Vasodilating Effects of Antispasmodic Agents and Their Cytotoxicity in Vascular Smooth Muscle Cells and Endothelial Cells—Potential Application in Microsurgery
Abstract
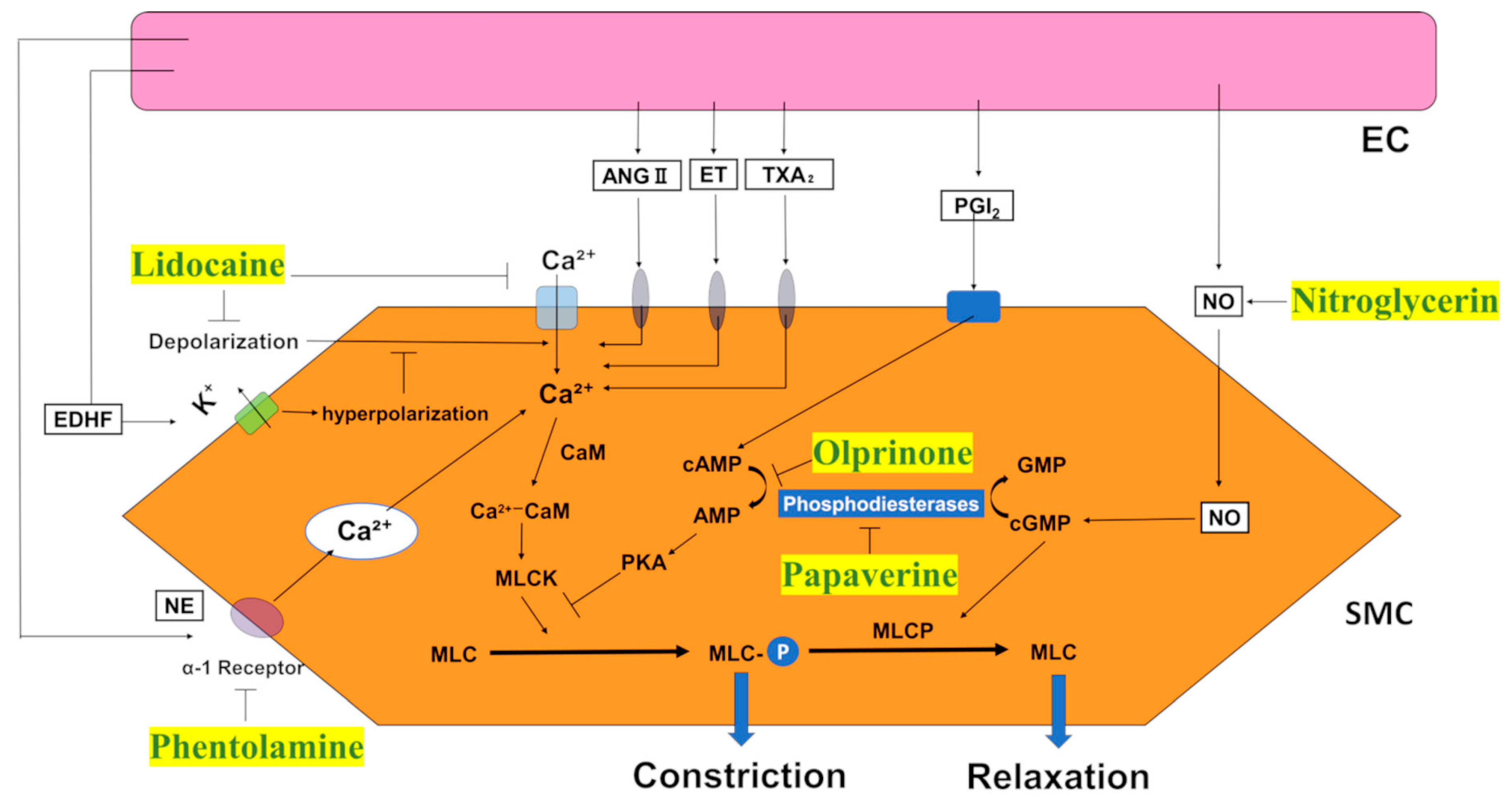
1. Introduction
2. Results
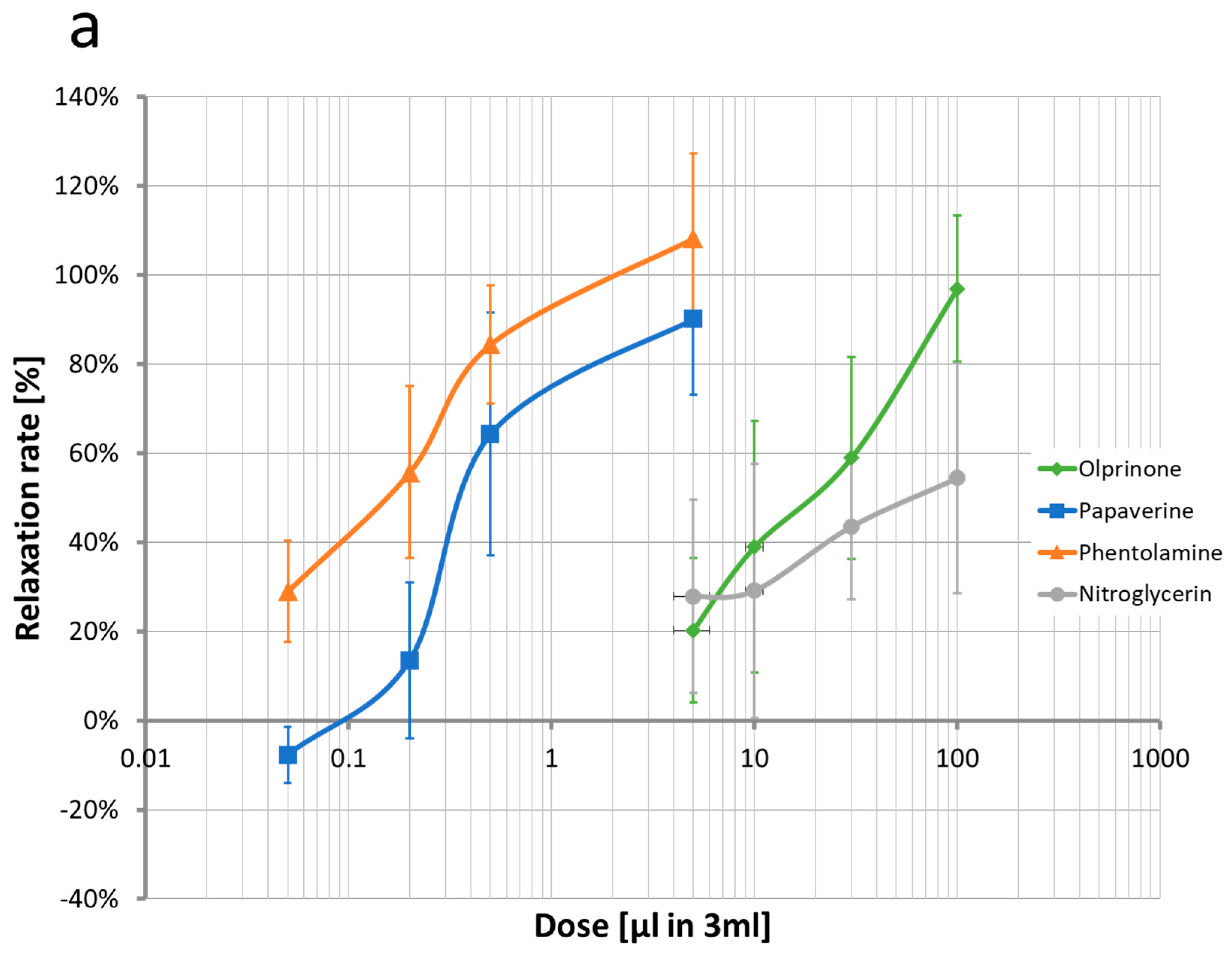
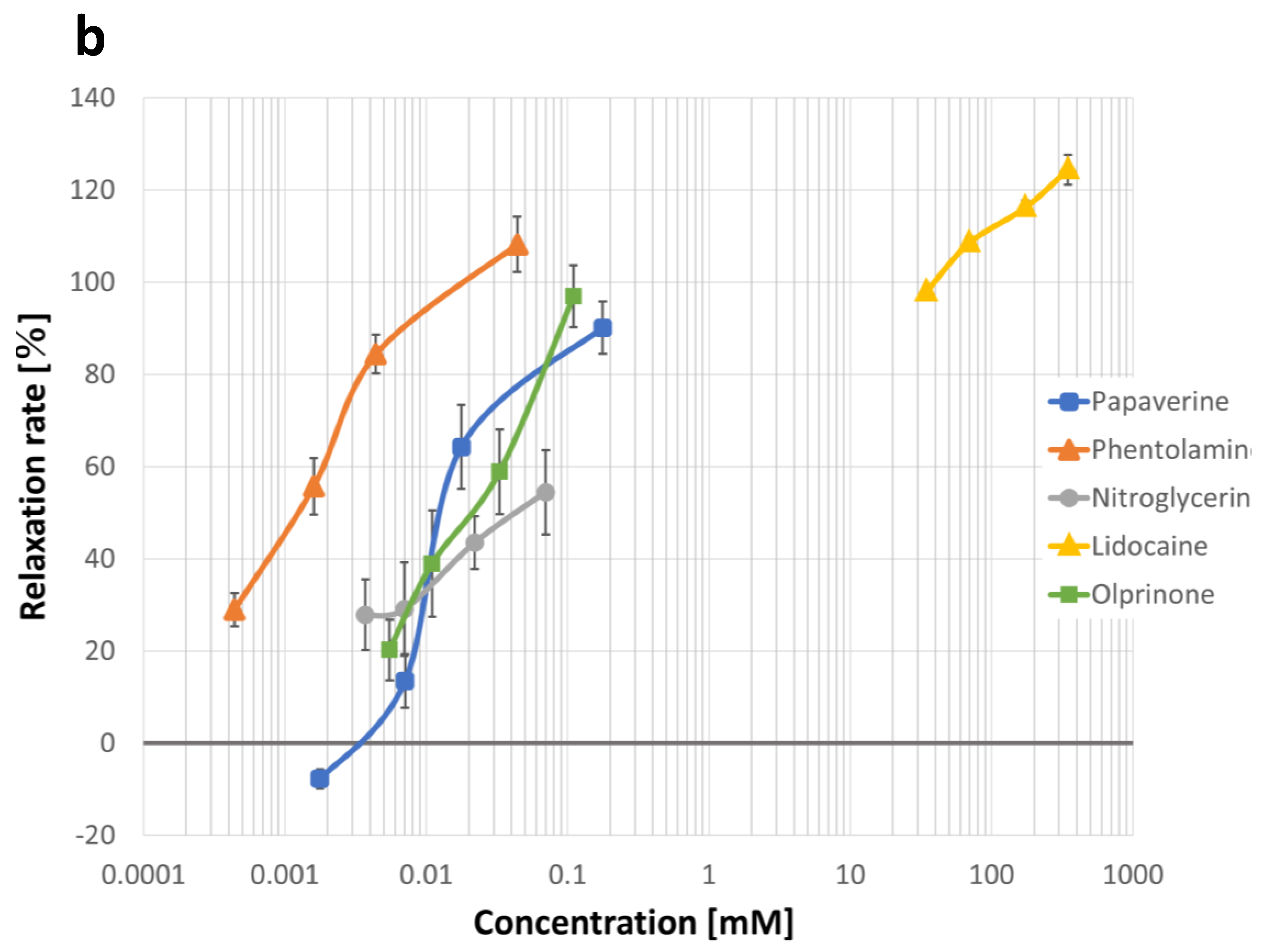
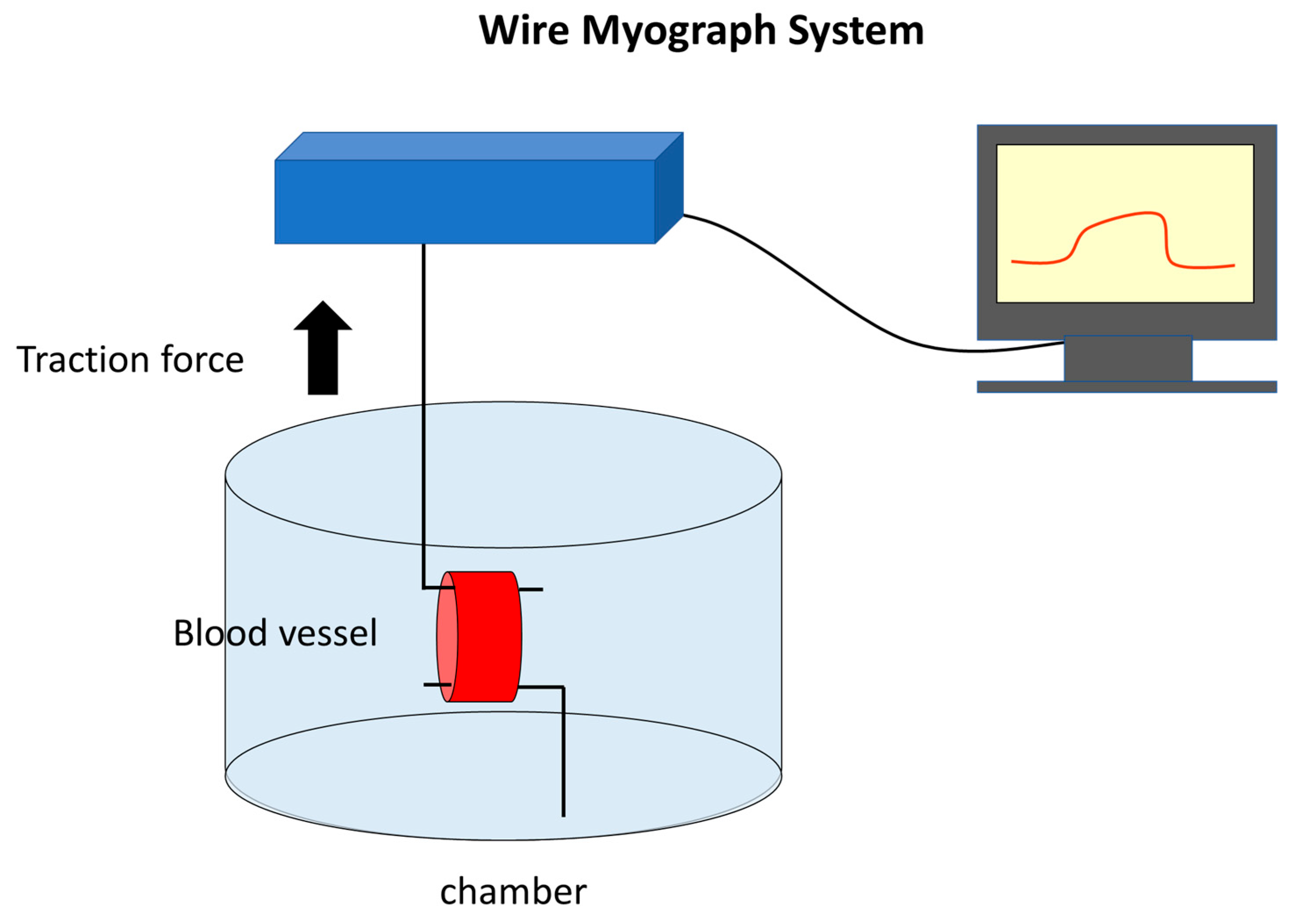
2.1. Quantification of Concentration-Vasodilation Relationship Using the Wire Myograph System
2.2. Measurement of IC50 Values for Each Drug
2.2.1. Lidocaine
2.2.2. Papaverine
2.2.3. Nitroglycerine
2.2.4. Phentolamine
2.2.5. Olprinone
3. Discussion
3.1. Lidocaine
3.2. Papaverine
3.3. Nitroglycerin
3.4. Phentolamine
3.5. Olprinone
4. Materials and Methods
4.1. Animals
4.2. Wire Myograph System
4.3. Cell Culture
4.4. Cell Viability Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigdeli, A.K.; Gazyakan, E.; Schmidt, V.J.; Bauer, C.; Germann, G.; Radu, C.A.; Kneser, U.; Hirche, C. Long-Term Outcome after Successful Lower Extremity Free Flap Salvage. J. Reconstr. Microsurg. 2019, 35, 263–269. [Google Scholar] [CrossRef]
- Bui, D.T.; Cordeiro, P.G.; Hu, Q.Y.; Disa, J.J.; Pusic, A.; Mehrara, B.J. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast. Reconstr. Surg. 2007, 119, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Devine, J.C.; Magennis, P.; Sillifant, P.; Rogers, S.N.; Vaughan, E.D. Factors that influence the outcome of salvage in free tissue transfer. Br. J. Oral Maxillofac. Surg. 2003, 41, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Yii, N.W.; Evans, G.R.; Miller, M.J.; Reece, G.P.; Langstein, H.; Chang, D.; Kroll, S.S.; Wang, B.; Robb, G.L. Thrombolytic therapy: What is its role in free flap salvage? Ann. Plast. Surg. 2001, 46, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.R.; Iorio, M.L.; Lee, B.T. A Systematic Review of Topical Vasodilators for the Treatment of Intraoperative Vasospasm in Reconstructive Microsurgery. Plast. Reconstr. Surg. 2015, 136, 411–422. [Google Scholar] [CrossRef]
