Abstract
Cytochrome P450 monooxygenases (CYPs/P450s) and their redox partners, ferredoxins, are ubiquitous in organisms. P450s have been studied in biology for over six decades owing to their distinct catalytic activities, including their role in drug metabolism. Ferredoxins are ancient proteins involved in oxidation-reduction reactions, such as transferring electrons to P450s. The evolution and diversification of P450s in various organisms have received little attention and no information is available for archaea. This study is aimed at addressing this research gap. Genome-wide analysis revealed 1204 P450s belonging to 34 P450 families and 112 P450 subfamilies, where some families and subfamilies are expanded in archaea. We also identified 353 ferredoxins belonging to the four types 2Fe-2S, 3Fe-4S, 7Fe-4S and 2[4Fe-4S] in 40 archaeal species. We found that bacteria and archaea shared the CYP109, CYP147 and CYP197 families, as well as several ferredoxin subtypes, and that these genes are co-present on archaeal plasmids and chromosomes, implying the plasmid-mediated lateral transfer of these genes from bacteria to archaea. The absence of ferredoxins and ferredoxin reductases in the P450 operons suggests that the lateral transfer of these genes is independent. We present different scenarios for the evolution and diversification of P450s and ferredoxins in archaea. Based on the phylogenetic analysis and high affinity to diverged P450s, we propose that archaeal P450s could have diverged from CYP109, CYP147 and CYP197. Based on this study’s results, we propose that all archaeal P450s are bacterial in origin and that the original archaea had no P450s.
1. Introduction
Cytochrome P450 monooxygenases (CYPs/P450s) are a superfamily of heme-containing enzymes discovered nearly six decades ago [1,2,3,4,5]. In the name, “cytochrome” indicates a heme-bound protein, “450” refers to the feature that these reduced proteins with carbon-monoxide bound absorb light at 450 nm wavelength and “monooxygenases” indicates their enzymatic reaction, i.e., incorporation of one oxygen atom into the substrates [1,2,3,4,5]. Although these enzymes are named monooxygenases, research has shown they perform diverse enzymatic reactions with regio- and stereo-selectivity [6,7,8]. These unique catalytic properties led to the exploration of the applications of these enzymes in all areas of biology [9,10,11,12,13].
A unique nomenclature and classification system has been developed for P450s [14,15,16,17]. The nomenclature system begins with the prefix “CYP” for cytochrome P450 monooxygenase, followed by an Arabic numeral designating the family, a capital letter representing the subfamily and an Arabic digit specifying the individual P450 in a family. The annotation/classification criteria include assigning family and subfamily with >40% identity belonging to the same family and all P450s with >55% identity belonging to the same subfamily [14,15,16,17].
P450s play an important role in the primary and secondary metabolism of organisms; thus, they can be found in archaea, bacteria and eukarya (Table 1) [18,19]. Unexpectedly, P450s were found in non-living entities such as viruses (Table 1) [20,21]. The evolution of P450s is linked to sterol biosynthesis, where the CYP51-catalyzed demethylation reaction is widely accepted as an ancestral reaction and thus CYP51 is a possible ancestor of all known eukaryotic P450s [9]. A recent study has shown that CYP51 evolved in bacteria and then moved into eukarya [22]. The evolution of viral P450s has also been investigated, where the apparent donors of the P450s are unclear [21]. Information on the evolution of P450s in bacteria, eukarya and viruses is available, but not in archaea. Archaea have P450s and one of the P450s, CYP119 from Sulfolobus solfataricus, has been subjected to intense investigation due to its thermophilic properties [23,24,25,26,27,28].

Table 1.
Comparative analysis of P450s and their families in species across biological kingdoms.
All P450s, except for self-sufficient P450s, need electrons for their enzymatic action, which are transferred by redox proteins [29]. Studies indicated the presence of functional ferredoxins and their reductase, known as 2-oxoacid: ferredoxin oxidoreductase (OFOR) in archaea [30]. The OFOR consists of alpha and beta subunits and the genes encoding these subunits often reside in proximity [31]. Ferredoxins are iron-sulfur (Fe-S) cluster proteins that evolved during early chemical evolution [32,33]. These proteins are involved in the transfer of electrons in oxidation-reduction reactions, including P450 reactions [29]. Ferredoxins, like other Fe-S cluster proteins, are classified into different types based on the number of Fe-atoms in their cluster, such as 2Fe-2S, 3Fe-4S, 4Fe-4S, 7Fe-8S (3Fe-4S and 4Fe-4S) and 2[4Fe-4S]) [29]. Ferredoxins are further classified into subtypes based on the characteristic spacing between the cysteine amino acids of the Fe-S binding motif [34]. A recent study provided preliminary information on ferredoxins in archaea [34]. The research showed that several archaeal ferredoxin subtypes are also present in bacteria and eukarya [34].
Considering there is no information concerning the evolution of archaeal P450s and their redox partners, this study is aimed at addressing this research gap. In this study, we performed genome-wide data mining, annotation and phylogenetic analysis of P450s and their redox partners in archaea. Also, we provided evidence on the evolution of archaeal P450s from bacteria, possibly by lateral gene transfer via plasmids.
2. Results and Discussion
2.1. Archaea Has the Lowest P450 Diversity
Genome data mining and annotation of P450s in archaeal sequences available at Joint Genome Institute Integrated Microbial Genomes and Microbiomes (JGI IMG/M) [35] revealed the presence of 1209 P450s, including five short protein sequences without P450 motifs (known as P450 fragments) (Figure 1 and Table S1). All 1204 P450s, except for five P450 fragments, can be grouped into 34 P450 families and 112 P450 subfamilies (Figure 2 and Table S2). The number of P450 families found in archaea is the lowest compared to bacteria and eukarya (Table 1) [18], indicating that archaea have the lowest P450 diversity. A P450 family saturation analysis revealed that P450 families are nearing saturation in archaea (Figure S1). The lowest P450 diversity and P450 family saturation in archaea are somewhat surprising, as this was not the case for bacteria and eukarya [18].
