The Role of Neutrophils in Spondyloarthritis: A Journey across the Spectrum of Disease Manifestations
Abstract
1. Introduction
Neutrophils Overview
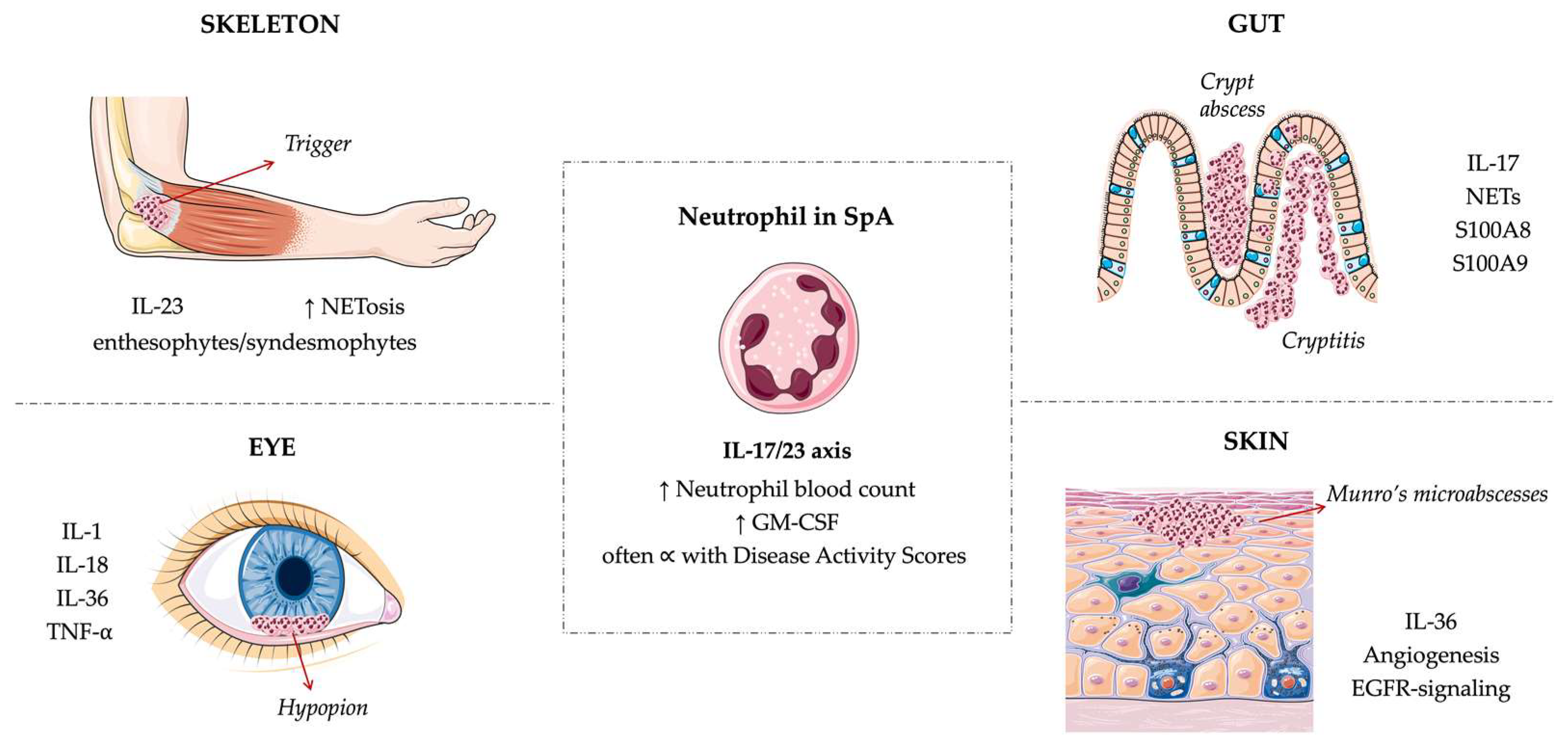
2. Neutrophils across SpA Manifestations
2.1. Articular Involvement
2.1.1. Enthesis
2.1.2. Peripheral and Axial Joints
2.2. Gut Involvement
2.3. Psoriasis
2.4. Uveitis
3. Therapeutics: Neutrophil-Targeted Therapies
| Strategies | Approaches | Target | Drugs and Diseases |
|---|---|---|---|
| Reducing neutrophil numbers | Targeting production | - GM-CSF receptor | Mavrilimumab in GCA and RA [127,128,129] |
| - GM-CSF | Otilimab in RA [130]; Namilumab in RA [131], PsO [132] and SpA (NCT03622658); Gimsilumab in AS (NCT04205851; NCT04351243) | ||
| - IL-23/IL-17 axis (a regulator of G-CSF production) | IL-17 inhibitors in PsO, PsA, ax-SpA; IL-12/23 inhibitors in PsO, PsA [133], CD, and SLE; IL-23 inhibitors in CD, UC, PsO, and PsA | ||
| Inducing depletion | - Circulating neutrophils | -Extracorporeal granulocytapheresis in RA [134], CD, and RCU [135] | |
| Interfering with neutrophil recruitment and chemotaxis | Selectin and integrin blockers | - α4β1-integrin | -Natalizumab in CD [136] |
| - Selectins | -TBC1269 (and others) in PsO [137] | ||
| Blocking complement | - C5a and C5a receptor | -Eculizumab and Avacopan in AAV [138,139]; NNC0215-0384 in RA (NCT01611688) | |
| Blocking leucotriens | - LTB4 | -CP-195543 in RA (NCT00424294) | |
| Blocking neutrophils activation | Signal transduction blockade (cytokine signaling in neutrophils) | - JAK | -Jak-inhibitors in PsA, RA, UC, AS [140] |
| - SYK | -Fostamatinib in RA [141] and SLE [142] | ||
| - PDE4 | -Apremilast in PsO, PsA [143], SLE (NCT00708916) and AS [144] | ||
| Blocking cytokines whose receptors are also on neutrophils | - TNF-α | -TNF-α inhibitors in RA, PsA, PsO, SpA, AS, CD, UC, uveitis [145] | |
| - IL-6 | -IL-6 inhibitors in RA, AS, SSc, vasculitis, SLE, AOSD [146] | ||
| Blocking neutrophil-derived mediators | Neutrophil granule enzymes | - MMP9 | -andecaliximab in UC and CD [147,148] |
| NETs (blocking activity of enzymes critical for NETs formation) | - NADPH, MPO, PAD4, DNase Inhibitor | Not yet elucidated in humans affected by autoimmune diseases. | |
| Others | Blocking neutrophil function | - Neutrophil inflammasome | -β-hydroxybutyrate in gout flares [149] -IL-1β inhibitors in RA, SpA, PsA, AS, AOSD, uveitis, GCA, vasculitis [150] |
