Association between NLRP3 rs10754558 and CARD8 rs2043211 Variants and Susceptibility to Chronic Kidney Disease
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Cases and Control Subjects
2.2. Association of NLRP3 and CARD8 Gene Polymorphisms with CKD and ESRD
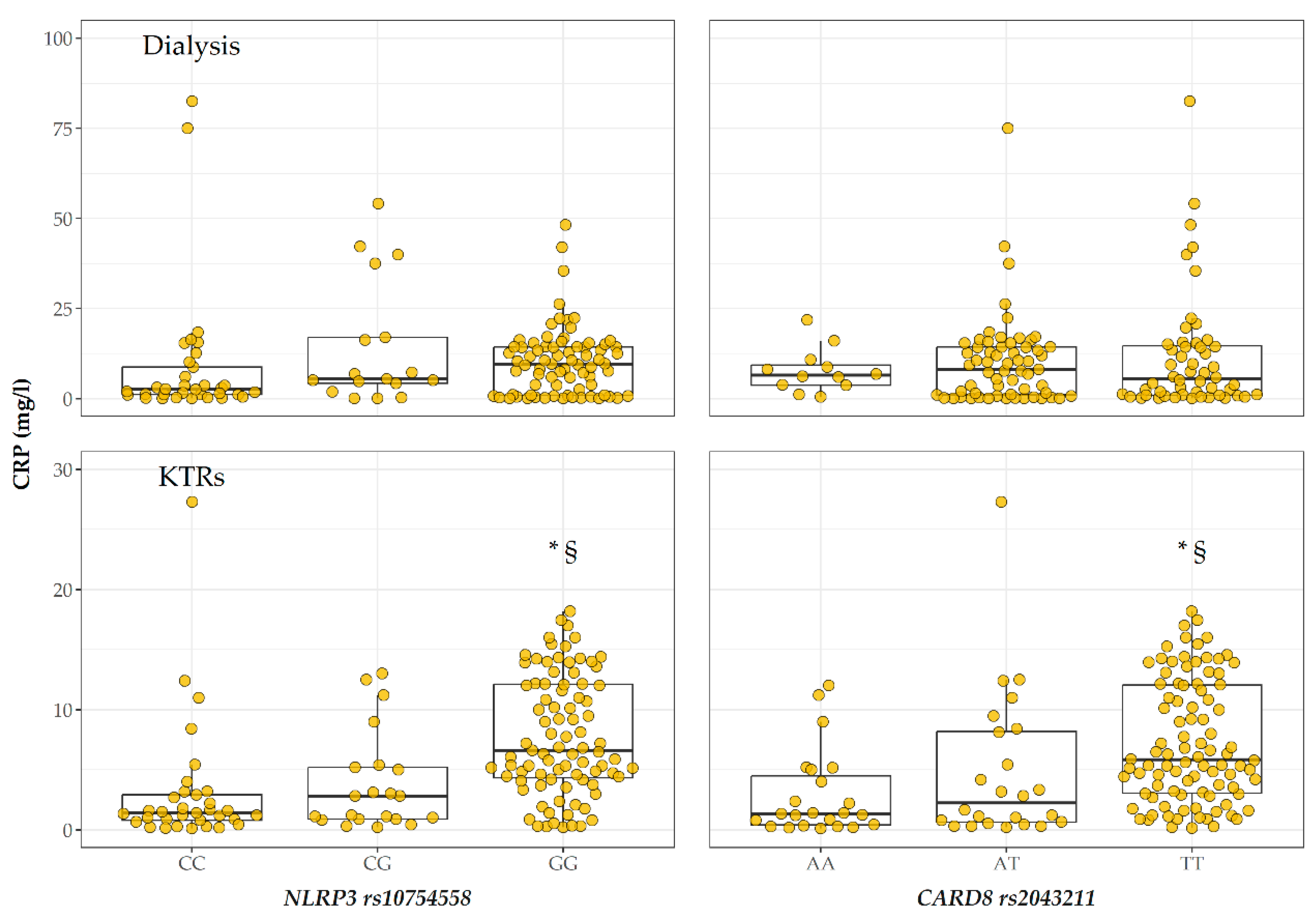
2.3. CRP Levels According to Genotype
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. SNP Genotyping
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory Processes in Renal Fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Attley, J.; Cuzzocrea, S. Targeting Inflammation: New Therapeutic Approaches in Chronic Kidney Disease (CKD). Pharmacol. Res. 2014, 81, 91–102. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.-D. The Cell Biology of Inflammasomes: Mechanisms of Inflammasome Activation and Regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; de Alba, E. Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. Int. J. Mol. Sci. 2021, 22, E872. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like Receptors and Inflammasomes: A Review of Their Canonical and Non-Canonical Signaling Pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Bouchier-Hayes, L.; Conroy, H.; Egan, H.; Adrain, C.; Creagh, E.M.; MacFarlane, M.; Martin, S.J. CARDINAL, a Novel Caspase Recruitment Domain Protein, Is an Inhibitor of Multiple NF-Kappa B Activation Pathways. J. Biol. Chem. 2001, 276, 44069–44077. [Google Scholar] [CrossRef]
- Ito, S.; Hara, Y.; Kubota, T. CARD8 Is a Negative Regulator for NLRP3 Inflammasome, but Mutant NLRP3 in Cryopyrin-Associated Periodic Syndromes Escapes the Restriction. Arthritis Res. Ther. 2014, 16, R52. [Google Scholar] [CrossRef] [PubMed]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R. Multifactorial Functions of the Inflammasome Component NLRP3 in Pathogenesis of Chronic Kidney Diseases. Kidney Int. 2019, 96, 58–66. [Google Scholar] [CrossRef]
- Vesey, D.A.; Cheung, C.; Cuttle, L.; Endre, Z.; Gobe, G.; Johnson, D.W. Interleukin-1beta Stimulates Human Renal Fibroblast Proliferation and Matrix Protein Production by Means of a Transforming Growth Factor-Beta-Dependent Mechanism. J. Lab. Clin. Med. 2002, 140, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Liu, H.-F.; Yao, C.-W.; Liu, H.-Y.; Huang-Fu, C.-M.; Chen, X.-W.; Du, S.-H.; Chen, X.-W. Effects of Interleukin 18 on Injury and Activation of Human Proximal Tubular Epithelial Cells. Nephrology (Carlton) 2007, 12, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bani-Hani, A.H.; Leslie, J.A.; Asanuma, H.; Dinarello, C.A.; Campbell, M.T.; Meldrum, D.R.; Zhang, H.; Hile, K.; Meldrum, K.K. IL-18 Neutralization Ameliorates Obstruction-Induced Epithelial-Mesenchymal Transition and Renal Fibrosis. Kidney Int. 2009, 76, 500–511. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, S.-M.; Kim, K.-P.; Lee, S.-H.; Moon, J.-Y. The Role of Inflammasome-Dependent and Inflammasome-Independent NLRP3 in the Kidney. Cells 2019, 8, 1389. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Li, L.; Ma, T.; Shi, M.; Yang, Y.; Fan, Q. A Small Molecule Inhibitor MCC950 Ameliorates Kidney Injury in Diabetic Nephropathy by Inhibiting NLRP3 Inflammasome Activation. Diabetes Metab. Syndr. Obes. 2019, 12, 1297–1309. [Google Scholar] [CrossRef]