- Ueda, K.; Harii, K. Comparative study of topical use of vasodilating solutions. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2003, 37, 201–207. [Google Scholar] [CrossRef]
- Ricci, J.A.; Koolen, P.G.; Shah, J.; Tobias, A.M.; Lee, B.T.; Lin, S.J. Comparing the Outcomes of Different Agents to Treat Vasospasm at Microsurgical Anastomosis during the Papaverine Shortage. Plast. Reconstr. Surg. 2016, 138, 401e–408e. [Google Scholar] [CrossRef]
- Rinkinen, J.; Halvorson, E.G. Topical Vasodilators in Microsurgery: What Is the Evidence? J. Reconstr. Microsurg. 2017, 33, 1–7. [Google Scholar] [CrossRef]
- Gutterman, D.D. Adventitia-dependent influences on vascular function. Am. J. Physiol. 1999, 277, H1265–H1272. [Google Scholar] [CrossRef]
- Schwartz, B.G.; Economides, C.; Mayeda, G.S.; Burstein, S.; Kloner, R.A. The endothelial cell in health and disease: Its function, dysfunction, measurement and therapy. Int. J. Impot. Res. 2010, 22, 77–90. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Wang, Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediat. Inflamm. 2017, 2017, 8135934. [Google Scholar] [CrossRef]
- Rensen, S.S.; Doevendans, P.A.; van Eys, G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Le Brocq, M.; Leslie, S.J.; Milliken, P.; Megson, I.L. Endothelial dysfunction: From molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 1631–1674. [Google Scholar] [CrossRef]
- Ogawa, H.; Kusumoto, J.; Nomura, T.; Hashikawa, K.; Terashi, H.; Sakakibara, S. Wire Myography for Continuous Estimation of the Optimal Concentration of Topical Lidocaine as a Vasodilator in Microsurgery. J. Reconstr. Microsurg. 2021, 37, 541–550. [Google Scholar] [CrossRef]