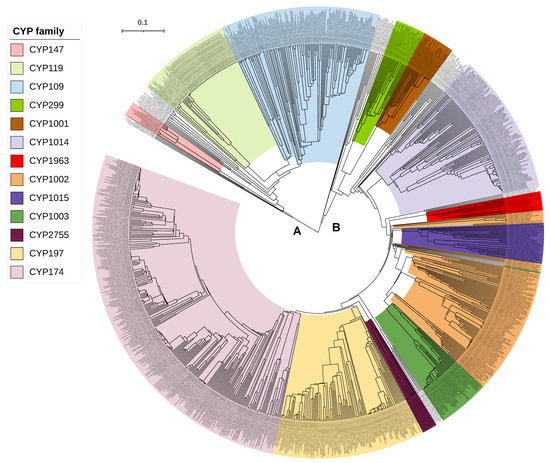
Figure 1.
Phylogenetic analysis of archaeal P450s. Major P450 families and the P450 families that are common between bacteria and archaea are displayed in different colors. The tree branch length is indicated as the scale bar in the upper left corner. A and B represent the two main branches of the tree. The tree is constructed with P450 sequences presented in Table S1 by the alignment of MAFFT v6.864 [36] and inferred by the Trex web server [37].
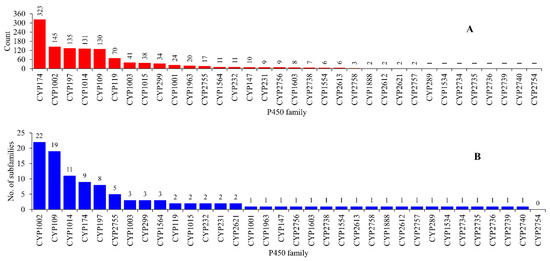
Figure 2.
Comparative analysis of P450 families (A) and subfamilies (B) in archaea. The numbers next to the bars represent the member count for that family or subfamily. Detailed information is provided in Table S2.
2.2. Some P450 Families and Subfamilies Are Expanded in Archaea
Among the P450 families found in archaea, CYP174 had the highest number of members (323 P450s), followed by CYP1002 (145 P450s), CYP197 (135 P450s), CYP1014 (131 P450s) and CYP109 (130 P450s) (Figure 2 and Table S2). A total of 19 P450 families have less than 10 members and 10 families have between 10–70 members (Figure 2 and Table S2). This suggests that CYP174, CYP1002, CYP197, CYP1014 and CYP109 are expanded (the presence of the same P450 in many species) in archaea and, thus, possibly play an important role in these species. This phenomenon is also observed at the subfamily level, where members belonging to a particular subfamily are more highly populated in archaea (Figure 2 and Table S2). Among P450 families, CYP1002 has the highest number of subfamilies (22), followed by CYP109 (19), CYP1014 (11) and CYP174 (9) (Figure 2 and Table S2). Despite having the highest number of members, CYP174 had only nine subfamilies, with subfamilies CYP174B and CYP174A expanded with 182 and 96 members (Table S2). Subfamily A is expanded with 60 members in CYP119. This suggests that these subfamilies possibly play an important role and thus are expanded in archaea. Overall, the subfamily level diversity observed for archaea is lowest compared to bacteria and eukarya [18].
2.3. Plasmid-Mediated Lateral Transfer of P450s from Bacteria to Archaea
P450 comparisons revealed three P450 families are shared by archaea and bacteria but no P450 family is shared by archaea and eukarya. The commonly shared archaeal and bacterial P450 families are CYP109, CYP147 and CYP197. The copresence of these P450 families was observed in both the archaea and bacteria (Table S3). CYP109 and CYP197 are found in the same species 35 times, CYP109 and CYP147 are found in the same species two times and CYP197 and CYP147 are located in the same species three times (Table S3). Furthermore, the genus Myxococcus has all three families but not all three in the same species (Table S3). The copresence of these P450 families in both archaea and bacteria indicates the possibility that these P450 families came from bacteria to archaea by lateral gene transfer.
It is well-known that plasmids play a role in carrying genes from one organism to another and archaeal plasmids have been known to shuttle genes from bacteria and eukarya [38]. To find out if any P450s are on archaeal plasmids, we analyzed archaeal plasmids for P450s (Figure 3 and Table S4). In total, 63 P450s were found on plasmids belonging to 40 archaeal species (Figure 3 and Tables S4 and S5). The Halocatena sp. AD-1 plasmid (unnamed3: NZ_CP096022.1/CP096022.1) had the highest number of members (four), followed by three members on Halorussus halophilus ZS-3 plasmid (unnamed1: NZ_CP044524.1/CP044524.1) (Figure 3 and Table S5). A total of 11 P450 families (out of 34 P450 families in archaea) are found on the archaeal plasmids (Figure 3 and Table S5). Among archaeal plasmid P450 families, CYP1014 had the most members (19), followed by CYP197 and CYP109 (15 members each), CYP1002 (four members), CYP174 (3 members), CYP2755 (2 members) and P450 families CYP299A18, CYP2758A1, CYP2756A5, CYP1963A15 and CYP1015B2 had a single member (Figure 3 and Table S5).
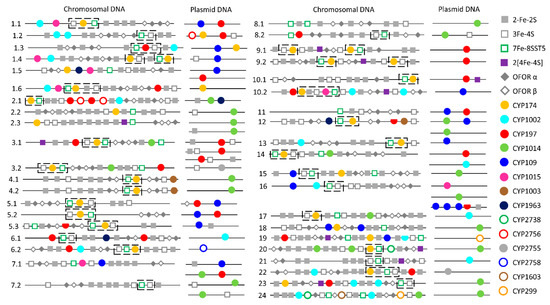
Figure 3.
Schematic representation of P450s, ferredoxins and ferredoxin reductase (OFOR) on chromosomal and plasmid DNA from 40 archaeal species. The numbers 1 to 24 represent archaeal species belonging to different genera and subsection numbers such as 1.1, 2.1, etc., represent species in a genus. P450s are presented with circles (filled or empty), where a half-filled circle indicates a P450 fragment. Ferredoxins are represented per their iron-sulfur cluster type and OFOR with alpha and beta subunits. A dashed line box shows the association between CYP174, 3Fe-4SST1 and 7Fe-8SST5. P450s, ferredoxins and ferredoxin reductase alpha and beta subunits. Detailed information is presented in Tables S5 and S6.
