Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina
Abstract
1. Introduction
2. Results
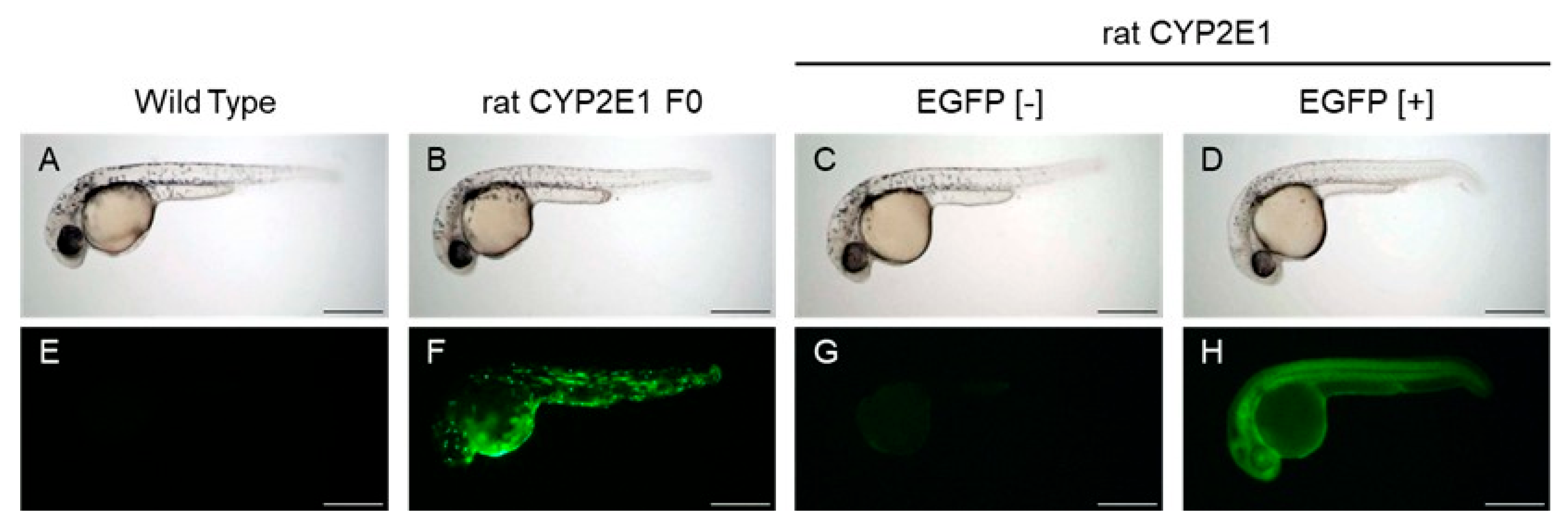
2.1. Generation of Transgenic Zebrafish Expressing Rat CYP2E1
2.2. Measurement of CYP2E1 Activity in Transgenic Zebrafish Expressing rat CYP2E1
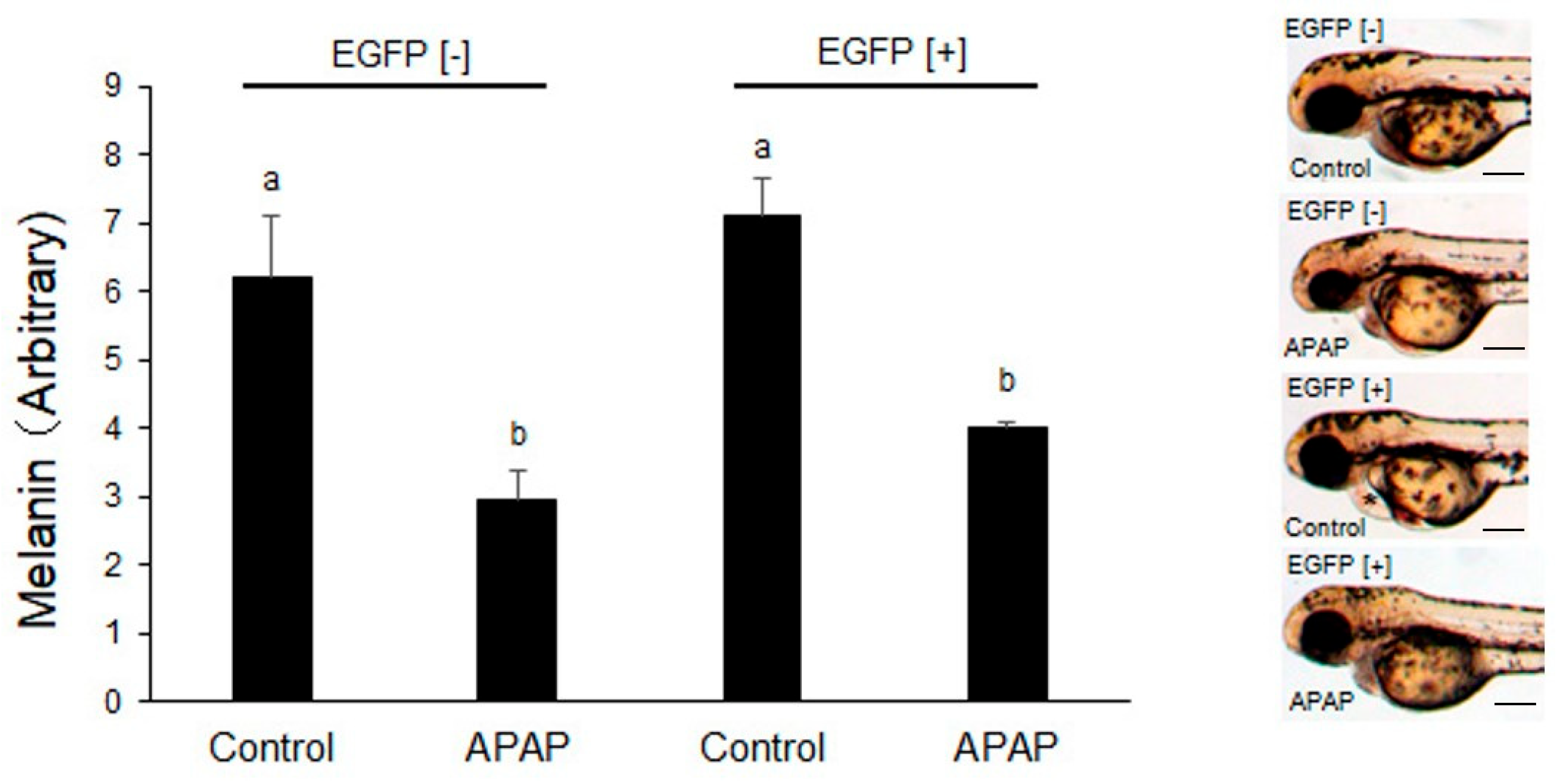
2.3. Effects of Earlier Exposure to Acetaminophen on Zebrafish Embryos/Larvae
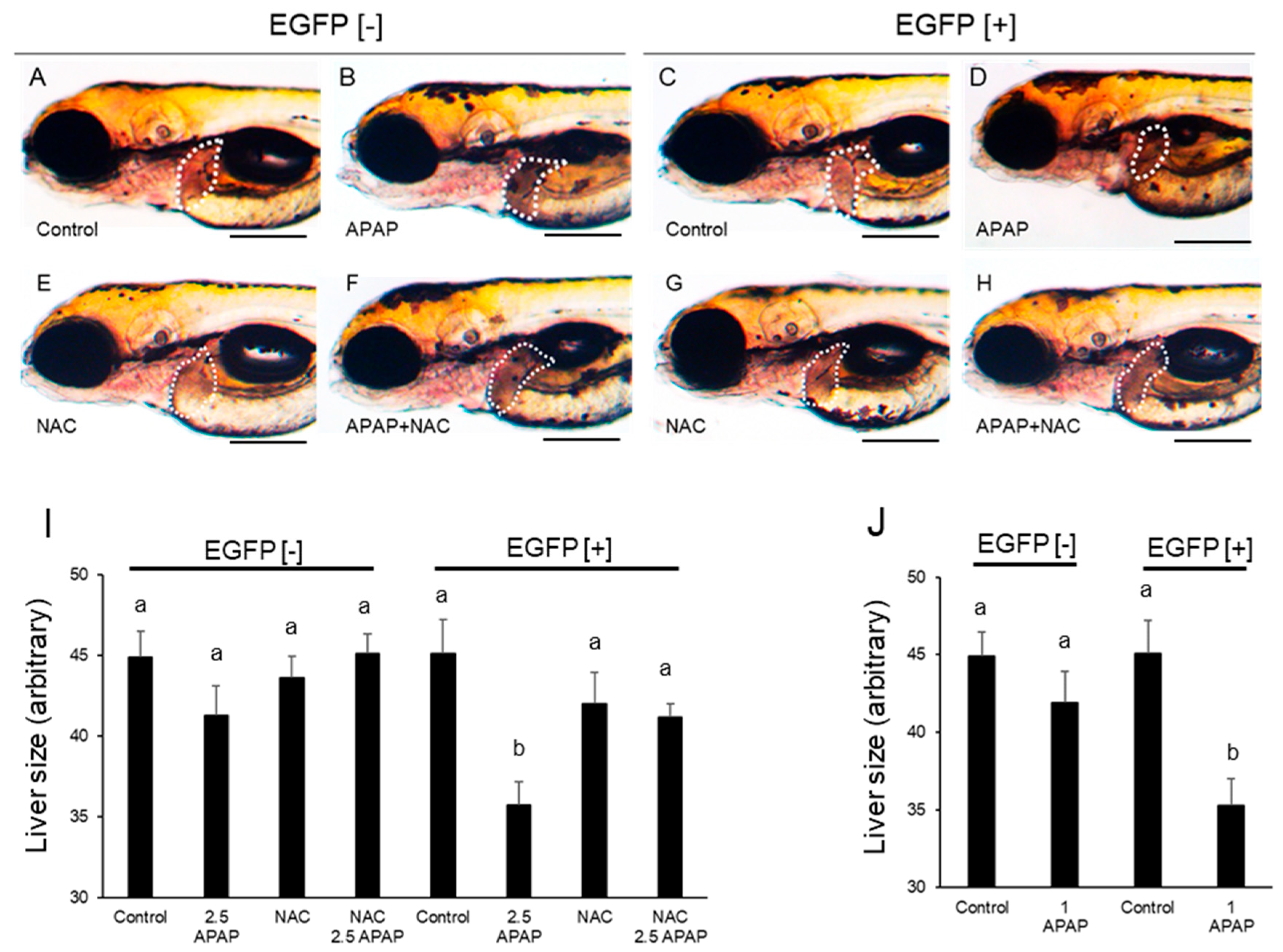
2.4. Effects of Later Exposure to Acetaminophen on Larval Zebrafish
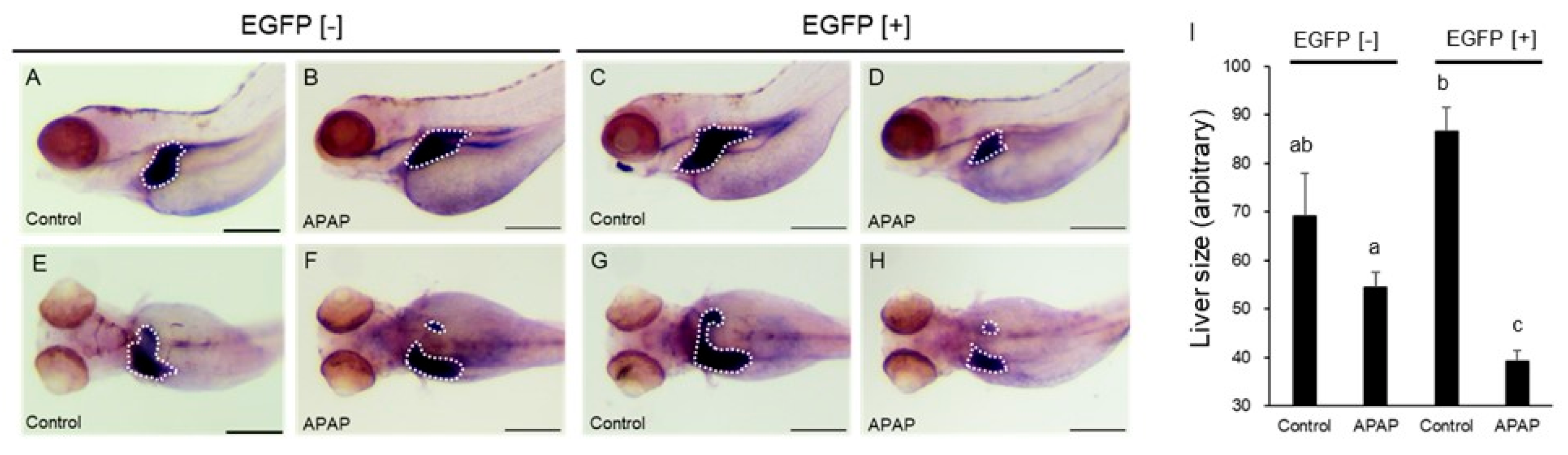
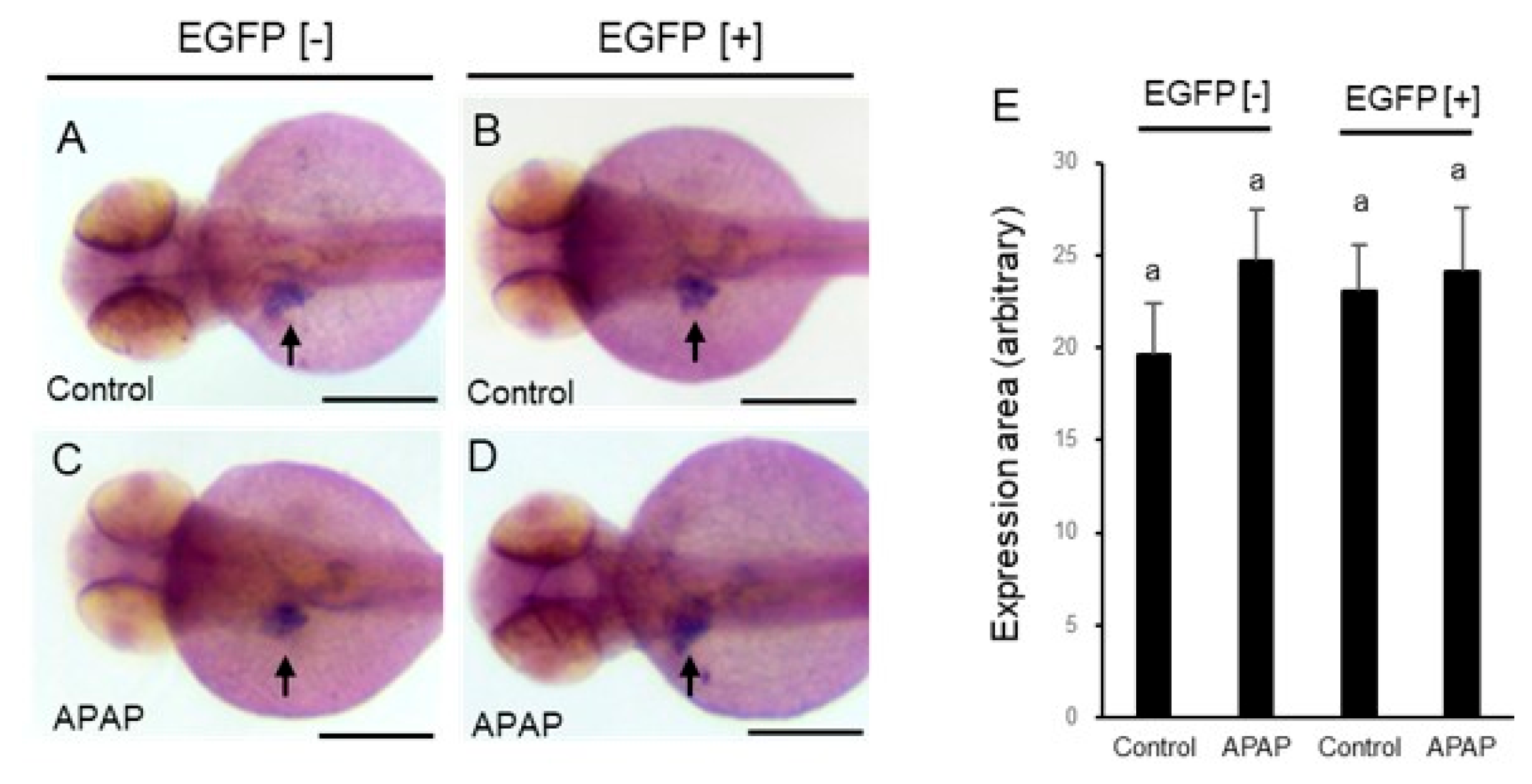
2.5. Effects of APAP on Markers for Hepatocytes and Liver Primordium
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Transgenesis of Rat CYP2E1 into the Zebrafish Genome
4.3. Measurement of Rat CYP2E1 Activity
4.4. Zebrafish and Chemical Treatment
4.5. Measurement of the Size of the Retina and Liver
4.6. Measurement of Melanin
4.7. Whole-Mount In Situ Hybridization
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioannides, C.; Lewis, D.F.V. Cytochromes P450 in the bioactivation of chemicals. Curr. Top. Med. Chem. 2004, 4, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Feng, S. Role of Metabolic Enzymes P450 (CYP) on Activating Procarcinogen and their Polymorphisms on the Risk of Cancers. Curr. Drug Metab. 2015, 16, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 and Chemical Toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.; Tamburic, S. A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics 2022, 9, 90. [Google Scholar] [CrossRef]