- Afridi, N.S.; Paletz, J.; Morris, S. Free Flap Failures: What to Do Next? Can. J. Plast. Surg. 2000, 8, 30–32. [Google Scholar] [CrossRef]
- Yu, J.T.; Patel, A.J.; Malata, C.M. The use of topical vasodilators in microvascular surgery. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 226–228. [Google Scholar] [CrossRef]
- Hyza, P.; Streit, L.; Schwarz, D.; Kubek, T.; Vesely, J. Vasospasm of the flap pedicle: The effect of 11 of the most often used vasodilating drugs. Comparative study in a rat model. Plast. Reconstr. Surg. 2014, 134, 574e–584e. [Google Scholar] [CrossRef]
- Zhang, J.; Lipa, J.E.; Black, C.E.; Huang, N.; Neligan, P.C.; Ling, F.T.; Levine, R.H.; Semple, J.L.; Pang, C.Y. Pharmacological characterization of vasomotor activity of human musculocutaneous perforator artery and vein. J. Appl. Physiol. 2000, 89, 2268–2275. [Google Scholar] [CrossRef]
- Demaria, R.G.; Vernhet, H.; Aya, G.; Oliva-Lauraire, M.C.; Juan, J.M.; Dauzat, M.M. Experimental model for comparative evaluation of pharmacologically induced vasodilation of arterial wall mechanical properties. J. Cardiovasc. Pharmacol. 2003, 42, 389–394. [Google Scholar] [CrossRef]
- Hou, S.M.; Seaber, A.V.; Urbaniak, J.R. Relief of blood-induced arterial vasospasm by pharmacologic solutions. J. Reconstr. Microsurg. 1987, 3, 147–151. [Google Scholar] [CrossRef]
- Beekman, W.H.; Sluimers, J.E.; Kort, W.J.; van der Meulen, J.C. Resolution of experimental microvascular vasoconstriction in rats by topical application of lidocaine hydrochloride in varying concentrations. Ann. Plast. Surg. 1988, 21, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.W.; Butterworth, J.F. Local anesthetic systemic toxicity: Update on mechanisms and treatment. Curr. Opin. Anaesthesiol. 