and live/dead cell staining, immunofluorescence staining, Western blotting, RT-PCR, histological analysis, and cell migration assay | Icariin+PEG hydrogel resulted in faster healing and formation of new hair follicles; induced a higher level of M2 phenotypic transformation of macrophages; reduced the invasion of inflammation, excessive deposition of collagen, and immoderate activation of myofibroblasts; and increased the expression of BMP4 and Smad1/5 phosphorylation in skin wounds. | BMP4; Smad1/5 | [101] |
| Quercetin | In vitro: RNA analysis In vivo: Wild-type C57BL/6J mice were treated with quercetin (0.5, 1, 5, or 50 mM). Corneal scarring was assessed for 3 weeks by slit lamp imaging and clinically scored. In a separate animal study, six New Zealand White rabbits underwent lamellar keratectomy surgery, followed by treatment with 5 mM quercetin or vehicle twice daily for three days. Stromal backscattering was assessed at week 3 by in vivo confocal microscopy. | In vitro: Human corneal fibroblasts showed that quercetin modulated select factors of the transforming growth factor-beta (TGF-beta) signaling pathway. These results provide evidence that quercetin may inhibit corneal scarring. In vivo: In mice, a single dose of 5 mM quercetin reduced corneal scar formation. In rabbits, stromal backscattering was lower in two out of three animals in the quercetin-treated group. | TGF-β2 and SMAD7 | [102] | |
| Hedgehog, bone morphogenetic protein (BMP), and Wnt signaling | Propolis | In vivo: GC-MS analysis and antioxidant activity testing; blood glucose levels testing; real-time polymerase chain reaction method. | Phenols and flavonoids from the ethanolic extract of propolis (EEP) can improve the caudal fin regeneration of hyperglycemic zebrafish. | Shha, Igf2a, Bmp2b, and Col1a2 | [103] |
| PI3K/AKT/mTOR | Isoliquiritigenin (ISL) | In vitro: MTT assay, wound-healing assay, quantitative real-time PCR, western blot and immunofluorescence assay. | The results showed that ISL inhibited TGF-beta 1-induced proliferation and migration and down-regulated the expressions of alpha-smooth muscle actin (α-SMA), collagen type I alpha 1 (COLIA1), and fibronectin (FN). ISL treatment led to up-regulation of microtubule-associated protein light chain 3 (LC3) in TGF-beta 1-treated MRC-5 cells, accompanied by a significant decrease in the phosphorylation levels of phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), and mammalian target of rapamycin (mTOR). | α-SMA, COLIA1 and FN | [104] |
| Mitogen-activated protein (MAP) kinases, such as ERK1,2 and Jun N-terminal kinase (JNK) 1,2 activation and c-Jun phosphorylation | Oleanolic acid (OA)—pentacyclic triterpene | In vitro: Scratch assay in two epithelial cell lines of different linage: non-malignant mink lung epithelial cells, Mv1Lu, and human breast cancer cells, MDA-MB-231. | OA enhanced cell migration for in vitro scratch closure; MDA-MB-231 cells treated with OA displayed an altered gene expression profile affecting transcription factor genes (c-JUN) as well as proteins involved in migration and ECM dynamics (PAI1); OA treatment served changes in the epidermal growth factor receptor (EGFR) subcellular localization. | phospho-c-Jun, c-Jun, phospho-ERK1/2 and phospho-JNK1/2 proteins | [105] |
| Notch, AKT, and MAPK signaling pathways. | Total flavonoids from Semen Cuscutae (TFSC), the main estrogenic active constituent of the plant, which includes hyperin, rutin, quercitrin, quercetin, and isorhamnetin | In vitro: HTR-8 cells migration and invasion functions were analyzed using wound healing and transwell assays. The regulatory effect of TFSC on MMP9 expression and relevant signaling. pathways were analyzed by western blot. | TFSC significantly promoted the migration of EVT cells in a dose and time-dependent manner compared to control group. The migration and invasion of EVT cells were maximized at the highest dosage of 5 μg/mL of TFSC. The expression of MMP9 in EVT cells was significantly increased after TFSC treatment. Furthermore, cells treated with TFSC significantly upregulated protein expressions in Notch, AKT, and p38/MAPK signaling pathways. | MMP9 | [106] |
| EGFR/PI3K/AKT | Chinese medicine Huiyang Shengji decoction (HYSJD) main components were flavonoids, terpenes, alkaloids, phenylpropanoids, and carbohydrates | In vivo: Microarray analysis, GO and KEGG enrichment analysis, ELISA assays, and western blot analysis | HYSJD was found to increase the wound-healing rate in chronic skin ulcers in mice at days 3, 7, and 14 post-wound formation and promote the proliferation of epidermal cells. Two proteins that were differentially expressed between the different groups, i.e., IGF-1 and EGFR, were further validated. Serum ELISA assays showed that serum EGFR in the HYSJD treatment group was significantly increased. KEGG pathway analysis suggested that the PI3K/AKT pathway involved in HYSJD promoting the proliferation of epidermal cells in chronic wounds in mice. | IGF-1 and EGFR | [107] |
| MAPK and PI3K/Akt | Pinocembrin | Cell viability assay; direct measurement of cell number; cellular wound-healing activity assay; western blot analysis; immunofluorescence study | Pinocembrin induced an increase in HaCaT cell number and significantly triggered ERK1/2 and Akt activation. Pinocembrin induces keratinocyte proliferation mainly by activating MAPK and PI3K/Akt kinases. | pAKT pERK | [108] |