- European Medicine Agency. News 29/09/2021. Available online: https://www.ema.europa.eu/en/news/ema-implements-new-measures-minimise-animal-testing-during-medicines-development (accessed on 26 November 2022).
- European Parliament. Directive 2010/63/EU. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 26 November 2022).
- Hill, J.A.; Teraoka, H.; Heideman, W.; Peterson, E.R. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Kimmel, B.C.; Ballard, W.W.; Kimmel, R.S.; Ullmann, B.; Schilling, F.T. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Loerracher, A.K.; Braunbeck, T. Cytochrome P450-dependent biotransformation capacities in embryonic, juvenile and adult stages of zebrafish (Danio rerio)—A state-of-the-art review. Arch. Toxicol. 2021, 95, 2299–2334. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef]
- Corley-Smith, G.E.; Su, H.-T.; Wang-Buhler, J.-L.; Tseng, H.-P.; Hu, C.-H.; Hoang, T.; Chung, W.-G.; Buhler, D.R. CYP3C1, the first member of a new cytochrome P450 subfamily found in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2006, 340, 1039–1046. [Google Scholar] [CrossRef]
- Tsedensodnom, O.; Vacaru, A.M.; Howarth, D.L.; Yin, C.; Sadler, K.C. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis. Models Mech. 2013, 6, 1213–1226. [Google Scholar]
- Lai, M.W.; Klein-Schwartz, W.; Rodgers, G.C.; Abrams, J.Y.; Haber, D.A.; Bronstein, A.C.; Wruk, K.M. Annual Report of the American Association of Poison Control Centers’ national poisoning and exposure database. Clin. Toxicol. 2005, 44, 803–932. [Google Scholar] [CrossRef]
