From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children
Abstract
1. Introduction
1.1. Conceptual Framework for This Review
1.2. Overview of Innate Immune System Receptors
1.3. TLR and NLR Synergisms and Antagonisms
1.4. Hyperinflammation, Bacterial Co- and Super-Infections and Autoimmunity in COVID-19
2. TLR and NLR Activation in SARS-CoV-2, Severe COVID-19 and Its Autoimmune Complications
2.1. Overview
2.2. Innate Receptor Activation by SARS-CoV-2 and Its Vaccines
2.3. Innate Receptor Activation in Severe COVID-19
2.4. Innate Receptor Activation in MIS-C and KD
2.5. Innate Receptor Activation in Autoimmune Myocarditis
2.6. Innate Receptor Activation in Autoimmune Coagulopathies
2.7. Comparison of Innate Receptor Activation Patterns in COVID-19-Related Diseases
3. Receptor Synergisms May Hyper-Activate Innate Immunity
3.1. Neutrophil and Monocyte Activation in Severe COVID-19
3.2. Synergistic Innate Receptor Activation as a Cause of Cytokine Over-Production and Hyperinflammation in COVID-19
3.3. Innate Receptor Synergies in MIS-C and KD
3.4. Innate Receptor Synergisms in APS
3.5. SARS-CoV-2 Vaccines and Risks of Autoimmune Complications
3.6. Possible Roles of Underlying Diseases That Predispose Severe COVID-19
4. Future Directions for Further Research and Implications for Prevention and Treatment of COVID-19-Associated Autoimmune Diseases
5. Materials and Methods
Funding
Conflicts of Interest
Appendix A
- Broering, R.; Montag, M.; Jiang, M.; Lu, M.; Sowa, J.P.; Kleinehr, K.; Gerken, G.; Schlaak, J.F. Corticosteroids shift the Toll-like receptor response pattern of primary-isolated murine liver cells from an inflammatory to an anti-inflammatory state. Int. Immunol. 2011, 23, 537–544. https://doi.org/10.1093/intimm/dxr048.
- Acharya, A.P.; Carstens, M.R.; Lewis, J.S.; Dolgova, N.; Xia, C.Q.; Clare-Salzler, M.J.; Keselowsky, B.G. A cell-based microarray to investigate combinatorial effects of microparticle-encapsulated adjuvants on dendritic cell activation. J. Mater. Chem. B 2016, 4, 1672–1685. https://doi.org/10.1039/C5TB01754H.
- Becker, C.E.; O’Neill, L.A. Inflammasomes in inflammatory disorders: The role of TLRs and their interactions with NLRs. Semin. Immunopathol. 2007, 29, 239–248.
- Caron, G.; Duluc, D.; Frémaux, I.; Jeannin, P.; David, C.; Gascan, H.; Delneste, Y. Direct stimulation of human T cells via TLR5 and TLR7/8: Flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 2005, 175, 1551–1557. https://doi.org/10.4049/jimmunol.175.3.1551. PMID: 16034093.
- Conforti-Andreoni, C.; Beretta, O.; Licandro, G.; Qian, H.L.; Urbano, M.; Vitulli, F.; Ricciardi-Castagnoli, P.; Mortellaro, A. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. J. Leukoc. Biol. 2010, 88, 1207–1216. https://doi.org/10.1189/jlb.1009652.
- Dabbagh, K.; Lewis, D.B. Toll-like Receptors and T-helper-1/T-helper-2 Responses. Curr. Opin. Infect. Dis. 2003, 16, 199–204. https://doi.org/10.1097/00001432-200306000-00003.
- Dahiya, Y.; Pandey, R.K.; Sodhi, A. NOD2 downregulates TLR2/1 mediated IL1 beta gene expression in mouse peritoneal macrophages. PLoS ONE 2011, 6, e27828.
- Farzi, A.; Reichmann, F.; Meinitzer, A.; Mayerhofer, R.; Jain, P.; Hassan, A.M.; Fröhlich, E.E.; Wagner, K.; Painsipp, E.; Rinner, B.; et al. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav. Immun. 2015, 44, 106–120. https://doi.org/10.1016/j.bbi.2014.08.011.
- Fischetti, L.; Zhong, Z.; Pinder, C.L.; Tregoning, J.S.; Shattock, R.J. The synergistic effects of combining TLR ligand based adjuvants on the cytokine response are dependent upon p38/JNK signalling. Cytokine 2017, 99, 287–296. https://doi.org/10.1016/j.cyto.2017.08.009.
- Fritz, J.H.; Girardin, S.E.; Fitting, C.; Werts, C.; Mengin-Lecreulx, D.; Caroff, M.; Cavaillon, J.-M.; Philpott, D.J.; Adib-Conquy, M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 2005, 35, 2459–2470. https://doi.org/10.1002/eji.200526286.
- Ghosh, T.K.; Mickelson, D.J.; Solberg, J.C.; Lipson, K.E.; Inglefield, J.R.; Alkan, S.S. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int. Immun. 2007, 7, 1111–1121. https://doi.org/10.1016/j.intimp.2007.04.006.
- Giamarellos-Bourboulis, E.J.; Mouktaroudi, M.; Bodar, E.; van der Ven, J.; Kullberg, B.J.; Netea, M.G.; van der Meer, J.W. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann. Rheum. Dis. 2009, 68, 273–278. https://doi.org/10.1136/ard.2007.082222.
- Hotz, C.; Roetzer, L.C.; Huber, T.; Sailer, A.; Oberson, A.; Treinies, M.; Heidegger, S.; Herbst, T.; Endres, S.; Bourquin, C. TLR and RLR Signaling Are Reprogrammed in Opposite Directions after Detection of Viral Infection. J. Immunol. 2015, 195, 4387–4395. https://doi.org/10.4049/jimmunol.1500079.
- Jung, Y.O.; Cho, M.L.; Kang, C.M.; Jhun, J.Y.; Park, J.S.; Oh, H.J.; Min, J.K.; Park, S.H.; Kim, H.Y. Toll-like receptor 2 and 4 combination engagement upregulate IL-15 synergistically in human rheumatoid synovial fibroblasts. Immunol. Lett. 2007, 109, 21–27. https://doi.org/10.1016/j.imlet.2006.12.006.
- Køllgaard, T.; Enevold, C.; Bendtzen, K.; Hansen, P.R.; Givskov, M.; Holmstrup, P.; Nielsen, C.H. Cholesterol crystals enhance TLR2- and TLR4-mediated pro-inflammatory cytokine responses of monocytes to the proatherogenic oral bacterium Porphyromonas gingivalis. PLoS ONE 2017, 12, e0172773. https://doi.org/10.1371/journal.pone.0172773.
- Krumbiegel, D.; Zepp, F.; Meyer, C.U. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum. Immunol. 2007, 68, 813–822. https://doi.org/10.1016/j.humimm.2007.08.001.
- Lacroix-Lamandé, S.; d’Andon, M.F.; Michel, E.; Ratet. G.; Philpott, D.J.; Girardin, S.E.; Boneca, I.G.; Vandewalle, A.; Werts, C. Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome. J. Immunol. 2012, 188, 2805–2814. https://doi.org/10.4049/jimmunol.1101987.
- Lantier, L.; Drouet, F.; Guesdon, W.; Mancassola, R.; Metton, C.; Lo-Man, R.; Werts, C.; Laurent, F.; Lacroix-Lamandé, S. Poly(I:C)-induced protection of neonatal mice against intestinal Cryptosporidium parvum infection requires an additional TLR5 signal provided by the gut flora. J. Infect. Dis. 2014, 209, 457–467. https://doi.org/10.1093/infdis/jit432. Epub 2013 Sep 6. PMID: 24014881.
- Lee, B.R.; Jeong, S.K.; Ahn, B.C.; Lee, B.J.; Shin, S.J.; Yum, J.S.; Ha, S.J. Combination of TLR1/2 and TLR3 ligands enhances CD4(+) T cell longevity and antibody responses by modulating type I IFN production. Sci. Rep. 2016, 6, 32526. https://doi.org/10.1038/srep32526.
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. https://doi.org/10.1007/s12016-013-8402-y.
- Mäkeläm, S.M.; Strengell, M.; Pietilä, T.E.; Osterlund, P.; Julkunen, I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 2009, 85, 664–672. https://doi.org/10.1189/jlb.0808503. [.
- Moen, S.H.; Ehrnström, B.; Kojen, J.F.; Yurchenko, M.; Beckwith, K.S.; Afset, J.E.; Damås, J.K.; Hu, Z.; Yin, H.; Espevik, T.; et al. Human Toll-like Receptor 8 (TLR8) Is an Important Sensor of Pyogenic Bacteria; and Is Attenuated by Cell Surface TLR Signaling. Front. Immunol. 2019, 10, 1209. https://doi.org/10.3389/fimmu.2019.01209.
- Mohammad Hosseini, A.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-like receptors in the pathogenesis of autoimmune diseases. Adv. Pharm. Bull. 2015, 5 (Suppl. 1), 605. https://doi.org/10.15171/apb.2015.082.
- Franchi, L.; Park, J.H.; Shaw, M.H.; Marina-Garcia, N.; Chen, G.; Kim, Y.G.; Núñez, G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008, 10, 1–8. doi: 10.1111/j.1462-5822.2007.01059.x. Epub 2007 Oct 18. PMID: 17944960.
- Napolitani, G.; Rinaldi, A.; Bertoni, F.; Sallusto, F.; Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005, 6, 769–776. https://doi.org/10.1038/ni1223.
- Nasirudeen, A.M.A.; Wong, H.H.; Thien, P.; Xu, S.; Lam, K.-P.; Liu, D.X. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl. Trop. Dis. 2011, 5, e926. https://doi.org/10.1371/journal.pntd.0000926.
- Nouri-Shirazi, M.; Tamjidi, S.; Nourishirazi, E.; Guinet, E. TLR8 combined withTLR3 or TLR4 agonists enhances DC-NK driven effector Tc1 cells. Immunol. Lett. 2017, 193, 58–66. https://doi.org/10.1016/j.imlet.2017.10.015.
- Oviedo-Boyso, J.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Collaborative Action of Toll-Like and Nod-Like Receptors as Modulators of the Inflammatory Response to Pathogenic Bacteria. Mediat. Inflamm. 2014, 2014, 432785. https://doi.org/10.1155/2014/432785.
- Pandey, S.; Gruenbaum, A.; Kanashova, T.; Mertins, P.; Cluzel, P.; Chevrier, N. Pairwise Stimulations of Pathogen-Sensing Pathways Predict Immune Responses to Multi-adjuvant Combinations. Cell Syst. 2020, 11, 495–508.e10. https://doi.org/10.1016/j.cels.2020.10.001. Epub 2020 Oct 27. PMID: 33113356, PMCID: PMC7677225.
- Pashenkov, M.V.; Murugina, N.E.; Budikhina, A.S.; Pinegin, B.V. Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications. J. Leukoc. Biol. 2019, 105, 669–680. https://doi.org/10.1002/JLB.2RU0718-290R. Epub 2018 Dec 5. PMID: 30517768.
- Re, F.; Strominger, J.L. IL-10 Released by Concomitant TLR2 Stimulation Blocks the Induction of a Subset of Th1 Cytokines That Are Specifically Induced by TLR4 or TLR3 in Human Dendritic Cells. J. Immunol. 2004, 173, 7548–7555. https://doi.org/10.4049/jimmunol.173.12.7548.
- Sato, S.; Nomura, F.; Kawai, T.; Takeuchi, O.; Mühlradt, P.F.; Takeda, K.; Akira, S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 2000, 165, 7096–7101. https://doi.org/10.4049/jimmunol.165.12.7096.
- Seydoux, E.; Liang, H.; Dubois Cauwelaert, N.; Archer, M.; Rintala, N.D.; Kramer, R.; Carter, D.; Fox, C.B.; Orr, M.T. Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses. J. Immunol. 2018, 201, 98–112. https://doi.org/10.4049/jimmunol.1701604.
- Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, Sugawara S, Takada H. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005, 7, 53–61. https://doi.org/10.1111/j.1462-5822.2004.00433.x. PMID: 15617523..
- Takada, H.; Uehara, A. Enhancement of TLR-mediated innate immune responses by peptidoglycans through NOD signaling. Curr. Pharm. Des. 2006, 12, 4163–4172. https://doi.org/10.2174/138161206778743510.
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005, 23, 307–336. https://doi.org/10.1146/annurev.immunol.23.021704.115843.
- Tukhvatulin, A.I.; Gitlin, I.I.; Shcheblyakov, D.V.; Artemicheva, N.M.; Burdelya, L.G.; Shmarov, M.M.; Naroditsky, B.S.; Gudkov, A.V.; Gintsburg, A.L.; Logunov, D.Y. Combined stimulation of Toll-Like receptor 5 and NOD1 strongly potentiates activity of NF-B; resulting in enhanced innate immune reactions and resistance to Salmonella enterica Serovar Typhimurium infection. Infect. Immun. 2013, 81, 3855–3864, https://doi.org/10.1128/IAI.00525-13.
- Vanhoutte, F.; Paget, C.; Breuilh, L.; Fontaine, J.; Vendeville, C.; Goriely, S.; Ryffel, B.; Faveeuw, C.; Trottein, F. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol. Lett. 2008, 116, 86–94. https://doi.org/10.1016/j.imlet.2007.11.014.
- Watanabe, T.; Asano, N.; Murray, P.J.; Ozato, K.; Tailor, P.; Fuss, I.J.; Kitani, A.; Strober, W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Investig. 2008, 118, 545–559. https://doi.org/10.1172/JCI33145.
- Watanabe, T.; Kitani, A.; Murray, P.J.; Strober, W. NOD2 is a negative regulator of toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 2004, 5, 800–808.
- Wikén, M.; Grunewald, J.; Eklund, A.; Wahlström, J. Higher monocyte expression of TLR2 and TLR4, and enhanced pro-inflammatory synergy of TLR2 with NOD2 stimulation in sarcoidosis. J. Clin. Immunol. 2009, 9, 78–89. https://doi.org/10.1007/s10875-008-9225-0.
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2015, 82, 1173–1182. https://doi.org/10.1128/AEM.03044-15.
- Zhou, H.; Coveney, A.P.; Wu, M.; Huang, J.; Blankson, S.; Zhao, H.; O’Leary, D.P.; Bai, Z.; Li, Y.; Redmond, H.P.; et al. Activation of Both TLR and NOD Signaling Confers Host Innate Immunity-Mediated Protection Against Microbial Infection. Front. Immunol. 2019, 9, 3082. https://doi.org/10.3389/fimmu.2018.03082.