P450s of the same family were found on both plasmids and chromosomes and in species belonging to the same genus; some plasmids have a P450 and the same P450 is absent on plasmids but present on the chromosome in some species, indicating the transfer of P450s between plasmids and chromosomal DNA (Figure 3 and Tables S5 and S6). Comparative analysis of P450 families revealed a pattern where certain P450 families are expanded after being transferred from plasmids to chromosomes in archaeal species (Figure 4). CYP174 and CYP1002 have three and four members on plasmids and 32 and 18 members on chromosomes (Figure 4). In archaea, these two P450 families have the highest number of members (Figure 2), suggesting members of these two families indeed expanded after transfer from plasmids to chromosomes.
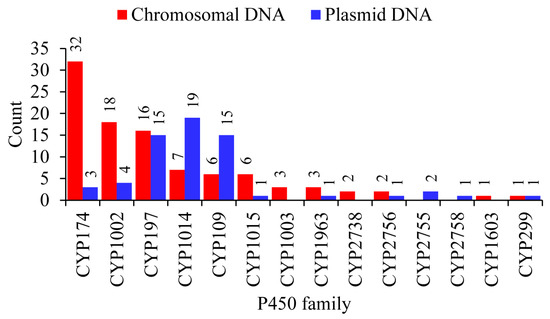
Figure 4.
Comparative analysis of P450 families between chromosomal and plasmid DNA from 40 archaeal species. The numbers next to the bars represent the member count for that family. Detailed information is provided in Tables S5 and S6.
Among the P450 families shared between archaea and bacteria, CYP109 and CYP197 P450s are copresent on four different plasmids of four different archaeal species, indicating that these P450s might have come together as they are copresent in bacterial species as well (Figure 3 and Table S3). However, the third P450 family shared by these groups, CYP147, is not found on archaeal plasmids. In order to understand the origin of CYP147 in archaea, we analyzed the CYP147 family across living organisms (Figure 5 and Table S7). The analysis revealed that only nine bacterial genera (Myxococcus, Streptomyces, Rhodococcus, Ktedonobacterales, Magnetospirillum, Methylobacterium, Mycobacterium, Frankia and Chondromyces) have the CYP147 P450 family belonging to the subfamilies ranging from A–D and F–L (Table S7) [18]. Most of the CYP147 P450s belonging to the same subfamily were found to be orthologs indicating their origin from a common ancestor in these genera [18]. Methanosarcina of archaea has CYP147 subfamily E, which is not present in bacteria. All CYP147s of Methanosarcina belong to this same subfamily E and all share >85% sequence identity, strongly indicating their common ancestral origin. Interestingly, if one indel is removed, all CYP147E P450s of Methanosarcina are 60–61% identical to CYP147A1 of Myxococcus xanthus, suggesting they belong to the same subfamily. This was clear as the P450s of Methanosarcina and CYP147A1 of the Myxococcus xanthus group aligned next to each other on the phylogenetic tree (Figure 5). Based on these results, it seems probable that the CYP147E sequences of Methanosarcina originated in Myxococcus sp. Interestingly, the CYP147 gene was laterally transferred into M. barkeri after the acetate kinase (ackA) and phosphate acetyltransferase (Pta) genes were transferred about 250 million years ago as M. mazei and M. acetivorans have ackA and Pta genes [39] but not CYP147. Considering only a few CYP147 P450s exist in archaea, it is highly likely that this P450 also came via plasmids like CYP109 and CYP197 P450s.
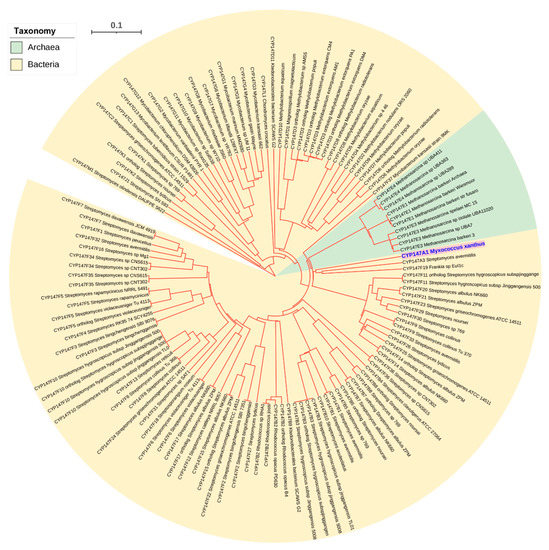
Figure 5.
Phylogenetic analysis of the CYP147 P450 family members from archaea and bacteria. Different colors highlighted CYP147 members from Methanosarcina and CYP147A1 from Myxococcus xanthus. The tree branch length is indicated as the scale bar in the upper left corner. CYP147 members used in constructing the phylogenetic tree are presented in Table S7. The protein sequences were aligned by MAFFT v6.864 [36] and the tree was inferred by the Trex web server [37].
2.4. Archaeal P450s Are Bacterial in Origin, Not Vice Versa
The polarity of lateral transfer (from bacteria to archaea) is partly based on the diversity of the P450s inside archaea (34 P450 families) and outside in bacteria (1910 P450 families) (Table 1). There are 130 CYP109s in archaea in 19 subfamilies. There are 183 CYP109s in bacteria in 41 subfamilies [18]. CYP147E is the only subfamily in archaea and is only in Methanosarcina (Figure 5). Bacteria have 11 subfamilies and CYP147E should belong to CYP147A in Myxococcus (Figure 5). CYP197 has 135 sequences in eight subfamilies in archaea, but most of the sequences are in CYP197C (87 sequences, probable orthologs) and CYP197L (35 sequences, probable orthologs) (Table S2). The other subfamilies have three members (CYP197AK) or only one sequence of each or just pseudogene fragments (Table S2). Contrary to what was observed in archaea, CYP197 in bacteria has 27 subfamilies [18].
2.5. Lateral Transfer of Putative Redox Partners Is Independent of P450s
Genome-wide analysis revealed the presence of 352 ferredoxins belonging to four types such as 2Fe-2S, 3Fe-4S, 7Fe-4S and 2[4Fe-4S] in 40 archaeal species (Figure 6 and Tables S5 and S6). These 40 species were chosen because they have P450s on their plasmids (Table S4) and are thus appropriate for studying P450 and ferredoxin evolutionary links if any are present. Among ferredoxin types, 2Fe-2S had the highest number of members (199), followed by 3Fe-4S (94 members), 7Fe-4S (49 members) and 2[4Fe-4S] (Figure 6). Ferredoxin subtype analysis revealed archaeal species’ preference for specific subtypes (Figure 6), as observed in other microbes such as bacteria [34,40]. 2Fe-2S had 17 subtypes, where subtype 24 had the highest number of members (67) and 3Fe-4S had 15 subtypes, where subtype 11 had the highest number of members (41) (Figure 6). OFOR subunits can be found in archaeal genomes with 82 alpha and 78 beta subunits (Figure 6 and Tables S5 and S6). As indicated in the literature [31], our study found that these units’ corresponding genes were next to each other (Table S6). Almost all OFOR subunits were found on the chromosomal DNA, with only one exception of a single beta subunit on the Haloprofundus salinisoli strain SQT7-1 plasmid (NZ_CP083664.1) (Figure 6).