- Chun, J.; Chung, H.; Wang, X.; Barry, R.; Taheri, Z.M.; Platnich, J.M.; Ahmed, S.B.; Trpkov, K.; Hemmelgarn, B.; Benediktsson, H.; et al. NLRP3 Localizes to the Tubular Epithelium in Human Kidney and Correlates With Outcome in IgA Nephropathy. Sci. Rep. 2016, 6, 24667. [Google Scholar] [CrossRef]
- Chi, K.; Geng, X.; Liu, C.; Cai, G.; Hong, Q. Research Progress on the Role of Inflammasomes in Kidney Disease. Mediat. Inflamm. 2020, 2020, 8032797. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Liang, T. Association of NLRP3 Rs35829419 and Rs10754558 Polymorphisms With Risks of Autoimmune Diseases: A Systematic Review and Meta-Analysis. Front. Genet. 2021, 12, 690860. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, Y.; Ebisawa, M.; Tomikawa, M.; Imai, T.; Komata, T.; Hirota, T.; Harada, M.; Sakashita, M.; Suzuki, Y.; Shimojo, N.; et al. Associations of Functional NLRP3 Polymorphisms with Susceptibility to Food-Induced Anaphylaxis and Aspirin-Induced Asthma. J. Allergy Clin. Immunol. 2009, 124, 779–785.e6. [Google Scholar] [CrossRef]
- Fontalba, A.; Gutiérrez, O.; Llorca, J.; Mateo, I.; Berciano, J.; Fernández-Luna, J.L.; Combarros, O. Deficiency of CARD8 Is Associated with Increased Alzheimer’s Disease Risk in Women. Dement. Geriatr. Cogn. Disord. 2008, 26, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.D.; Kellis, M. HaploReg: A Resource for Exploring Chromatin States, Conservation, and Regulatory Motif Alterations within Sets of Genetically Linked Variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Zhang, Q.-B.; Qing, Y.-F.; He, Y.-L.; Xie, W.-G.; Zhou, J.-G. Association of NLRP3 Polymorphisms with Susceptibility to Primary Gouty Arthritis in a Chinese Han Population. Clin. Rheumatol. 2018, 37, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Razmara, M.; Srinivasula, S.M.; Wang, L.; Poyet, J.-L.; Geddes, B.J.; DiStefano, P.S.; Bertin, J.; Alnemri, E.S. CARD-8 Protein, a New CARD Family Member That Regulates Caspase-1 Activation and Apoptosis. J. Biol. Chem. 2002, 277, 13952–13958. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhou, X.; Xie, X.; Chen, X.; Jie, Z.; Zou, Q.; Hu, H.; Zhu, L.; Cheng, X.; et al. Cell Intrinsic Role of NF-ΚB-Inducing Kinase in Regulating T Cell-Mediated Immune and Autoimmune Responses. Sci. Rep. 2016, 6, 22115. [Google Scholar] [CrossRef]
- Fontalba, A.; Martinez-Taboada, V.; Gutierrez, O.; Pipaon, C.; Benito, N.; Balsa, A.; Blanco, R.; Fernandez-Luna, J.L. Deficiency of the NF-KappaB Inhibitor Caspase Activating and Recruitment Domain 8 in Patients with Rheumatoid Arthritis Is Associated with Disease Severity. J. Immunol. 2007, 179, 4867–4873. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, X.; Li, C.; Xing, S.; Fu, Z.; Yuan, Y.; Wang, R.; Wang, Y.; Lv, W. CARD8 Rs2043211 Polymorphism Is Associated with Gout in a Chinese Male Population. Cell. Physiol. Biochem. 2015, 35, 1394–1400. [Google Scholar] [CrossRef]