| - Neutrophils alarmins (S100A8/S100A9) | -Paquinimod in SLE (NCT00997100) |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dougados, M.; Baeten, D. Spondyloarthritis. Lancet 2011, 377, 2127–2137. [Google Scholar] [CrossRef]
- Benjamin, M.; McGonagle, D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 2001, 199, 503–526. [Google Scholar] [CrossRef]
- Bengtsson, K.; Forsblad-D’Elia, H.; Deminger, A.; Klingberg, E.; Dehlin, M.; Exarchou, S.; Lindström, U.; Askling, J.; Jacobsson, L.T.H. Incidence of extra-articular manifestations in ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis: Results from a national register-based cohort study. Rheumatology 2021, 60, 2725–2734. [Google Scholar] [CrossRef]
- Ronneberger, M.; Schett, G. Pathophysiology of Spondyloarthritis. Curr. Rheumatol. Rep. 2011, 13, 416–420. [Google Scholar] [CrossRef]
- Generali, E.; Bose, T.; Selmi, C.; Voncken, J.W.; Damoiseaux, J.G. Nature versus nurture in the spectrum of rheumatic diseases: Classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun. Rev. 2018, 17, 935–941. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Mauro, D.; Simone, D.; Bucci, L.; Ciccia, F. Novel immune cell phenotypes in spondyloarthritis pathogenesis. Semin. Immunopathol. 2021, 43, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Macleod, T.; Bridgewood, C.; McGonagle, D. Role of neutrophil interleukin-23 in spondyloarthropathy spectrum disorders. Lancet Rheumatol. 2023, 5, e47–e57. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Eash, K.J.; Greenbaum, A.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2018.00113 (accessed on 31 January 2023). [CrossRef] [PubMed]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Khoyratty, T.E.; Ai, Z.; Ballesteros, I.; Eames, H.L.; Mathie, S.; Martín-Salamanca, S.; Wang, L.; Hemmings, A.; Willemsen, N.; von Werz, V.; et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat. Immunol. 2021, 22, 1093–1106. [Google Scholar] [CrossRef]
- Montaldo, E.; Lusito, E.; Bianchessi, V.; Caronni, N.; Scala, S.; Basso-Ricci, L.; Cantaffa, C.; Masserdotti, A.; Barilaro, M.; Barresi, S.; et al. Cellular and transcriptional dynamics of human neutrophils at steady state and upon stress. Nat. Immunol. 2022, 23, 1470–1483. [Google Scholar] [CrossRef]
- Enthesitis: From Pathophysiology to Treatment—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29158573/ (accessed on 2 November 2022).