References
- Root-Bernstein, R. Synergistic Activation of Toll-Like and NOD Receptors by Complementary Antigens as Facilitators of Autoimmune Disease: Review, Model and Novel Predictions. Int. J. Mol. Sci. 2020, 21, 4645. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Innate Receptor Activation Patterns Involving TLR and NLR Synergisms in COVID-19, ALI/ARDS and Sepsis Cytokine Storms: A Review and Model Making Novel Predictions and Therapeutic Suggestions. Int. J. Mol. Sci. 2021, 22, 2108. [Google Scholar] [CrossRef]
- Chen, K.; Huang, J.; Gong, W.; Iribarren, P.; Dunlop, N.M.; Wang, J.M. Toll-like receptors in inflammation, infection and cancer. Int. Immunopharmacol. 2007, 7, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.; Remijn, T.; Oosting, M.; de Jong, D.J.; Diavatopoulos, D.A.; Hermans, P.W.; Ferwerda, G. Respiratory syncytial virus infection augments NOD2 signaling in an IFN-β-dependent manner in human primary cells. Eur. J. Immunol. 2012, 42, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Selvanantham, T.; Escalante, N.K.; Cruz Tleugabulova, M.; Fiévé, S.; Girardin, S.E.; Philpott, D.J.; Mallevaey, T. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J. Immunol. 2013, 191, 5646–5654. [Google Scholar] [CrossRef]
- Schwarz, H.; Posselt, G.; Wurm, P.; Ulbing, M.; Duschl, A.; Horejs-Hoeck, J. TLR8 and NOD signaling synergistically induce the production of IL-1β and IL-23 in monocyte-derived DCs and enhance the expression of the feedback inhibitor SOCS2. Immunobiology 2013, 218, 533–542. [Google Scholar] [CrossRef]
- Tada, H.; Aiba, S.; Shibata, K.; Ohteki, T.; Takada, H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef]
- Knudsen, M.L.; Johansson, D.X.; Kostic, L.; Nordström, E.K.; Tegerstedt, K.; Pasetto, A.; Applequist, S.E.; Ljungberg, K.; Sirard, J.C.; Liljeström, P. The adjuvant activity of alphavirus replicons is enhanced by incorporating the microbial molecule flagellin into the replicon. PLoS ONE 2013, 8, e65964. [Google Scholar] [CrossRef]
- Kamaladasa, A.; Gomes, L.; Jeewandara, C.; Shyamali, N.L.; Ogg, G.S.; Malavige, G.N. Lipopolysaccharide acts synergistically with the dengue virus to induce monocyte production of platelet activating factor and other inflammatory mediators. Antivir. Res. 2016, 133, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Heeringa, P.; Jongman, R.M.; Zwiers, P.J.; Niemarkt, A.E.; Yan, R.; de Graaf, I.A.; Li, R.; Ravasz Regan, E.; Kümpers, P.; et al. Intracellular RIG-I Signaling Regulates TLR4-Independent Endothelial Inflammatory Responses to Endotoxin. J. Immunol. 2016, 196, 4681–4691. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Huang, W.; Li, M.; Luo, G.; Wu, X.; Su, B.; Zhao, L.; Zhang, S.; Chen, X.; Jia, M.; Zhu, J.; et al. The Inflammatory Factors Associated with Disease Severity to Predict COVID-19 Progression. J. Immunol. 2021, 206, 1597–1608. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Pneumococcal and Influenza Vaccination Rates and Pneumococcal Invasive Disease Rates Set Geographical and Ethnic Population Susceptibility to Serious COVID-19 Cases and Deaths. Vaccines 2021, 9, 474. [Google Scholar] [CrossRef]
- Root-Bernstein, R. COVID-19 coagulopathies: Human blood proteins mimic SARS-CoV-2 virus, vaccine proteins and bacterial co-infections inducing autoimmunity: Combinations of bacteria and SARS-CoV-2 synergize to induce autoantibodies targeting cardiolipin, cardiolipin-binding proteins, platelet factor 4, prothrombin, and coagulation factors. Bioessays 2021, 43, e2100158. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Wu, X.; Pang, B.; Yang, F.; Zheng, W.; Liu, C.; Zhang, J. Comorbidities and complications of COVID-19 associated with disease severity, progression, and mortality in China with centralized isolation and hospitalization: A systematic review and meta-analysis. Front. Public Health 2022, 10, 923485. [Google Scholar] [CrossRef]
- Udompornpitak, K.; Bhunyakarnjanarat, T.; Chindamporn, A.; Tovichayathamrong, P.; Torvorapanit, P.; Chiewchengchol, D.; Chancharoenthana, W.; Leelahavanichkul, A. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-β-D-glucan in Blood from Gut Translocation. Cells 2022, 11, 1103. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M.; et al. Microbial co-infections in COVID-19: Associated microbiota and underlying mechanisms of pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, K.; Batra, P.; Vinayaraj, E.V.; Bhatia, R.; SaiKiran, K.; Singh, V.; Singh, S.; Verma, N.; Singh, U.B.; Mohan, A.; et al. Coinfections with Other Respiratory Pathogens among Patients with COVID-19. Microbiol. Spectr. 2021, 9, e0016321. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Elabbadi, A.; Turpin, M.; Gerotziafas, G.T.; Teulier, M.; Voiriot, G.; Fartoukh, M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021, 49, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Metzemaekers, M.; de Carvalho, A.C.; Nooyens, A.; Jacobs, C.; Vanderbeke, L.; Malengier-Devlies, B.; Gouwy, M.; Heylen, E.; Meersseman, P.; et al. Atypical response to bacterial coinfection and persistent neutrophilic bronchoalveolar inflammation distinguish critical COVID-19 from influenza. JCI Insight 2022, 7, e155055. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tamiazzo, S.; Stobbione, P.; Agatea, L.; De Gaspari, P.; Stecca, A.; Lauritano, E.C.; Roveta, A.; Tozzoli, R.; Guaschino, R.; et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin. Transl. Sci. 2021, 14, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Stjepanovic, M.I.; Stojanovic, M.R.; Stankovic, S.; Cvejic, J.; Dimic-Janjic, S.; Popevic, S.; Buha, I.; Belic, S.; Djurdjevic, N.; Stjepanovic, M.M.; et al. Autoimmune and immunoserological markers of COVID-19 pneumonia: Can they help in the assessment of disease severity. Front. Med. 2022, 9, 934270. [Google Scholar] [CrossRef] [PubMed]
- Cavallieri, F.; Sellner, J.; Zedde, M.; Moro, E. Neurologic complications of coronavirus and other respiratory viral infections. Handb. Clin. Neurol. 2022, 189, 331–358. [Google Scholar] [CrossRef]
- Schirinzi, T.; Landi, D.; Liguori, C. COVID-19: Dealing with a potential risk factor for chronic neurological disorders. J. Neurol. 2021, 268, 1171–1178. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Rojas, M.; Beltrán, S.; Polo, F.; Camacho-Domínguez, L.; Morales, S.D.; Gershwin, M.E.; Anaya, J.M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022, 132, 102898. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. Rheumatol. 2021, 17, 731–748. [Google Scholar] [CrossRef]
- McMurray, J.C.; May, J.W.; Cunningham, M.W.; Jones, O.Y. Multisystem Inflammatory Syndrome in Children (MIS-C), a Post-viral Myocarditis and Systemic Vasculitis-A Critical Review of Its Pathogenesis and Treatment. Front. Pediatr. 2020, 8, 626182. [Google Scholar] [CrossRef]
- Bukulmez, H. Current Understanding of Multisystem Inflammatory Syndrome (MIS-C) Following COVID-19 and Its Distinction from Kawasaki Disease. Curr. Rheumatol. Rep. 2021, 23, 58. [Google Scholar] [CrossRef]
- Xu, Y.M.; Chu, Y.Q.; Wang, H. Correlation Analysis of Anti-Cardiolipin Antibody/D Dimer/C-Reactive Protein and Coronary Artery Lesions/Multiple-Organ Damage in Children with Kawasaki Disease. Front. Pediatr. 2021, 9, 704929. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Jung, J.; Kim, Y.E.; Huh, K.; Hong, J.; Kim, D.W.; Kim, M.Y.; Jung, S.Y.; Kim, J.H.; Ahn, J.G. Temporal Correlation Between Kawasaki Disease and Infectious Diseases in South Korea. JAMA Netw. Open. 2022, 5, e2147363. [Google Scholar] [CrossRef]
- Shirato, K.; Imada, Y.; Kawase, M.; Nakagaki, K.; Matsuyama, S.; Taguchi, F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014, 86, 2146–2153. [Google Scholar] [CrossRef]
- Shahbaz, F.F.; Martins, R.S.; Umair, A.; Ukrani, R.D.; Jabeen, K.; Sohail, M.R.; Khan, E. A Review of Coronaviruses Associated With Kawasaki Disease: Possible Implications for Pathogenesis of the Multisystem Inflammatory Syndrome Associated with COVID-19. Clin. Med. Insights Pediatr. 2022, 16, 11795565221075319. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Fukaya, T. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr. Opin. Infect. Dis. 2007, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Min, D.E.; Kim, D.H.; Han, M.Y.; Cha, S.H.; Yoon, K.L. High antistreptolysin O titer is associated with coronary artery lesions in patients with Kawasaki disease. Korean J. Pediatr. 2019, 62, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Malhotra, M.; Viteri-Jackson, A.; Thomas, W.; Zabriskie, J.B. Antibodies to highly conserved peptide sequence of staphylococcal and streptococcal superantigens in Kawasaki disease. Exp. Mol. Pathol. 2004, 76, 117–121. [Google Scholar] [CrossRef]
- Kornitzer, J.; Johnson, J.; Yang, M.; Pecor, K.W.; Cohen, N.; Jiang, C.; Ming, X. A Systematic Review of Characteristics Associated with COVID-19 in Children with Typical Presentation and with Multisystem Inflammatory Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 8269. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 355, 1074–1087. [Google Scholar] [CrossRef]
- Lazova, S.; Dimitrova, Y.; Hristova, D.; Tzotcheva, I.; Velikova, T. Cellular, Antibody and Cytokine Pathways in Children with Acute SARS-CoV-2 Infection and MIS-C-Can We Match the Puzzle? Antibodies 2022, 11, 25. [Google Scholar] [CrossRef]