- McKinney, C.; Stamp, L.K.; Dalbeth, N.; Topless, R.K.; Day, R.O.; Kannangara, D.R.; Williams, K.M.; Janssen, M.; Jansen, T.L.; Joosten, L.A.; et al. Multiplicative Interaction of Functional Inflammasome Genetic Variants in Determining the Risk of Gout. Arthritis Res. Ther. 2015, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Nie, S.; Jiang, G.; Zhou, Y.; Zhou, M.; Zhao, Y.; Li, S.; Wang, F.; Lv, Q.; Huang, Y.; et al. Regulation of CARD8 Expression by ANRIL and Association of CARD8 Single Nucleotide Polymorphism Rs2043211 (p.C10X) with Ischemic Stroke. Stroke 2014, 45, 383–388. [Google Scholar] [CrossRef]
- Yang, S.-K.; Kim, H.; Hong, M.; Lim, J.; Choi, E.; Ye, B.D.; Park, S.-K.; Song, K. Association of CARD8 with Inflammatory Bowel Disease in Koreans. J. Hum. Genet. 2011, 56, 217–223. [Google Scholar] [CrossRef]
- Tsetsos, F.; Roumeliotis, A.; Tsekmekidou, X.; Alexouda, S.; Roumeliotis, S.; Theodoridis, M.; Thodis, E.; Panagoutsos, S.; Papanas, N.; Papazoglou, D.; et al. Genetic Variation in CARD8, a Gene Coding for an NLRP3 Inflammasome-Associated Protein, Alters the Genetic Risk for Diabetic Nephropathy in the Context of Type 2 Diabetes Mellitus. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120970892. [Google Scholar] [CrossRef]
- Dessing, M.C.; Kers, J.; Damman, J.; Navis, G.J.; Florquin, S.; Leemans, J.C. Donor and Recipient Genetic Variants in NLRP3 Associate with Early Acute Rejection Following Kidney Transplantation. Sci. Rep. 2016, 6, 36315. [Google Scholar] [CrossRef] [PubMed]
- Arbiol-Roca, A.; Padró-Miquel, A.; Vidal-Alabró, A.; Hueso, M.; Fontova, P.; Bestard, O.; Rama, I.; Torras, J.; Grinyó, J.M.; Alía-Ramos, P.; et al. ANRIL as a Genetic Marker for Cardiovascular Events in Renal Transplant Patients—An Observational Follow-up Cohort Study. Transpl. Int. 2018, 31, 1018–1027. [Google Scholar] [CrossRef]
- Andersen, K.; Eltrich, N.; Lichtnekert, J.; Anders, H.-J.; Vielhauer, V. The NLRP3/ASC Inflammasome Promotes T-Cell-Dependent Immune Complex Glomerulonephritis by Canonical and Noncanonical Mechanisms. Kidney Int. 2014, 86, 965–978. [Google Scholar] [CrossRef]
- Lichtnekert, J.; Kulkarni, O.P.; Mulay, S.R.; Rupanagudi, K.V.; Ryu, M.; Allam, R.; Vielhauer, V.; Muruve, D.; Lindenmeyer, M.T.; Cohen, C.D.; et al. Anti-GBM Glomerulonephritis Involves IL-1 but Is Independent of NLRP3/ASC Inflammasome-Mediated Activation of Caspase-1. PLoS ONE 2011, 6, e26778. [Google Scholar] [CrossRef] [PubMed]