2011, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Jernbeck, J.; Samuelson, U.E. Effects of lidocaine and calcitonin gene-related peptide (CGRP) on isolated human radial arteries. J. Reconstr. Microsurg. 1993, 9, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Gherardini, G.; Samuelson, U.; Jernbeck, J.; Aberg, B.; Sjöstrand, N. Comparison of vascular effects of ropivacaine and lidocaine on isolated rings of human arteries. Acta Anaesthesiol. Scand. 1995, 39, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Beekman, W.H.; Dammeijer, P.F.; Sluimers, J.E.; Kort, W.J.; van der Meulen, J.H. Improvement in blood flow and diameter of the postanastomotic rat tail artery by topical application of lidocaine in varying concentrations. Ann. Plast. Surg. 1990, 24, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, S.; Nakatsuka, T.; Ohura, N.; Sato, Y.; Harii, K. Topical application of amrinone (a selective phosphodiesterase III inhibitor) for relief of vasospasm. J. Surg. Res. 2000, 93, 149–155. [Google Scholar] [CrossRef]
- Ohta, I.; Kawai, H.; Kawabata, H.; Masada, K.; Ono, K. Topical use of Xylocaine for relieving vasospasm: Effect of concentration. J. Reconstr. Microsurg. 1991, 7, 205–208. [Google Scholar] [CrossRef]
- Johnstone, R.E.; Wax, M.K.; Bishop, D.J.; Chafin, J.B. Large doses of topical lidocaine during microvascular surgery are not associated with toxic blood concentrations. Anesthesiology 1995, 82, 593–596. [Google Scholar] [CrossRef]
- Hou, S.M.; Liu, T.K.; Yu, H.Y. Absorption of lidocaine following topical application in microvascular procedures on rabbits. J. Orthop. Res. 1991, 9, 545–549. [Google Scholar] [CrossRef]