- Lee, S.S.; Buters, J.T.; Pineau, T.; Fernandez-Salguero, P.; Gonzalez, F.J. Role of CYP2E1 in the Hepatotoxicity of Acetaminophen. J. Biol. Chem. 1996, 271, 12063–12067. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Ge, W.; Yan, R. Metabolic competency of larval zebrafish in drug-induced liver injury: A case study of acetaminophen poisoning. Toxicol Sci. 2022, 189, 175–185. [Google Scholar] [CrossRef]
- Jagtap, U.; Basu, S.; Lokhande, L.; Bharti, N.; Sachidanandan, C. BML-257, a small molecule that protects against drug induced liver injury in zebrafish. Chem. Res. Toxicol. 2022, 35, 1393–1399. [Google Scholar] [CrossRef]
- North, T.E.; Babu, I.R.; Vedder, L.M.; Lord, A.M.; Wishnok, J.S.; Tannenbaum, S.R.; Zon, L.I.; Goessling, W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA 2010, 107, 17315–17320. [Google Scholar] [CrossRef]
- Köster, R.W.; Fraser, S.E. Tracing transgene expression in living zebrafish embryos. Dev. Biol. 2001, 233, 329–346. [Google Scholar] [CrossRef]
- Harrison, P.M.; Keays, R.; Bray, G.P.; Alexander, G.J.M.; Williams, R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 1990, 335, 1572–1573. [Google Scholar] [CrossRef]
- Dudziak, K.; Nowak, M.; Sozoniuk, M. One Host-Multiple Applications: Zebrafish (Danio rerio) as Promising Model for Studying Human Cancers and Pathogenic Diseases. Int. J. Mol. Sci. 2022, 23, 10255. [Google Scholar] [CrossRef]
- Poon, K.L.; Wang, X.; Ng, A.S.; Goh, W.H.; McGinnis, C.; Fowler, S.; Carney, T.J.; Wang, H.; Ingham, P.W. Humanizing the zebrafish liver shifts drug metabolic profiles and improves pharmacokinetics of CYP3A4 substrates. Arch. Toxicol. 2017, 91, 1187–1197. [Google Scholar] [CrossRef]