- Deplano, S.; Cook, H.T.; Russell, R.; Franchi, L.; Schneiter, S.; Bhangal, G.; Unwin, R.J.; Pusey, C.D.; Tam, F.W.K.; Behmoaras, J. P2X7 Receptor-Mediated Nlrp3-Inflammasome Activation Is a Genetic Determinant of Macrophage-Dependent Crescentic Glomerulonephritis. J. Leukoc. Biol. 2013, 93, 127–134. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Q.; Liang, T.; Zhang, J.; Bai, J.; Yuan, M.; Shen, P. TRIM40 Inhibits IgA1-Induced Proliferation of Glomerular Mesangial Cells by Inactivating NLRP3 Inflammasome through Ubiquitination. Mol. Immunol. 2021, 140, 225–232. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-Inflammasome Activation in Non-Myeloid-Derived Cells Aggravates Diabetic Nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive Oxygen Species Promote Tubular Injury in Diabetic Nephropathy: The Role of the Mitochondrial Ros-Txnip-Nlrp3 Biological Axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Lima, C.A.D.; Vajgel, G.; Sandrin-Garcia, P. The Role of NLRP3 Inflammasome in Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 12476. [Google Scholar] [CrossRef]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium Oxalate Crystals Induce Renal Inflammation by NLRP3-Mediated IL-1β Secretion. J. Clin. Investig. 2013, 123, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Caiello, I.; Cherqui, S.; Whisenant, T.; Petrini, S.; Emma, F.; De Benedetti, F. Inflammasome Activation by Cystine Crystals: Implications for the Pathogenesis of Cystinosis. J. Am. Soc. Nephrol. 2014, 25, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Darisipudi, M.N.; Thomasova, D.; Mulay, S.R.; Brech, D.; Noessner, E.; Liapis, H.; Anders, H.-J. Uromodulin Triggers IL-1β-Dependent Innate Immunity via the NLRP3 Inflammasome. J. Am. Soc. Nephrol. 2012, 23, 1783–1789. [Google Scholar] [CrossRef]
- Wen, L.; Yang, H.; Ma, L.; Fu, P. The Roles of NLRP3 Inflammasome-Mediated Signaling Pathways in Hyperuricemic Nephropathy. Mol. Cell. Biochem. 2021, 476, 1377–1386. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological Inhibition of the NLRP3 Inflammasome Reduces Blood Pressure, Renal Damage, and Dysfunction in Salt-Sensitive Hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Dowling, J.K.; Ling, Y.H.; Diep, H.; Chan, C.T.; Ferens, D.; Kett, M.M.; Pinar, A.; Samuel, C.S.; Vinh, A.; et al. Inflammasome Activity Is Essential for One Kidney/Deoxycorticosterone Acetate/Salt-Induced Hypertension in Mice. Br. J. Pharmacol. 2016, 173, 752–765. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, M.; Ding, G.; Zhang, Y.; Huang, S.; Jia, Z.; Zhang, A. Angiotensin II Stimulates the NLRP3 Inflammasome to Induce Podocyte Injury and Mitochondrial Dysfunction. Kidney Dis. 2018, 4, 83–94. [Google Scholar] [CrossRef]
- Xiang, H.; Zhu, F.; Xu, Z.; Xiong, J. Role of Inflammasomes in Kidney Diseases via Both Canonical and Non-Canonical Pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar] [CrossRef]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 Inflammasome Activation in Dialyzed Chronic Kidney Disease Patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Zhang, Y.; Ma, Y. Chronic Kidney Disease and NLRP3 Inflammasome: Pathogenesis, Development and Targeted Therapeutic Strategies. Biochem. Biophys. Rep. 2023, 33, 101417. [Google Scholar] [CrossRef] [PubMed]
- Rs10754558 RefSNP Report—DbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs10754558 (accessed on 15 January 2022).