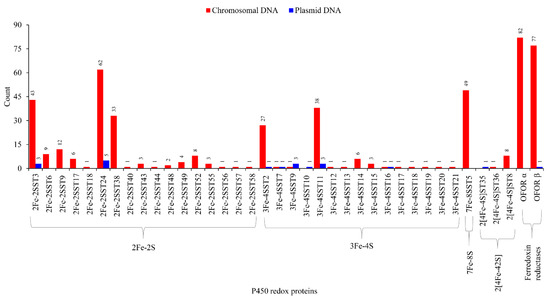
Figure 6.
Comparative analysis of ferredoxins and ferredoxin reductase (OFOR) between chromosomal and plasmid DNA. Ferredoxin types and subtypes and OFOR alpha and beta subunits were presented in the figure. Detailed information is provided in Tables S5 and S6.
Ferredoxin subtype comparative analysis revealed plasmids and chromosomes share eight subtypes (Figure 6). Although archaea contained the same six ferredoxin types as bacteria, archaea had a low ferredoxin subtype diversity (Table S8). The ferredoxin subtype count between archaea vs. bacteria is as follows: 2Fe-2S 27 vs. 35; 3Fe-4S 8 vs. 16; 4Fe-4S 4 vs. 11; 7Fe-8S 4 vs. 6; 2[4Fe-4S] 21 vs. 21; 2[4Fe-4S]Alv 2 vs. 10 (Table S8).
The operonic analysis revealed that many P450s, both on the plasmids and chromosomes, are part of operons (Table 2). However, redox partners (ferredoxin or OFOR) are not typically part of these operons (Table 2). One interesting observation is the association between CYP174, 3Fe-4SST1 and 7Fe-8SST5 (Figure 3). In most archaeal genomes, CYP174 is located with 3Fe-4SST1 and 7Fe-8SST5 and, in some cases, one of these ferredoxins (Figure 3). In a few archaeal genomes, other P450s and redox partners were found between them (Figure 3).

Table 2.
P450 operon analysis in 40 archaeal species. Arrows indicate gene orientation.
2.6. CYP109, CYP147 and CYP197 Gave Rise to Archaeal P450s
From the results presented in this study, it is clear that only three P450s are common between bacteria and archaea, indicating a possibility that these three P450s gave rise to all P450s in archaea. However, we need phylogenetic evidence to conclude that these three P450 families led to the evolution of other archaeal P450 families. Thus, we have performed a phylogenetic analysis of archaeal P450s (Figure 1). The structure of the tree shows two main branches. CYP109 and CYP147 are on Branch A and CYP197 is on Branch B. Each branch could have diverged from a single donor (CYP197 in Branch B) or two (CYP109 and CYP147 in Branch A). BLAST analysis of Branch B P450s at the National Center for Biotechnology Information (NCBI) [41] gave top non-archaeal hits that are the best BLAST hits to multiple CYP197 sequences of bacteria (results not shown, see methods), suggesting P450s of Branch B are indeed descendants of CYP197 from bacteria.
One would anticipate that if a P450 is a progenitor for other P450s, it should appear deepest on the branch. These three P450 families, however, do not belong to the deepest branches (Figure 1). Initially, the deepest branches seem to be the donors. However, when you consider that gene duplication followed by the acquisition of new functions leads to divergence in the sequence, deeper branches can be evolved from branches later in the tree. One P450 example we can give is the CYP51 and CYP61/CYP710 families. These are both in the sterol biosynthesis pathway. CYP51 is a demethylase [9,42] and CYP61/710 is a desaturase that acts later in the pathway [43,44]. It is highly likely that CYP61/710 evolved from a gene duplication of the CYP51 as the sterol pathway was evolving [45,46]. Today these are in different families. Even CYP61 (fungi) and CYP710 of plants and some protists were initially placed in separate families, though they are now recognized as homologs.
2.7. Most of the Archaeal P450s Are Orphans with No Known Function
The catalytic activity of only one archaeal P450, CYP119, has been described. CYP119 is found to be catalytically diverse, including its peroxidase activity utilizing H2O2 [26]. CYP119 catalyzes the oxidation of lauric acid [47,48], epoxidation of styrene [26], chemical dehalogenation [49], electrochemical reduction of nitrite, nitric oxide and nitrous oxide [50] and peroxidation of Amplex® Red [51]. CYP119 fused to proliferating cell nuclear antigen (PCNA) was shown to be more active in the hydroxylation of lauric acid due to the localization of ferredoxin and ferredoxin reductase via PCNA [52]. However, the natural substrates of CYP119 are not identified. Based on the characterized homologs in bacteria, CYP109 family members are involved in the oxidation of substrates such as n-alkanes, fatty acids, primary n-alcohols, terpenoids, testosterone and norisoprenoids [53,54,55]. CYP109B1 from Bacillus subtilis oxidizes saturated fatty acids along with their methyl and ethyl esters [54]. CYP109C2 and CYP109D1 of Sorangium cellulosum (delta proteobacteria) accomplish subterminal hydroxylation of saturated fatty acids [53]. CYP109D1 of S. cellulosum was also shown to have highly regioselective hydroxylation of norisoprenoids, alpha- and beta-ionone [56]. CYP109E1 from Bacillus megaterium catalyzes a cholesterol and vitamin D2 two-step hydroxylation at positions C24 and C25 [57,58].
CYP147G1 from Mycobacterium marinum has activity against fatty acids, specifically linear and ω-2 methyl branched fatty acids at the ω-1 position [59]. CYP147F1 from Streptomyces peucetius is an efficient long-chain fatty acid hydroxylase [60]. Many CYP147 and CYP197 members have been shown to be part of biosynthetic gene clusters in mycobacterial-, streptomyces- and firmicutes species, indicating their role in the biosynthesis of natural metabolites [61,62,63,64]. Apart from its involvement in natural metabolite biosynthesis, nothing is known about the functions of CYP197. The commonality seems to be fatty acids are preferred substrates.