- Kerschner, J.E.; Futran, N.D. The effect of topical vasodilating agents on microvascular vessel diameter in the rat model. Laryngoscope 1996, 106, 1429–1433. [Google Scholar] [CrossRef]
- Swartz, W.M.; Brink, R.R.; Buncke, H.J. Prevention of thrombosis in arterial and venous microanastomoses by using topical agents. Plast. Reconstr. Surg. 1976, 58, 478–481. [Google Scholar] [CrossRef]
- Evans, G.R.; Gherardini, G.; Gürlek, A.; Langstein, H.; Joly, G.A.; Cromeens, D.M.; Sukumaran, A.V.; Williams, J.; Kilbourn, R.G.; Wang, B.; et al. Drug-induced vasodilation in an in vitro and in vivo study: The effects of nicardipine, papaverine, and lidocaine on the rabbit carotid artery. Plast. Reconstr. Surg. 1997, 100, 1475–1481. [Google Scholar] [CrossRef]
- Gherardini, G.; Gürlek, A.; Cromeens, D.; Joly, G.A.; Wang, B.G.; Evans, G.R. Drug-induced vasodilation: In vitro and in vivo study on the effects of lidocaine and papaverine on rabbit carotid artery. Microsurgery 1998, 18, 90–96. [Google Scholar] [CrossRef]
- Huang, G.K.; Li, H.Q. Relief of vasospasm by drugs: An experimental study. Eur. J. Plast. Surg. 1991, 14, 10–14. [Google Scholar] [CrossRef]
- Cooper, G.J.; Gillot, T.; Parry, E.A.; Kennedy, A.; Wilkinson, G.A. Papaverine injures the endothelium of the internal mammary artery. Cardiovasc. Surg. 1995, 3, 553–555. [Google Scholar] [CrossRef]
- Gao, Y.J.; Stead, S.; Lee, R.M. Papaverine induces apoptosis in vascular endothelial and smooth muscle cells. Life Sci. 2002, 70, 2675–2685. [Google Scholar] [CrossRef]
- Ricci, J.A.; Singhal, D.; Fukudome, E.Y.; Tobias, A.M.; Lin, S.J.; Lee, B.T. Topical nitroglycerin for the treatment of intraoperative microsurgical vasospasm. Microsurgery 2018, 38, 524–529. [Google Scholar] [CrossRef]