- Rs2043211 RefSNP Report—DbSNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2043211 (accessed on 15 January 2022).
- Lee, Y.H.; Bae, S.-C. Association between Functional NLRP3 Polymorphisms and Susceptibility to Autoimmune and Inflammatory Diseases: A Meta-Analysis. Lupus 2016, 25, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Kleber, M.E.; März, W.; Pang, S.; Zewinger, S.; Triem, S.; Ege, P.; Reichert, M.C.; Krawczyk, M.; Weber, S.N.; et al. Genetically Determined NLRP3 Inflammasome Activation Associates with Systemic Inflammation and Cardiovascular Mortality. Eur. Heart J. 2021, 42, 1742–1756. [Google Scholar] [CrossRef]
- Wang, S.; Fang, F.; Jin, W.B.; Wang, X.; Zheng, X.S. Investigation into the Association between NLRP3 Gene Polymorphisms and Susceptibility to Type 2 Diabetes Mellitus. Genet. Mol. Res. 2015, 14, 17447–17452. [Google Scholar] [CrossRef]
- Da Cruz, H.L.A.; Cavalcanti, C.A.J.; de Azêvedo Silva, J.; de Lima, C.A.D.; Fragoso, T.S.; Barbosa, A.D.; Dantas, A.T.; de Ataíde Mariz, H.; Duarte, A.L.B.P.; Pontillo, A.; et al. Differential Expression of the Inflammasome Complex Genes in Systemic Lupus Erythematosus. Immunogenetics 2020, 72, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, N.; Zare Rafie, M.; Esmaeilzadeh, E.; Dehani, M.; Davar, S.; Mosallaei, M.; Pakzad, B.; Ghorashi, T.; Darvish, H.; Soosanabadi, M. Three Functional Variants in the NLRP3 Gene Are Associated with Susceptibility and Clinical Characteristics of Systemic Lupus Erythematosus. Lupus 2021, 30, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, D.; Zhang, L.; Fu, M.; Zeng, Y.; Russell, R. Variants of NLRP3 Gene Are Associated with Insulin Resistance in Chinese Han Population with Type-2 Diabetes. Gene 2013, 530, 151–154. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Montesanto, A.; Lagani, V.; Martino, C.; Dato, S.; De Rango, F.; Berardelli, M.; Corsonello, A.; Mazzei, B.; Mari, V.; Lattanzio, F.; et al. A Novel, Population-Specific Approach to Define Frailty. Age 2010, 32, 385–395. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kuhn, M.; Wickham, H. Tidymodels: A Collection of Packages for Modeling and Machine Learning Using Tidyverse Principles. 2020. Available online: https://www.tidymodels.org (accessed on 15 September 2022).
- Moreno, V.; Gonzalez, J.R. SNPassoc: SNPs-Based Whole Genome Association Studies. 2021. Available online: https://github.com/isglobal-brge/SNPassoc (accessed on 15 September 2022).