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. J. Exp. Med. 2001, 194, 519–528. [Google Scholar] [CrossRef]
- Yu, J.J.; Ruddy, M.J.; Wong, G.C.; Sfintescu, C.; Baker, P.J.; Smith, J.B.; Evans, R.T.; Gaffen, S.L. An essential role for IL-17 in preventing pathogen-initiated bone destruction: Recruitment of neutrophils to inflamed bone requires IL-17 receptor–dependent signals. Blood 2007, 109, 3794–3802. [Google Scholar] [CrossRef]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F.; et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL-23. J. Leukoc. Biol. 2019, 105, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Takahashi, T.; Hata, H.; Nomura, T.; Sakaguchi, N. SKG mice, a new genetic model of rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, 10. [Google Scholar] [CrossRef]
- Stavre, Z.; Bridgewood, C.; Zhou, Q.; Maeda, Y.; Huang, T.-T.; Karman, J.; Khan, A.; Giryes, S.; Sharif, K.; McGonagle, D.; et al. A role for neutrophils in early enthesitis in spondyloarthritis. Arthritis Res. Ther. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.A.N.; McLean, M.; Kitson, S.M.; Melchor, E.G.; Patommel, K.; Cao, H.M.; Reilly, J.H.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; et al. S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci. Rep. 2019, 9, 1463. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximénez-Embún, P.; Guío-Carrión, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100A8-S100A9 Protein Complex Mediates Psoriasis by Regulating the Expression of Complement Factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef]
- Turina, M.C.; Sieper, J.; Yeremenko, N.; Conrad, K.; Haibel, H.; Rudwaleit, M.; Baeten, D.; Poddubnyy, D. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann. Rheum. Dis. 2014, 73, 1746–1748. [Google Scholar] [CrossRef]
- Pruenster, M.; Kurz, A.R.M.; Chung, K.-J.; Cao-Ehlker, X.; Bieber, S.; Nussbaum, C.F.; Bierschenk, S.; Eggersmann, T.K.; Rohwedder, I.; Heinig, K.; et al. Extracellular MRP8/14 is a regulator of β2 integrin-dependent neutrophil slow rolling and adhesion. Nat. Commun. 2015, 6, 6915. [Google Scholar] [CrossRef]
- De Wilde, K.; Martens, A.; Lambrecht, S.; Jacques, P.; Drennan, M.B.; Debusschere, K.; Govindarajan, S.; Coudenys, J.; Verheugen, E.; Windels, F.; et al. A20 inhibition of STAT1 expression in myeloid cells: A novel endogenous regulatory mechanism preventing development of enthesitis. Ann. Rheum. Dis. 2017, 76, 585–592. [Google Scholar] [CrossRef]
- McGonagle, D.; Marzo-Ortega, H.; O’Connor, P.; Gibbon, W.; Hawkey, P.; Henshaw, K.; Emery, P. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann. Rheum. Dis. 2002, 61, 534–537. [Google Scholar] [CrossRef]
- Pachowsky, M.L.; Raimondo, M.G.; Xu, C.; Rauber, S.; Tascilar, K.; Labinsky, H.; Vogg, M.; Saad, M.S.A.; Simon, D.; Rech, J.; et al. Concise report: A minimal-invasive method to retrieve and identify entheseal tissue from psoriatic arthritis patients. Ann. Rheum. Dis. 2022, 81, 1131–1135. [Google Scholar] [CrossRef]
- Van Tubergen, A.; Weber, U. Diagnosis and classification in spondyloarthritis: Identifying a chameleon. Nat. Rev. Rheumatol. 2012, 8, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zheng, N.; Chen, S.-B.; Xiao, Z.-Y.; Wu, M.-Y.; Liu, Y.; Zeng, Q.-Y. Ten years’ experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum. 2012, 64, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef]
- Papagoras, C.; Tsiami, S.; Chrysanthopoulou, A.; Mitroulis, I.; Baraliakos, X. Serum granulocyte-macrophage colony-stimulating factor (GM-CSF) is increased in patients with active radiographic axial spondyloarthritis and persists despite anti-TNF treatment. Arthritis Res. Ther. 2022, 24, 195. [Google Scholar] [CrossRef] [PubMed]
- Regan-Komito, D.; Swann, J.W.; Demetriou, P.; Cohen, E.S.; Horwood, N.J.; Sansom, S.N.; Griseri, T. GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat. Commun. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Al-Osami, M.H.; Awadh, N.I.; Khalid, K.B.; Awadh, A.I. Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with Ankylosing spondylitis: A case-control study. Hortic. Bras. Rheumatol. 2020, 60, 13. [Google Scholar] [CrossRef] [PubMed]
- Karow, F.; Smiljanovic, B.; Grün, J.R.; Poddubnyy, D.; Proft, F.; Talpin, A.; Hue, C.; Boland, A.; Deleuze, J.-F.; Garchon, H.-J.; et al. Monocyte transcriptomes from patients with axial spondyloarthritis reveal dysregulated monocytopoiesis and a distinct inflammatory imprint. Arthritis Res. Ther. 2021, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Limon, P.; Ladehesa-Pineda, M.L.; Castro-Villegas, M.D.C.; Abalos-Aguilera, M.D.C.; Lopez-Medina, C.; Lopez-Pedrera, C.; Barbarroja, N.; Espejo-Peralbo, D.; González-Reyes, J.A.; Villalba, J.M.; et al. Enhanced NETosis generation in radiographic axial spondyloarthritis: Utility as biomarker for disease activity and anti-TNF-α therapy effectiveness. J. Biomed. Sci. 2020, 27, 54. [Google Scholar] [CrossRef]