- Ding, R.; Feng, W.; Li, H.; Wang, L.; Li, D.; Cheng, Z.; Guo, J.; Hu, D. A comparative study on in vitro and in vivo effects of topical vasodilators in human internal mammary, radial artery and great saphenous vein. Eur. J. Cardiothorac. Surg. 2008, 34, 536–541. [Google Scholar] [CrossRef]
- Sasson, L.; Cohen, A.J.; Hauptman, E.; Schachner, A. Effect of topical vasodilators on internal mammary arteries. Ann. Thorac. Surg. 1995, 59, 494–496. [Google Scholar] [CrossRef]
- Taylor, S.H.; Sutherland, G.R.; Mackenzie, G.J.; Staunton, H.P.; Donald, K.W. The Circulatory Effects of Intravenous Phentolamine in Man. Circulation 1965, 31, 741–754. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, D.; Gong, R.; Chen, S.; Fang, F.; Zhuang, Y. Mechanically Induced Vasospasm-Evaluation of Spasmolytic Efficacy of 10 Pharmaceutical Agents Using Laser Speckle Contrast Imaging. Lasers Surg. Med. 2021, 53, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Salmerón, R.J.; Mora, R.; Masotti, M.; Betriu, A. Assessment of the efficacy of phentolamine to prevent radial artery spasm during cardiac catheterization procedures: A randomized study comparing phentolamine vs. verapamil. Catheter. Cardiovasc. Interv. 2005, 66, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Olprinone: A phosphodiesterase III inhibitor with positive inotropic and vasodilator effects. Cardiovasc. Drug. Rev. 2002, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Kusagawa, M.; Shimomura, A.; Suga, T.; Ito, M.; Konishi, T.; Nakano, T. Ca2+(-)dependent and Ca2+(-)independent vasorelaxation induced by cardiotonic phosphodiesterase inhibitors. Eur. J. Pharmacol. 1993, 240, 57–66. [Google Scholar] [CrossRef]
- Adachi, H.; Kamata, S.; Kodama, K.; Nagakura, T. Vasorelaxant effect of a phosphodiesterase 3 inhibitor, olprinone, on isolated human radial artery. Eur. J. Pharmacol. 2000, 396, 43–47. [Google Scholar] [CrossRef]