- Son, M.B.F.; Friedman, K. COVID-19: Multisystem Inflammatory Syndrome in Children (MIS-C) Clinical Features, Evaluation, and Diagnosis UpToDate, Literature Review Current through: August 2022.|This Topic Last Updated: 28 April 2022. Available online: https://www.uptodate.com/contents/covid-19-multisystem-inflammatory-syndrome-in-children-mis-c-clinical-features-evaluation-and-diagnosis#H3359371996 (accessed on 22 September 2022).
- Ching, L.L.; Nerurkar, V.R.; Lim, E.; Shohet, R.V.; Melish, M.E.; Bratincsak, A. Elevated Levels of Pentraxin 3 Correlate With Neutrophilia and Coronary Artery Dilation During Acute Kawasaki Disease. Front. Pediatr. 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliț, L.E.; Gozar, L.; Mărginean, C.D.; Mărginean, M.O. Incomplete Refractory Kawasaki Disease in an Infant—A Case Report and a Review of the Literature. Front. Pediatr. 2018, 6, 210. [Google Scholar] [CrossRef]
- Maggio, M.C.; Corsello, G.; Prinzi, E.; Cimaz, R. Kawasaki disease in Sicily: Clinical description and markers of disease severity. Ital. J. Pediatr. 2016, 42, 92. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, T.; Nakashima, Y.; Murata, K.; Kanno, S.; Nishio, H.; Saito, M.; Tanaka, T.; Yamamura, K.; Sakai, Y.; Takada, H.; et al. Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms. PLoS ONE 2014, 9, e113054. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, Y.; Wang, H.; Gao, Z.; Wang, Y.; Fang, M.; Shi, S.; Zhang, P.; Wang, H.; Su, Y.; et al. Toll-like Receptor Signaling in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Innate Immune Responses and the Potential Application Value of Toll-Like Receptor Immunomodulators in Patients With Coronavirus Disease 2019. Front. Microbiol. 2022, 13, 948770. [Google Scholar] [CrossRef]
- Wang, W.T.; He, M.; Shimizu, C.; Croker, B.A.; Hoffman, H.M.; Tremoulet, A.H.; Burns, J.C.; Shyy, J.Y. Inflammasome Activation in Children With Kawasaki Disease and Multisystem Inflammatory Syndrome. Arter. Thromb. Vasc. Biol. 2021, 41, 2509–2511. [Google Scholar] [CrossRef] [PubMed]
- Goitein, O.; Sabag, A.; Koperstein, R.; Hamdan, A.; Di Segni, E.; Konen, E.; Matetzky, S. Role of C reactive protein in evaluating the extent of myocardial inflammation in acute myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17 (Suppl. 1), P291. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Vonck, J.; Podufaly, A. Antigenic complementarity between coxsackie virus and streptococcus in the induction of rheumatic heart disease and autoimmune myocarditis. Autoimmunity 2009, 42, 1–16. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Rethinking Molecular Mimicry in Rheumatic Heart Disease and Autoimmune Myocarditis: Laminin, Collagen IV, CAR, and B1AR as Initial Targets of Disease. Front. Pediatr. 2014, 2, 85. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fairweather, D. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J. Theor. Biol. 2015, 375, 101–123. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fairweather, D. Complexities in the relationship between infection and autoimmunity. Curr. Allergy Asthma Rep. 2014, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- García-Salido, A.; Vicente, J.C.D.C.; Hofheinz, S.B.; Ramírez, J.B.; Barrio, M.S.; Gordillo, I.L.; Yuste, A.H.; Pardellans, C.G.; Tejedor, M.C.-M.; Labarga, B.H.; et al. Severe manifestations of SARS-CoV-2 in children and adolescents: From COVID-19 pneumonia to multisystem inflammatory syndrome: A multicentre study in pediatric intensive care units in Spain. Crit. Care 2020, 24, 666. [Google Scholar] [CrossRef]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pré, P.; Mohammad, Z.; et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health 2020, 4, 669–677. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Subashchandran, V.; Yuriditsky, E.; Horowitz, J.M.; Reynolds, H.R.; Hochman, J.S.; Berger, J.S. Thrombosis in hospitalized patients with viral respiratory infections versus COVID-19. Am. Heart J. 2021, 231, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Kong, J.; Wang, W.; Wu, M.; Yao, L.; Wang, Z.; Jin, J.; Wu, D.; Yu, X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb. Res. 2020, 192, 3–8. [Google Scholar] [CrossRef]
- Borghi, M.O.; Beltagy, A.; Garrafa, E.; Curreli, D.; Cecchini, G.; Bodio, C.; Grossi, C.; Blengino, S.; Tincani, A.; Franceschini, F.; et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front. Immunol. 2020, 11, 584241. [Google Scholar] [CrossRef]
- Dabit, J.Y.; Valenzuela-Almada, M.O.; Vallejo-Ramos, S.; Duarte-García, A. Epidemiology of Antiphospholipid Syndrome in the General Population. Curr. Rheumatol. Rep. 2022, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Emmenegger, M.; Kumar, S.S.; Emmenegger, V.; Malinauskas, T.; Buettner, T.; Rose, L.; Schierack, P.; Sprinzl, M.F.; Sommer, C.J.; Lackner, K.J.; et al. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. PLoS Pathog. 2021, 17, e1010118. [Google Scholar] [CrossRef]
- Liu, T.; Dai, J.; Yang, Z.; Yu, X.; Xu, Y.; Shi, X.; Wei, D.; Tang, Z.; Xu, G.; Xu, W.; et al. Inactivated SARS-CoV-2 vaccine does not influence the profile of prothrombotic antibody nor increase the risk of thrombosis in a prospective Chinese cohort. Sci. Bull. 2021, 66, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Gkrouzman, E.; Barbhaiya, M.; Erkan, D.; Lockshin, M.D. Reality Check on Antiphospholipid Antibodies in COVID-19-Associated Coagulopathy. Arthritis Rheumatol. 2021, 73, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Huber, J.; Ziehl, A. Complementary Sets of Autoantibodies Induced by SARS-CoV-2, Adenovirus and Bacterial Antigens Cross-React with Human Blood Protein Antigens in COVID-19 Coagulopathies. Int. J. Mol. Sci. 2022, 23, 11500. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, A.; Fortunati, V.; Cherubini, F.; Bernardini, S.; Nuccetelli, M. Anti-phospholipids antibodies and immune complexes in COVID-19 patients: A putative role in disease course for anti-annexin-V antibodies. Clin. Rheumatol. 2021, 40, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Brodard, J.; Kremer Hovinga, J.A.; Fontana, P.; Studt, J.D.; Gruel, Y.; Greinacher, A. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J. Thromb. Haemost. 2021, 19, 1294–1298. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Henry, B.M.; Lippi, G. The complicated relationships of heparin-induced thrombocytopenia and platelet factor 4 antibodies with COVID-19. Int. J. Lab. Hematol. 2021, 43, 547–558. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Potential association between bacterial infections and ischemic stroke based on fifty case-control studies: A systematic review and meta-analysis. New Microbes New Infect. 2022, 47, 100980. [Google Scholar] [CrossRef]
- Budzyński, J.; Wiśniewska, J.; Ciecierski, M.; Kędzia, A. Association between bacterial infection and peripheral vascular disease: A review. Int. J. Angiol. 2016, 25, 3–13. [Google Scholar]
- Lucchese, G.; Flöel, A.; Stahl, B. Cross-reactivity as a mechanism linking infections to stroke. Front. Neurol. 2019, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Krause, I.; Fridkin, M.; Keller, N.; Kopolovic, J.; Goldberg, I.; Tobar, A.; Shoenfeld, Y. Bacterial induction of autoantibodies to β2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J. Clin. Investig. 2002, 109, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Sène, D.; Piette, J.C.; Cacoub, P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmun. Rev. 2008, 7, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pinto, C.; García-Carrasco, M.; Cervera, R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant). Curr. Rheumatol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, R.M.; Cham, L.B.; Gris-Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cémalovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 mediate distinct immunopathological and antiviral plasmacytoid dendritic cell responses to SARS-CoV-2 infection. EMBO J. 2022, 41, e109622. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Umar, S.; Palasiewicz, K.; Meyer, A.; Kumar, P.; Prabhakar, B.S.; Volin, M.V.; Rahat, R.; Al-Awqati, M.; Chang, H.J.; Zomorrodi, R.K.; et al. Inhibition of IRAK4 dysregulates SARS-CoV-2 spike protein-induced macrophage inflammatory and glycolytic reprogramming. Cell. Mol. Life Sci. 2022, 79, 301. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. SARS-CoV-2 takes its Toll. Nat. Immunol. 2021, 22, 801–802. [Google Scholar] [CrossRef]
- Cinquegrani, G.; Spigoni, V.; Iannozzi, N.T.; Parello, V.; Bonadonna, R.C.; Dei Cas, A. SARS-CoV-2 Spike protein is not pro-inflammatory in human primary macrophages: Endotoxin contamination and lack of protein glycosylation as possible confounders. Cell Biol. Toxicol. 2022, 38, 667–678. [Google Scholar] [CrossRef]
- Ouyang, W.; Xie, T.; Fang, H.; Gao, C.; Stantchev, T.; Clouse, K.A.; Yuan, K.; Ju, T.; Frucht, D.M. Variable Induction of Pro-Inflammatory Cytokines by Commercial SARS CoV-2 Spike Protein Reagents: Potential Impacts of LPS on In Vitro Modeling and Pathogenic Mechanisms In Vivo. Int. J. Mol. Sci. 2021, 22, 7540. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. An Overview of Recent Insights into the Response of TLR to SARS-CoV-2 Infection and the Potential of TLR Agonists as SARS-CoV-2 Vaccine Adjuvants. Viruses 2021, 13, 2302. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021, 31, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Strazzabosco, G.; Fernandez, M.; Caccuri, F.; Caruso, A.; Rizzo, R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms 2021, 9, 1820. [Google Scholar] [CrossRef]