- Papagoras, C.; Chrysanthopoulou, A.; Mitsios, A.; Ntinopoulou, M.; Tsironidou, V.; Batsali, A.K.; Papadaki, H.A.; Skendros, P.; Ritis, K. IL-17A expressed on neutrophil extracellular traps promotes mesenchymal stem cell differentiation toward bone-forming cells in ankylosing spondylitis. Eur. J. Immunol. 2021, 51, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Mitsios, A.; Arampatzioglou, A.; Arelaki, S.; Mitroulis, I.; Ritis, K. NETopathies? Unraveling the Dark Side of Old Diseases through Neutrophils. Front. Immunol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.J.; Farr, M.; Meynell, M.J.; Hawkins, C.F. Synovial fluid in ankylosing spondylitis. Ann. Rheum. Dis. 1973, 32, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, E.; Baeten, D.; De Rycke, L.; Vandooren, B.; Foell, D.; Roth, J.; Cañete, J.D.; Boots, A.M.; Veys, E.M.; De Keyser, F. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R569–R580. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Kruithof, E.; De Rycke, L.; Boots, A.M.; Mielants, H.; Veys, E.M.; De Keyser, F. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res. Ther. 2005, 7, R359–R369. [Google Scholar] [CrossRef]
- Kruithof, E.; De Rycke, L.; Vandooren, B.; De Keyser, F.; FitzGerald, O.; McInnes, I.; Tak, P.P.; Bresnihan, B.; Veys, E.M.; Baeten, D.; et al. Identification of synovial biomarkers of response to experimental treatment in early-phase clinical trials in spondylarthritis. Arthritis Rheum. 2006, 54, 1795–1804. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Yang, X.; Song, Z.; Wang, Y.; Zhang, Z. NETosis in Psoriatic Arthritis: Serum MPO–DNA Complex Level Correlates With Its Disease Activity. Front. Immunol. 2022, 13, 911347. [Google Scholar] [CrossRef]
- Lambert, S.; Hambro, C.A.; Johnston, A.; Stuart, P.E.; Tsoi, L.C.; Nair, R.P.; Elder, J.T. Neutrophil Extracellular Traps Induce Human Th17 Cells: Effect of Psoriasis-Associated TRAF3IP2 Genotype. J. Investig. Dermatol. 2019, 139, 1245–1253. [Google Scholar] [CrossRef]
- De Benedittis, G.; Latini, A.; Conigliaro, P.; Triggianese, P.; Bergamini, A.; Novelli, L.; Ciccacci, C.; Chimenti, M.S.; Borgiani, P. A multilocus genetic study evidences the association of autoimmune-related genes with Psoriatic Arthritis in Italian patients. Immunobiology 2022, 227, 152232. [Google Scholar] [CrossRef]
- Bierkarre, H.; Harder, J.; Cuthbert, R.; Emery, P.; Leuschner, I.; Mrowietz, U.; Hedderich, J.; McGonagle, D.; Gläser, R. Differential expression of antimicrobial peptides in psoriasis and psoriatic arthritis as a novel contributory mechanism for skin and joint disease heterogeneity. Scand. J. Rheumatol. 2016, 45, 188–196. [Google Scholar] [CrossRef]
- Frasca, L.; Palazzo, R.; Chimenti, M.S.; Alivernini, S.; Tolusso, B.; Bui, L.; Botti, E.; Giunta, A.; Bianchi, L.; Petricca, L.; et al. Anti-LL37 Antibodies Are Present in Psoriatic Arthritis (PsA) Patients: New Biomarkers in PsA. Front. Immunol. 2018, 9, 1936. [Google Scholar] [CrossRef]
- Gracey, E.; Vereecke, L.; McGovern, D.; Fröhling, M.; Schett, G.; Danese, S.; De Vos, M.; Bosch, F.V.D.; Elewaut, D. Revisiting the gut–joint axis: Links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 2020, 16, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Mielants, H.; Veys, E.M.; Cuvelier, C.; De Vos, M.; Goemaere, S.; De Clercq, L.; Schatteman, L.; Gyselbrecht, L.; Elewaut, D. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J. Rheumatol. 1995, 22, 2279–2784. [Google Scholar] [PubMed]
- Mielants, H.; Veys, E.M.; Cuvelier, C.; De Vos, M.; Goemaere, S.; De Clercq, L.; Schatteman, L.; Elewaut, D. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J. Rheumatol. 1995, 22, 2273–2278. [Google Scholar] [PubMed]
- Lin, E.; Lai, H.-J.; Cheng, Y.-K.; Leong, K.-Q.; Cheng, L.-C.; Chou, Y.-C.; Peng, Y.-C.; Hsu, Y.-H.; Chiang, H.-S. Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis. Biomedicines 2020, 8, 275. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Alessandro, R.; Luchetti, M.M.; Milling, S.; Saieva, L.; Cypers, H.; Stampone, T.; Di Benedetto, P.; et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2017, 76, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ferrante, A.; Guggino, G.; Ciccia, F. Gut inflammation in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 863–876. [Google Scholar] [CrossRef]
- Tester, A.M.; Cox, J.H.; Connor, A.R.; Starr, A.E.; Dean, R.A.; Suarez-Puente, X.; López-Otín, C.; Overall, C.M. LPS Responsiveness and Neutrophil Chemotaxis In Vivo Require PMN MMP-8 Activity. PLoS ONE 2007, 2, e312. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Proost, P.; Wuyts, A.; Van Damme, J.O.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000, 96, 2673–2681. [Google Scholar] [CrossRef]