- Hartman, J.H.; Kozal, J.S.; Di Giulio, R.T.; Meyer, J.N. Zebrafish have an ethanol-inducible hepatic 4-nitrophenol hydroxylase that is not CYP2E1-like. Environ. Toxicol. Pharmacol. 2017, 54, 142–145. [Google Scholar] [CrossRef]
- Raunio, H.; Pentikäinen, O.; Juvonen, R.O. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 4708. [Google Scholar] [CrossRef] [PubMed]
- Loerracher, A.K.; Braunbeck, T. Inducibility of cytochrome P450-mediated 7-methoxycoumarin-O-demethylase activity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2020, 225, 105540. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Chung, H.-Y.; Su, H.-T.; Tseng, H.-P.; Tzou, W.-S.; Chin-Hwa Hu, C.-H. Regulation of zebrafish CYP3A65 transcription by AHR2. Toxicol. Appl. Pharmacol. 2013, 270, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Auriola, S.; Pasanen, M.; Juvonen, R.O. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 2009, 39, 11–21. [Google Scholar] [CrossRef]
- Philippot, G.; Hosseini, K.; Yakub, A.; Mhajar, Y.; Hamid, M.; Buratovic, S.; Fredriksson, R. Paracetamol (Acetaminophen) and its Effect on the Developing Mouse Brain. Front. Toxicol. 2022, 4, 867748. [Google Scholar] [CrossRef]
- Cedron, V.P.; Weiner, A.M.; Vera, M.; Sanchez, L. Acetaminophen affects the survivor, pigmentation and development of craniofacial structures in zebrafish (Danio rerio) embryos. Biochem. Pharmacol. 2020, 174, 113816. [Google Scholar] [CrossRef]