- Tripathi, U.; Nchioua, R.; Prata, L.G.P.L.; Zhu, Y.; Gerdes, E.O.W.; Giorgadze, N.; Pirtskhalava, T.; Parker, E.; Xue, A.; Espindola-Netto, J.M.; et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 2021, 13, 21838–21854. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhuang, M.W.; Deng, J.; Zheng, Y.; Zhang, J.; Nan, M.L.; Zhang, X.J.; Gao, C.; Wang, P.H. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J. Med. Virol. 2021, 93, 5376–5389. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Nguyen, H.O.; Sozio, F.; Schioppa, T.; Gaudenzi, C.; Laffranchi, M.; Scapini, P.; Passari, M.; Barbazza, I.; Tiberio, L.; et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight 2021, 6, e150542. [Google Scholar] [CrossRef]
- Severa, M.; Diotti, R.A.; Etna, M.P.; Rizzo, F.; Fiore, S.; Ricci, D.; Iannetta, M.; Sinigaglia, A.; Lodi, A.; Mancini, N.; et al. Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021, 17, e1009878. [Google Scholar] [CrossRef]
- Shah, A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front. Immunol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Sakai, K.; Gotoh, S.; Miorin, L.; de Jesus, P.D.; et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef] [PubMed]
- Thorne, L.G.; Reuschl, A.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021, 40, e107826. [Google Scholar] [CrossRef]
- Kouwaki, T.; Nishimura, T.; Wang, G.; Oshiumi, H. RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Front. Immunol. 2021, 12, 700926. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.G.; Chauveau, L.; Hertzog, J.; Bridgeman, A.; Fowler, G.; Moonen, J.P.; Dupont, M.; Russell, R.A.; Noerenberg, M.; Rehwinkel, J. The RNA sensor MDA5 detects SARS-CoV-2 infection. Sci. Rep. 2021, 11, 13638. [Google Scholar] [CrossRef]
- Campbell, G.R.; To, R.K.; Hanna, J.; Spector, S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 2021, 24, 102295. [Google Scholar] [CrossRef]
- Kucia, M.; Ratajczak, J.; Bujko, K.; Adamiak, M.; Ciechanowicz, A.; Chumak, V.; Brzezniakiewicz-Janus, K.; Ratajczak, M.Z. An evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia 2021, 35, 3026–3029. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Lepiarz-Raba, I.; Al-Hindawi, A.A. Induction of Exaggerated Cytokine Production in Human Peripheral Blood Mononuclear Cells by a Recombinant SARS-CoV-2 Spike Glycoprotein S1 and Its Inhibition by Dexamethasone. Inflammation 2021, 44, 1865–1877. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawabata, K.; Koizumi, N.; Sakurai, F.; Nakashima, K.; Sakurai, H.; Sasaki, T.; Okada, N.; Yamanishi, K.; Mizuguchi, H. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 2007, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Iacobelli-Martinez, M.; Nemerow, G.R. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007, 81, 1305–1312. [Google Scholar] [CrossRef]
- Fejer, G.; Freudenberg, M.; Greber, U.F.; Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. (Bp) 2011, 1, 279–288. [Google Scholar] [CrossRef]
- Lindsay, R.W.; Darrah, P.A.; Quinn, K.M.; Wille-Reece, U.; Mattei, L.M.; Iwasaki, A.; Kasturi, S.P.; Pulendran, B.; Gall, J.G.; Spies, A.G.; et al. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 2010, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.; Ocheretina, O.; Murphy, M.; Falck-Pedersen, E. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J. Virol. 2009, 83, 4081–4091. [Google Scholar] [CrossRef] [PubMed]
- Anchim, A.; Raddi, N.; Zig, L.; Perrieau, P.; Le Goffic, R.; Ryffel, B.; Benihoud, K. Humoral Responses Elicited by Adenovirus Displaying Epitopes Are Induced Independently of the Infection Process and Shaped by the Toll-Like Receptor/MyD88 Pathway. Front. Immunol. 2018, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Hosseinabadi, Z.; Rezazadeh Zarandi, E.; Mirabzadeh, M.; Amiri, A.; Abbasifard, M. mRNA expression of toll-like receptors 3, 7, 8, and 9 in the nasopharyngeal epithelial cells of coronavirus disease 2019 patients. BMC Infect. Dis. 2022, 22, 448. [Google Scholar] [CrossRef]
- Fallerini, C.; Daga, S.; Mantovani, S.; Benetti, E.; Picchiotti, N.; Francisci, D.; Paciosi, F.; Schiaroli, E.; Baldassarri, M.; Fava, F.; et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: Findings from a nested case-control study. eLife 2021, 10, e67569. [Google Scholar] [CrossRef]
- Brauns, E.; Azouz, A.; Grimaldi, D.; Xiao, H.; Thomas, S.; Nguyen, M.; Olislagers, V.; Vu Duc, I.; Orte Cano, C.; Del Marmol, V.; et al. Functional reprogramming of monocytes in patients with acute and convalescent severe COVID-19. JCI Insight 2022, 7, e154183. [Google Scholar] [CrossRef]
- Yang, C.A.; Huang, Y.L.; Chiang, B.L. Innate immune response analysis in COVID-19 and kawasaki disease reveals MIS-C predictors. J. Formos. Med. Assoc. 2022, 121, 623–632. [Google Scholar] [CrossRef]
- Parackova, Z.; Zentsova, I.; Bloomfield, M.; Vrabcova, P.; Smetanova, J.; Klocperk, A.; Mesežnikov, G.; Casas Mendez, L.F.; Vymazal, T.; Sediva, A. Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils’ but Impaired Monocytes’ and Dendritic Cells’ Responsiveness. Cells 2020, 9, 2206. [Google Scholar] [CrossRef]
- Dutta, D.; Liu, J.; Xiong, H. NLRP3 inflammasome activation and SARS-CoV-2-mediated hyperinflammation, cytokine storm and neurological syndromes. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 138–160. [Google Scholar]
- Aymonnier, K.; Ng, J.; Fredenburgh, L.E.; Zambrano-Vera, K.; Münzer, P.; Gutch, S.; Fukui, S.; Desjardins, M.; Subramaniam, M.; Baron, R.M.; et al. Inflammasome activation in neutrophils of patients with severe COVID-19. Blood Adv. 2022, 6, 2001–2013. [Google Scholar] [CrossRef]
- Henriques-Pons, A.; Beghini, D.G.; Silva, V.D.S.; Iwao Horita, S.; da Silva, F.A.B. Pulmonary Mesenchymal Stem Cells in Mild Cases of COVID-19 Are Dedicated to Proliferation; In Severe Cases, They Control Inflammation, Make Cell Dispersion, and Tissue Regeneration. Front. Immunol. 2022, 12, 780900. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.M.; Lee, S.-G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Paik, S.; et al. COVID-19 Patients Upregulate Toll-like Receptor 4-mediated Inflammatory Signaling That Mimics Bacterial Sepsis. J. Korean Med. Sci. 2020, 35, e343. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R.H.; Elesawy, B.H.; Ali, T.M.; Abdallah, M.; Assal, H.H.; Ahmed, A.E.; Ahmed, O.M. Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19. Vaccines 2022, 10, 1106. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, Y.; Wang, K.; Zhang, X.; Chen, W.; Sheng, J.; Qiu, Y.; Diao, H.; Li, L. Inflammation and Antiviral Immune Response Associated With Severe Progression of COVID-19. Front. Immunol. 2021, 12, 631226. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Wang, F.; Liu, R.; Yang, J.; Chen, B. New insights into genetic characteristics between multiple myeloma and COVID-19: An integrative bioinformatics analysis of gene expression omnibus microarray and the cancer genome atlas data. Int. J. Lab. Hematol. 2021, 43, 1325–1333. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef]
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J Allergy Clin Immunol. 2021, 148, 732–738.e1. [Google Scholar] [CrossRef]
- Abuhammour, W.; Yavuz, L.; Jain, R.; Abu Hammour, K.; Al-Hammouri, G.F.; El Naofal, M.; Halabi, N.; Yaslam, S.; Ramaswamy, S.; Taylor, A.; et al. Genetic and Clinical Characteristics of Patients in the Middle East With Multisystem Inflammatory Syndrome in Children. JAMA Netw. Open. 2022, 5, e2214985. [Google Scholar] [CrossRef]
- Schulert, G.S.; Blum, S.A.; Cron, R.Q. Host genetics of pediatric SARS-CoV-2 COVID-19 and multisystem inflammatory syndrome in children. Curr. Opin. Pediatr. 2021, 33, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.R.; Kuo, H.C.; Lee, Y.J.; Chi, H.; Li, S.C.; Lee, H.C.; Yang, K.D. Phenotype, Susceptibility, Autoimmunity, and Immunotherapy Between Kawasaki Disease and Coronavirus Disease-19 Associated Multisystem Inflammatory Syndrome in Children. Front. Immunol. 2021, 12, 632890. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Li, S.-C.; Huang, L.-H.; Chen, P.-C.; Lin, Y.-Y.; Lin, C.-C.; Kuo, H.-C. Identifying genetic hypomethylation and upregulation of Toll-like receptors in Kawasaki disease. Oncotarget 2017, 8, 11249–11258. [Google Scholar] [CrossRef]
- Lin, I.C.; Kuo, H.C.; Lin, Y.J.; Wang, F.S.; Wang, L.; Huang, S.C.; Chien, S.J.; Huang, C.F.; Wang, C.L.; Yu, H.R.; et al. Augmented TLR2 expression on monocytes in both human Kawasaki disease and a mouse model of coronary arteritis. PLoS ONE 2012, 7, e38635. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, N.S. Association of Toll-like receptor 2-positive monocytes with coronary artery lesions and treatment nonresponse in Kawasaki disease. Korean J. Pediatr. 2017, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.H.; Amin, R.; Alyasin, S.; Kashef, S.; Karimi, M.H.; Babaei, M.; Younesi, V. Down-regulation of TLR2, 3, 9 and Signaling Mediators, MyD88 and TRIF, Gene Transcript Levels in Patients with Kawasaki Disease Treated with IVIG. Iran. J. Allergy Asthma Immunol. 2015, 14, 188–197. [Google Scholar]
- Srivastava, P.; Bamba, C.; Pilania, R.K.; Kumari, A.; Kumrah, R.; Sil, A.; Singh, S. Exploration of Potential Biomarker Genes and Pathways in Kawasaki Disease: An Integrated in-Silico Approach. Front. Genet. 2022, 13, 849834. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Van Der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ma, W.; Lin, X.; Huang, S.; Yu, M. Identification of Key Genes and Underlying Mechanisms in Acute Kawasaki Disease Based on Bioinformatics Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930547. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Cao, Y.; Lin, Y.I.; Zhu, H.; Fu, Y.; Chen, X.; Zhang, Q. Association between toll-like receptor 6 expression and auxiliary T cells in the peripheral blood of pediatric patients with allergic purpura. Exp. Ther. Med. 2015, 10, 1536–1540. [Google Scholar] [CrossRef]