- Iking-Konert, C.; Ostendorf, B.; Sander, O.; Jost, M.; Wagner, C.; Joosten, L.; Schneider, M.; Hänsch, G.M. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: Evidence for activation by T cells. Ann. Rheum. Dis. 2005, 64, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Cypers, H.; Varkas, G.; Beeckman, S.; Debusschere, K.; Vogl, T.; Roth, J.; Drennan, M.; Lavric, M.; Foell, D.; Cuvelier, C.A.; et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann. Rheum. Dis. 2016, 75, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Guggino, G.; Ferrante, A.; Ciccia, F. Role of Subclinical Gut Inflammation in the Pathogenesis of Spondyloarthritis. Front. Med. 2018, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, L.; Mielants, H.; Veys, E.M.; Cuvelier, C.; De Vos, M.; Gyselbrecht, L.; Elewaut, D.; Goemaere, S. Gut inflammation in psoriatic arthritis: A prospective ileocolonoscopic study. J. Rheumatol. 1995, 22, 680–683. [Google Scholar]
- Macaluso, F.; Guggino, G.; Rizzo, A.; Ferrante, A.; Ciccia, F. Histopathology of the gut in rheumatic diseases. Reumatismo 2018, 70, 178–186. [Google Scholar] [CrossRef]
- Mielants, H.; Veys, E.M.; De Vos, M.; Cuvelier, C.; Goemaere, S.; De Clercq, L.; Schatteman, L.; Elewaut, D. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J. Rheumatol. 1995, 22, 2266–2272. [Google Scholar]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Voyich, J.M.; Whitney, A.R.; DeLeo, F.R. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J. Leukoc. Biol. 2005, 78, 1408–1418. [Google Scholar] [CrossRef]
- Arosa, L.; Camba-Gómez, M.; Conde-Aranda, J. Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future. Gastrointest. Disord. 2022, 4, 263–276. [Google Scholar] [CrossRef]
- Brannigan, A.E.; O’connell, R.P.; Hurley, H.; O’neill, A.; Brady, H.R.; Fitzpatrick, J.M.; William, R.; Watson, G. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock 2000, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kusugami, K.; Hosokawa, T.; Imada, A.; Shimizu, T.; Yamaguchi, T.; Ohsuga, M.; Kyokane, K.; Sakai, T.; Nishio, Y.; et al. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in Inflammatory Bowel disease. J. Gastroenterol. Hepatol. 1999, 14, 46–53. [Google Scholar] [CrossRef]
- Bassler, K.; Schulte-Schrepping, J.; Warnat-Herresthal, S.; Aschenbrenner, A.C.; Schultze, J.L. The Myeloid Cell Compartment—Cell by Cell. Annu. Rev. Immunol. 2019, 37, 269–293. [Google Scholar] [CrossRef]
- Qaiyum, Z.; Lim, M.; Inman, R.D. The gut-joint axis in spondyloarthritis: Immunological, microbial, and clinical insights. Semin. Immunopathol. 2021, 43, 173–192. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- FitzGerald, O.; Ogdie, A.; Chandran, V.; Coates, L.C.; Kavanaugh, A.; Tillett, W.; Leung, Y.Y.; Dewit, M.; Scher, J.U.; Mease, P.J. Psoriatic arthritis. Nat. Rev. Dis. Prim. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Czerwińska, J.; Owczarczyk-Saczonek, A. The Role of the Neutrophilic Network in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2022, 23, 1840. [Google Scholar] [CrossRef]
- Naik, H.B.; Natarajan, B.; Stansky, E.; Ahlman, M.A.; Teague, H.; Salahuddin, T.; Ng, Q.; Joshi, A.A.; Krishnamoorthy, P.; Dave, J.; et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arter. Thromb. Vasc. Biol. 2015, 35, 2667–2676. [Google Scholar] [CrossRef]
- Polat, M.; Bugdayci, G.; Kaya, H.; Oğuzman, H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol. Alp. Pannonica et Adriat. 2017, 26, 97–100. [Google Scholar] [CrossRef]
- Teague, H.L.; Varghese, N.J.; Tsoi, L.C.; Dey, A.K.; Garshick, M.S.; Silverman, J.I.; Baumer, Y.; Harrington, C.L.; Stempinski, E.; Elnabawi, Y.A.; et al. Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis. JACC Basic Transl. Sci. 2019, 4, 1–14. [Google Scholar] [CrossRef]
- Toichi, E.; Tachibana, T.; Furukawa, F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J. Am. Acad. Dermatol. 2000, 43, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Takahashi, H.; Suga, Y.; Eto, H.; Etoh, T.; Okuma, K.; Takahashi, K.; Kanbara, T.; Seishima, M.; Morita, A.; et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. J. Am. Acad. Dermatol. 2013, 68, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Umezawa, Y.; Yamagiwa, A.; Saeki, H.; Kondo, M.; Gabazza, E.; Nakagawa, H.; Mizutani, H. Biologic therapy improves psoriasis by decreasing the activity of monocytes and neutrophils. J. Dermatol. 2014, 41, 679–685. [Google Scholar] [CrossRef]
- Hwang, T.-L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 12. [Google Scholar]
- Bloomfield, F.J.; Young, M.M. Enhanced chemiluminescence production by phagocytosing neutrophils in psoriasis. Inflammation 1988, 12, 153–159. [Google Scholar] [CrossRef]
- Dilek, N.; Dilek, A.R.; Taşkın, Y.; Erkinüresin, T.; Yalçın, Ö.; Saral, Y. Contribution of myeloperoxidase and inducible nitric oxide synthase to pathogenesis of psoriasis. Postep. Dermatol. Allergol. 2016, 33, 435–439. [Google Scholar] [CrossRef]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U.; Wingertszahn, J.; Wiedow, O. Human Leukocyte Elastase Induces Keratinocyte Proliferation by Epidermal Growth Factor Receptor Activation. J. Investig. Dermatol. 2004, 123, 338–345. [Google Scholar] [CrossRef]
- Loss-of-Function Myeloperoxidase Mutations Are Associated with Increased Neutrophil Counts and Pustular Skin Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32758448/ (accessed on 2 November 2022).