| Akt and ERK | Icariin | In vitro: CCK-8 assay; transwell assay; western blot assay; RT-qPCR; ELISA. In vivo: hematoxylin and eosin (H&E) staining; epidermal thickness assessment. | In vitro shows that icariin significantly promoted the migration and proliferation of keratinocytes via the activation of AKT serine/threonine kinase 1 (AKT) and extracellular signal-regulated kinase (ERK). In addition, icariin inhibited the production of interleukin (IL)-6 and tumor necrosis factor (TNF)-α and induced the production of IL-10. In vivo shows icariin treatment accelerated the wound closure rate. | Cyclin D1 and D3 | [109] |
| ERK, P38, JNK and Akt | Artocarpin | In vitro: Cell viability; proliferation assays; wound healing assay. In vivo: BrdU incorporation assay; mouse cytokine array; immunohistochemical staining; immunoblotting analysis; Matrigel assay. Excisional wound model; Transmission electron microscopy (TEM). | Artocarpin accelerated inflammatory progression and subsequently decreased persistent inflammation. Artocarpin increased collagen production and increased human fibroblast proliferation and migration by activating the P38 and JNK pathways. Moreover, Artocarpin increased the proliferation and migration of human keratinocytes through the ERK and P38 pathways and augmented human endothelial cell proliferation and tube formation through the Akt and P38 pathways. | Akt, ERK, and P38 | [110] |
| Year | Flavonoids/Plants | Clinical Trial Design | Observation Time (Day) | Results | Refs. |
|---|---|---|---|---|---|
| 2020 | Quercetin (incorporated with oleic acid) | 56 patients (28 for both men and women) who applied the nano-hydrogel containing quercetin and oleic acid on the wound. | 8 months | Quercetin reduced skin lesion, improved tissue viscoelasticity, and could facilitate treatment in chronic wound. | [143] |
| 2009 | Micronized purified flavonoid fraction (MPFF) + compression treatment | 723 patients who took the MPFF orally. | 6 months | The combination of MPFF with the compression method sped up recovery of venous leg ulcer. | [145] |
| 2010 | Quercetin | 40 male volunteers were given cream containing quercetin to apply on their oral ulcer. | 10 days | Quercetin cream is able to relieve pain and induced complete healing within 2–4 days. | [148] |
| 2018 | Anthocyanin (water-soluble flavonoid) | 68 orthodontic patients were given anthocyanin gel to apply on their oral wound. | 7 days | The mucoadhesive gel containing anthocyanin promoted wound closure in acute oral wounds | [149] |
| 2021 | Anthocyanin | A double-blinded clinical trial on 60 volunteers was conducted with the application of anthocyanin gel onto oral wounds. | 7 days | The anthocyanin niosome gel accelerated wound closure, thus improving patients’ life quality in terms of relieving pain from the oral wound. | [150] |
| 2021 | Flavonoid class is not mentioned specifically | 33 patients for uncomplicated sacrococcygeal pilonidal sinus wound surgery. | 28 days | The treatment of 15% propolis water solution facilitated wound recovery by showing significant healing rate starting from the first week. | [153] |
| 2022 | Flavonoid class is not mentioned specifically | The study categorized the clinical trials into three groups: randomized, single-blinded, and double-blinded. 50 subjects for chronic venous leg ulcers | 8 weeks | The study analyzed 305 plant species with wound-healing properties. Among the plants, 25 compounds that contained substantial levels of flavonoids were isolated. Mimosa tenuiflora (Willd.) poir extract exerted the highest wound-healing activity by reducing the ulcer size to 93% in 8th weeks. | [155] |
| 2007 | Flavonoid class is not mentioned specifically | A double-blind clinical trial was continued by having 140 primiparous women as test subjects to study wound-healing activity on epiosomy incision | 14 days | The treatment can reduce pain, redness, and edema. No significant difference was found for both treatment and control groups in reducing the likelihood of wound dehiscence and secretion. The study suggested the importance of using appropriate dosage in the treatment. | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. https://doi.org/10.3390/ijms24054607
Zulkefli N, Che Zahari CNM, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS, Bunawan H, Baharum SN, Mediani A, Ahmed QU, et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. International Journal of Molecular Sciences. 2023; 24(5):4607. https://doi.org/10.3390/ijms24054607
Chicago/Turabian StyleZulkefli, Nabilah, Che Nur Mazadillina Che Zahari, Nor Hafiza Sayuti, Ammar Akram Kamarudin, Norazalina Saad, Hamizah Shahirah Hamezah, Hamidun Bunawan, Syarul Nataqain Baharum, Ahmed Mediani, Qamar Uddin Ahmed, and et al. 2023. "Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective" International Journal of Molecular Sciences 24, no. 5: 4607. https://doi.org/10.3390/ijms24054607
APA StyleZulkefli, N., Che Zahari, C. N. M., Sayuti, N. H., Kamarudin, A. A., Saad, N., Hamezah, H. S., Bunawan, H., Baharum, S. N., Mediani, A., Ahmed, Q. U., Ismail, A. F. H., & Sarian, M. N. (2023). Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. International Journal of Molecular Sciences, 24(5), 4607. https://doi.org/10.3390/ijms24054607