- Cai., Y.; Hu, W. Identifying differentially expressed genes and miRNAs in Kawasaki disease by bioinformatics analysis. Sci. Rep. 2022, 12, 21879. [Google Scholar] [CrossRef]
- Giordani, L.; Quaranta, M.G.; Marchesi, A.; Straface, E.; Pietraforte, D.; Villani, A.; Malorni, W.; Del Principe, D.; Viora, M. Increased frequency of immunoglobulin (Ig)A-secreting cells following Toll-like receptor (TLR)-9 engagement in patients with Kawasaki disease. Clin. Exp. Immunol. 2011, 163, 346–353. [Google Scholar] [CrossRef]
- Onoyama, S.; Ihara, K.; Yamaguchi, Y.; Ikeda, K.; Yamaguchi, K.; Yamamura, K.; Hoshina, T.; Mizuno, Y.; Hara, T. Genetic susceptibility to Kawasaki disease: Analysis of pattern recognition receptor genes. Hum. Immunol. 2012, 73, 654–660. [Google Scholar] [CrossRef]
- Ji, M.L.; Dong, J.Y.; Xu, Y.; Pan, Y.T.; Fan, Z.D.; Yu, H.G. Inositol-Triphosphate 3-Kinase C and DNA Methylation Involvement in NLRP3 Inflammasome Activation in Kawasaki Disease. Indian J. Pediatr. 2022, 90, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H.; et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Latz, E. Deciphering How NLRP3 Incites the Stromal Response in Kawasaki Vasculitis. Circ. Res. 2021, 129, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Kogan, E.; Berezovskiy, Y.; Blagova, O.; Kukleva, A.; Semyonova, L.; Gretsov, E.; Ergeshov, A. Morphologically, immunohistochemically and PCR proven lymphocytic viral peri-, endo-, myocarditis in patients with fatal COVID-19. Diagn. Pathol. 2022, 17, 31. [Google Scholar] [CrossRef]
- Heidecker, B.; Kittleson, M.M.; Kasper, E.K.; Wittstein, I.S.; Champion, H.C.; Russell, S.D.; Hruban, R.H.; Rodriguez, E.R.; Baughman, K.L.; Hare, J.M. Transcriptomic Biomarkers for the Accurate Diagnosis of Myocarditis. Circulation 2011, 123, 1174–1184. [Google Scholar] [CrossRef]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef] [PubMed]
- Gorbea, C.; Makar, K.A.; Pauschinger, M.; Pratt, G.; Bersola, J.L.; Varela, J.; David, R.M.; Banks, L.; Huang, C.H.; Li, H.; et al. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J. Biol. Chem. 2010, 285, 23208–23223. [Google Scholar] [CrossRef]
- Triantafilou, K.; Orthopoulos, G.; Vakakis, E.; Ahmed, M.A.E.; Golenbock, D.T.; Lepper, P.M.; Triantafilou, M. Human cardiac inflammatory responses triggered by coxsackie B Viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell. Microbiol. 2005, 7, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cox, C.J.; Alvarez, K.M.; Cunningham, M.W. Cutting edge: Cardiac myosin activates innate immune responses through TLRs. J. Immunol. 2009, 183, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Kim, T.J.; Moon, H.J.; Kim, Y.J.; Yoon, H.K.; Seong, S.Y. Cardiolipin activates antigen-presenting cells via TLR2-PI3K-PKN1-AKT/p38-NF-kB signaling to prime antigen-specific naïve T cells in mice. Eur. J. Immunol. 2018, 48, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Maeda, A.; Lee, J.S.; Mohammadyani, D.; Dar, H.H.; Jiang, J.F.; St Croix, C.M.; Watkins, S.; Tyurin, V.A.; Tyurina, Y.Y.; et al. Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity. Sci. Signal. 2015, 8, ra95. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.E.; Wenzel, T.J.; Simtchouk, S.; Greuel, B.K.; Gibon, J.; Klegeris, A. Extracellular Cardiolipin Modulates Select Immune Functions of Astrocytes in Toll-Like Receptor (TLR) 4-Dependent Manner. Mediat. Inflamm. 2022, 2022, 9946439. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Ranger, A.L.; McRae, S.A.; Klegeris, A. Extracellular cardiolipin modulates microglial phagocytosis and cytokine secretion in a toll-like receptor (TLR) 4-dependent manner. J. Neuroimmunol. 2021, 353, 577496. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; He, K.; Xu, M.; Gong, J.P. Cardiolipin inhibitor ameliorates the non-alcoholic steatohepatitis through suppressing NLRP3 inflammasome activation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8158–8167. [Google Scholar] [CrossRef]
- Pizzuto, M.; Pelegrin, P.; Ruysschaert, J.M. Lipid-protein interactions regulating the canonical and the non-canonical NLRP3 inflammasome. Prog. Lipid Res. 2022, 87, 101182. [Google Scholar] [CrossRef]
- Deguchi, H.; Fernandez, J.A.; Hackeng, T.M.; Banka, C.L.; Griffin, J.H. Cardiolipin is a normal component of human plasma lipoproteins. Proc. Natl. Acad. Sci. USA 2000, 97, 1743–1748. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- López-Lara, I.M.; Geiger, O. Bacterial lipid diversity. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1287–1299. [Google Scholar] [CrossRef]
- Cole, R.; Proulx, P. Further studies on the cardiolipin phosphodiesterase of Escherichia coli. Can. J. Biochem. 1977, 55, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; Linskens, E.A.; Benoit, D.; Peperstraete, H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J. Thromb. Haemost. 2020, 18, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, M.; Henes, J.; Saur, S. The Role of Antiphospholipid Antibodies in COVID-19. Curr. Rheumatol. Rep. 2021, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Foret, T.; Dufrost, V.; Salomon Du Mont, L.; Costa, P.; Lefevre, B.; Lacolley, P.; Regnault, V.; Zuily, S.; Wahl, D. Systematic Review of Antiphospholipid Antibodies in COVID-19 Patients: Culprits or Bystanders? Curr. Rheumatol. Rep. 2021, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Kelchtermans, H.; Pelkmans, L.; de Laat, B.; Devreese, K.M. IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: A critical review of their association with thrombosis. J. Thromb. Haemost. 2016, 14, 1530–1548. [Google Scholar] [CrossRef]
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Musiał, J.; Wahl, D.; de Laat, B.; Devreese, K.M.J. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J. Thromb. Haemost. 2018, 16, 2016–2023. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Thomas, R.R. Anticardiolipin (aCL) in sera from periodontitis subjects activate Toll-like receptor 4 (TLR4). PLoS ONE 2018, 13, e0203494. [Google Scholar] [CrossRef]
- Naranjo, L.; Stojanovich, L.; Djokovic, A.; Andreoli, L.; Tincani, A.; Maślińska, M.; Sciascia, S.; Infantino, M.; Garcinuño, S.; Kostyra-Grabczak, K.; et al. Circulating immune-complexes of IgG/IgM bound to B2-glycoprotein-I associated with complement consumption and thrombocytopenia in antiphospholipid syndrome. Front. Immunol. 2022, 13, 957201. [Google Scholar] [CrossRef]
- Martínez-Flores, J.A.; Serrano, M.; Pérez, D.; Cámara, A.G.; Lora, D.; Morillas, L.; Ayala, R.; Paz-Artal, E.; Morales, J.M.; Serrano, A. Circulating Immune Complexes of IgA Bound to Beta 2 Glycoprotein are Strongly Associated with the Occurrence of Acute Thrombotic Events. J. Atheroscler. Thromb. 2016, 23, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Cheng, C.Y.; Yang, Y.; Denas, G.; Pengo, V. Prevalence of aPhosphatidylserine/prothrombin antibodies and association with antiphospholipid antibody profiles in patients with antiphospholipid syndrome: A systematic review and meta-analysis. Thromb. Res. 2022, 214, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, A.; Chayoua, W.; de Laat, B.; Moore, G.W.; Musiał, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Added value of antiphosphatidylserine/prothrombin antibodies in the workup of thrombotic antiphospholipid syndrome: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2022, 20, 2136–2150. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.J.; Fickentscher, C.; Boehlen, F.; Kruithof, E.K.; De Moerloose, P. NF-kB is activated from endosomal compartments in antiphospholipid antibodies-treated human monocytes. J. Thromb. Haemost. 2014, 12, 779–791. [Google Scholar] [CrossRef]
- Gladigau, G.; Haselmayer, P.; Scharrer, I.; Munder, M.; Prinz, N.; Lackner, K.; Schild, H.; Stein, P.; Radsak, M.P. A role for Toll-like receptor mediated signals in neutrophils in the pathogenesis of the anti-phospholipid syndrome. PLoS ONE 2012, 7, e42176. [Google Scholar] [CrossRef]
- Satta, N.; Kruithof, E.K.; Fickentscher, C.; Dunoyer-Geindre, S.; Boehlen, F.; Reber, G.; Burger, D.; De Moerloose, P. Toll-like receptor 2 mediates the activation of human monocytes and endothelial cells by antiphospholipid antibodies. Blood 2011, 117, 5523–5531. [Google Scholar] [CrossRef]
- Mulla, M.J.; Brosens, J.J.; Chamley, L.W.; Giles, I.; Pericleous, C.; Rahman, A.; Joyce, S.K.; Panda, B.; Paidas, M.J.; Abrahams, V.M. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am. J. Reprod. Immunol. 2009, 62, 96–111. [Google Scholar] [CrossRef]
- Tong, M.; Kayani, T.; Jones, D.M.; Salmon, J.E.; Whirledge, S.; Chamley, L.W.; Abrahams, V.M. Antiphospholipid Antibodies Increase Endometrial Stromal Cell Decidualization, Senescence, and Inflammation via Toll-like Receptor 4, Reactive Oxygen Species, and p38 MAPK Signaling. Arthritis Rheumatol. 2022, 74, 1001–1012. [Google Scholar] [CrossRef]
- Hurst, J.; Prinz, N.; Lorenz, M.; Bauer, S.; Chapman, J.; Lackner, K.J.; von Landenberg, P. TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology 2009, 214, 683–691. [Google Scholar] [CrossRef]