- Wrześniok, D.; Oprzondek, M.; Hechmann, A.; Beberok, A. Effect of paracetamol on melanization process in human epidermal melanocytes. Acta Pol. Pharm. 2016, 73, 653. [Google Scholar]
- Glasco, D.M.; Wang, Z.; Kang, S.; Funkhouser, A.T. Acetaminophen Disrupts the Development of Pharyngeal Arch-Derived Cartilage and Muscle in Zebrafish. J. Dev. Biol. 2022, 10, 30. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mellgren, E.M.; Johnson, S.L. How the zebrafish gets its stripes. Dev. Biol. 2001, 240, 301–314. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirayama, I.; Takehana, M.; Kobayashi, S.; Tamura, H. Cytochrome P450 Expression in Rat Ocular Tissues and Its Induction by Phenobarbital. J. Health Sci. 2002, 48, 346–349. [Google Scholar] [CrossRef]
- Yin, H.-C.; Tseng, H.-P.; Chung, H.-Y.; Ko, C.-Y.; Tzou, W.-S.; Buhler, D.R.; Hu, C.-H. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol. Sci. 2008, 103, 158–168. [Google Scholar] [CrossRef]
- Nawaji, T.; Yamashita, N.; Umeda, H.; Zhang, S.; Mizoguchi, N.; Seki, M.; Kitazawa, T.; Teraoka, H. Cytochrome P450 Expression and Chemical Metabolic Activity before Full Liver Development in Zebrafish. Pharmaceuticals 2020, 13, 456. [Google Scholar] [CrossRef]
- Teraoka, H.; Dong, W.; Ogawa, S.; Tsukiyama, S.; Okuhara, Y.; Niiyama, M.; Ueno, N.; Peterson, R.E.; Hiraga, H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002, 65, 192–199. [Google Scholar] [CrossRef]
- Morgan, K.; French, S.W.; Morgan, T.R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 2002, 36, 122–134. [Google Scholar] [CrossRef]