- Sasaki, H. The right gastroepiploic artery in coronary artery bypass grafting. J. Card. Surg. 2008, 23, 398–407. [Google Scholar] [CrossRef]
- Sakakibara, S.; Ishida, Y.; Hashikawa, K.; Yamaoka, T.; Terashi, H. Intima/medulla reconstruction and vascular contraction-relaxation recovery for acellular small diameter vessels prepared by hyperosmotic electrolyte solution treatment. J. Artif. Organs 2014, 17, 169–177. [Google Scholar] [CrossRef]

| No.* | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lidocaine | 0 | 1.73 × 10−1 | 3.46 × 10−1 | 6.93 × 10−1 | 1.73 | 3.46 | 6.82 | 17.31 | 34.63 | 69.25 | 173.13 | 346.26 | |
| Papaverine | 0 | 3.55 × 10−4 | 7.10 × 10−4 | 1.77 × 10−3 | 3.55 × 10−3 | 7.10 × 10−3 | 1.77 × 10−2 | 3.55 × 10−2 | 7.10 × 10−2 | 1.78 × 10−1 | 3.55 × 10−1 | 7.10 × 10−1 | |
| Nitroglycerine | 0 | 1.10 × 10−4 | 2.20 × 10−4 | 5.50 × 10−4 | 1.10 × 10−3 | 2.20 × 10−3 | 5.50 × 10−3 | 1.10 × 10−2 | 2.20 × 10−2 | 5.50 × 10−2 | 1.10 × 10−1 | 2.20 × 10−1 | |
| Phentolamine | 0 | 4.42 × 10−5 | 8.83 × 10−5 | 2.21 × 10−5 | 4.42 × 10−4 | 8.83 × 10−4 | 2.21 × 10−4 | 4.42 × 10−3 | 8.83 × 10−3 | 2.21 × 10−3 | 4.42 × 10−2 | 8.83 × 10−2 | |
| Olprinone | 0 | 1.64 × 10−4 | 3.28 × 10−4 | 8.20 × 10−4 | 1.64 × 10−3 | 3.28 × 10−3 | 8.20 × 10−3 | 1.64 × 10−2 | 3.28 × 10−2 | 8.20 × 10−2 | 1.64 × 10−1 | 3.28 × 10−1 | [mM] |
| 1 | 5 | 10 | 30 | 60 | (min) | |
|---|---|---|---|---|---|---|
| Lidocaine | 55.3121 | 27.9377 | 16.693 | 10.106 | 7.1017 | |
| Papaverine | 0.09704 | 0.11823 | 0.01348 | 0.0159 | 0.00146 | |
| Nitroglycerine | ND | ND | ND | 0.1339 | 0.1093 | |
| Phentolamine | ND | ND | ND | ND | ND | |
| Olprinone | ND | ND | ND | ND | ND | [mM] |
| 1 | 5 | 10 | 30 | 60 | (min) | |
|---|---|---|---|---|---|---|
| Lidocaine | 24.4 | 16.8 | 13.5 | 10.3 | 7.64 | |