- Hau, C.S.; Kanda, N.; Tada, Y.; Shibata, S.; Uozaki, H.; Fukusato, T.; Sato, S.; Watanabe, S. Lipocalin-2 exacerbates psoriasiform skin inflammation by augmenting T-helper 17 response. J. Dermatol. 2016, 43, 785–794. [Google Scholar] [CrossRef]
- Schön, M.P.; Broekaert, S.M.C.; Erpenbeck, L. Sexy again: The renaissance of neutrophils in psoriasis. Exp. Dermatol. 2017, 26, 305–311. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Dang, E.; Xue, K.; Zhang, J.; Li, B.; Qiao, H.; Cao, T.; Zhuang, Y.; Shen, S.; et al. Neutrophil Extracellular Traps Promote Inflammatory Responses in Psoriasis via Activating Epidermal TLR4/IL-36R Crosstalk. Front. Immunol. 2019, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Yu, H.-S.; Yen, F.-L.; Lin, C.-L.; Chen, G.-S.; Lan, C.-C.E. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef]
- Wang, W.-M.; Jin, H.-Z. Role of Neutrophils in Psoriasis. J. Immunol. Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.-H.; Homey, B.; Cao, W.; Wang, Y.-H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, X.; Zhai, Y.; Li, L.; Li, H.; Sun, X.; Yu, P.; Xue, T.; Li, Y.; Hu, Y. Single cell transcriptional zonation of human psoriasis skin identifies an alternative immunoregulatory axis conducted by skin resident cells. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Rosenbaum, J.T. Uveitis in spondyloarthritis including psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Clin. Rheumatol. 2015, 34, 999–1002. [Google Scholar] [CrossRef]
- Rosenbaum, J.T. The eye in spondyloarthritis✰. Semin. Arthritis Rheum. 2019, 49, S29–S31. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Haroon, M.; Rosenbaum, J.T. Acute Anterior Uveitis and Spondyloarthritis: More Than Meets the Eye. Curr. Rheumatol. Rep. 2015, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, D.; Yates, W.; Amjadi, S.; McCluskey, P. HLA-B27 Anterior Uveitis: Immunology and Immunopathology. Ocul. Immunol. Inflamm. 2016, 24, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-C.; Li, Q. Immunopathology of uveitis. Br. J. Ophthalmol. 1998, 82, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, W.; Jiang, R.; Zhang, R.; Wang, Y.; Wang, L.; Gordon, L.; Chen, L. Expression profile of IL-1 family cytokines in aqueous humor and sera of patients with HLA-B27 associated anterior uveitis and idiopathic anterior uveitis. Exp. Eye Res. 2015, 138, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Repo, H.; Leirisalo-Repo, M.; Koivuranta-Vaara, P. Exaggerated inflammatory responsiveness plays a part in the pathogenesis of HLA-B27 linked diseases—Hypothesis. Ann. Clin. Res. 1984, 16, 47–50. [Google Scholar]
- Yamada, M.; Shichi, H.; Yuasa, T.; Tanouchi, Y.; Mimura, Y. Superoxide in ocular inflammation: Human and experimental uveitis. J. Free. Radicals Biol. Med. 1986, 2, 111–117. [Google Scholar] [CrossRef]
- Sedgwick, J.B.; Bergstresser, P.R.; Hurd, E.R. Increased Granulocyte Adherence in Psoriasis and Psoriatic Arthritis. J. Investig. Dermatol. 1980, 74, 81–84. [Google Scholar] [CrossRef]
- Iijima, S.; Iwata, M.; Otsuka, F. Psoriatic Arthritis and Hypopyon-lridocyclitis. Possible mechanism of the association of psoriasis and anterior uveitis. Dermatology 1996, 193, 295–299. [Google Scholar] [CrossRef]
- Chang, J.H.; Hampartzoumian, T.; Everett, B.; Lloyd, A.; McCluskey, P.J.; Wakefield, D. Changes in Toll-like Receptor (TLR)-2 and TLR4 Expression and Function but Not Polymorphisms Are Associated with Acute Anterior Uveitis. Investig. Opthalmology Vis. Sci. 2007, 48, 1711–1717. [Google Scholar] [CrossRef]
- Erridge, C.; Pridmore, A.; Eley, A.; Stewart, J.; Poxton, I.R. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via Toll-like receptor 2. J. Med Microbiol. 2004, 53, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; McCluskey, P.; Wakefield, D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 2006, 90, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ohashi, S.; Kawa, Y.; Takahama, H.; Ito, M.; Soma, Y.; Mizoguchi, M. Elevated Serum Granulocyte Colony-Stimulating Factor Levels in Patients With Active Phase of Sweet Syndrome and Patients With Active Behçet Disease: Implication in neutrophil apoptosis dysfunction. Arch. Dermatol. 2004, 140, 570–574. [Google Scholar] [CrossRef] [PubMed]
- The Antiapoptotic Protein Mcl-1 Is Essential for the Survival of Neutrophils but Not Macrophages—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17062731/ (accessed on 3 November 2022).