3. Materials and Methods
3.1. Species and Their Genome Database Information
Archaeal genomes available for public use at the Joint Genome Institute Integrated Microbial Genomes and Microbiomes (JGI IMG/M) [35] were used in the study (last accessed on August 2022). The genome sequences include complete genomes, uncultured archaeal sequences and sequences from metagenomic studies. Information on the archaeal species used in the study is provided in Table S1.
3.2. Genome Data Mining and Annotation of P450s
Genome data mining and identification of P450s in archaea were carried out following the protocol described elsewhere [63,65]. Each archaeal sequence available at JGI IMG/M [35] was searched for P450s using the InterPro code “IPR001128”. The hit protein sequences were then searched for the presence of P450 characteristic motifs such as EXXR and CXG [66,67]. Proteins with no motifs and a short amino acid sequence length (<350 amino acids in length) were considered P450 fragments. These P450 fragments were not included in further analysis. Only five fragments were identified in the study. The rest of the P450s (1204) were selected for assigning the family and subfamilies. Following the International P450 Nomenclature Committee rule [14,15,17], proteins with >40% identity and >55% identity will be grouped under the same family and subfamily, respectively. P450s with less than 40% identity were assigned to a new P450 family. Archaeal P450s identified in this study and their protein sequences, assigned names and species are presented in Table S1.
3.3. Analysis of P450s in Archaeal Plasmids
Each archaeal plasmid’s (Table S4) proteome was manually searched for P450s. When a P450 was found, it was assigned to a family and subfamily, as described in the above section.
3.4. Phylogenetic Analysis of P450s
Phylogenetic analysis of P450s was carried out following the procedure described elsewhere [68]. The phylogenetic tree of P450s was constructed using protein sequences (Table S1). CYP147F37 (formerly CYP147E2) of Mycobacterium kansasii was included in the analysis as a positive control to check the alignment with the same P450 families in archaea. Firstly, the protein sequences were aligned by the MAFFT v6.864 [36] in the Trex web server with default parameters [37]. The alignments were then automatically subjected to interpret the best tree using the Trex web server [37]. Finally, the best-inferred tree was visualized, colored and generated by the Interactive Tree Of Life (iTOL) [69]. This method was used for constructing trees for archaeal P450s (Figure 1) and CYP147 P450s (Figure 5).
3.5. BLAST Analysis of Archaeal P450 Families for Affinity to CYP109 or CYP197
Family representatives for each archaeal P450 family were blast searched against the NCBI nr database to find the best hit. This best hit was blast-searched against all named prokaryotic P450s (https://drnelson.uthsc.edu/p450seqs-dbs/, accessed on 18 February 2023) to find the strongest family match. For example, the sequences in Figure 1 between CYP147 and CYP109 have four families. One sequence from each family was searched as described. The result was that the strongest family affinity to named P450s was always CYP109. A similar approach was used for the families in the Branch B.
3.6. Comparative Analysis of P450s
For comparative analysis, P450s from bacteria and eukarya were retrieved from the published article [18] and used in the study. The prokaryotic P450s can be downloaded at the website: https://drnelson.uthsc.edu/p450seqs-dbs/, accessed on 18 February 2023.
3.7. Genome Data Mining and Annotation of Ferredoxins
Genome data mining and annotation of ferredoxins in 40 archaeal species were carried out following the methods described elsewhere [34] with slight modifications. Each archaeal proteome, including proteins on plasmids, was manually searched for iron-sulfur cluster proteins. The selected proteins were then subjected to protein BLAST at the National Center for Biotechnology and Information (NCBI) [70] against the Protein Data Bank (PDB) database [71] and analyzed for the presence of characteristic motif of ferredoxins using the InterPro database [72] and NCBI Conserved Domains Database (CDD) [73]. Proteins that had a hit against ferredoxins in the PDB database and have ferredoxin motifs, as indicated by different databases, were selected for further annotation. Annotation of ferredoxins (assigning Fe-S cluster subtypes) was carried out based on the characteristic spacing patterns between cysteine amino acids of the Fe-S cluster-binding motif, as described elsewhere [34]. Ferredoxins belonging to the new subtypes were assigned a unique subtype number that corresponded to the continuation of ferredoxin subtype numbers published for Bacteroidetes species [40]. Because P450s are found in plasmids from 40 different species, ferredoxin analysis was restricted to these species to provide a clear picture of the ferredoxin origin and, if any, the relationship to P450s.
3.8. Comparative Analysis of Ferredoxins
For comparative analysis, ferredoxins from different domains of life were retrieved from the published articles [34,40] and used in the study. Using the ferredoxin subtype data generated in this study and from the published studies [34,40], a heatmap showing the presence and absence of ferredoxin subtypes in bacteria, archaea and eukarya was produced (Table S8).
3.9. Genome Data Mining of Ferredoxin Reductases
Genome data mining and annotation of ferredoxin reductases in 40 archaeal species were carried out with a manual search through the proteome. The proteins that are described as potential ferredoxin reductases in the literature [29] were selected and presented as putative ferredoxin reductases. As a potential ferredoxin reductase capable of transferring electrons to ferredoxins, we only found 2-oxoacid ferredoxin oxidoreductase (OFOR). Thus, in this article, we presented the alpha and beta subunits of OFOR.
3.10. Retrieving Protein Identification Numbers from NCBI
JGI IMG/M uses different protein identification numbers (IDs). Due to this reason, in this study, we retrieved protein IDs for P450s that are common between archaea and bacteria from NCBI. The GenBank accession numbers for the genomes, plasmids, P450s, ferredoxins and ferredoxin reductases (alpha and beta subunits) are listed in the supplemental tables.
3.11. Operon Predictions
Operons in archaeal genomes (plasmids and chromosomal DNA) were analyzed using Operon-mapper [74]. The complete gene sequence in FASTA format was downloaded (chromosomal and plasmid DNA) from NCBI and submitted to the Operon-mapper web server for operon prediction. The predicted operons were searched for the presence of P450s. The genes in the operons with P450s were noted and presented in the table format (Table 2).