- Prinz, N.; Clemens, N.; Strand, D.; Pütz, I.; Lorenz, M.; Daiber, A.; Stein, P.; Degreif, A.; Radsak, M.; Schild, H.; et al. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood 2011, 118, 2322–2332. [Google Scholar] [CrossRef]
- Döring, Y.; Hurst, J.; Lorenz, M.; Prinz, N.; Clemens, N.; Drechsler, M.D.; Bauer, S.; Chapman, J.; Shoenfeld, Y.; Blank, M. Human antiphospholipid antibodies induce TNFalpha in monocytes via Toll-like receptor 8. Immunobiology 2010, 215, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Gysler, S.M.; Mulla, M.J.; Guerra, M.; Brosens, J.J.; Salmon, J.E.; Chamley, L.W.; Abrahams, V.M. Antiphospholipid antibody-induced miR-146a-3p drives trophoblast interleukin-8 secretion through activation of Toll-like receptor 8. Mol. Hum. Reprod. 2016, 22, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.J.; Pasternak, M.C.; Salmon, J.E.; Chamley, L.W.; Abrahams, V.M. Role of NOD2 in antiphospholipid antibody-induced and bacterial MDP amplification of trophoblast inflammation. J. Autoimmun. 2019, 98, 103–112. [Google Scholar] [CrossRef]
- Mulla, M.J.; Salmon, J.E.; Chamley, L.W.; Brosens, J.J.; Boeras, C.M.; Kavathas, P.B.; Abrahams, V.M. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1β production by human first trimester trophoblast. PLoS ONE 2013, 8, e65237. [Google Scholar] [CrossRef]
- Raschi, E.; Chighizola, C.B.; Grossi, C.; Ronda, N.; Gatti, R.; Meroni, P.L.; Borghi, M.O. β2-glycoprotein I, lipopolysaccharide and endothelial TLR4: Three players in the two hit theory for anti-phospholipid-mediated thrombosis. J. Autoimmun. 2014, 55, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.-E.; Gaillard, F.; Daridon, C.; Shoenfeld, Y.; Jamin, C.; Youinou, P. TLR2 Is One of the Endothelial Receptors for β2-Glycoprotein I. J. Immunol. 2010, 185, 1550–1557. [Google Scholar] [CrossRef]
- Benhamou, Y.; Bellien, J.; Armengol, G.; Brakenhielm, E.; Adriouch, S.; Iacob, M.; Remy-Jouet, I.; Le Cam-Duchez, V.; Monteil, C.; Renet, S.; et al. Role of Toll-like Receptors 2 and 4 in mediating endothelial dysfunction and arterial remodeling in primary arterial antiphospholipid syndrome. Arthritis Rheumatol. 2014, 66, 3210–3220. [Google Scholar] [CrossRef]
- Meroni, P.L.; Raschi, E.; Testoni, C.; Parisio, A.; Borghi, M.O. Innate immunity in the antiphospholipid syndrome: Role of toll-like receptors in endothelial cell activation by antiphospholipid antibodies. Autoimmun. Rev. 2004, 3, 510–515. [Google Scholar] [CrossRef]
- Borghi, M.O.; Raschi, E.; Grossi, C.; Chighizola, C.B.; Meroni, P.L. Toll-like receptor 4 and β2 glycoprotein I interaction on endothelial cells. Lupus 2014, 23, 1302–1304. [Google Scholar] [CrossRef]
- Raschi, E.; Borghi, M.O.; Grossi, C.; Broggini, V.; Pierangeli, S.; Meroni, P.L. Toll-like receptors: Another player in the pathogenesis of the anti-phospholipid syndrome. Lupus 2008, 17, 937–942. [Google Scholar] [CrossRef]
- Sorice, M.; Longo, A.; Capozzi, A.; Garofalo, T.; Misasi, R.; Alessandri, C.; Conti, F.; Buttari, B.; Riganò, R.; Ortona, E.; et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007, 56, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sheng, L.; Wang, H.; Xie, H.; Mu, Y.; Wang, T.; Yan, J. Anti-β2GPI/β2GPI stimulates activation of THP-1 cells through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb. Res. 2013, 132, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kong, X.; Xie, Y.; He, C.; Wang, T.; Zhou, H. Role of TLR-4 in anti-β2-glycoprotein I-induced activation of peritoneal macrophages and vascular endothelial cells in mice. Mol. Med. Rep. 2019, 19, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Petrlova, J.; Petruk, G.; Huber, R.G.; McBurnie, E.W.; van der Plas, M.J.A.; Bond, P.J.; Puthia, M.; Schmidtchen, A. Thrombin-derived C-terminal fragments aggregate and scavenge bacteria and their proinflammatory products. J. Biol. Chem. 2020, 295, 3417–3430. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, R.; Bulder, I.; de Groot, E.R.; Aarden, L.A.; Finkelman, M.A. Potentiation of Toll-like receptor-induced cytokine production by (1-->3)-beta-D-glucans: Implications for the monocyte activation test. J. Endotoxin. Res. 2007, 13, 140–149. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Puthia, M.; Tanner, L.; Petruk, G.; Schmidtchen, A. Experimental Model of Pulmonary Inflammation Induced by SARS-CoV-2 Spike Protein and Endotoxin. ACS Pharmacol. Transl. Sci. 2022, 5, 141–148. [Google Scholar] [CrossRef]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Stromdahl, A.C.; Cerps, S.; Uller, L.; Kjellstrom, S.; Bond, P.J.; Schmidtchen, A.A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2021, 12, 916–932. [Google Scholar] [CrossRef]
- Tumpara, S.; Gründing, A.R.; Sivaraman, K.; Wrenger, S.; Olejnicka, B.; Welte, T.; Wurm, M.J.; Pino, P.; Kiseljak, D.; Wurm, F.M.; et al. Boosted Pro-Inflammatory Activity in Human PBMCs by Lipopolysaccharide and SARS-CoV-2 Spike Protein Is Regulated by α-1 Antitrypsin. Int. J. Mol. Sci. 2021, 22, 7941. [Google Scholar] [CrossRef]
- Loke, M.F.; Yadav, I.; Lim, T.K.; van der Maarel, J.R.C.; Sham, L.T.; Chow, V.T. SARS-CoV-2 Spike Protein and Mouse Coronavirus Inhibit Biofilm Formation by Streptococcus pneumoniae and Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 3291. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Subedi, K.; Liu, T.; Khalasawi, N.; Pretto-Kernahan, C.D.; Wotring, J.W.; Wang, J.; Yin, C.; Jiang, A.; Fu, C.; et al. Surface translocation of ACE2 and TMPRSS2 upon TLR4/7/8 activation is required for SARS-CoV-2 infection in circulating monocytes. Cell Discov. 2022, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Gómez-Mejia, A.; Schweizer, T.A.; Huemer, M.; Chang, C.C.; Acevedo, C.; Bergada-Pijuan, J.; Vulin, C.; Hofmaenner, D.A.; Scheier, T.C.; et al. Hyperinflammatory environment drives dysfunctional myeloid cell effector response to bacterial challenge in COVID-19. PLoS Pathog. 2022, 18, e1010176. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, I.; Giroux, N.; Olson, L.; Morrison, S.A.; Llanga, T.; Akinade, T.O.; Zhu, Y.; Zhong, Y.; Bose, S.; Arvai, S.; et al. DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID-19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials 2022, 283, 121393. [Google Scholar] [CrossRef]
- Bär, J.; Boumasmoud, M.; Mairpady Shambat, S.; Vulin, C.; Huemer, M.; Schweizer, T.A.; Gómez-Mejia, A.; Eberhard, N.; Achermann, Y.; Zingg, P.O.; et al. Quantification of within-patient Staphylococcus aureus phenotypic heterogeneity as a proxy for the presence of persisters across clinical presentations. Clin. Microbiol Infect. 2022, 28, 1022.e1–1022.e7. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef]
- Larionova, R.; Byvaltsev, K.; Kravtsova, O.; Takha, E.; Petrov, S.; Kazarian, G.; Valeeva, A.; Shuralev, E.; Mukminov, M.; Renaudineau, Y.; et al. SARS-Cov2 acute and post-active infection in the context of autoimmune and chronic inflammatory diseases. J. Transl. Autoimmun. 2022, 5, 100154. [Google Scholar] [CrossRef]
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M.; et al. Pathological Evidence for SARS-CoV-2 as a Cause of Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 314–325. [Google Scholar] [CrossRef]
- Kumar, N.P.; Venkataraman, A.; Hanna, L.E.; Putlibai, S.; Karthick, M.; Rajamanikam, A.; Sadasivam, K.; Sundaram, B.; Babu, S. Systemic Inflammation and Microbial Translocation Are Characteristic Features of SARS-CoV-2-Related Multisystem Inflammatory Syndrome in Children. Open Forum Infect. Dis. 2021, 8, ofab279. [Google Scholar] [CrossRef]
- Avramovic, M.Z.; Emersic, N.; Kopitar, A.N.; Korva, M.; Avsic-Zupanc, T.; Ihan, A.; Avcin, T. POS0072 comprehensive immune profiling of 20 children with multisystem inflammatory syndrome. Ann. Rheum. Dis. 2021, 80, 242–243. [Google Scholar] [CrossRef]
- Ebrahim, F.A.; Moturi, G.; Mongare, N.; Shah, R. Unusual Presentation of Multisystemic Inflammatory Syndrome. Case Rep. Med. 2022, 2022, 8442855. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.R.; Tozman, E.C. Antineutrophil cytoplasmic antibodies: Major autoantigens, pathophysiology, and disease associations. Semin. Arthritis Rheum. 1995, 25, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Batu, E.D.; Sener, S.; Ozen, S. COVID-19 associated pediatric vasculitis: A systematic review and detailed analysis of the pathogenesis. Semin. Arthritis Rheum. 2022, 55, 152047. [Google Scholar] [CrossRef]
- Bryant, M.C.; Spencer, L.T.; Yalcindag, A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr. Rheumatol. Online J. 2022, 20, 65. [Google Scholar] [CrossRef]
- Pavoni, V.; Gianesello, L.; Horton, A. Antiphospholipid antibodies in critically ill COVID-19 patients with thromboembolism: Cause of disease or epiphenomenon? J. Thromb. Thrombolysis 2021, 52, 542–552. [Google Scholar] [CrossRef]
- Andreoli, L.; Fredi, M.; Nalli, C.; Piantoni, S.; Reggia, R.; Dall’Ara, F.; Franceschini, F.; Tincani, A. Clinical significance of IgA anti-cardiolipin and IgA anti-β2glycoprotein I antibodies. Curr. Rheumatol. Rep. 2013, 15, 343. [Google Scholar] [CrossRef]
- Bnina, A.B.; Dhia, R.B.; Gnaba, S.; Annabi, A.; Chouchane, S.; Naija, W.; Said, H.; Oueslati, A.; Bouatay, A. Assessment of antiphospholipid antibodies profiles based on severity of COVID-19 pneumonia. Pan Afr. Med. J. 2022, 42, 110. [Google Scholar] [CrossRef]