- Increased Production of Pro-Inflammatory Cytokines and Enhanced T Cell Responses after Activation of Human Dendritic Cells with IL-1 and CD40 Ligand—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12385649/ (accessed on 3 November 2022).
- Goldberg, G.L.; Cornish, A.L.; Murphy, J.; Pang, E.S.; Lim, L.L.; Campbell, I.K.; Scalzo-Inguanti, K.; Chen, X.; McMenamin, P.G.; Maraskovsky, E.; et al. G-CSF and Neutrophils Are Nonredundant Mediators of Murine Experimental Autoimmune Uveoretinitis. Am. J. Pathol. 2016, 186, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Iwata, M.; Otsuka, F. Rheological analysis of peripheral blood neutrophils in a patient with cutaneous and arthropathic psoriasis accompanying hypopyon-iridocyclitis. Australas. J. Dermatol. 1996, 37 (Suppl. 1), S40–S41. [Google Scholar] [CrossRef]
- Morton, A.M.; Sefik, E.; Upadhyay, R.; Weissleder, R.; Benoist, C.; Mathis, D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 6696–6701. [Google Scholar] [CrossRef]
- Hysa, E.; Cutolo, C.A.; Gotelli, E.; Pacini, G.; Schenone, C.; Kreps, E.O.; Smith, V.; Cutolo, M. Immunopathophysiology and clinical impact of uveitis in inflammatory rheumatic diseases: An update. Eur. J. Clin. Investig. 2021, 51, e13572. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- De Benedetti, F.; Ruperto, N.; Baildam, E.; Burgos-Vargas, R.; Horneff, G.; Huppertz, H.I.; Minden, K.; Myones, B.L.; Onel, K.; Wang, J.; et al. A14: Neutropenia With Tocilizumab Treatment Is Not Associated With Increased Infection Risk in Patients with Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2014, 66, S23–S24. [Google Scholar] [CrossRef]
- Shovman, O.; Shoenfeld, Y.; Langevitz, P. Tocilizumab-induced neutropenia in rheumatoid arthritis patients with previous history of neutropenia: Case series and review of literature. Immunol. Res. 2015, 61, 164–168. [Google Scholar] [CrossRef]
- Strand, V.; Dikranian, A.; Beal, J.; Kwok, K.; Krishnaswami, S.; Wood, S.; Nduaka, C. Analysis of early neutropenia, clinical response, and serious infection events in patients receiving tofacitinib for rheumatoid arthritis. In Arthritis & Rheumatology; Wiley-Blackwell 111 River ST: Hoboken, NJ, USA, 2014; pp. S1086–S1087. [Google Scholar]
- Wigerblad, G.; Cao, Q.; Brooks, S.; Naz, F.; Gadkari, M.; Jiang, K.; Gupta, S.; O’Neil, L.; Dell’Orso, S.; Kaplan, M.J.; et al. Single-Cell Analysis Reveals the Range of Transcriptional States of Circulating Human Neutrophils. J. Immunol. 2022, 209, 772–782. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.; Miranda, P.; Korkosz, M.; Vencovsky, J.; Rubbert-Roth, A.; Mysler, E.; Sleeman, M.A.; Godwood, A.; et al. A randomised phase IIb study of mavrilimumab, a novel GM–CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1020–1030. [Google Scholar] [CrossRef]
- Cid, M.C.; Unizony, S.H.; Blockmans, D.; Brouwer, E.; Dagna, L.; Dasgupta, B.; Hellmich, B.; Molloy, E.; Salvarani, C.; Trapnell, B.C.; et al. Efficacy and safety of mavrilimumab in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2022, 81, 653–661. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovsky, J.; Guo, X.; White, W.I.; Ryan, P.C.; Godwood, A.; Albulescu, M.; et al. A Randomized Phase II b Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 49–59. [Google Scholar] [CrossRef]
- Buckley, C.D.; Simón-Campos, J.A.; Zhdan, V.; Becker, B.; Davy, K.; Fisheleva, E.; Gupta, A.; Hawkes, C.; Inman, D.; Layton, M.; et al. Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: A randomised, phase 2b, dose-ranging study. Lancet Rheumatol. 2020, 2, e677–e688. [Google Scholar] [CrossRef]
- Taylor, P.C.; for the NEXUS Study Group; Saurigny, D.; Vencovsky, J.; Takeuchi, T.; Nakamura, T.; Matsievskaia, G.; Hunt, B.; Wagner, T.; Souberbielle, B. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: A randomized, controlled trial. Arthritis Res. Ther. 2019, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Gooderham, M.; Jenkins, R.; Vender, R.; Szepietowski, J.; Wagner, T.; Hunt, B.; Souberbielle, B.; on behalf of the NEPTUNE investigators; The NEPTUNE investigators. Granulocyte–macrophage colony-stimulating factor (GM-CSF) as a therapeutic target in psoriasis: Randomized, controlled investigation using namilumab, a specific human anti-GM-CSF monoclonal antibody. Br. J. Dermatol. 2019, 180, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.V.C.; Chiva, L.M.; Villarino, M.R. Granulocyte and monocyte/macrophage apheresis for the treatment of immune-mediated inflammatory arthropathies: Case reports. Drugs Context 2021, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Mao, J.-W.; Wang, Y.-D. Selective granulocyte and monocyte apheresis in inflammatory bowel disease: Its past, present and future. World J. Gastrointest. Pathophysiol. 