4. Conclusions
Based on archaea having the lowest P450 diversity, saturation of P450 families and three common P450 families (CYP109, CYP147 and CYP197) between archaea and bacteria, we propose that archaea inherited P450s from bacteria by lateral gene transfer and it did not have any P450s originally. Only 34 P450 families are identified in archaea compared to over 1900 P450 families in bacteria (Table 1). One interpretation of this skewed abundance is that P450s came late to archaea, so they have not had as much time to diverge. This shows that archaeal P450s are of bacterial origin, not vice versa. The co-occurrence of CYP109 and CYP197 on the same plasmids raises the potential that both families might have been transferred to archaea at the same time. The presence and absence of CYP147 in Methanosarcina species may provide a date of transfer after the end Permian extinction event 250 million years ago. Phylogenetic analysis and the high affinity of diverged P450 families for one of the three families shared with bacteria indicates that CYP109, CYP147 and CYP197 gave rise to all archaeal P450s. Given the presence of ferredoxins of the same subtype on archaeal plasmids and chromosomes, as well as the same ferredoxin in bacteria and the low ferredoxin subtype diversity in archaea compared to bacteria, it is highly likely that ferredoxins were transferred laterally from bacteria to archaea, most likely via plasmids. However, annotating ferredoxin subtypes in all archaeal and bacterial species will reveal a clear picture of the genesis of ferredoxins in archaea. P450 redox partners (ferredoxins or OFOR) were not found to be part of P450 operons, indicating independent evolution of P450s and redox partners in archaea.
The emergence and divergence of P450s and ferredoxins in archaea can result in a number of scenarios: (i) plasmid-mediated direct transfer of these three P450 families (at least CYP109 and CYP197) and ferredoxins; (ii) the transferred P450s and ferredoxins gave rise to new P450s and new ferredoxins, either on plasmids or after transfer to chromosomal DNA; and (iii) subsequent divergence of new P450s and new ferredoxins resulted in the formation of all P450s and ferredoxins in archaea (Figure 7). A point to be noted is that all these scenarios are presented based on the available data as at the time of this publication. Thus, we do not rule out other lateral gene transfer mechanisms, but such mechanisms need evidence.
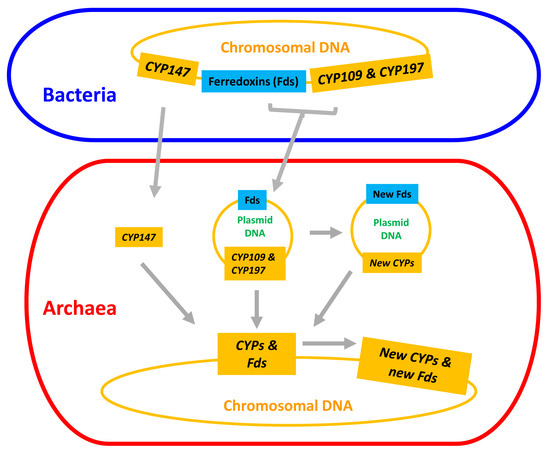
Figure 7.
Schematic representation of evolution and diversification of P450s/CYPs and ferredoxins in archaea.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms24044161/s1.
Author Contributions
Conceptualization, K.S.; methodology, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; software, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; validation, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; formal analysis, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; investigation, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; resources, D.R.N. and K.S.; data curation, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; writing—original draft preparation, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; writing—review and editing, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; visualization, P.E.N., B.V.Z.N., W.C., D.R.N. and K.S.; supervision, K.S.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
Khajamohiddin Syed expresses sincere gratitude to the University of Zululand, South Africa, for funding his research (Grant number C686). Master student Bridget Valeria Zinhle Nkosi thanked the National Research Foundation (NRF), South Africa, for postgraduate scholarships (Reference number MND200527525406).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Garfinkel, D. Studies on pig liver microsomes. I. Enzymic and pigment composition of different microsomal fractions. Arch. Biochem. Biophys. 1958, 77, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Pigments of rat liver microsomes. Arch. Biochem. Biophys. 1958, 75, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Omura, T. Recollection of the early years of the research on cytochrome P450. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 617–640. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sato, R. A new cytochrome in liver microsomes. J. Biol. Chem. 1962, 237, 1375–1376. [Google Scholar] [CrossRef]
- White, R.E.; Coon, M.J. Oxygen activation by cytochrome P-450. Annu. Rev. Biochem. 1980, 49, 315–356. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-containing oxygenases. Chem. Rev. 1996, 96, 2841–2888. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, J.; Hu, G.; Gao, C.; Guo, L.; Chen, X.; Liu, L.; Song, W. Current state and future perspectives of cytochrome P450 enzymes for C–H and C = C oxygenation. Synth. Syst. Biotechnol. 2022, 7, 887–899. [Google Scholar] [CrossRef]
- Kelly, S.L.; Kelly, D.E. Microbial cytochromes P450: Biodiversity and biotechnology. Where do cytochromes P450 come from, what do they do and what can they do for us? Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2013, 368, 20120476. [Google Scholar] [CrossRef]
- Girvan, H.M.; Munro, A.W. Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology. Curr. Opin. Chem. Biol. 2016, 31, 136–145. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Friggeri, L.; Waterman, M.R. CYP51 as drug targets for fungi and protozoan parasites: Past, present and future. Parasitology 2018, 145, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Nelson, D.R. Cytochrome P450 nomenclature. Methods Mol. Biol. 1998, 107, 15–24. [Google Scholar]
- Nelson, D.R. Cytochrome P450 nomenclature, 2004. Methods Mol. Biol. 2006, 320, 1–10. [Google Scholar]
- Nelson, D.R. The cytochrome p450 homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar] [CrossRef]