- Rauch, J.; Dieudé, M.; Subang, R.; Levine, J.S. The dual role of innate immunity in the antiphospholipid syndrome. Lupus 2010, 19, 347–353. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Cifù, A.; Domenis, R.; Pistis, C.; Curcio, F.; Fabris, M. Anti-β2-glycoprotein I and anti-phosphatidylserine/prothrombin antibodies exert similar pro-thrombotic effects in peripheral blood monocytes and endothelial cells. Auto Immun. Highlights 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Laplante, P.; Amireault, P.; Subang, R.; Dieudé, M.; Levine, J.S.; Rauch, J. Interaction of β2-glycoprotein I with lipopolysaccharide leads to Toll-like receptor 4 (TLR4)-dependent activation of macrophages. J. Biol. Chem. 2011, 286, 42494–42503. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, J.; Toppila, I.; Härma, M.; Palviainen, T.; Salminen, A.; Sandholm, N.; Pietiäinen, M.; Kopra, E.; de Barros, J.P.; Lassenius, M.I.; et al. Genetic Profile of Endotoxemia Reveals an Association With Thromboembolism and Stroke. J. Am. Heart Assoc. 2021, 10, e022482. [Google Scholar] [CrossRef]
- Nilsson, M.; Wasylik, S.; Morgelin, M.; Olin, A.I.; Meijers, J.; Derksen, R.H.; De Groot, P.G.; Herwald, H. The antibacterial activity of peptides derived from human β2 glycoprotein I is inhibited by protein H and M1 protein from Streptococcus pyogenes. Mol. Microbiol. 2008, 67, 482–492. [Google Scholar] [CrossRef]
- Agar, C.; de Groot, P.G.; Mörgelin, M.; Monk, S.D.; van Os, G.; Levels, J.H.; de Laat, B.; Urbanus, R.T.; Herwald, H.; van der Poll, T.; et al. β2-glycoprotein I: A novel component of innate immunity. Blood 2011, 117, 6939–6947. [Google Scholar] [CrossRef]
- Ağar, Ç.; de Groot, P.G.; Marquart, J.A.; Meijers, J.C. Evolutionary conservation of the lipopolysaccharide binding site of β₂-glycoprotein I. Thromb. Haemost. 2011, 106, 1069–1075. [Google Scholar] [CrossRef]
- Kivity, S.; Agmon-Levin, N.; Blank, M.; Shoenfeld, Y. Infections and autoimmunity—Friends or foes? Trends Immunol. 2009, 30, 409–414. [Google Scholar] [CrossRef]
- McGonagle, D.; De Marco, G.; Bridgewood, C. Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection. J. Autoimmun. 2021, 121, 102662. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Churchill, E.; Oliverio, S. T Cell Receptor Sequences Amplified during Severe COVID-19 and Multisystem Inflammatory Syndrome in Children Mimic SARS-CoV-2, Its Bacterial Co-Infections and Host Autoantigens. Int. J. Mol. Sci. 2023, 24, 1335. [Google Scholar] [CrossRef]
- Petrušić, V.; Todorović, N.; Živković, I.; Dimitrijević, R.; Muhandes, L.; Rajnpreht, I.; Dimitrijević, L. Autoantibody response and pregnancy-related pathology induced by combined LPS and tetanus toxoid hyperimmunization in BALB/c and C57BL/6 mice. Autoimmunity 2015, 48, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, L.; Živković, I.; Stojanović, M.; Petrušić, V.; Živančević-Simonović, S. Vaccine model of antiphospholipid syndrome induced by tetanus vaccine. Lupus 2012, 21, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immun. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front. Immunol. 2021, 11, 617089. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Vetrei, C.; Amato, F.; De Lorenzo, C. Interactions of spike-RBD of SARS-CoV-2 and Platelet Factor 4: New insights in the etiopathogenesis of thrombosis. Int. J. Mol. Sci. 2021, 22, 8562. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. Thromboses and hemostasis disorders associated with COVID-19: The possible causal role of cross-reactivity and immunological imprinting. Glob. Med. Genet. 2021, 8, 162–170. [Google Scholar] [CrossRef]
- Casey, L.M.; Kakade, S.; Decker, J.T.; Rose, J.A.; Deans, K.; Shea, L.D.; Pearson, R.M. Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials 2019, 218, 119333. [Google Scholar] [CrossRef]
- Platton, S.; Bartlett, A.; MacCallum, P.; Makris, M.; McDonald, V.; Singh, D.; Scully, M.; Pavord, S. Evaluation of laboratory assays for anti-Platelet Factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J. Thromb. Haemost. 2021, 19, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Sørvoll, I.H.; Horvei, K.D.; Ernstsen, S.L.; Laegreid, I.J.; Lund, S.; Grønli, R.H.; Olsen, M.K.; Jacobsen, H.K.; Eriksson, A.; Halstensen, A.M.; et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.; Ulm, L.; Holtfreter, S.; Schönborn, L.; Kuhn, S.O.; Scheer, C.; Warkentin, T.E.; Bröker, B.; Becker, K.; Aurich, K.; et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162β. Blood 2021, 138, 299–303. [Google Scholar] [CrossRef]
- Terpos, E.; Politou, M.; Ntanasis-Stathopoulos, I.; Karalis, V.; Merkouri, E.; Fotiou, D.; Gavriatopoulou, M.; Malandrakis, P.; Kastritis, E.; Trougakos, I.P.; et al. High Prevalence of Anti-PF4 Antibodies Following ChAdOx1 nCov-19 (AZD1222) Vaccination Even in the Absence of Thrombotic Events. Vaccines 2021, 9, 712. [Google Scholar] [CrossRef]
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Thurm, C.; Reinhold, A.; Borucki, K.; Kahlfuss, S.; Feist, E.; Schreiber, J.; Reinhold, D.; Schraven, B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines 2022, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.O.; Bombaci, M.; Bodio, C.; Lonati, P.A.; Gobbini, A.; Lorenzo, M.; Torresani, E.; Dubini, A.; Bulgarelli, I.; Solari, F.; et al. Anti-Phospholipid Antibodies and Coronavirus Disease 2019: Vaccination Does Not Trigger Early Autoantibody Production in Healthcare Workers. Front. Immunol. 2022, 15, 930074. [Google Scholar] [CrossRef]
- Krashias, G.; Pafiti, A.; Deeba, E.; Christodoulou, C.; Pantzaris, M.; Lambrianides, A. SARS CoV- 2 vaccination induces antibodies against cardiolipin. BMC Res. Notes 2022, 15, 292. [Google Scholar] [CrossRef]
- Pan, H.; Tang, Z.; Teng, J.; Sun, Y.; Liu, H.; Cheng, X.; Su, Y.; Ye, J.; Hu, Q.; Chi, H.; et al. COVID-19 vaccine affects neither prothrombotic antibody profile nor thrombosis in primary antiphospholipid syndrome: A prospective study. Rheumatology 2022, 62, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, F.; Balbi, G.G.M.; Aikawa, N.E.; Silva, C.A.; Kupa, L.V.K.; Medeiros-Ribeiro, A.C.; Yuki, E.F.; Pasoto, S.G.; Saad, C.G.; Borba, E.F.; et al. Immunogenicity, safety, and antiphospholipid antibodies after SARS-CoV-2 vaccine in patients with primary antiphospholipid syndrome. Lupus 2022, 31, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Othman, M.; Labelle, A.; Mazzetti, I.; Elbatarny, H.S.; Lillicrap, D. Adenovirus-induced thrombocytopenia: The role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007, 109, 2832–2839. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune responses to viral gene therapy vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Jonsson, M.I.; Lenman, A.E.; Frängsmyr, L.; Nyberg, C.; Abdullahi, M.; Arnberg, N. Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells. J. Virol. 2009, 83, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Hofherr, S.E.; Mok, H.; Gushiken, F.C.; Lopez, J.A.; Barry, M.A. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum. Gene Ther. 2007, 18, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Raddi, N.; Vigant, F.; Wagner-Ballon, O.; Giraudier, S.; Custers, J.; Hemmi, S.; Benihoud, K. Pseudotyping serotype 5 adenovirus with the fiber from other serotypes uncovers a key role of the fiber protein in adenovirus 5-induced thrombocytopenia. Hum. Gene Ther. 2016, 27, 193–201. [Google Scholar] [CrossRef]
- Baker, A.T.; Boyd, R.J.; Sarkar, D.; Teijeira-Crespo, A.; Chan, C.K.; Bates, E.; Waraich, K.; Vant, J.; Wilson, E.; Truong, C.D.; et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021, 7, eabl8213. [Google Scholar] [CrossRef] [PubMed]
- Fejér, G.; Szalay, K.; Gyory, I.; Fejes, M.; Kúsz, E.; Nedieanu, S.; Páli, T.; Schmidt, T.; Siklódi, B.; Lázár, G., Jr.; et al. Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock. J. Immunol. 2005, 175, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, Y.C.; Chang, T.K.; Huang, C.H.; Lu, Y.C.; Huang, C.H.; Chen, M.J. Enhanced expression of coxsackievirus and adenovirus receptor in lipopolysaccharide-induced inflammatory macrophages is through TRIF-dependent innate immunity pathway. Life Sci. 2021, 265, 118832. [Google Scholar] [CrossRef]
- Shannon, O.; Herwald, H.; Oehmcke, S. Modulation of the coagulation system during severe streptococcal disease. Curr. Top. Microbiol. Immunol. 2013, 368, 189–205. [Google Scholar] [CrossRef]
- Steinert, M.; Ramming, I.; Bergmann, S. Impact of Von Willebrand Factor on bacterial pathogenesis. Front. Med. 2020, 7, 543. [Google Scholar] [CrossRef]
- Lukomski, S.; Bachert, B.A.; Squeglia, F.; Berisio, R. Collagen-like proteins of pathogenic streptococci. Mol. Microbiol. 2017, 103, 919–930. [Google Scholar] [CrossRef]
- Thomas, S.; Arora, S.; Liu, W.; Churion, K.; Wu, Y.; Höök, M. vhp is a fibrinogen-binding protein related to vWbp in Staphylococcus aureus. mBio 2021, 12, e0116721. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. T cell mimicry in inflammatory heart disease. Mol. Immunol. 2004, 40, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; McGrath, L.J.; Andersen, K.M.; Khan, F.; Malhotra, D.; Alfred, T.; Nguyen, J.L.; Puzniak, L.; Thoburn, E.; Jodar, L.; et al. COVID-19 severity and risk of subsequent cardiovascular events. Clin. Infect. Dis. 2022, ciac661. [Google Scholar] [CrossRef] [PubMed]
- Haabeth, O.A.W.; Lohmeyer, J.J.K.; Sallets, A.; Blake, T.R.; Sagiv-Barfi, I.; Czerwinski, D.K.; McCarthy, B.; Powell, A.E.; Wender, P.A.; Waymouth, R.M.; et al. An mRNA SARS-CoV-2 Vaccine Employing Charge-Altering Releasable Transporters with a TLR-9 Agonist Induces Neutralizing Antibodies and T Cell Memory. ACS Cent. Sci. 2021, 7, 1191–1204. [Google Scholar] [CrossRef]