2020, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.; Wang, J.; Buckman, C.A.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Zollner, T.M.; Boehncke, W.-H. The Molecular Basis of Lymphocyte Recruitment to the Skin: Clues for Pathogenesis and Selective Therapies of Inflammatory Disorders. J. Investig. Dermatol. 2003, 121, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.R.; Merkel, P.A.; Schall, T.J.; Bekker, P. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2021, 384, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, N.; Zonozi, R.; Rosenthal, J.; Laliberte, K.; Niles, J.L.; Cortazar, F.B. Treatment of Aggressive Antineutrophil Cytoplasmic Antibody–Associated Vasculitis With Eculizumab. Kidney Int. Rep. 2020, 5, 542–545. [Google Scholar] [CrossRef]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Tanaka, Y.; Millson, D.; Iwata, S.; Nakayamada, S. Safety and efficacy of fostamatinib in rheumatoid arthritis patients with an inadequate response to methotrexate in phase II OSKIRA-ASIA-1 and OSKIRA-ASIA-1X study. Rheumatology 2021, 60, 2884–2895. [Google Scholar] [CrossRef]
- Walker, A.; Erwig, L.; Foster, K.; Nevin, K.; Wenzel, J.; Worm, M.; Williams, N.; Ratia, N.; Hoang, B.; Schneider-Merck, T.; et al. Safety, pharmacokinetics and pharmacodynamics of a topical SYK inhibitor in cutaneous lupus erythematosus: A double-blind Phase Ib study. Exp. Dermatol. 2021, 30, 1686–1692. [Google Scholar] [CrossRef]
- Keating, G.M. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2017, 77, 459–472. [Google Scholar] [CrossRef]
- Taylor, P.C.; van der Heijde, D.; Landewé, R.; McCue, S.; Cheng, S.; Boonen, A. A Phase III Randomized Study of Apremilast, an Oral Phosphodiesterase 4 Inhibitor, for Active Ankylosing Spondylitis. J. Rheumatol. 2021, 48, 1259–1267. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: http://www.ncbi.nlm.nih.gov/books/NBK482425/ (accessed on 26 October 2022).
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Bhandari, B.R.; Randall, C.; Younes, Z.H.; Romanczyk, T.; Xin, Y.; Wendt, E.; Chai, H.; McKevitt, M.; Zhao, S.; et al. Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease. J. Crohns Colitis 2018, 12, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Siegel, C.A.; Friedenberg, K.A.; Younes, Z.H.; Seidler, U.; Bhandari, B.R.; Wang, K.; Wendt, E.; McKevitt, M.; Zhao, S.; et al. A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. β-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef]
- Stefania, S.; Colia, R.; Cinzia, R.; Corrado, A.; Cantatore, F.P. Off-label use of anti-IL-1 drugs in rheumatic diseases. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211006584. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Zeng, F.; Nakamura, S.; Reid, K.T.; Gracey, E.; Lim, M.; Leng, L.; Jo, S.; Park, Y.-S.; Kusuda, M.; et al. Macrophage migration inhibitory factor drives pathology in a mouse model of spondyloarthritis and is associated with human disease. Sci. Transl. Med. 2021, 13, eabg1210. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: Recent advances and controversies—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31278139/ (accessed on 2 November 2022).
- Tamassia, N.; e Silva, F.A.; Calzetti, F.; Lonardi, S.; Gasperini, S.; Gardiman, E.; Bianchetto-Aguilera, F.; Gatta, L.B.; Girolomoni, G.; Mantovani, A.; et al. A Reappraisal on the Potential Ability of Human Neutrophils to Express and Produce IL-17 Family Members In Vitro: Failure to Reproducibly Detect It. Front. Immunol. 2018, 9, 795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coletto, L.A.; Rizzo, C.; Guggino, G.; Caporali, R.; Alivernini, S.; D’Agostino, M.A. The Role of Neutrophils in Spondyloarthritis: A Journey across the Spectrum of Disease Manifestations. Int. J. Mol. Sci. 2023, 24, 4108. https://doi.org/10.3390/ijms24044108
Coletto LA, Rizzo C, Guggino G, Caporali R, Alivernini S, D’Agostino MA. The Role of Neutrophils in Spondyloarthritis: A Journey across the Spectrum of Disease Manifestations. International Journal of Molecular Sciences. 2023; 24(4):4108. https://doi.org/10.3390/ijms24044108
Chicago/Turabian StyleColetto, Lavinia Agra, Chiara Rizzo, Giuliana Guggino, Roberto Caporali, Stefano Alivernini, and Maria Antonietta D’Agostino. 2023. "The Role of Neutrophils in Spondyloarthritis: A Journey across the Spectrum of Disease Manifestations" International Journal of Molecular Sciences 24, no. 4: 4108. https://doi.org/10.3390/ijms24044108
APA StyleColetto, L. A., Rizzo, C., Guggino, G., Caporali, R., Alivernini, S., & D’Agostino, M. A. (2023). The Role of Neutrophils in Spondyloarthritis: A Journey across the Spectrum of Disease Manifestations. International Journal of Molecular Sciences, 24(4), 4108. https://doi.org/10.3390/ijms24044108