- Nelson, D.R.; Kamataki, T.; Waxman, D.J.; Guengerich, F.P.; Estabrook, R.W.; Feyereisen, R.; Gonzalez, F.J.; Coon, M.J.; Gunsalus, I.C.; Gotoh, O.; et al. The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993, 12, 1–51. [Google Scholar] [CrossRef]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Lamb, D.C.; Lei, L.; Warrilow, A.G.; Lepesheva, G.I.; Mullins, J.G.; Waterman, M.R.; Kelly, S.L. The first virally encoded cytochrome p450. J. Virol. 2009, 83, 8266–8269. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Follmer, A.H.; Goldstone, J.V.; Nelson, D.R.; Warrilow, A.G.; Price, C.L.; True, M.Y.; Kelly, S.L.; Poulos, T.L.; Stegeman, J.J. On the occurrence of cytochrome P450 in viruses. Proc. Natl. Acad. Sci. USA 2019, 116, 12343–12352. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Hargrove, T.Y.; Zhao, B.; Wawrzak, Z.; Goldstone, J.V.; Nes, W.D.; Kelly, S.L.; Waterman, M.R.; Stegeman, J.J.; Lepesheva, G.I. Concerning P450 evolution: Structural analyses support bacterial origin of sterol 14α-demethylases. Mol. Biol. Evol. 2021, 38, 952–967. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.A.; Maves, S.A.; Weiss, K.E.; Krepich, S.; Sligar, S.G. Characterization of a cytochrome P450 from the acidothermophilic Archaea Sulfolobus solfataricus. Biochem. Biophys. Res. Commun. 1998, 252, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.K.; Koo, L.S.; Schuller, D.J.; Li, H.; de Montellano, P.R.O.; Poulos, T.L. Crystal structure of a thermophilic cytochrome P450 from the archaeon Sulfolobus solfataricus. J. Biol. Chem. 2000, 275, 31086–31092. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Yamane, K.; Adachi, S.-i.; Shiro, Y.; Weiss, K.E.; Maves, S.A.; Sligar, S.G. Thermophilic cytochrome P450 (CYP119) from Sulfolobus solfataricus: High resolution structure and functional properties. J. Inorg. Biochem. 2002, 91, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.S.; Kiko, K.; Niemeyer, C.M. Characterization of the peroxidase activity of CYP119, a thermostable P450 from Sulfolobus acidocaldarius. Chem. Bio. Chem. 2008, 9, 420–425. [Google Scholar] [CrossRef]
- Basudhar, D.; Madrona, Y.; Kandel, S.; Lampe, J.N.; Nishida, C.R.; de Montellano, P.R.O. Analysis of cytochrome P450 CYP119 ligand-dependent conformational dynamics by two-dimensional NMR and X-ray crystallography. J. Biol. Chem. 2015, 290, 10000–10017. [Google Scholar] [CrossRef]
- Sakalli, T.; Surmeli, N.B. Functional characterization of a novel CYP119 variant to explore its biocatalytic potential. Biotechnol. Appl. Biochem. 2021, 69, 1741–1756. [Google Scholar] [CrossRef]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 systems--biological variations of electron transport chains. Biochim. Et Biophys. Acta 2007, 1770, 330–344. [Google Scholar] [CrossRef]
- Puchkaev, A.V.; Wakagi, T.; Ortiz de Montellano, P.R. CYP119 Plus a Sulfolobus tokodaii Strain 7 ferredoxin and 2-oxoacid: Ferredoxin oxidoreductase constitute a high-temperature cytochrome P450 catalytic system. J. Am. Chem. Soc. 2002, 124, 12682–12683. [Google Scholar] [CrossRef]
- Zhang, Q.; Iwasaki, T.; Wakagi, T.; Oshima, T. 2-Oxoacid: Ferredoxin oxidoreductase from the thermoacidophilic archaeon, Sulfolobus sp. strain 7. J. Biochem. 1996, 120, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Venkateswara Rao, P.; Holm, R. Synthetic analogues of the active sites of iron−sulfur proteins. Chem. Rev. 2004, 104, 527–560. [Google Scholar] [CrossRef]
- Cammack, R. Evolution and diversity in the iron-sulphur proteins. Chem. Scr. 1983, 21, 87–95. [Google Scholar]
- Nzuza, N.; Padayachee, T.; Chen, W.; Gront, D.; Nelson, D.R.; Syed, K. Diversification of ferredoxins across living organisms. Curr. Issues Mol. Biol. 2021, 43, 1374–1390. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-M.A.; Chu, K.; Palaniappan, K.; Ratner, A.; Huang, J.; Huntemann, M.; Hajek, P.; Ritter, S.; Varghese, N.; Seshadri, R. The IMG/M data management and analysis system v. 6.0: New tools and advanced capabilities. Nucleic Acids Res. 2021, 49, D751–D763. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Boc, A.; Diallo, A.B.; Makarenkov, V. T-REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012, 40, W573–W579. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Rothman, D.H.; Fournier, G.P.; French, K.L.; Alm, E.J.; Boyle, E.A.; Cao, C.; Summons, R.E. Methanogenic burst in the end-Permian carbon cycle. Proc. Natl. Acad. Sci. USA 2014, 111, 5462–5467. [Google Scholar] [CrossRef]
- Nkosi, B.V.Z.; Padayachee, T.; Gront, D.; Nelson, D.R.; Syed, K. Contrasting health effects of Bacteroidetes and Firmicutes lies in their genomes: Analysis of P450s, ferredoxins, and secondary metabolite clusters. Int. J. Mol. Sci. 2022, 23, 5057. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Baldwin, B.C.; Corran, A.J.; Kelly, D.E. Characterization of Saccharomyces cerevisiae CYP61, sterol delta22-desaturase, and inhibition by azole antifungal agents. J. Biol. Chem. 1997, 272, 9986–9988. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Mizutani, M.; Aoki, N.; Watanabe, B.; Saga, H.; Saito, S.; Oikawa, A.; Suzuki, H.; Sakurai, N.; Shibata, D. Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. Plant Cell. 2006, 18, 1008–1022. [Google Scholar] [CrossRef]
- Nelson, D.R. Cytochrome P450 and the individuality of species. Arch. Biochem. Biophys 1999, 369, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lee, M.-K.; Jefcoate, C.; Kim, S.-C.; Chen, F.; Yu, J.-H. Fungal cytochrome p450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome. Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-R.; Eun, C.-Y.; Park, H.-G.; Han, S.-H.; Han, J.-S.; Cho, K.-S.; Chun, Y.-J.; Kim, D.-H. Regioselective oxidation of lauric acid by CYP119, an orphan cytochrome P450 from Sulfolobus acidocaldarius. J. Microbiol. Biotechnol. 2010, 20, 574–578. [Google Scholar]