| Controls (n = 85) | Cases (n = 303) | p | |
|---|---|---|---|
| Age (years) | 72.06 ± 6.19 | 62.10 ± 14.88 | <0.001 |
| Sex (males) | 51 (60.00) | 199 (65.68) | 0.402 |
| Glucose (mg/dL) | 109.06 ± 39.61 | 105.45 ± 38.44 | 0.455 |
| Urea (mg/dL) | 32.46 ± 9.43 | 54.84 ± 30.60 | <0.001 |
| Uric Acid (mg/dL) | 4.63 ± 1.39 | 5.92 ± 1.50 | <0.001 |
| Ca (mg/dL) | 9.45 ± 0.50 | 9.43 ± 0.83 | 0.845 |
| Ph (mg/dL) | 3.39 ± 0.58 | 4.01 ± 1.52 | <0.001 |
| Total Cholesterol (mg/dL) | 206.38 ± 39.88 | 180.69 ± 48.39 | <0.001 |
| Triglycerides (mg/dL) | 131.44 ± 65.74 | 176.66 ± 101.63 | <0.001 |
| HDL Cholesterol (mg/dL) | 59.02 ± 16.37 | 49.51 ± 17.60 | <0.001 |
| LDL Cholesterol (mg/dL) | 121.04 ± 34.21 | 95.22 ± 40.24 | <0.001 |
| Ferritinemy (ng/mL) | 72.00 (42.00–161.00) | 135.00 (45.50–391.50) | <0.001 |
| CRP (mg/L) | 2.46 (1.56–4.79) | 5.18 (1.10–12.23) | 0.01 |
| Serum Albumin (gr/dL) | 3.95 ± 0.32 | 3.72 ± 0.62 | <0.001 |
| Hgb (gr/dL) | 14.21 ± 1.54 | 12.37 ± 1.90 | <0.001 |
| Type 2 Diabetes | 13 (15.29) | 63(20.79) | 0.330 |
| Hypertension | 49 (57.65) | 209 (68.98) | 0.068 |
| Controls (n = 85) | Cases (n = 303) | AOR (95% CI) | p | |
|---|---|---|---|---|
| NLRP3 rs10754558 | ||||
| CC | 39 (45.88) | 77 (25.41) | 1 | |
| CG | 31 (36.47) | 44 (14.52) | 0.64 (0.32–1.24) | 0.18 |
| GG | 15 (17.65) | 182 (60.07) | 6.04 (3.05–12.53) | <0.001 |
| C allele | 109 (64.12) | 198 (32.67%) | 1 | |
| G allele | 61 (35.88) | 408 (67.33%) | 3.56 (2.43–5.26) | <0.001 |
| CARD8 rs2043211 | ||||
| AA | 44 (51.76) | 39 (12.87) | 1 | |
| TA | 29 (34.12) | 99 (32.67) | 4.41 (2.29–8.71) | <0.001 |
| TT | 12 (14.12) | 165 (54.46) | 14.34 (6.80–32.28) | <0.001 |
| A allele | 117 (68.82) | 177 (29.21%) | 1 | |
| T allele | 53 (31.18) | 429 (70.79%) | 5.06 (3.42–7.57) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Russa, A.; Lofaro, D.; Montesanto, A.; La Russa, D.; Zaza, G.; Granata, S.; Di Dio, M.; Serra, R.; Andreucci, M.; Bonofiglio, R.; et al. Association between NLRP3 rs10754558 and CARD8 rs2043211 Variants and Susceptibility to Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 4184. https://doi.org/10.3390/ijms24044184
La Russa A, Lofaro D, Montesanto A, La Russa D, Zaza G, Granata S, Di Dio M, Serra R, Andreucci M, Bonofiglio R, et al. Association between NLRP3 rs10754558 and CARD8 rs2043211 Variants and Susceptibility to Chronic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(4):4184. https://doi.org/10.3390/ijms24044184
Chicago/Turabian StyleLa Russa, Antonella, Danilo Lofaro, Alberto Montesanto, Daniele La Russa, Gianluigi Zaza, Simona Granata, Michele Di Dio, Raffaele Serra, Michele Andreucci, Renzo Bonofiglio, and et al. 2023. "Association between NLRP3 rs10754558 and CARD8 rs2043211 Variants and Susceptibility to Chronic Kidney Disease" International Journal of Molecular Sciences 24, no. 4: 4184. https://doi.org/10.3390/ijms24044184
APA StyleLa Russa, A., Lofaro, D., Montesanto, A., La Russa, D., Zaza, G., Granata, S., Di Dio, M., Serra, R., Andreucci, M., Bonofiglio, R., & Perri, A. (2023). Association between NLRP3 rs10754558 and CARD8 rs2043211 Variants and Susceptibility to Chronic Kidney Disease. International Journal of Molecular Sciences, 24(4), 4184. https://doi.org/10.3390/ijms24044184