- Stuckenholz, C.; Lu, L.; Thakur, P.; Kaminski, N.; Bahary, N. FACS-Assisted Microarray Profiling Implicates Novel Genes and Pathways in Zebrafish Gastrointestinal Tract Development. Gastroenterology. 2009, 137, 1321–1332. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, H.; Wang, C.; He, L.; Guo, C.; Peng, C.; Li, Y. Hepatoprotective effect of forsythiaside against acetaminophen-induced liver injury in zebrafish: Coupling network pharmacology with biochemical pharmacology. J. Ethnopharmacol. 2021, 271, 113890. [Google Scholar] [CrossRef]
- Shwartz, A.; Goessling, W.; Yin, C. Macrophages in Zebrafish Models of Liver Diseases. Front. Immunol. 2019, 10, 2840. [Google Scholar] [CrossRef]
- Kawakami, K. Transposon tools and methods in zebrafish. Dev. Dyn. 2005, 234, 244–254. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 1993. [Google Scholar]
- Kim, J.-H.; Lee, S.M.; Myung, C.H.; Lee, K.L.; Hyun, S.M.; Lee, J.E.; Park, Y.S.; Jeon, S.R.; Park, J.K.; Chang, S.E.; et al. Melanogenesis inhibition of β-lapachone, a natural product from Tabebuia avellanedae, with effective in vivo lightening potency. Arch. Dermatol. Res. 2015, 307, 229–238. [Google Scholar] [CrossRef]
- Miller, M.S.; Warner, S.P.; Jorquera, R.; Castonguay, A.; Schüller, H.M. Expression of the cytochrome P4502E and 2B gene families in the lungs and livers of nonpregnant, pregnant, and fetal hamsters. Biochem. Pharmacol. 1992, 44, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.P.; Lasker, J.M.; Raucy, J.L. Expression, induction, and catalytic activity of the ethanol-inducible cytochrome P450 (CYP2E1) in human fetal liver and hepatocytes. Mol. Pharmacol. 1996, 49, 260–268. [Google Scholar] [PubMed]