- Koo, L.S.; Immoos, C.E.; Cohen, M.S.; Farmer, P.J.; Ortiz de Montellano, P.R. Enhanced electron transfer and lauric acid hydroxylation by site-directed mutagenesis of CYP119. J. Am. Chem. Soc. 2002, 124, 5684–5691. [Google Scholar] [CrossRef] [PubMed]
- Blair, E.; Greaves, J.; Farmer, P.J. High-temperature electrocatalysis using thermophilic P450 CYP119: Dehalogenation of CCl4 to CH4. J. Am. Chem. Soc. 2004, 126, 8632–8633. [Google Scholar] [CrossRef]
- Immoos, C.E.; Chou, J.; Bayachou, M.; Blair, E.; Greaves, J.; Farmer, P.J. Electrocatalytic reductions of nitrite, nitric oxide, and nitrous oxide by thermophilic cytochrome P450 CYP119 in film-modified electrodes and an analytical comparison of its catalytic activities with myoglobin. J. Am. Chem. Soc. 2004, 126, 4934–4942. [Google Scholar] [CrossRef]
- Başlar, M.S.; Sakallı, T.; Güralp, G.; Kestevur Doğru, E.; Haklı, E.; Surmeli, N.B. Development of an improved Amplex Red peroxidation activity assay for screening cytochrome P450 variants and identification of a novel mutant of the thermophilic CYP119. JBIC J. Biol. Inorg. Chem. 2020, 25, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Hirakawa, H.; Nagamune, T. Electron donation to an archaeal cytochrome P450 is enhanced by PCNA-mediated selective complex formation with foreign redox proteins. Biotechnol. J. 2014, 9, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Khatri, Y.; Hannemann, F.; Girhard, M.; Kappl, R.; Même, A.; Ringle, M.; Janocha, S.; Leize-Wagner, E.; Urlacher, V.B.; Bernhardt, R. Novel family members of CYP109 from Sorangium cellulosum So ce56 exhibit characteristic biochemical and biophysical properties. Biotechnol. Appl. Biochem. 2013, 60, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Girhard, M.; Klaus, T.; Khatri, Y.; Bernhardt, R.; Urlacher, V.B. Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis. Appl. Microbiol. Biotechnol. 2010, 87, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Nishi, T.; Shibata, D.; Suzuki, H.; Ohta, D.; Kino, K. Characterization of orphan monooxygenases by rapid substrate screening using FT-ICR mass spectrometry. Chem. Biol. 2008, 15, 563–572. [Google Scholar] [CrossRef]
- Khatri, Y.; Girhard, M.; Romankiewicz, A.; Ringle, M.; Hannemann, F.; Urlacher, V.B.; Hutter, M.C.; Bernhardt, R. Regioselective hydroxylation of norisoprenoids by CYP109D1 from Sorangium cellulosum So ce56. Appl. Microbiol. Biotechnol. 2010, 88, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Putkaradze, N.; König, L.; Kattner, L.; Hutter, M.C.; Bernhardt, R. Highly regio-and stereoselective hydroxylation of vitamin D2 by CYP109E1. Biochem. Biophys. Res. Commun. 2020, 524, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Putkaradze, N.; Litzenburger, M.; Hutter, M.C.; Bernhardt, R. CYP109E1 from Bacillus megaterium acts as a 24-and 25-hydroxylase for cholesterol. Chem. Bio. Chem. 2019, 20, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Child, S.A.; Rossi, V.P.; Bell, S.G. Selective ϖ-1 oxidation of fatty acids by CYP147G1 from Mycobacterium marinum. Biochim. Et. Biophys. Acta. Gen. Subj. 2019, 1863, 408–417. [Google Scholar] [CrossRef]
- Bhattarai, S.; Liou, K.; Oh, T.-J. Hydroxylation of long chain fatty acids by CYP147F1, a new cytochrome P450 subfamily protein from Streptomyces peucetius. Arch. Biochem. Biophys. 2013, 539, 63–69. [Google Scholar] [CrossRef]
- Padayachee, T.; Nzuza, N.; Chen, W.; Nelson, D.R.; Syed, K. impact of lifestyle on cytochrome P450 monooxygenase repertoire is clearly evident in the bacterial phylum Firmicutes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Senate, L.M.; Tjatji, M.P.; Pillay, K.; Chen, W.; Zondo, N.M.; Syed, P.R.; Mnguni, F.C.; Chiliza, Z.E.; Bamal, H.D.; Karpoormath, R.; et al. Similarities, variations, and evolution of cytochrome P450s in Streptomyces vs. Mycobacterium. Sci. Rep. 2019, 9, 3962. [Google Scholar]
- Mnguni, F.C.; Padayachee, T.; Chen, W.; Gront, D.; Yu, J.-H.; Nelson, D.R.; Syed, K. More P450s are involved in secondary metabolite biosynthesis in Streptomyces compared to Bacillus, Cyanobacteria and Mycobacterium. Int. J. Mol. Sci. 2020, 21, 4814. [Google Scholar] [CrossRef] [PubMed]
- Zondo, N.M.; Padayachee, T.; Nelson, D.R.; Syed, K. Saprophytic to pathogenic mycobacteria: Loss of cytochrome P450s vis a vis their prominent involvement in natural metabolite biosynthesis. Int. J. Mol. Sci. 2022, 24, 149. [Google Scholar] [CrossRef] [PubMed]
- Malinga, N.A.; Nzuza, N.; Padayachee, T.; Syed, P.R.; Karpoormath, R.; Gront, D.; Nelson, D.R.; Syed, K. An unprecedented number of cytochrome P450s are involved in secondary metabolism in Salinispora Species. Microorganisms 2022, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Mashele, S.S. Comparative analysis of P450 signature motifs EXXR and CXG in the large and diverse kingdom of fungi: Identification of evolutionarily conserved amino acid patterns characteristic of P450 family. PLoS ONE 2014, 9, e95616. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 1992, 267, 83–90. [Google Scholar] [CrossRef]
- Msomi, N.N.; Padayachee, T.; Nzuza, N.; Syed, P.R.; Kryś, J.D.; Chen, W.; Gront, D.; Nelson, D.R.; Syed, K. In silico analysis of P450s and their role in secondary metabolism in the bacterial class Gammaproteobacteria. Molecules 2021, 26, 1538. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021, 49, D10. [Google Scholar] [CrossRef]
- wwPDB consortium. Protein Data Bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019, 47, D520–D528. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.-Y.; El-Gebali, S.; Fraser, M.I. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Taboada, B.; Estrada, K.; Ciria, R.; Merino, E. Operon-mapper: A web server for precise operon identification in bacterial and archaeal genomes. Bioinformatics 2018, 34, 4118–4120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).