| Papaverine | 0.056 | 0.00404 | 0.00429 | 0.005 | 0.00648 | |
| Nitroglycerine | ND | ND | 0.0351 | 0.038 | 0.0388 | |
| Phentolamine | ND | ND | ND | ND | ND | |
| Olprinone | ND | ND | ND | ND | ND | [mM] |
| Lidocaine | Papaverine | Nitroglycerin | Phentolamine | Olprinone | |
|---|---|---|---|---|---|
| Cost (Yen) | 118 | 1.59 | 4.99 | 0.18 | 201.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, M.; Hirayama, Y.; Ogawa, H.; Nomura, T.; Terashi, H.; Sakakibara, S. Vasodilating Effects of Antispasmodic Agents and Their Cytotoxicity in Vascular Smooth Muscle Cells and Endothelial Cells—Potential Application in Microsurgery. Int. J. Mol. Sci. 2023, 24, 10850. https://doi.org/10.3390/ijms241310850
Ueda M, Hirayama Y, Ogawa H, Nomura T, Terashi H, Sakakibara S. Vasodilating Effects of Antispasmodic Agents and Their Cytotoxicity in Vascular Smooth Muscle Cells and Endothelial Cells—Potential Application in Microsurgery. International Journal of Molecular Sciences. 2023; 24(13):10850. https://doi.org/10.3390/ijms241310850
Chicago/Turabian StyleUeda, Misato, Yasuki Hirayama, Haruo Ogawa, Tadashi Nomura, Hiroto Terashi, and Shunsuke Sakakibara. 2023. "Vasodilating Effects of Antispasmodic Agents and Their Cytotoxicity in Vascular Smooth Muscle Cells and Endothelial Cells—Potential Application in Microsurgery" International Journal of Molecular Sciences 24, no. 13: 10850. https://doi.org/10.3390/ijms241310850
APA StyleUeda, M., Hirayama, Y., Ogawa, H., Nomura, T., Terashi, H., & Sakakibara, S. (2023). Vasodilating Effects of Antispasmodic Agents and Their Cytotoxicity in Vascular Smooth Muscle Cells and Endothelial Cells—Potential Application in Microsurgery. International Journal of Molecular Sciences, 24(13), 10850. https://doi.org/10.3390/ijms241310850