- Babai, S.; Auclert, L.; Le-Louët, H. Safety data and withdrawal of hepatotoxic drugs. Therapie 2021, 76, 715–723. [Google Scholar] [CrossRef] [PubMed]

| p-nitropheonl | p-nitrocatechol | |
|---|---|---|
| Wild type | 1.577 ± 0.191 a | 0.052 ± 0.015 a |
| EGFP [+] | 1.560 ± 0.123 a | 0.525 ± 0.113 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Dong, W.; Nakamura, T.; Mizoguchi, N.; Nawaji, T.; Nishikawa, M.; Onaga, T.; Ikushiro, S.; Kobayashi, M.; Teraoka, H. Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina. Int. J. Mol. Sci. 2023, 24, 4013. https://doi.org/10.3390/ijms24044013
Sato Y, Dong W, Nakamura T, Mizoguchi N, Nawaji T, Nishikawa M, Onaga T, Ikushiro S, Kobayashi M, Teraoka H. Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina. International Journal of Molecular Sciences. 2023; 24(4):4013. https://doi.org/10.3390/ijms24044013
Chicago/Turabian StyleSato, Yoshinori, Wenjing Dong, Tatsuro Nakamura, Naohiro Mizoguchi, Tasuku Nawaji, Miyu Nishikawa, Takenori Onaga, Shinichi Ikushiro, Makoto Kobayashi, and Hiroki Teraoka. 2023. "Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina" International Journal of Molecular Sciences 24, no. 4: 4013. https://doi.org/10.3390/ijms24044013
APA StyleSato, Y., Dong, W., Nakamura, T., Mizoguchi, N., Nawaji, T., Nishikawa, M., Onaga, T., Ikushiro, S., Kobayashi, M., & Teraoka, H. (2023). Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina. International Journal of Molecular Sciences, 24(4), 4013. https://doi.org/10.3390/ijms24044013