- Atalis, A.; Keenum, M.C.; Pandey, B.; Beach, A.; Pradhan, P.; Vantucci, C.; O’Farrell, L.; Noel, R.; Jain, R.; Hosten, J.; et al. Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine. J. Control Release 2022, 347, 476–488. [Google Scholar] [CrossRef]
- Bakkari, M.A.; Valiveti, C.K.; Kaushik, R.S.; Tummala, H. Toll-like Receptor-4 (TLR4) Agonist-Based Intranasal Nanovaccine Delivery System for Inducing Systemic and Mucosal Immunity. Mol. Pharm. 2021, 18, 2233–2241. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef]
- Adamczak, D.M.; Nowak, J.K.; Frydrychowicz, M.; Kaczmarek, M.; Sikora, J. The role of Toll-like receptors and vitamin D in diabetes mellitus type 1--a review. Scand J. Immunol. 2014, 80, 75–84. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martínez, M.S.; Chacín, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C.; et al. Microbiota and Diabetes Mellitus: Role of Lipid Mediators. Nutrients. 2020, 12, 3039. [Google Scholar] [CrossRef]
- Naveen, K.V.; Saravanakumar, K.; Sathiyaseelan, A.; MubarakAli, D.; Wang, M.H. Human Fungal Infection, Immune Response, and Clinical Challenge-a Perspective During COVID-19 Pandemic. Appl. Biochem. Biotechnol. 2022, 194, 4244–4257. [Google Scholar] [CrossRef]
- Nambiar, M.; Varma, S.R.; Jaber, M.; Sreelatha, S.V.; Thomas, B.; Nair, A.S. Mycotic infections—Mucormycosis and oral candidiasis associated with Covid-19: A significant and challenging association. J. Oral Microbiol. 2021, 13, 1967699. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int. Immunopharmacol. 2020, 89 Pt B, 107087. [Google Scholar] [CrossRef]
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate Immune Receptors, Key Actors in Cardiovascular Diseases. JACC Basic Transl. Sci. 2020, 5, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Witte, S.; Aich, A. Role of Mitochondrial Nucleic Acid Sensing Pathways in Health and Patho-Physiology. Front. Cell Dev. Biol. 2022, 10, 796066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Pierce, J.; Stevens, M.P. COVID-19 and antimicrobial stewardship: Lessons learned, best practices, and future implications. Int. J. Infect. Dis. 2021, 113, 103–108. [Google Scholar] [CrossRef]
- Hashad, N.; Stewart, D.; Perumal, D.; Abdulrazzaq, N.; Tonna, A.P. The impact of COVID-19 on antimicrobial stewardship programme implementation in hospitals—An exploration informed by the Consolidated Framework for Implementation Research. J. Hosp. Infect. 2022, 129, 144–152. [Google Scholar] [CrossRef]
- Rothe, K.; Feihl, S.; Schneider, J.; Wallnöfer, F.; Wurst, M.; Lukas, M.; Treiber, M.; Lahmer, T.; Heim, M.; Dommasch, M.; et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: A retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Thindwa, D.; Quesada, M.G.; Liu, Y.; Bennett, J.; Cohen, C.; Knoll, M.D.; von Gottberg, A.; Hayford, K.; Flasche, S. Use of seasonal influenza and pneumococcal polysaccharide vaccines in older adults to reduce COVID-19 mortality. Vaccine 2020, 38, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Cutland, C.L.; Klugman, K.P.; Madhi, S.A. Pneumococcal Conjugate Vaccine Protection against Coronavirus-Associated Pneumonia Hospitalization in Children Living with and without HIV. mBio 2021, 12, e02347-20. [Google Scholar] [CrossRef] [PubMed]
- Jehi, L.; Ji, X.; Milinovich, A.; Erzurum, S.; Rubin, B.P.; Gordon, S.; Young, J.B.; Kattan, M.W. Individualizing Risk Prediction for Positive Coronavirus Disease 2019 Testing. Chest 2020, 158, 1364–1375. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef]
- Noale, M.; Trevisan, C.; Maggi, S.; Incalzi, R.A.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The Association between Influenza and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines 2020, 8, 471. [Google Scholar] [CrossRef]
- Lewnard, A.J.; Bruxvoort, K.J.; Fischer, H.; Hong, V.X.; Grant, L.R.; Jódar, L.; Gessner, B.D.; Tartof, S.Y. Prevention of COVID-19 among older adults receiving pneumococcal conjugate vaccine suggests interactions between Streptococcus pneumoniae and SARS-CoV-2 in the respiratory tract. J. Infect. Dis. 2021, 225, 1710–1720. [Google Scholar] [CrossRef]
- Sumbul, B.; Sumbul, H.E.; Okyay, R.A.; Gülümsek, E.; ¸Sahin, A.R.; Boral, B.; Koçyiğit, B.F.; Alfishawy, M.; Gold, J.; Tasdogan, A.M. Is there a link between pre-existing antibodies acquired due to childhood vaccinations or past infections and COVID-19? A case control study. PeerJ 2021, 9, e10910. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Age and Location in Severity of COVID-19 Pathology: Do Lactoferrin and Pneumococcal Vaccination Explain Low Infant Mortality and Regional Differences? BioEssays 2020, 42, 2000076. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Possible Cross-Reactivity between SARS-CoV-2 Proteins, CRM197 and Proteins in Pneumococcal Vaccines May Protect Against Symptomatic SARS-CoV-2 Disease and Death. Vaccines 2020, 8, 559. [Google Scholar] [CrossRef]
- Blasi, F.; Di Pasquale, M.; Gramegna, A.; Viale, P.; Iacobello, C.; Gori, A.; Tumbarello, M.; Esposito, S.; Richeldi, L.; Bassetti, M. A new call for influenza and pneumococcal vaccinations during COVID-19 pandemic in Italy: A SIP/IRS (Italian Respiratory Society) and SITA (Italian Society of Antiinfective therapy) statement. Respir. Med. 2021, 190, 106674. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Munch, M.W.; Andersen-Ranberg, N.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Venkatesh, B.; Jha, V.; Rubenson, R.W.; Jakob, S.M.; Cioccari, L.; et al. Heterogenous treatment effects of dexamethasone 12 mg vs. 6 mg in patients with COVID-19 and severe hypoxaemia—Post hoc exploratory analyses of the COVID STEROID 2 trial. Acta Anaesthesiol. Scand. 2022, 67, 195–205. [Google Scholar] [CrossRef]
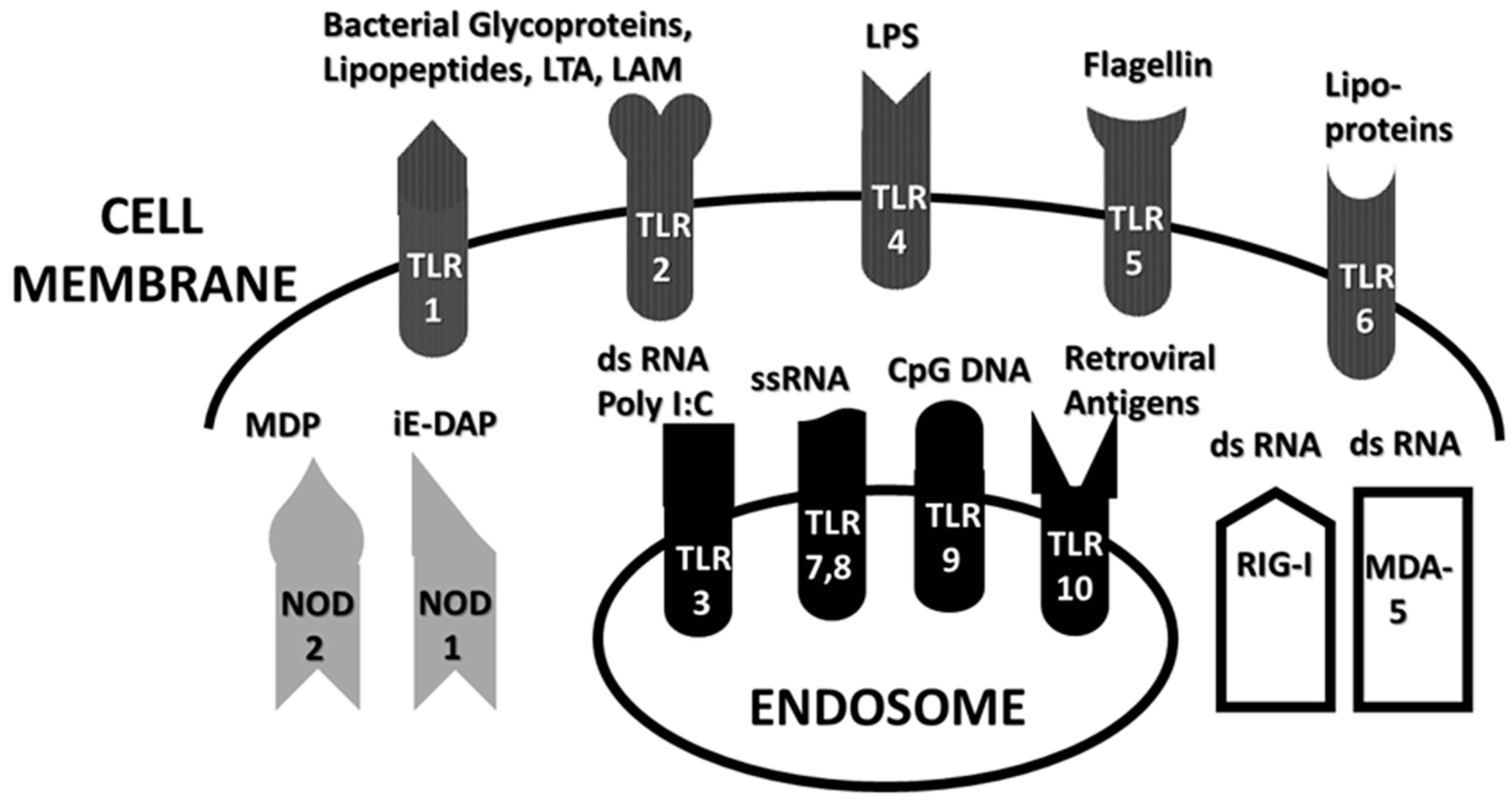
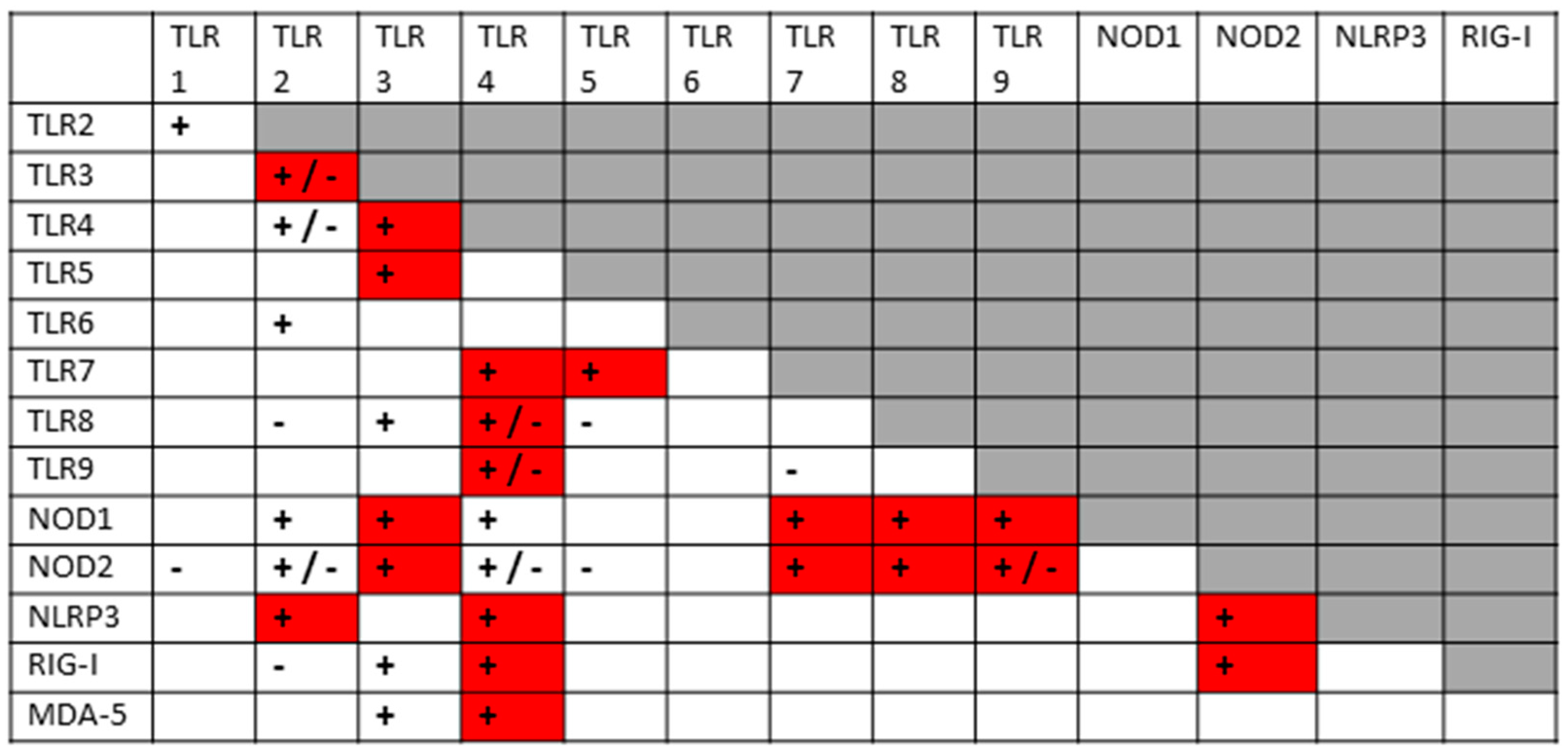
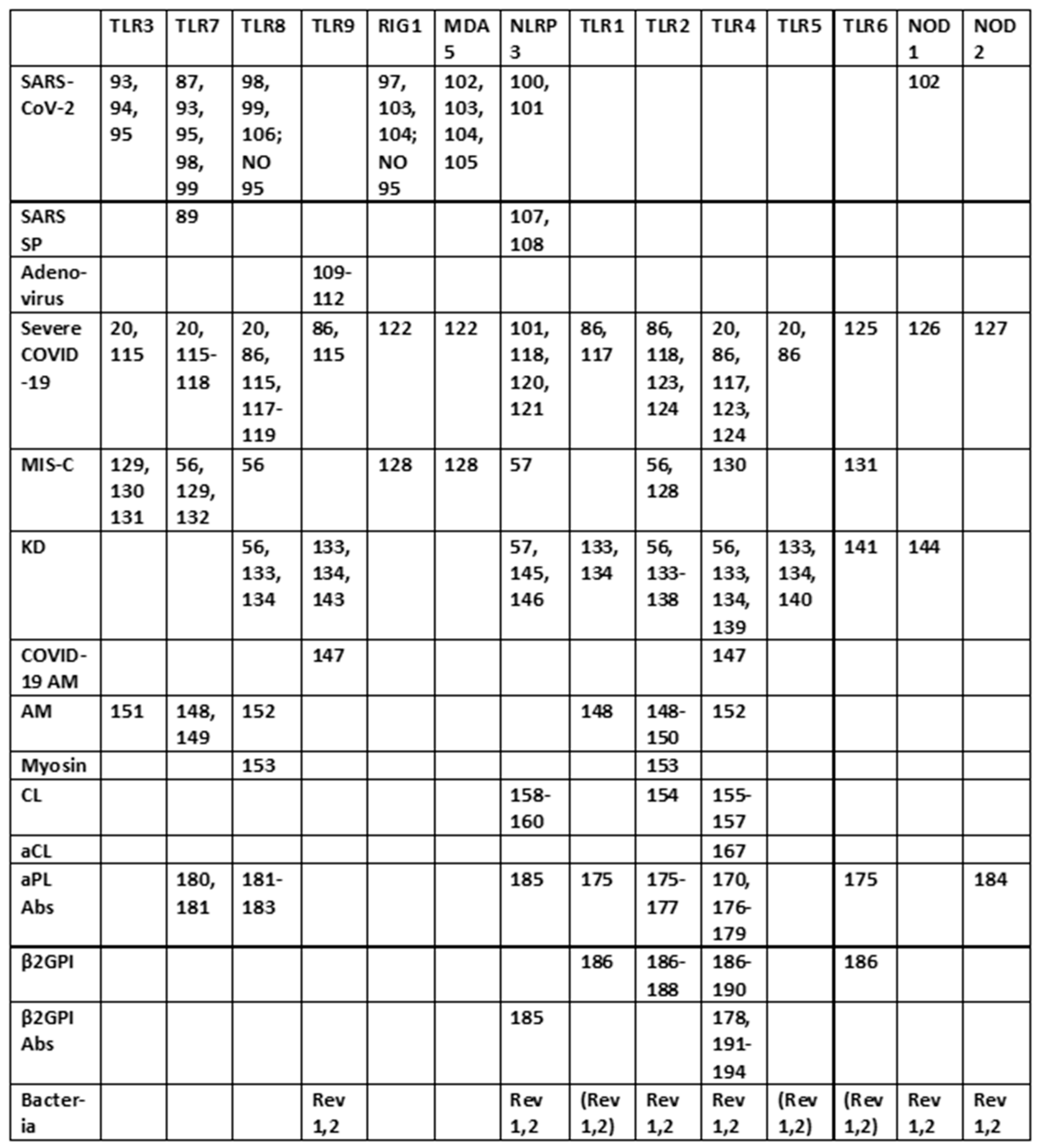
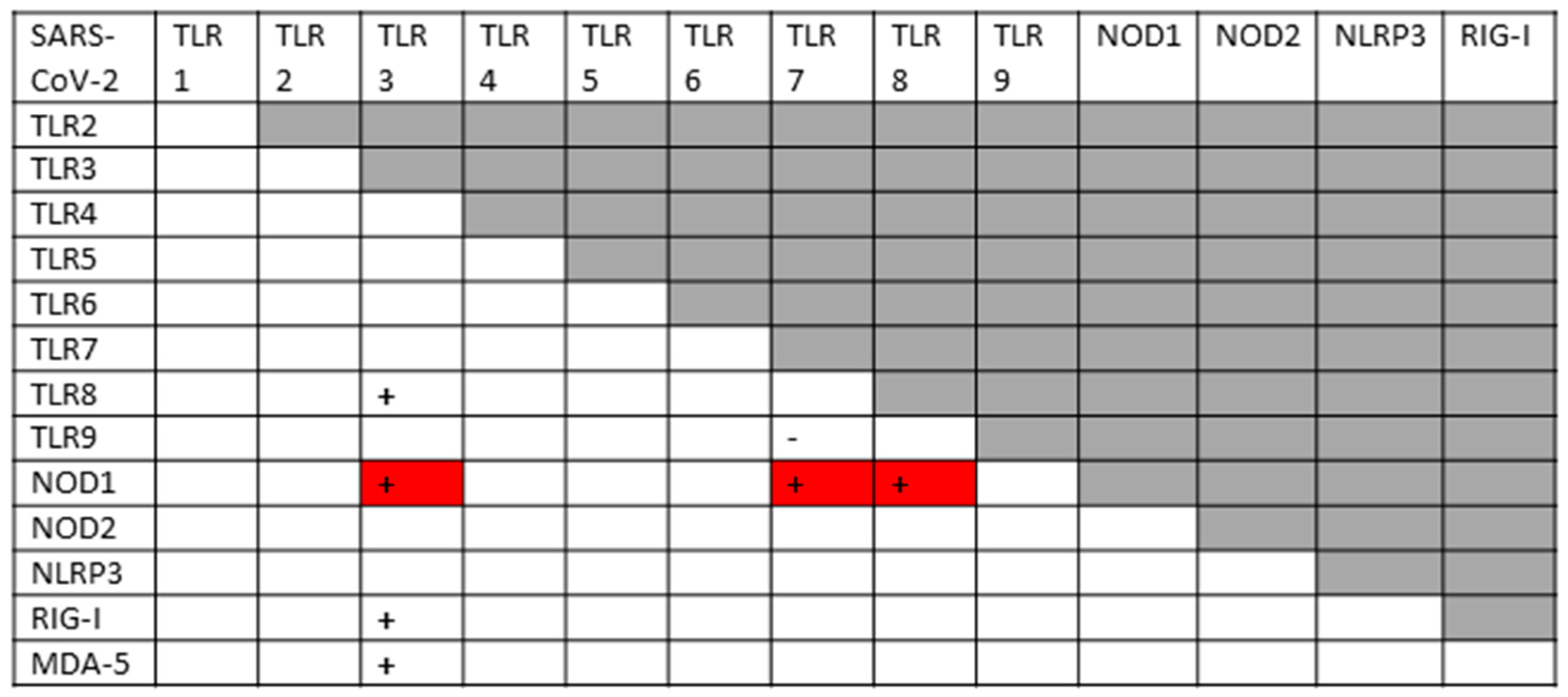

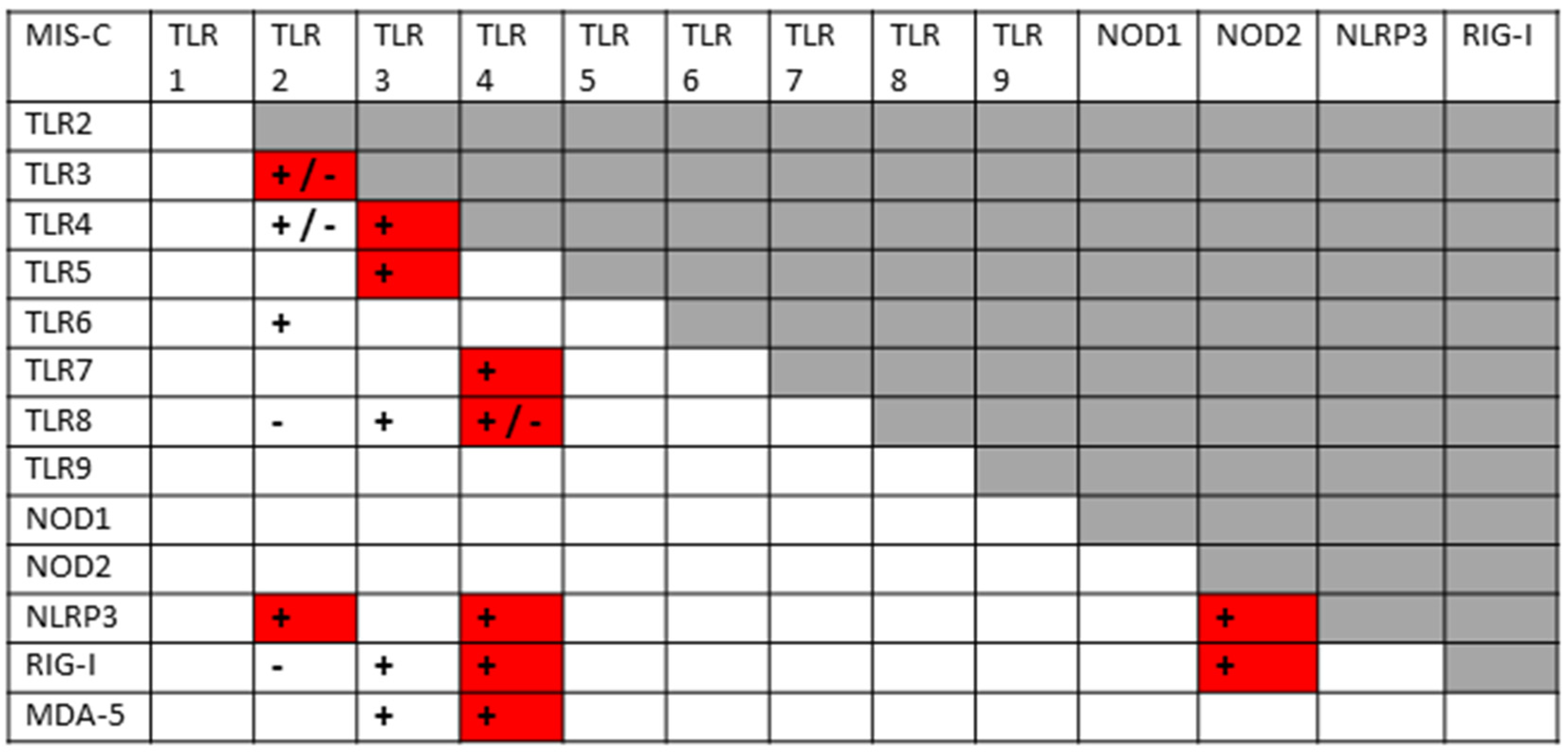
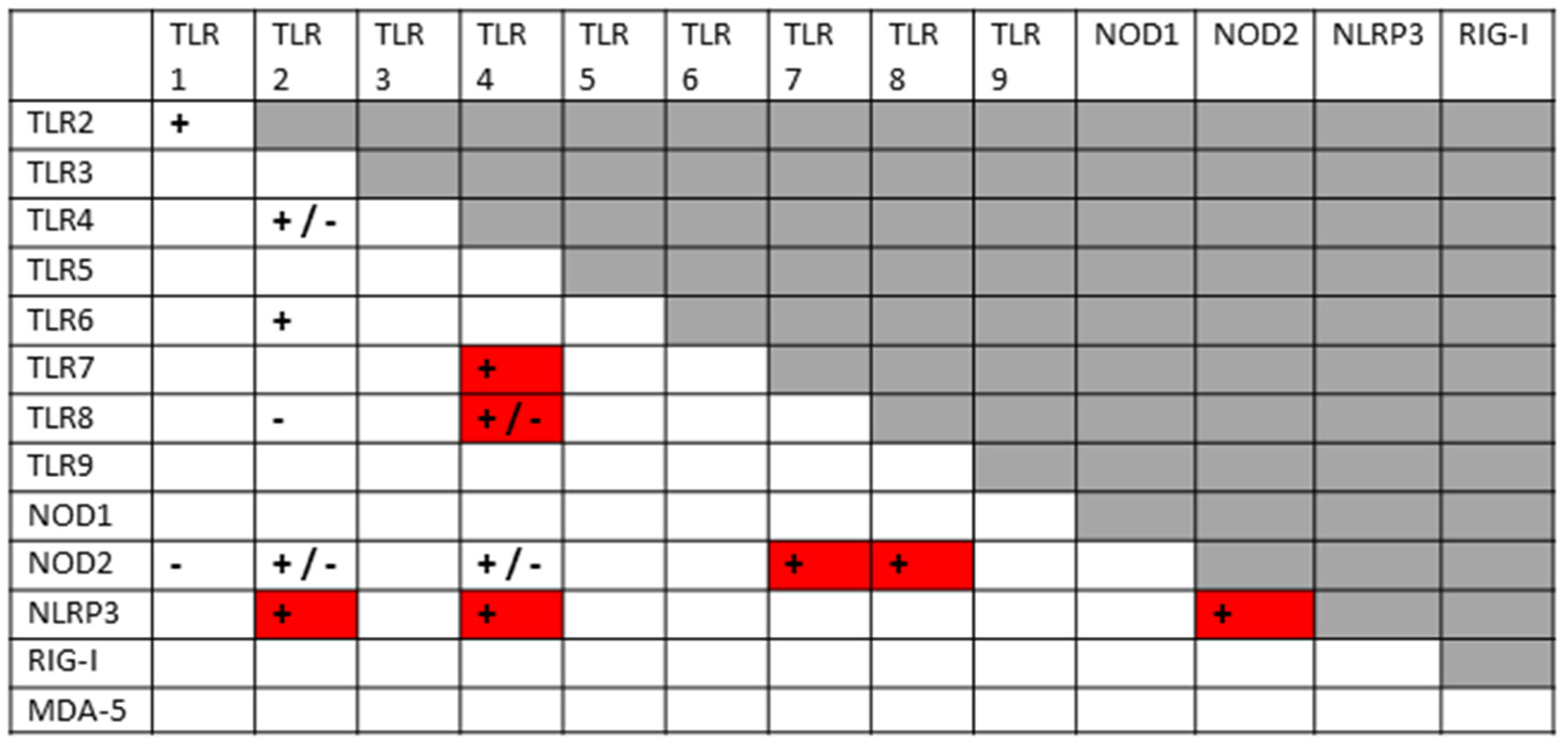

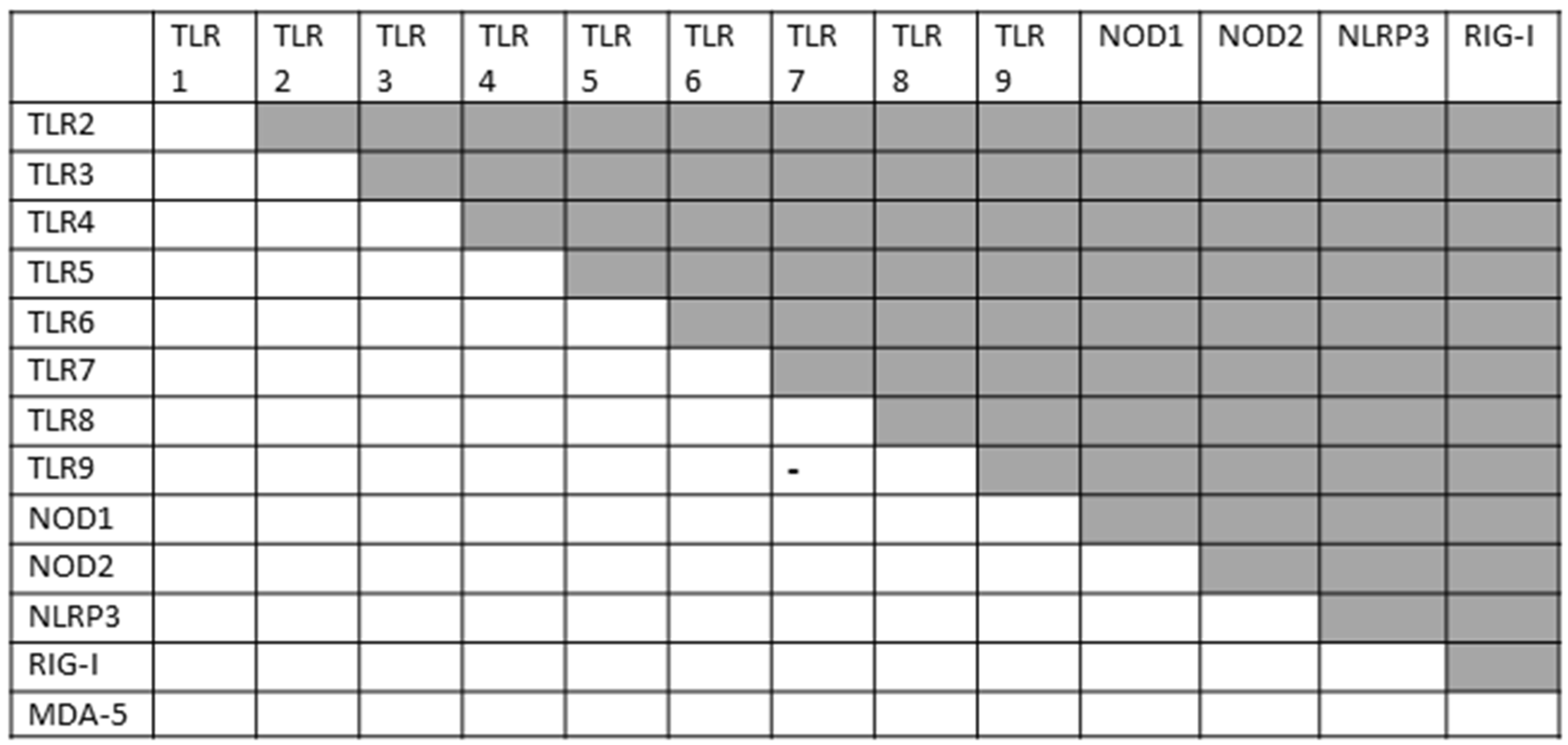
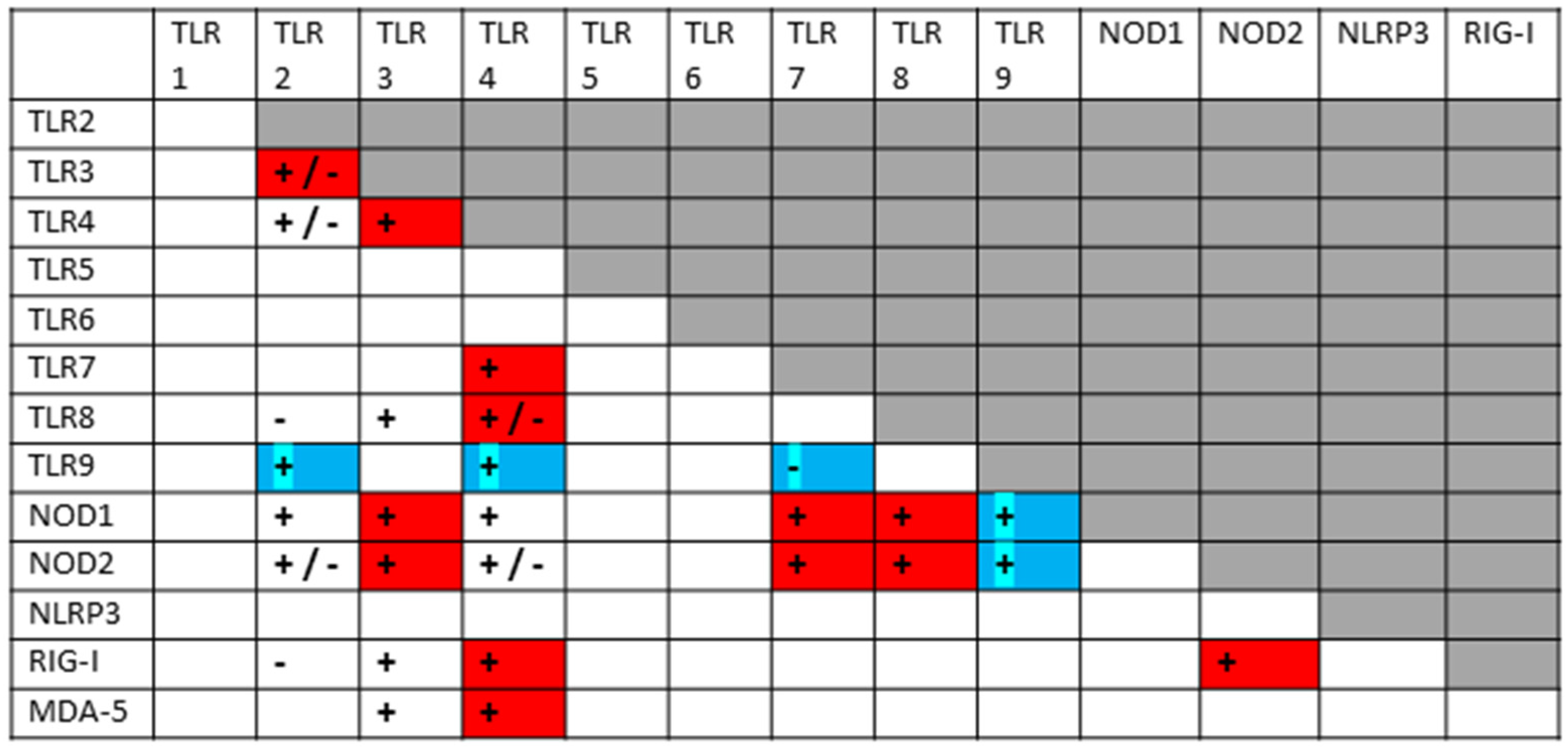
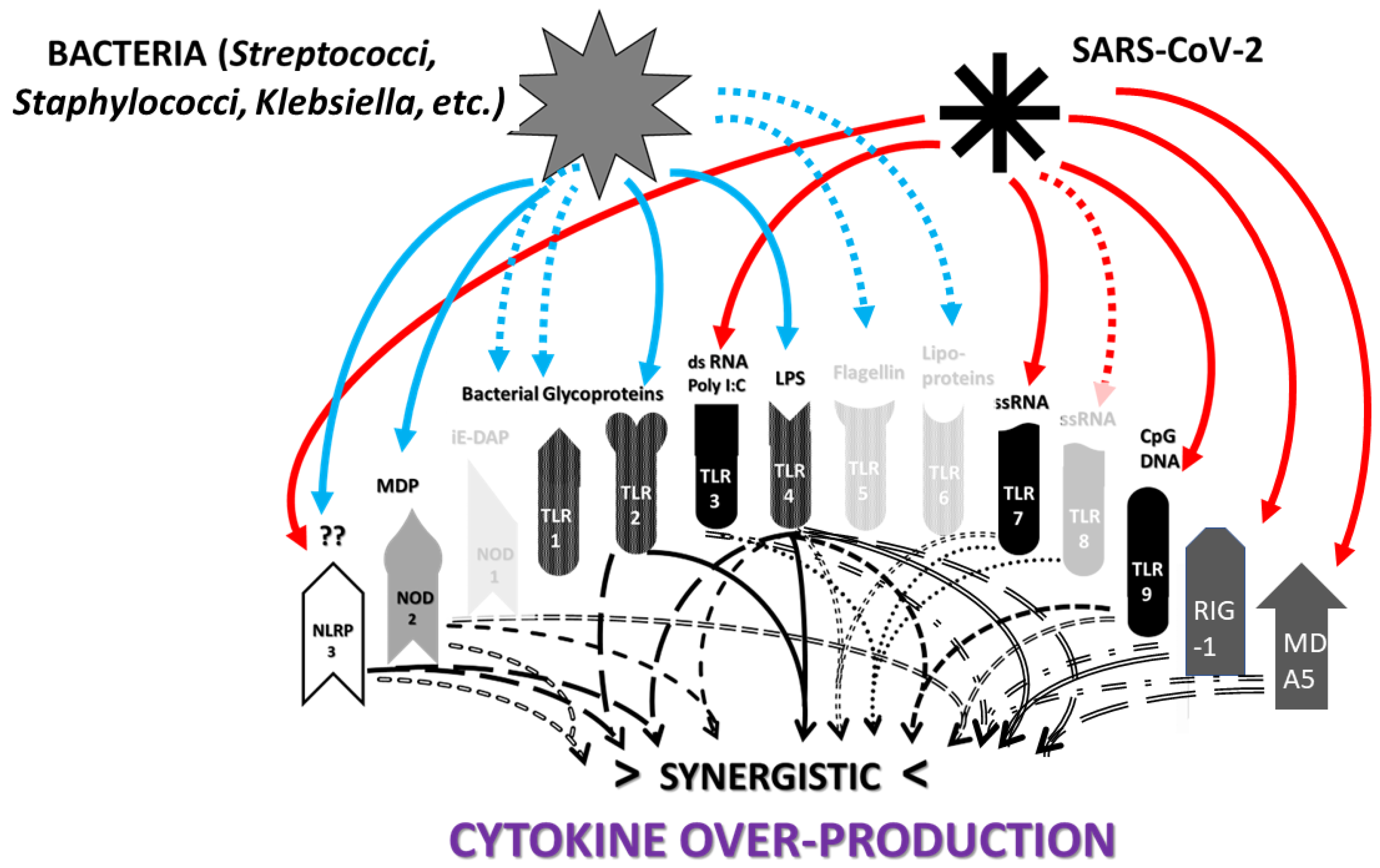
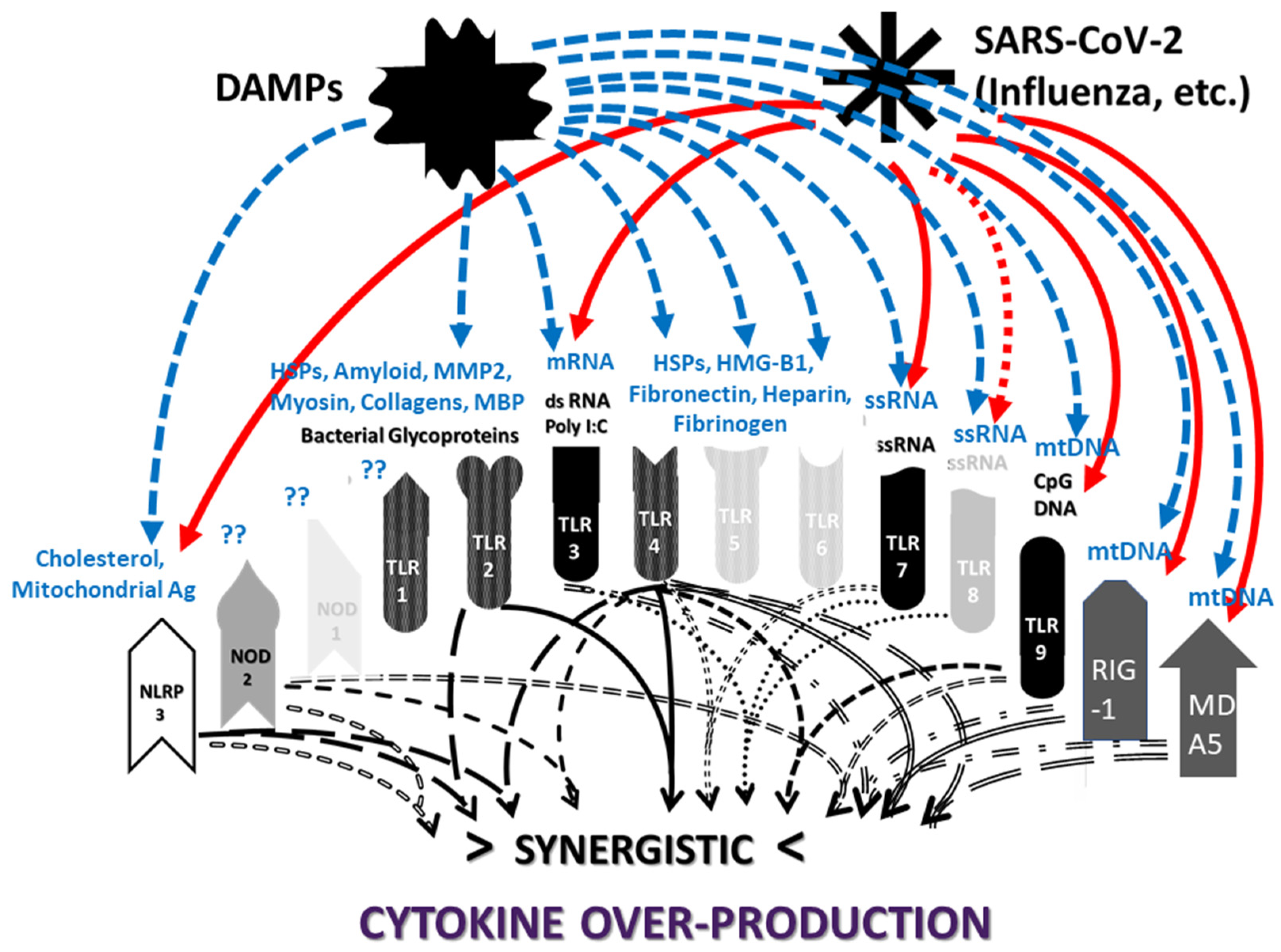
| MIS-C [51] (n > 1116) * | MIS-C [48] (n = 543) | Mild–Moderate COVID-19 [48] (n = 4268) | Severe COVID-19 [49,63] (n = 1190) | |
|---|---|---|---|---|
| Neutrophilia | 68–90% | 0 = | 33.2–25% = | |
| Lymphopenia | 80–95% | 42.5% | 1.5% | 24–45% = |
| Diarrhea | 60–100% | 53.2% | 3% | 11.5% |
| Nausea/Vomiting | 60–100% | 57.3% | 3% | 17.4% |
| Persistent Fever | 100% | 97.6% | 45.8% | 64.3–82.7% = |
| Septic Shock | 32–76% | 21.4% (84 ^–90% #) | 0.3% | 13.8% |
| Rash | 45–76% | 19.5% | 0.2% | 0 |
| D-dimer ↑ | 67–100% | 3% = | 12% = | |
| Ferritin ↑ | 55–76% | |||
| Fibrinogen ↑ | 80–100% | 0 = | 60% = | |
| Troponin ↑ | 50–90% | 2% | ||
| Procalcitonin ↑ | 80–95% | |||
| C-Reactive Protein ↑ | 90–100% | 12% = | 23.5–58.7% = | |
| Thrombocytopenia | 31–80% | 12% = | ||
| Met KD Criteria | 22–64% | 0 | 0 | |
| Syncope | 0.2% | 0 | ||
| Ischemic Stroke | 0.2% | 0.05–1.5% ~ | 16% + |
| Neutrophilia, NETs, CIC, Lymphopenia | Cytokine Over-Pro- duction | Virus Infection | Bacterial Infection | Virus– Bacterium Co-Infection | Auto- Antibodies | |
|---|---|---|---|---|---|---|
| SARS-CoV-2 Vaccination | Extremely Rare | Extremely Rare | Rare | Rare | Extremely Rare | Rare |
| Mild COVID-19 | Rare | Rare | Always | Rare | Rare | Rare |
| Severe COVID-19 | Frequent | Frequent | Always | Frequent | Frequent | Frequent |
| MIS-C | Frequent | Frequent | Always | Frequent | Frequent | Always |
| Kawasaki Disease | Frequent | Frequent | Possible | Possible | Unknown | Always |
| Autoimmune Myocarditis | Frequent | Frequent | Frequent | Frequent | Often | Always |
| COVID-19 Coagulopathies | Frequent | Frequent | Always | Frequent | Frequent | Always |
| Anti-Phospholipid Syndrome (APS) | Frequent | Frequent | Frequent | Frequent | Probable | Always |
| CL | β2GPI | PT | F VIII | F IX | vWF | PF4 | PDE | PL | Coll | Actin | Myosin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viruses | ||||||||||||
| SARS SP | 230 | 235, NOT 230 | 230 | 234 | 230, 233, 234, | 233, 234 | 233, 234 | |||||
| SARS-CoV-2 | 230 | 230 | 230, 235 | 230 | 234 | 230, 233, 234, | 233, 234 | 233, 234 | ||||
| Adeno | 230 | 230 | 230 | 230 | 230 | |||||||
| Infl A | ||||||||||||
| Bacteria | ||||||||||||
| GAS | 230 | 230 | 230 | 230 | 230 | 230 | 230 | 59, 60, 230 | 59, 60 | |||
| E. coli | 230 | 230 | 230 | 230 | ||||||||
| Staph | 230 | 230 | ||||||||||
| Klebs | 230 | 230 | ||||||||||
| Clost. | 230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Root-Bernstein, R. From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children. Int. J. Mol. Sci. 2023, 24, 3001. https://doi.org/10.3390/ijms24033001
Root-Bernstein R. From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children. International Journal of Molecular Sciences. 2023; 24(3):3001. https://doi.org/10.3390/ijms24033001
Chicago/Turabian StyleRoot-Bernstein, Robert. 2023. "From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children" International Journal of Molecular Sciences 24, no. 3: 3001. https://doi.org/10.3390/ijms24033001
APA StyleRoot-Bernstein, R. (2023). From Co-Infections to Autoimmune Disease via Hyperactivated Innate Immunity: COVID-19 Autoimmune Coagulopathies, Autoimmune Myocarditis and Multisystem Inflammatory Syndrome in Children. International Journal of Molecular Sciences, 24(3), 3001. https://doi.org/10.3390/ijms24033001







